STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy
Abstract
1. Introduction
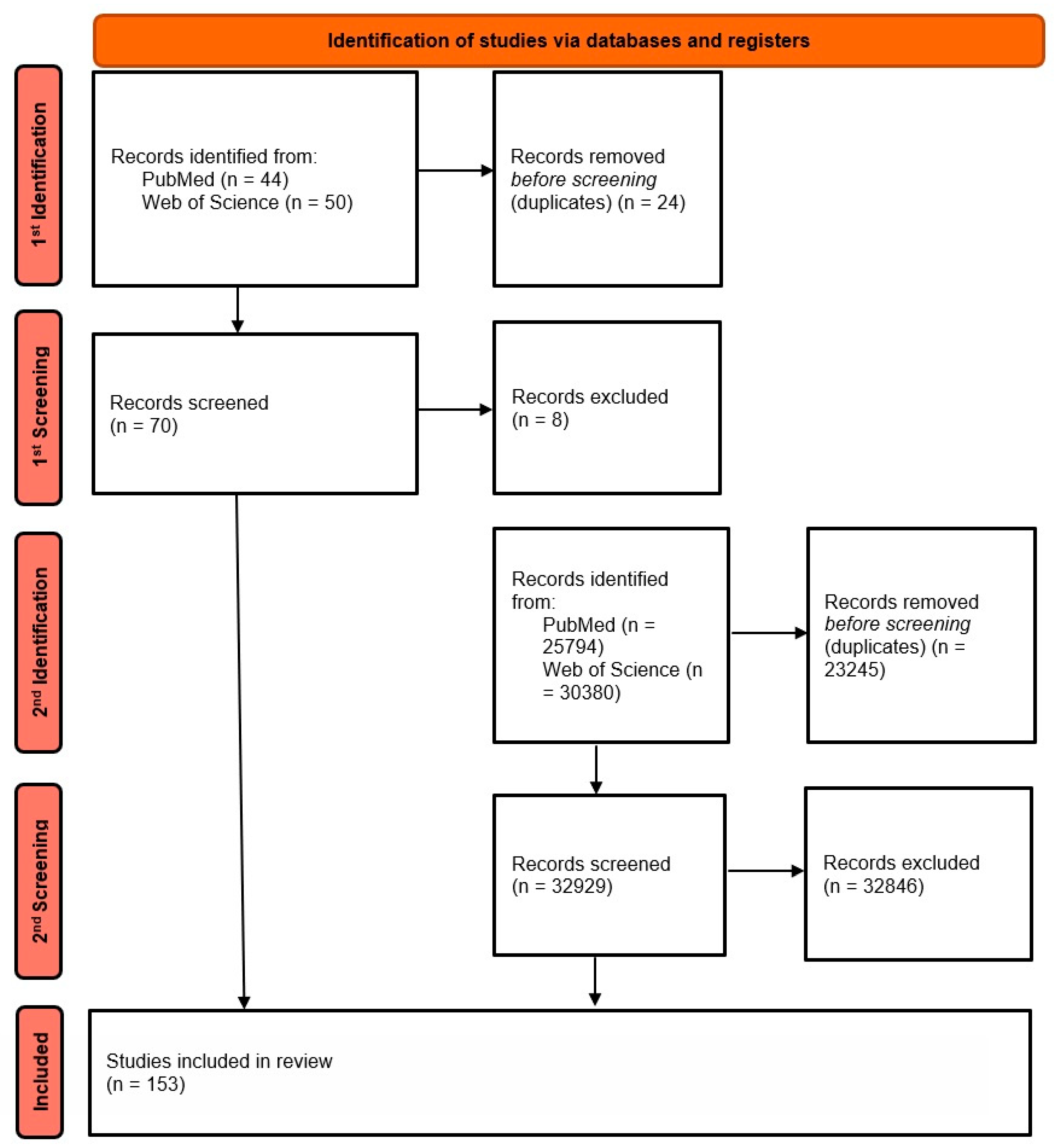
2. Materials and Methods
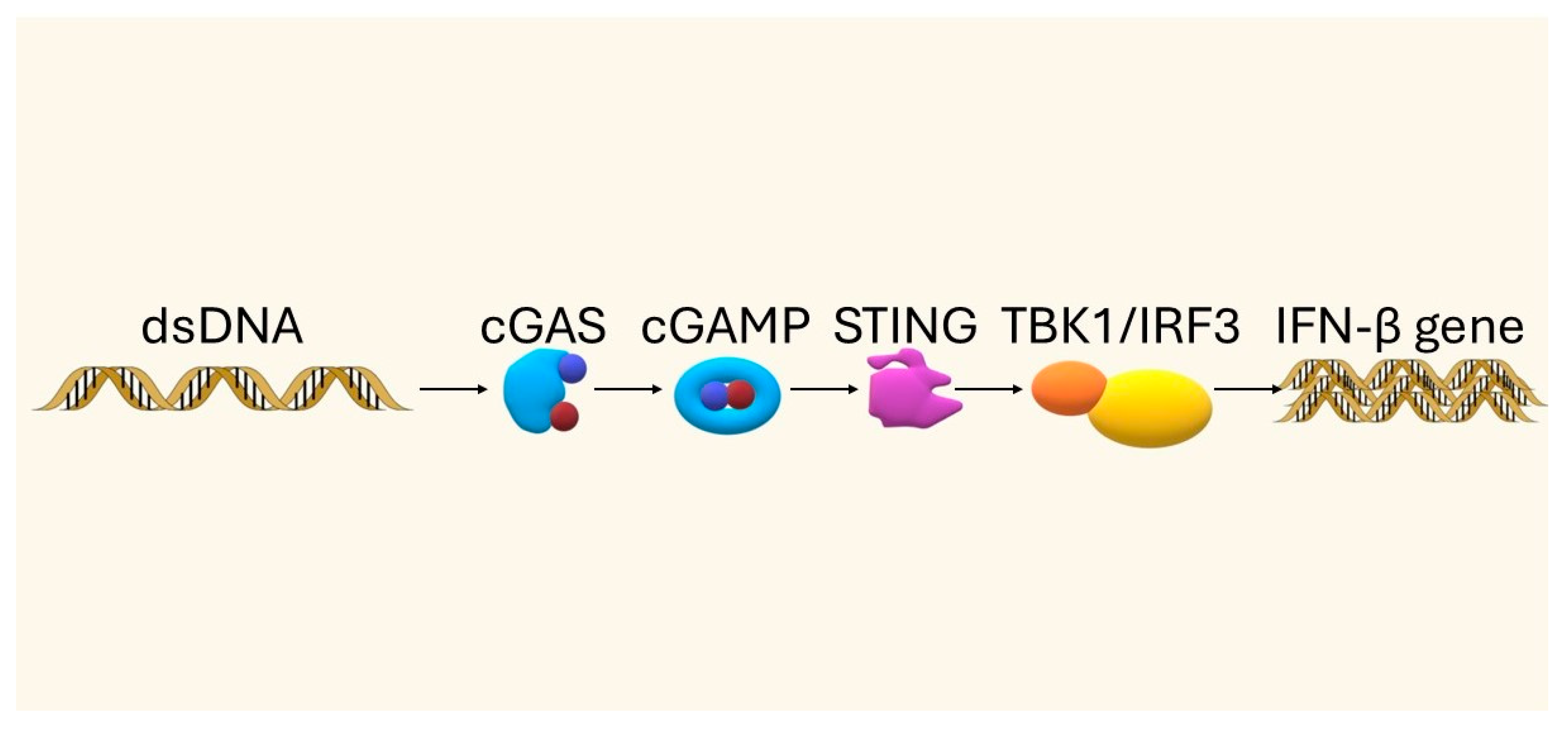
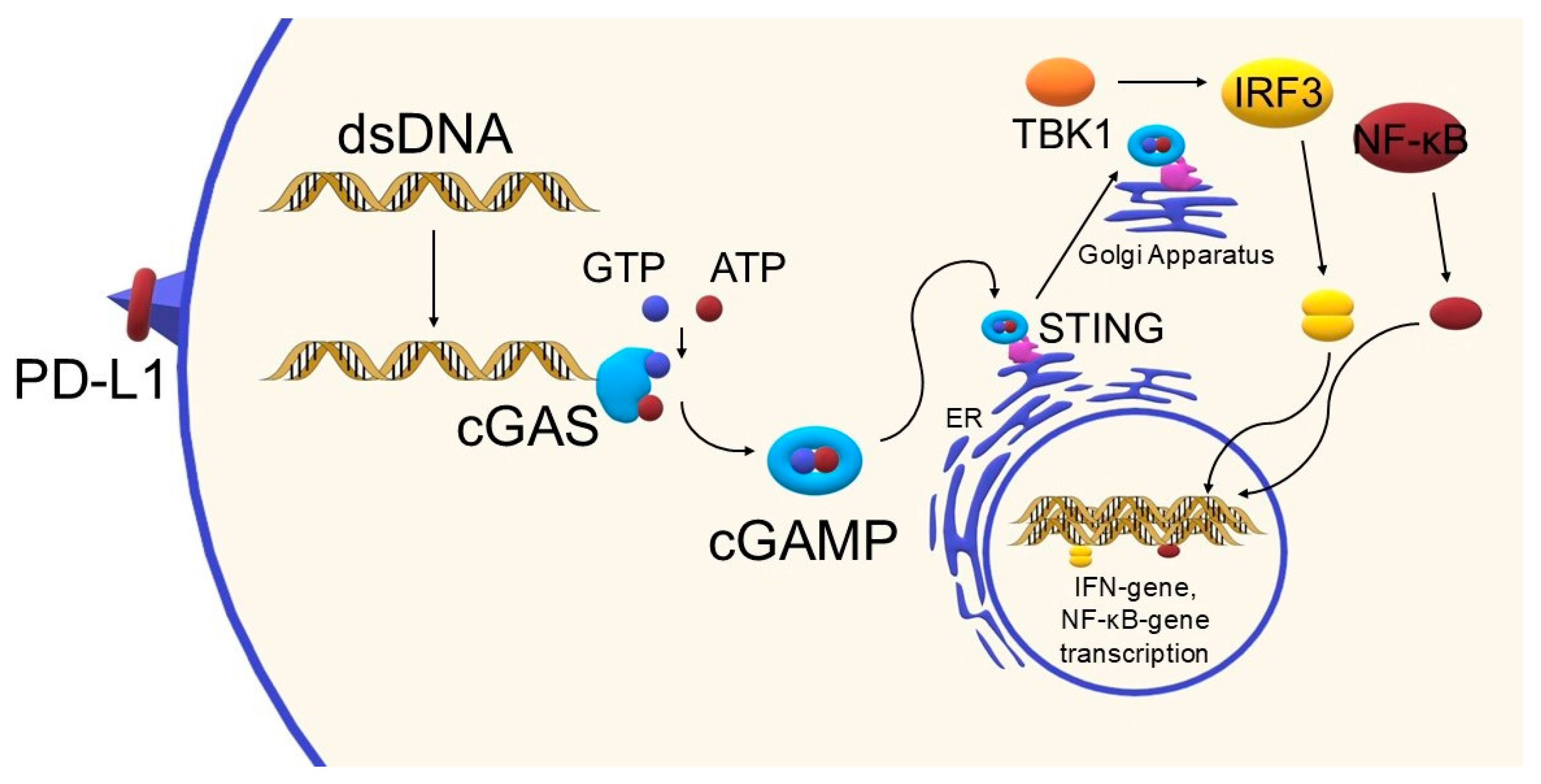
3. The STING Pathway—Mechanism and Importance in the Immune Response
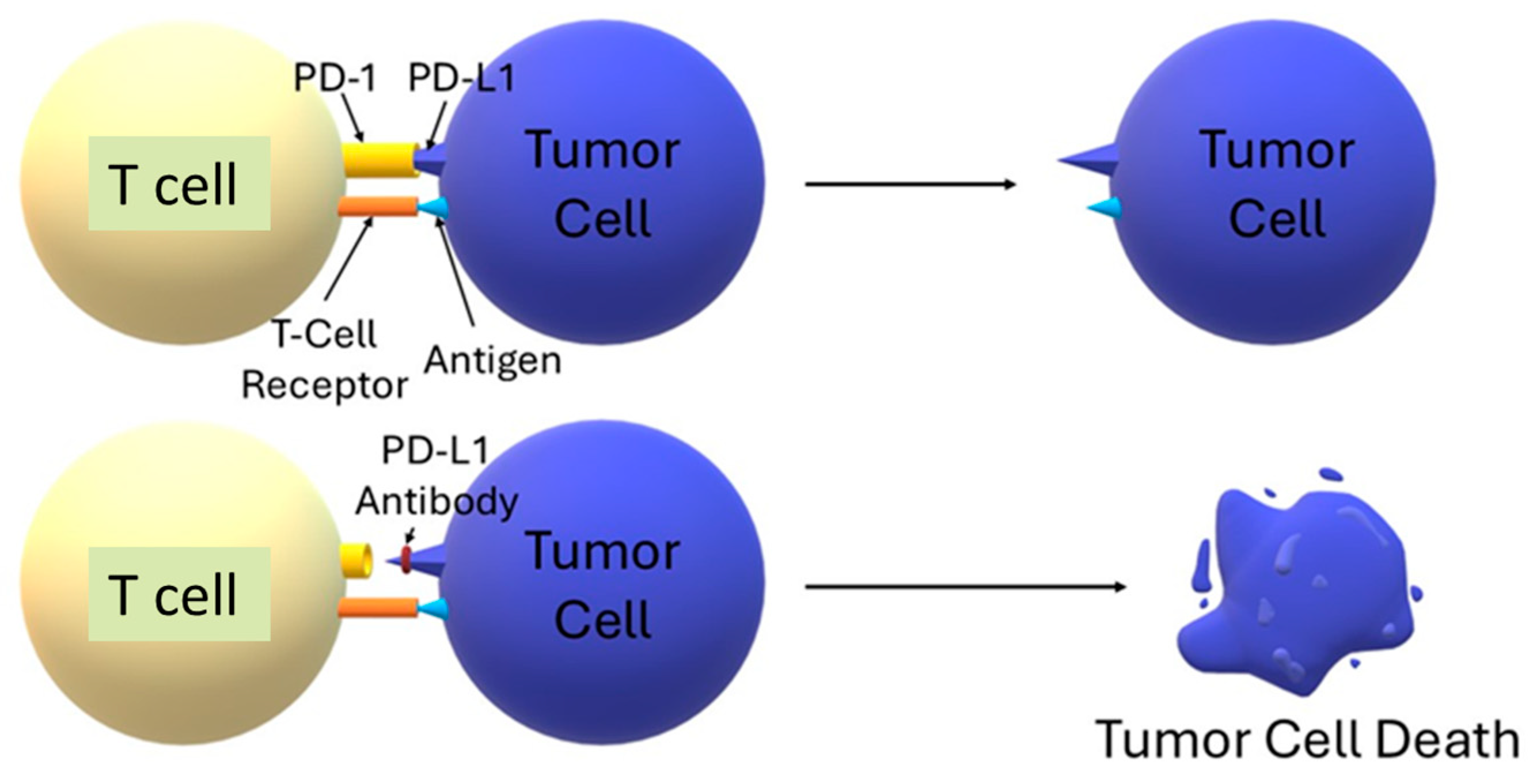
4. Anti-PD-L1 Immunotherapy—Limitations and the Need to Enhance Action
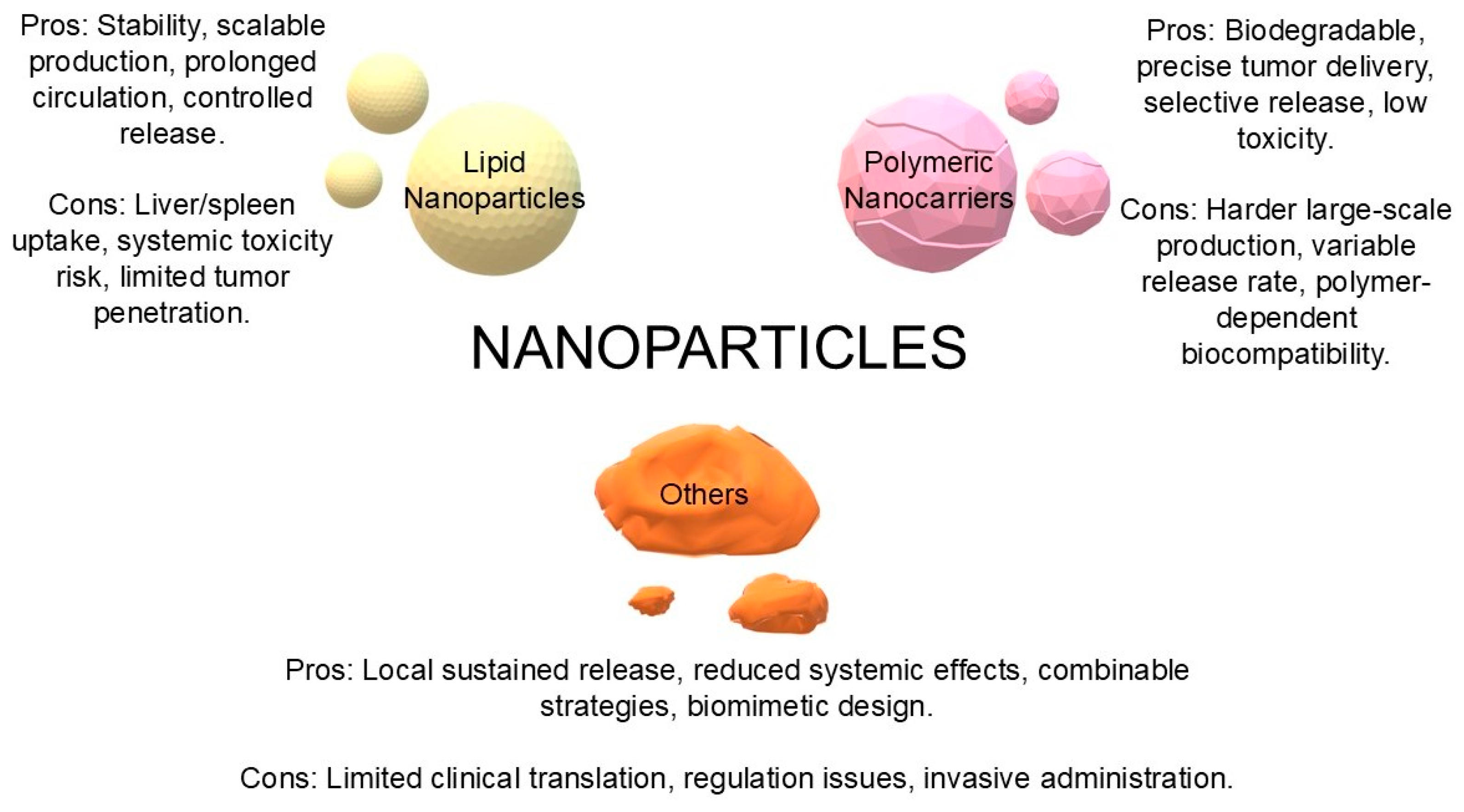
5. Nanoparticles as Carriers of STING Agonists
5.1. Lipid Nanoparticles (LNPs, Liposomes)
5.2. Polymeric Nanocarriers
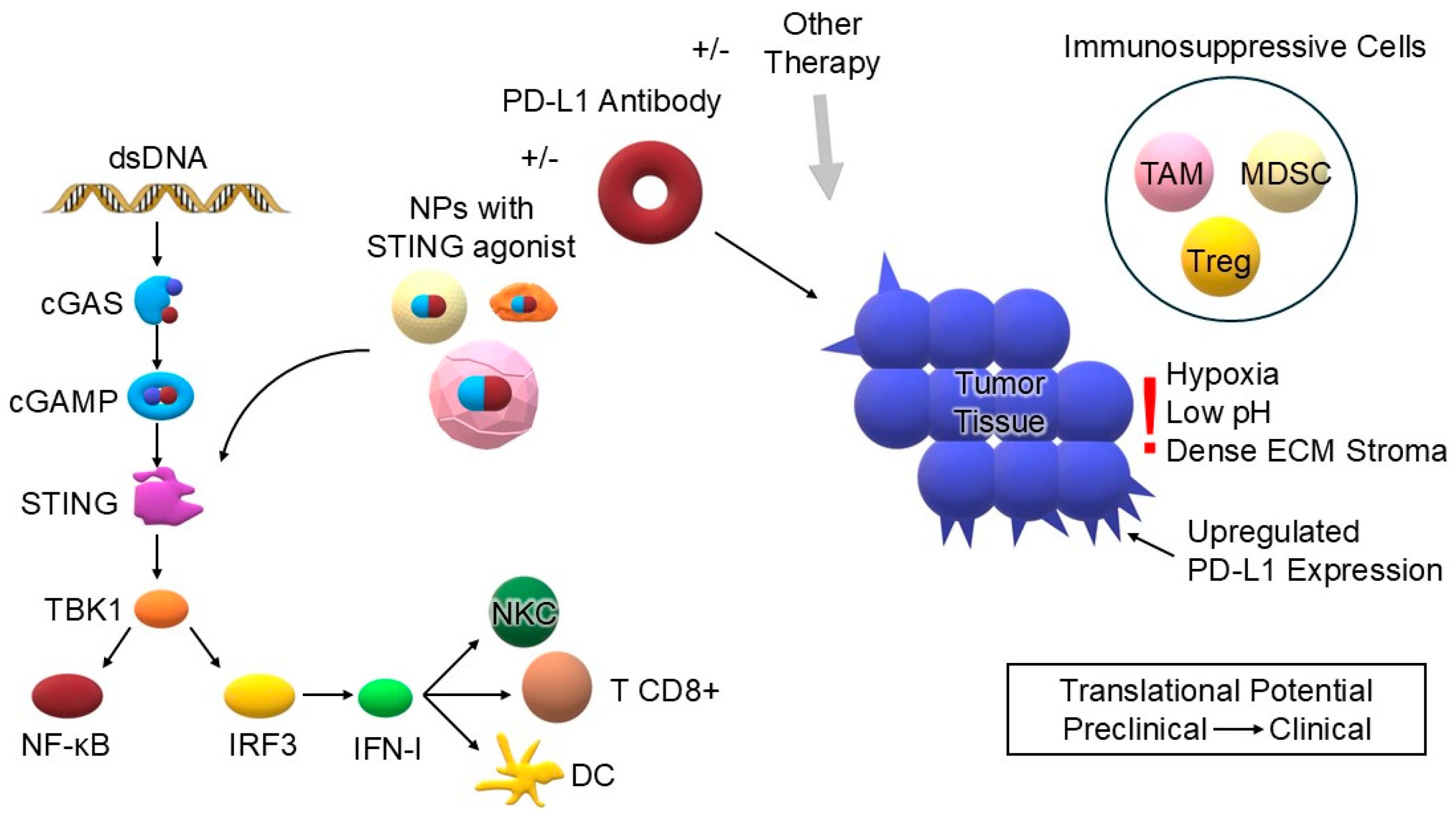
6. Combination of STING Agonists and Anti-PD-1/PD-L1 Therapy
6.1. Mechanism of Synergy
6.2. Lipid and Polymeric Nanocarriers
6.3. Biomimetic Platforms
6.4. Manganese as a STING Agonist
7. Other Combinations Using STING Agonists and Anti-PD-L1 Therapy
7.1. Chemotherapy
7.2. Radiotherapy
7.3. PDT
7.4. SDT
7.5. Provision of Nucleic Acids
7.6. Cuproptosis
7.7. Nanovaccines
7.8. Other Approaches
8. Clinical Data and Translational Aspects
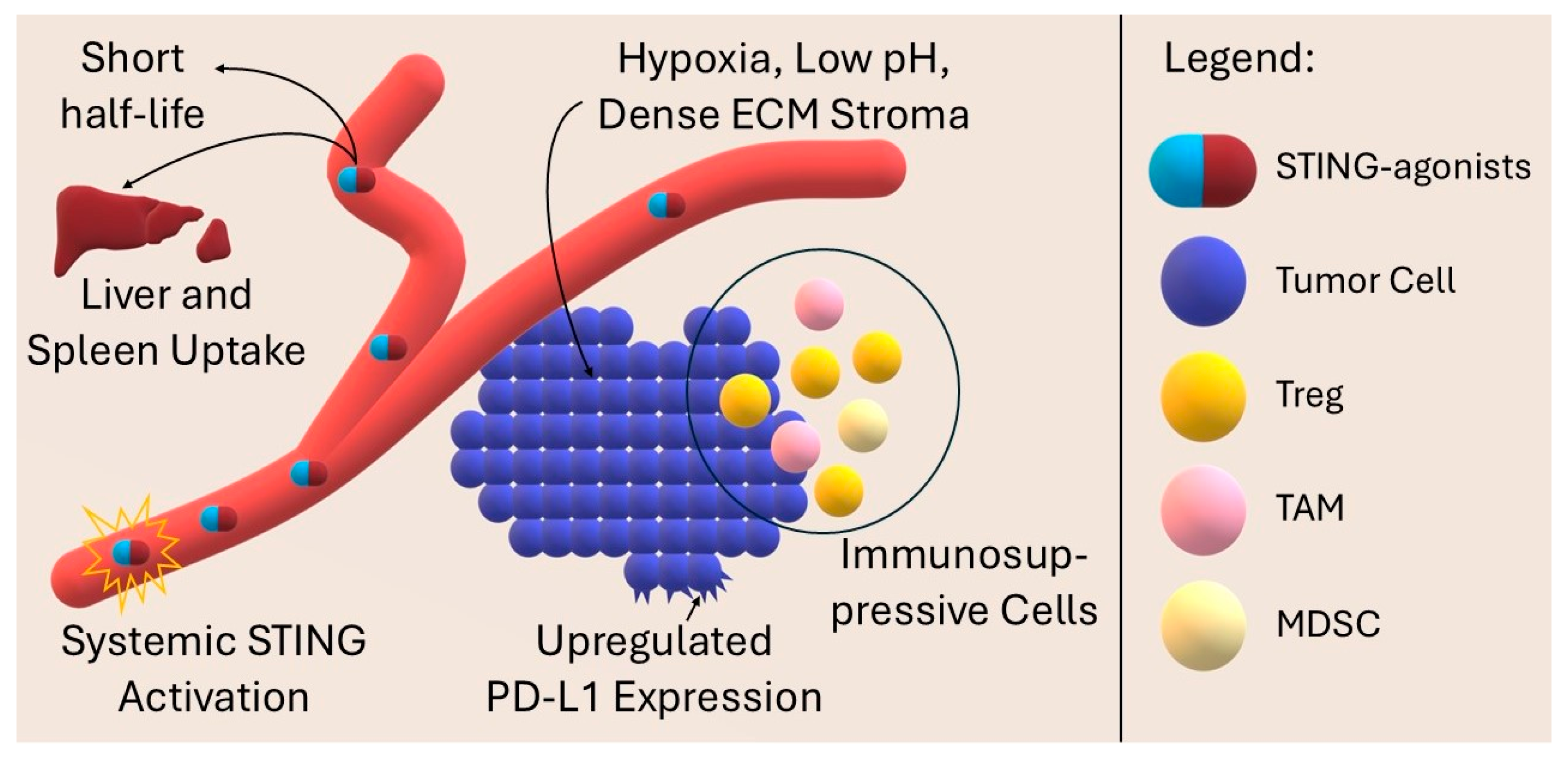
9. Challenges and Current Limitations
10. Prospects and Future Directions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and Function of the cGAS-STING Pathway of Cytosolic DNA Sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.-P.; Hornung, V. Molecular Mechanisms and Cellular Functions of cGAS-STING Signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.-C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Alu, A.; Han, X.; Wei, Y.; Wei, X. cGAS-STING Pathway in Cancer Biotherapy. Mol. Cancer 2020, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, L.; Yao, J.; Li, C.; Wang, X. Agonists and Inhibitors of the cGAS-STING Pathway. Molecules 2024, 29, 3121. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING Agonist Loaded Lipid Nanoparticles Overcome Anti-PD-1 Resistance in Melanoma Lung Metastasis via NK Cell Activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, X.; Lu, X.; Su, D.; Wang, Y.; Wu, H.; Zhang, Z.; Long, C.; Su, L.; Wang, Y.; et al. PD-L1 Antibody-Modified Plant-Derived Nanovesicles Carrying a STING Agonist for the Combinational Immunotherapy of Melanoma. Biomaterials 2025, 322, 123396. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X.; et al. A STING-Activating Nanovaccine for Cancer Immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, Y.; Li, T.; Ge, Z. Nanoparticle-Mediated STING Agonist Delivery for Enhanced Cancer Immunotherapy. Macromol. Biosci. 2021, 21, e2100133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the cGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Saha, S.; Li, J.; Montrose, D.C.; Martinez, L.A. P53 Engages the cGAS/STING Cytosolic DNA Sensing Pathway for Tumor Suppression. Mol. Cell 2023, 83, 266–280.e6. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, H. Conjugated STING Agonists. Mol. Ther. Nucleic Acids 2025, 36, 102530. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, W.; Jiang, H.; Meng, X.; Tang, D.; Liu, D. Clinical Applications of STING Agonists in Cancer Immunotherapy: Current Progress and Future Prospects. Front. Immunol. 2024, 15, 1485546. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, W.-M.; Cui, Q.-R.; Zhou, J.; Lu, G.-D. Metabolic Regulation of cGAS-STING Signaling in the Tumor Microenvironment: Dual Immune Roles and Therapeutic Implications. Cytokine Growth Factor Rev. 2025. [Google Scholar] [CrossRef]
- Samson, N.; Ablasser, A. The cGAS-STING Pathway and Cancer. Nat. Cancer 2022, 3, 1452–1463. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A Snapshot of the PD-1/PD-L1 Pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Córdova-Bahena, L.; Velasco-Velázquez, M.A. Anti-PD-1 And Anti-PD-L1 Antibodies as Immunotherapy Against Cancer: A Structural Perspective. Rev. Investig. Clin. 2020, 73, 8–16. [Google Scholar] [CrossRef]
- Lee, D.; Cho, M.; Kim, E.; Seo, Y.; Cha, J.-H. PD-L1: From Cancer Immunotherapy to Therapeutic Implications in Multiple Disorders. Mol. Ther. 2024, 32, 4235–4255. [Google Scholar] [CrossRef]
- Liang, C.; Ding, X.; Li, X.; Jiang, X.; Yang, H.; Yang, H.; Liu, K.; Hou, L. In Situ Self-Reassembling Nanosystem Enhances PD-L1 Blockade for Cancer Immunotherapy. J. Control. Release 2025, 377, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Tunger, A.; Sommer, U.; Wehner, R.; Kubasch, A.S.; Grimm, M.-O.; Bachmann, M.P.; Platzbecker, U.; Bornhäuser, M.; Baretton, G.; Schmitz, M. The Evolving Landscape of Biomarkers for Anti-PD-1 or Anti-PD-L1 Therapy. J. Clin. Med. 2019, 8, 1534. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.M.M.; Fathi, M.; Kazemi, F.; Bazregari, G.; Ghasemian, A. STING Pathway as a Cancer Immunotherapy: Progress and Challenges in Activating Anti-Tumor Immunity. Mol. Biol. Rep. 2024, 51, 487. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Imani, M.; Pourfarzi, F.; Jafari, N.; AbedianKenari, S.; Safarzadeh, E. Combination of IFN-Gamma with STING Agonist and PD-1 Immune Checkpoint Blockade: A Potential Immunotherapy for Gastric Cancer. Med. Oncol. 2024, 41, 110. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Zhu, A.X.; Wang, Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020, 11, 598877. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. Nanoparticles for Glioblastoma Treatment. Pharmaceutics 2025, 17, 688. [Google Scholar] [CrossRef]
- Wehbe, M.; Wang-Bishop, L.; Becker, K.W.; Shae, D.; Baljon, J.J.; He, X.; Christov, P.; Boyd, K.L.; Balko, J.M.; Wilson, J.T. Nanoparticle Delivery Improves the Pharmacokinetic Properties of Cyclic Dinucleotide STING Agonists to Open a Therapeutic Window for Intravenous Administration. J. Control. Release 2021, 330, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Lorkowski, M.E.; Atukorale, P.U.; Bielecki, P.A.; Tong, K.H.; Covarrubias, G.; Zhang, Y.; Loutrianakis, G.; Moon, T.J.; Santulli, A.R.; Becicka, W.M.; et al. Immunostimulatory Nanoparticle Incorporating Two Immune Agonists for the Treatment of Pancreatic Tumors. J. Control. Release 2021, 330, 1095–1105. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, M.; Wang, Z.; Zhou, R.; Ai, K. Enhancing Radiotherapy-Induced Anti-Tumor Immunity via Nanoparticle-Mediated STING Agonist Synergy. Mol. Cancer 2025, 24, 176. [Google Scholar] [CrossRef] [PubMed]
- Carozza, J.A.; Böhnert, V.; Nguyen, K.C.; Skariah, G.; Shaw, K.E.; Brown, J.A.; Rafat, M.; von Eyben, R.; Graves, E.E.; Glenn, J.S.; et al. Extracellular cGAMP Is a Cancer Cell-Produced Immunotransmitter Involved in Radiation-Induced Anti-Cancer Immunity. Nat. Cancer 2020, 1, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid Nanoparticles for siRNA Delivery in Cancer Treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Shaji, S.G.; Patel, P.; Cheng, K. Abstract 384: Lipid Nanoparticle Aided Delivery of a STING Agonist to Treat Pancreatic Cancer. Cancer Res. 2022, 82, 384. [Google Scholar] [CrossRef]
- Feng, B.; Lu, X.N.; Zhang, G.; Zhao, L.; Mei, D.Y. STING Agonist Delivery by Lipid Calcium Phosphate Nanoparticles Enhances Immune Activation for Neuroblastoma. Acta Mater. Med. 2023, 2, 216–227. [Google Scholar] [CrossRef]
- Dane, E.L.; Belessiotis-Richards, A.; Backlund, C.; Wang, J.; Hidaka, K.; Milling, L.E.; Bhagchandani, S.; Melo, M.B.; Wu, S.; Li, N.; et al. STING Agonist Delivery by Tumour-Penetrating PEG-Lipid Nanodiscs Primes Robust Anticancer Immunity. Nat. Mater. 2022, 21, 710–720. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Li, X.; Zhao, N.; Zhou, C.; Qiao, S.; Wang, J.; Song, S.; Pan, M. Shape-Tunable Hollow Polysiloxane Nanoparticles Based on a Surfactant-Free Soft Templating Method and Their Application as a Drug Carrier. ACS Appl. Mater. Interfaces 2024, 16, 2672–2682. [Google Scholar] [CrossRef]
- Peng, S.; Hou, X.; Liu, J.; Huang, F. Advances in Polymer Nanomaterials Targeting cGAS-STING Pathway for Enhanced Cancer Immunotherapy. J. Control. Release 2025, 381, 113560. [Google Scholar] [CrossRef]
- Podojil, J.R.; Cogswell, A.C.; Chiang, M.-Y.; Eaton, V.; Ifergan, I.; Neef, T.; Xu, D.; Meghani, K.A.; Yu, Y.; Orbach, S.M.; et al. Biodegradable Nanoparticles Induce cGAS/STING-Dependent Reprogramming of Myeloid Cells to Promote Tumor Immunotherapy. Front. Immunol. 2022, 13, 887649. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Li, Z.; Ren, L.; Pan, D.; Gong, Q.; Gu, Z.; Cai, H.; Luo, K. Branched Glycopolymer Prodrug-Derived Nanoassembly Combined with a STING Agonist Activates an Immuno-Supportive Status to Boost Anti-PD-L1 Antibody Therapy. Acta Pharm. Sin. B 2024, 14, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, W.; Su, L.; Chen, Q.; Liu, W.; Dai, M.; Ran, H. Biomimetic Nanoadjuvant-Enhanced Ultrasound-Induced STING Activation Potentiates aPD-L1 Therapy to Overcome Cancer Recurrence and Metastasis. Chem. Eng. J. 2025, 511, 161735. [Google Scholar] [CrossRef]
- Vasiyani, H.; Wadhwa, B. STING Activation and Overcoming the Challenges Associated with STING Agonists Using ADC (Antibody-Drug Conjugate) and Other Delivery Systems. Cell Signal. 2025, 128, 111647. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Liu, Y.; Xu, Z.; Xiao, B.; Cai, C.; Shi, C.; Liu, X.; Xu, G. Specific Activation of the STING Pathway by Engineering Piezoelectric Hydrogel Microspheres for Boosting Implant Infection Immunotherapy. ACS Nano 2025, 19, 16383–16404. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Qiao, Y.; Wei, L.; Su, Y.; Tan, Q.; Yang, X.; Li, S. Nanoparticle-Based Strategies to Enhance the Efficacy of STING Activators in Cancer Immunotherapy. Int. J. Nanomed. 2025, 20, 5429–5456. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, B.; Xia, H.; Pan, M.; Zhao, R.; Zhou, J.; Yin, Q.; Wan, F.; Yan, Y.; Fu, C.; et al. pH-Gated Nanoparticles Selectively Regulate Lysosomal Function of Tumour-Associated Macrophages for Cancer Immunotherapy. Nat. Commun. 2023, 14, 5888. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Jiang, X.; Zhou, Z.; Xiong, W.; Xue, F.; Liu, Y.; Xu, H.; Fan, B.; Li, Y.; Shen, J. A Hybrid Nanoadjuvant Simultaneously Depresses PD-L1/TGF-Β1 and Activates cGAS-STING Pathway to Overcome Radio-Immunotherapy Resistance. Adv. Mater. 2024, 36, 2304328. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, Y.; Wang, W.; Chao, M.; Sun, W.; Kong, Y.; Jiang, G.; Gao, Y.; Gao, F. An Anisotropic Gold-Palladium Heterostructured Nanosystem for Synergistically Overcoming Radioresistance and Enhancing Melanoma Radioimmunotherapy. Adv. Sci. 2025, 12, e00492. [Google Scholar] [CrossRef]
- Khalifa, A.M.; Nakamura, T.; Sato, Y.; Sato, T.; Hyodo, M.; Hayakawa, Y.; Harashima, H. Interval- and Cycle-Dependent Combined Effect of STING Agonist Loaded Lipid Nanoparticles and a PD-1 Antibody. Int. J. Pharm. 2022, 624, 122034. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ye, Y.; Liu, L.; Sha, Q.; Wang, X.; Jiao, T.; Zhang, L.; Wang, J. The Lipid Platform Increases the Activity of STING Agonists to Synergize Checkpoint Blockade Therapy against Melanoma. Biomater. Sci. 2021, 9, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Wang-Bishop, L.; Wehbe, M.; Shae, D.; James, J.; Hacker, B.C.; Garland, K.; Chistov, P.P.; Rafat, M.; Balko, J.M.; Wilson, J.T. Potent STING Activation Stimulates Immunogenic Cell Death to Enhance Antitumor Immunity in Neuroblastoma. J. Immunother. Cancer 2020, 8, e000282. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Li, J.; Sun, N.; Cheng, P.; Huang, J.; Li, S.; Kuai, R. Single-Dose Physically Cross-Linked Hyaluronic Acid and Lipid Hybrid Nanoparticles Containing Cyclic Guanosine Monophosphate-Adenosine Monophosphate Eliminate Established Tumors. ACS Nano 2024, 18, 29942–29955. [Google Scholar] [CrossRef]
- Ding, B.; Yue, J.; Zheng, P.; Ma, P.; Lin, J. Manganese Oxide Nanomaterials Boost Cancer Immunotherapy. J. Mater. Chem. B 2021, 9, 7117–7131. [Google Scholar] [CrossRef]
- Fan, Q.; Kuang, L.; Wang, B.; Yin, Y.; Dong, Z.; Tian, N.; Wang, J.; Yin, T.; Wang, Y. Multiple Synergistic Effects of the Microglia Membrane-Bionic Nanoplatform on Mediate Tumor Microenvironment Remodeling to Amplify Glioblastoma Immunotherapy. ACS Nano 2024, 18, 14469–14486. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zu, Z.; Lu, Y.; Sha, X.; Li, Y.; Liu, Y.; Lu, S.; Luo, X.; Zhou, Y.; Tao, J.; et al. Mitochondria-Targeted Manganese-Based Mesoporous Silica Nanoplatforms Trigger cGAS-STING Activation and Sensitize Anti PD-L1 Therapy in Triple-Negative Breast Cancer. Acta Biomater. 2025, 199, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Y.; Wang, P.; Kan, W.; Wang, M.; Li, H.; Wang, X.; Yuan, P.; Ma, Y.; Zhang, J.; et al. Biosynthetic MnSe Nanobomb with Low Mn Content Activates the cGAS-STING Pathway and Induces Immunogenic Cell Death to Enhance Antitumour Immunity. Acta Biomater. 2024, 184, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, M.; Li, R.; Sun, Y.; Ye, P.; Fang, K.; Wang, C.; Shi, S.; Dong, C. A Responsive Cocktail Nano-Strategy Breaking the Immune Excluded State Enhances Immunotherapy for Triple Negative Breast Cancer. Nanoscale 2025, 17, 4610–4623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Y.; Li, G.; He, M.; Li, Y.; Liu, Z.; Wang, H.; Shen, M.; Shi, X. Dendrimer-Mediated Generation of a Metal-Phenolic Network for Antibody Delivery to Elicit Improved Tumor Chemo/Chemodynamic/Immune Therapy. ACS Appl. Mater. Interfaces 2025, 17, 4662–4674. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, X.; Du, Y.; Tian, J. RGD Targeted Magnetic Ferrite Nanoparticles Enhance Antitumor Immunotherapeutic Efficacy by Activating STING Signaling Pathway. iScience 2024, 27, 109062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, G.; Zhu, J.; Ai, C.; Wang, W.; Zhao, Y.; Han, X.; Qi, Y.; Duan, J.; Yu, D. Lipid Acid Metabolism Reprogramming Nanoagent Induces Ferroptosis Storm and cGAS-STING Activation for Metal-Immunotherapy of Triple Negative Breast Cancer. Chem. Eng. J. 2025, 511, 162048. [Google Scholar] [CrossRef]
- Sun, L.; Gao, H.; Wang, H.; Zhou, J.; Ji, X.; Jiao, Y.; Qin, X.; Ni, D.; Zheng, X. Nanoscale Metal-Organic Frameworks-Mediated Degradation of Mutant P53 Proteins and Activation of cGAS-STING Pathway for Enhanced Cancer Immunotherapy. Adv. Sci. 2024, 11, e2307278. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, K.; Li, X.; Shen, M.; Lu, S.; Tang, D.; Han, H.; Yu, Y. Reduction Sensitive Polymers Delivering Cationic Platinum Drugs as STING Agonists for Enhanced Chemo-Immunotherapy. Adv. Funct. Mater. 2022, 32, 2204589. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Zhang, L.; Wang, M.; Yin, L.; Dong, X.; Xiao, H.; Xing, N. Targeting DNA Damage and Repair Machinery via Delivering WEE1 Inhibitor and Platinum (IV) Prodrugs to Stimulate STING Pathway for Maximizing Chemo-Immunotherapy in Bladder Cancer. Adv. Mater. 2024, 36, e2308762. [Google Scholar] [CrossRef]
- Crumrine, N.; Reda, M.; Kumar, P.; Wang, R.; Watcharawittayakul, T.; Gouw, V.; Khalil, S.; Ngamcherdtrakul, W.; Yantasee, W. Development of PD-L1 and STING-Targeted Nanoparticles for Lung Cancer Treatment. Cancer Immunol. Res. 2025, 13, A052. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, F.; Zhang, J.; Liao, L.; Jiang, M.; Huang, Y.; Liu, Y.; Lei, L.; Tao, Z.; Yu, C.-Y.; et al. Facile Integration of a Binary Nano-Prodrug with αPD-L1 as a Translatable Technology for Potent Immunotherapy of TNBC. Acta Biomater. 2025, 194, 373–384. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Qi, Y.; Li, M.; Zhang, R.; Gao, Z.; Cui, J.; Yu, D. Metal-Phenolic Vehicles Potentiate Cycle-Cascade Activation of Pyroptosis and cGAS-STING Pathway for Tumor Immunotherapy. ACS Nano 2024, 18, 23727–23740. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Yang, Y.; Lu, X.; Xu, J.; Lu, S.; Pan, H.; Zhou, W.; Li, W.; Chen, S. Mitigating Doxorubicin-Induced Cardiotoxicity and Enhancing Anti-Tumor Efficacy with a Metformin-Integrated Self-Assembled Nanomedicine. Adv. Sci. 2025, 12, e2415227. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Cang, J.; Yan, X.; Wu, F.; Sun, X.; Zhang, W. Synergistic Chemo-Immunotherapy for Osteosarcoma via a pH-Responsive Multi-Component Nanoparticle System. Front. Pharmacol. 2025, 16, 1584245. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shu, L.; Wang, M.; Yao, J.; Yao, Q.; Bian, S.; Chen, X.; Wan, J.; Zhang, F.; Zheng, S.; et al. Triple-Combination Immunogenic Nanovesicles Reshape the Tumor Microenvironment to Potentiate Chemo-Immunotherapy in Preclinical Cancer Models. Adv. Sci. 2023, 10, e2204890. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Ji, Z.; Shen, M.; Zhu, M.; Ren, Y.; Guo, L.; Yang, K.; Liu, T.; Yi, X. Tumor Vascular Destruction and cGAS-STING Activation Induced by Single Drug-Loaded Nano-Micelles for Multiple Synergistic Therapies of Cancer. Small 2023, 19, e2303517. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, J.; Zhang, J.; Zeng, G.; Zeng, B.; Song, S.; Lao, Z.; Chen, H.; Wen, Z.; Yang, Z.; et al. Nanosized Shikonin Disrupts Tumor-Cell Mismatch Repair and Synergizes with Manganese to Sensitize Squamous Carcinoma to Immunotherapy. ACS Nano 2025, 19, 13889–13905. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Zheng, Y.; Li, Y.; Tang, X.; Chen, Q.; Mao, W.; Li, W.; Liu, X.; Zhu, J. Combination of Irinotecan Silicasome Nanoparticles with Radiation Therapy Sensitizes Immunotherapy by Modulating the Activation of the cGAS/STING Pathway for Colorectal Cancer. Mater. Today Bio 2023, 23, 100809. [Google Scholar] [CrossRef]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The Use of Photodynamic Therapy in Medical Practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Yakub, Z.A.; Dynarowicz, K.; Myśliwiec, A.; Mytych, W.; Komosińska-Vassev, K.; Misiołek, M.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy in Glioma Cell Culture. Oncologie 2024, 26, 885–897. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Guo, L.; Huang, L.; Gao, W. STING-Activating Photo-Vaccination with Glutamine Metabolism Reprogramming and Cascade Mitochondrial Dysfunction for Robust Immune Landscape Remodeling. Chem. Eng. J. 2024, 502, 157744. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Li, Y.; Duan, D.; Liu, K.; Lei, M.; Zhang, L.; Zhu, Y. Carrier-Free Nanotherapeutics Unleashed: Ce6/siPD-L1 Co-Delivery System Synergizes Photodynamic and RNAi Therapies to Combat Breast Cancer. Int. J. Biol. Macromol. 2025, 318, 144962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, H.; Zhang, X.; Nice, E.C.; Huang, C.; Zhang, H.; Zheng, S. Copper-Coordination Driven Brain-Targeting Nanoassembly for Efficient Glioblastoma Multiforme Immunotherapy by Cuproptosis-Mediated Tumor Immune Microenvironment Reprogramming. J. Nanobiotechnol. 2024, 22, 801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, J.; Zhang, Y.; Wang, Y.; Li, W.; Zhang, B.; Cui, W.; Li, Y.; Wang, Y.; Yang, Z.; et al. In Situ Oxidation-Responsive Nanovaccine Coordinates Photosensitizer and STING Agonist for Cancer Photo-Immunotherapy. Nano Today 2025, 62, 102726. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, J.; Li, Z.; Yan, W.; Guo, Y. Nanomotors Activating Both cGAS-STING Pathway and Immune Checkpoint Blockade for Tumor Therapy and Bioimaging. Talanta 2025, 284, 127258. [Google Scholar] [CrossRef]
- Du, S.; Chen, C.; Qu, S.; Song, H.; Yang, J.; Li, Y.; Liu, K.; Lu, Q.; Luo, W.; Wang, R.; et al. DNAzyme-Assisted Nano-Herb Delivery System for Multiple Tumor Immune Activation. Small 2022, 18, e2203942. [Google Scholar] [CrossRef]
- Pu, H.; Huang, J.; Gui, B.; Chen, Y.; Guo, Y.; Lian, Y.; Pan, J.; Hu, Y.; Jiang, N.; Deng, Q.; et al. Ultrasound-Responsive Nanobubbles for Breast Cancer: Synergistic Sonodynamic, Chemotherapy, and Immune Activation through the cGAS-STING Pathway. ACS Appl. Mater. Interfaces 2025, 17, 19317–19334. [Google Scholar] [CrossRef]
- Xia, F.; Lu, Y.; Gong, Z.; Tu, Q.; Liang, S.; Wang, C.; Yao, H.; Zhong, L.; Fu, Y.; Guo, P.; et al. Cancer Immunotherapy Based on the Bidirectional Reprogramming of the Tumor Microenvironment by a “Brakes Off/ Step on the Accelerator” Core-Shell Manganese Phosphate/siPD-L1 Modulator. Exploration 2025, 5, 270009. [Google Scholar] [CrossRef]
- Huang, K.-W.; Hsu, F.-F.; Qiu, J.T.; Chern, G.-J.; Lee, Y.-A.; Chang, C.-C.; Huang, Y.-T.; Sung, Y.-C.; Chiang, C.-C.; Huang, R.-L.; et al. Highly Efficient and Tumor-Selective Nanoparticles for Dual-Targeted Immunogene Therapy against Cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, A.; Liu, J.; Xu, M.; Mi, P. Tumor-Targeting Nanocarriers Amplified Immunotherapy of Cold Tumors by STING Activation and Inhibiting Immune Evasion. Sci. Adv. 2025, 11, eadr1728. [Google Scholar] [CrossRef]
- Tao, J.; Dong, Y.; Wang, B.; Wang, T.; Zhang, A.; Li, S.; Chen, R.; Su, Y.; Jiang, T.; Zhao, X. Dual Metal Nanoflower Oxygen Pump Microneedles Based on Cuproptosis and STING Pathway Activation for Cancer Immunotherapy. Small 2025, 21, 2409187. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X.; Gao, S.; Yan, Y.; Li, X.; Wang, H. Tumor Microenvironment-Responsive DNA-Based Nanomedicine Triggers Innate Sensing for Enhanced Immunotherapy. J. Nanobiotechnol. 2023, 21, 382. [Google Scholar] [CrossRef]
- Chen, C.; Du, S.; Lu, Q.; Liu, K.; Pan, Y.; Jiang, Y.; Yang, J.; Han, X.; Song, Y. Tumor-Associated miRNAs Activated HCR-DNAzyme Theranostic Nanosystem to Trigger Innate- and Adaptive-Immune Responses for Long-Term Immunotherapy. Chem. Eng. J. 2023, 473, 145192. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, M.; Hao, Z.; Li, L.; Zhang, Y.; Fang, B.; Shao, M.; Ren, G.; Wang, K.; Liu, H.; et al. Zinc-Copper Bimetallic Nanoplatforms Trigger Photothermal-Amplified Cuproptosis and cGAS-STING Activation for Enhancing Triple-Negative Breast Cancer Immunotherapy. J. Nanobiotechnol. 2025, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Lv, H.; Feng, Y.; Li, Y.; Zhao, Z. Inhalable Nanoparticles with Enhanced Cuproptosis and cGAS-STING Activation for Synergistic Lung Metastasis Immunotherapy. Acta Pharm. Sin. B 2024, 14, 3697–3710. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Hu, Q.; Sun, Z.; Wang, N.; He, H.; Tang, Z.; Chen, W. A Booster for Radiofrequency Ablation: Advanced Adjuvant Therapy via In Situ Nanovaccine Synergized with Anti-Programmed Death Ligand 1 Immunotherapy for Systemically Constraining Hepatocellular Carcinoma. ACS Nano 2023, 17, 19441–19458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hou, B.; Wang, D.; Sun, F.; Song, R.; Shao, Q.; Wang, H.; Yu, H.; Li, Y. Engineering Polymeric Prodrug Nanoplatform for Vaccination Immunotherapy of Cancer. Nano Lett. 2020, 20, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, Y.; Liu, L.; Xu, Y.; Zhao, C.; Liu, W.; Rao, L. Genetically Edited Cascade Nanozymes for Cancer Immunotherapy. ACS Nano 2024, 18, 12295–12310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Liu, Z.; Jiang, B.; He, Z.; Liu, S.; Wu, Y.; Wu, Z.; Zhang, T.; Liu, M.; et al. Peroxidase-Like Nanozyme Activates the cGAS-STING Pathway via ROS-Induced mtDNA Release for Cancer Immunotherapy. Adv. Funct. Mater. 2024, 34, 2401576. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Li, J.; Liu, T.; Bao, Q.; Li, X.; Long, J.; Fu, L.; Zhang, Z.; Huang, S.; et al. Cholesterol Removal Improves Performance of a Model Biomimetic System to Co-Deliver a Photothermal Agent and a STING Agonist for Cancer Immunotherapy. Nat. Commun. 2023, 14, 5111. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Wang, Z.; Yan, F.; Shi, Z.; Feng, S. Glutathione-Responsive Metal-Organic-Framework-Derived MnxOy/(A/R)TiO2 Nanoparticles for Enhanced Synergistic Sonodynamic/Chemodynamic/Immunotherapy. ACS Nano 2025, 19, 885–899. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, S.; Li, L.; Liu, G.; Ma, Q.; Liu, Z.; Zhao, Y.; Wang, Q. Grapefruit Extracellular-Vesicle-Derived Nanodrug Loading Three-Functional Platinum(IV) Conjugates with Enhanced Targeting and TME Modulating Properties. J. Med. Chem. 2025, 68, 11928–11947. [Google Scholar] [CrossRef]
- Lu, S.; Mi, Z.; Liu, P.; Ding, J.; Ma, Y.; Yang, J.; Rong, P.; Zhou, W. Repolarizing Neutrophils via MnO2 Nanoparticle-Activated STING Pathway Enhances Salmonella-Mediated Tumor Immunotherapy. J. Nanobiotechnol. 2024, 22, 443. [Google Scholar] [CrossRef]
- Zhu, T.; Xiao, Y.; Chen, Z.; Ding, H.; Chen, S.; Jiang, G.; Huang, X. Inhalable Nanovesicles Loaded with a STING Agonist Enhance CAR-T Cell Activity against Solid Tumors in the Lung. Nat. Commun. 2025, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, B.; Liao, H.; Chen, X.; Yu, X.; Hu, J.; Lin, Q.; Cao, T.; Xu, K.; Zhou, Q.; et al. Nanodrug Modulates Premetastatic Niche and Suppresses Metastatic Lung Adenocarcinoma via Programmed Cell Death Ligand 1 Blockade and STING Pathway Activation. ACS Nano 2025, 19, 23893–23907. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Sweis, R.F.; Hodi, F.S.; Messersmith, W.A.; Andtbacka, R.H.I.; Ingham, M.; Lewis, N.; Chen, X.; Pelletier, M.; Chen, X.; et al. Phase I Dose-Escalation Trial of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res. 2022, 28, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Sweis, R.F.; Kasper, S.; Hamid, O.; Bhatia, S.; Dummer, R.; Stradella, A.; Long, G.V.; Spreafico, A.; Shimizu, T.; et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res. 2023, 29, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Haralambieva, I.H.; Ovsyannikova, I.G.; Voigt, E.A.; Larrabee, B.R.; Schaid, D.J.; Zimmermann, M.T.; Oberg, A.L.; Poland, G.A. Polymorphisms in STING Affect Human Innate Immune Responses to Poxviruses. Front. Immunol. 2020, 11, 567348. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Finan, J.M.; Guo, Y.; Goodyear, S.M.; Brody, J.R. Challenges and Opportunities in Targeting the Complex Pancreatic Tumor Microenvironment. JCO Oncol. Adv. 2024, 1, e2400050. [Google Scholar] [CrossRef] [PubMed]
- Carozza, J.A.; Cordova, A.F.; Brown, J.A.; AlSaif, Y.; Böhnert, V.; Cao, X.; Mardjuki, R.E.; Skariah, G.; Fernandez, D.; Li, L. ENPP1′s Regulation of Extracellular cGAMP Is a Ubiquitous Mechanism of Attenuating STING Signaling. Proc. Natl. Acad. Sci. USA 2022, 119, e2119189119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Böhnert, V.; Joseph, A.J.; Sudaryo, V.; Skariah, G.; Swinderman, J.T.; Yu, F.B.; Subramanyam, V.; Wolf, D.M.; Lyu, X.; et al. ENPP1 Is an Innate Immune Checkpoint of the Anticancer cGAMP–STING Pathway in Breast Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2313693120. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, S.; Huntoon, K.; Lux, J. Putting the Sting Back in STING Therapy: Novel Delivery Vehicles for Improved STING Activation. Front. Chem. Biol. 2024, 3, 1386220. [Google Scholar] [CrossRef]
- Pyclik, M.; Durslewicz, J.; Papinska, J.A.; Deshmukh, U.S.; Bagavant, H. STING Agonist-Induced Skin Inflammation Is Exacerbated with Prior Systemic Innate Immune Activation. Int. J. Mol. Sci. 2023, 24, 4128. [Google Scholar] [CrossRef]
- Hines, J.B.; Kacew, A.J.; Sweis, R.F. The Development of STING Agonists and Emerging Results as a Cancer Immunotherapy. Curr. Oncol. Rep. 2023, 25, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, X.; Peng, Q.; Oyang, L.; Ren, Z.; Wang, J.; Peng, M.; Zhou, Y.; Deng, X.; Liao, Q. The cGAS-STING Pathway in Cancer Immunity: Mechanisms, Challenges, and Therapeutic Implications. J. Hematol. Oncol. 2025, 18, 40. [Google Scholar] [CrossRef]
- Karimi, S.; Bakhshali, R.; Bolandi, S.; Zahed, Z.; Mojtaba Zadeh, S.S.; Kaveh Zenjanab, M.; Jahanban Esfahlan, R. For and against Tumor Microenvironment: Nanoparticle-Based Strategies for Active Cancer Therapy. Mater. Today Bio 2025, 31, 101626. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Mu, D.; Ni, H.; Wang, X.; Zhang, T.; Chang, X.; He, S.; Zhou, D. Regulation of the CD8+ T Cell and PDL1/PD1 Axis in Gastric Cancer: Unraveling the Molecular Landscape. Crit. Rev. Oncol./Hematol. 2025, 212, 104750. [Google Scholar] [CrossRef] [PubMed]
- Zarour, H.M. Reversing T-Cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- van Gulijk, M.; van Krimpen, A.; Schetters, S.; Eterman, M.; van Elsas, M.; Mankor, J.; Klaase, L.; de Bruijn, M.; van Nimwegen, M.; van Tienhoven, T.; et al. PD-L1 Checkpoint Blockade Promotes Regulatory T Cell Activity That Underlies Therapy Resistance. Sci. Immunol. 2023, 8, eabn6173. [Google Scholar] [CrossRef]
- Kumar, M.; Kulkarni, P.; Liu, S.; Chemuturi, N.; Shah, D.K. Nanoparticle Biodistribution Coefficients: A Quantitative Approach for Understanding the Tissue Distribution of Nanoparticles. Adv. Drug Deliv. Rev. 2023, 194, 114708. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Nanoparticles Evading The Reticuloendothelial System: Role of The Supported Bilayer. Biochim. Biophys. Acta 2009, 1788, 2259–2266. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.-M. Platelet-Membrane-Biomimetic Nanoparticles for Targeted Antitumor Drug Delivery. J. Nanobiotechnol. 2019, 17, 60. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering Nanoparticles to Lungs While Avoiding Liver and Spleen through Adsorption on Red Blood Cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, H.; Radosz, M.; Van Kirk, E.; Murdoch, W.J. pH-Responsive Nanoparticles for Cancer Drug Delivery. Methods Mol. Biol. 2008, 437, 183–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, X.; Zhu, D.; Fu, W.; Liu, Y.; Zheng, L.; Chen, P.; Gong, C.; Liu, X. Implantable Immunostimulant Microneedle Patch for Post-Surgical Prevention of Cancer Recurrence and Distant Tumor Inhibition. ACS Appl. Mater. Interfaces 2025, 17, 22362–22374. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Hong, X.; Zhang, X.; Chen, H.; Wen, X.; Lin, F.; Liu, J.; Yang, C.; Cheng, B.; Zhu, H.; et al. X-Ray-Responsive Dissolving Microneedles Mediate STING Pathway Activation to Potentiate Cutaneous Melanoma Radio-Immunotherapy. Theranostics 2025, 15, 6919–6937. [Google Scholar] [CrossRef]
- Molina, A.H.; Swain, J.; Sunga, G.M.; Dharmaraj, N.; Hussein, N.I.; Rangel, R.; Sikora, A.G.; Hartgerink, J.D.; Young, S. 748 Localized STING Agonist Delivery via Liposome-MDP Hydrogel Composites plus Systemic α-PD-1 and α-CTLA-4 Improves Immunotherapy Responses. J. Immunother. Cancer 2024, 12. [Google Scholar] [CrossRef]
- Nigam, S.; Wilson, D. Advancements in AI-Driven Smart Nanocarriers for Controlled and Sustained Drug Release in Cancer Therapy. Int. J. Artif. Intell. Cybersecur. 2025, 1. [Google Scholar]
- Noury, H.; Rahdar, A.; Romanholo Ferreira, L.F.; Jamalpoor, Z. AI-Driven Innovations in Smart Multifunctional Nanocarriers for Drug and Gene Delivery: A Mini-Review. Crit. Rev. Oncol./Hematol. 2025, 210, 104701. [Google Scholar] [CrossRef]
- Zhou, L.; Yi, W.; Zhang, Z.; Shan, X.; Zhao, Z.; Sun, X.; Wang, J.; Wang, H.; Jiang, H.; Zheng, M.; et al. STING Agonist-Boosted mRNA Immunization via Intelligent Design of Nanovaccines for Enhancing Cancer Immunotherapy. Natl. Sci. Rev. 2023, 10, nwad214. [Google Scholar] [CrossRef] [PubMed]
- Samathoti, P.; Kumarachari, R.K.; Bukke, S.P.N.; Rajasekhar, E.S.K.; Jaiswal, A.A.; Eftekhari, Z. The Role of Nanomedicine and Artificial Intelligence in Cancer Health Care: Individual Applications and Emerging Integrations—A Narrative Review. Discov. Oncol. 2025, 16, 697. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-Tumor Genomic Biomarkers for PD-1 Checkpoint Blockade-Based Immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Koh, Y.; Shibaki, R.; Harutani, Y.; Akamatsu, H.; Hayata, A.; Sugimoto, T.; Kitamura, Y.; Fukuoka, J.; Nakanishi, M.; et al. Uncovering the Role of Tumor cGAS Expression in Predicting Response to PD-1/L1 Inhibitors in Non-Small Cell Lung Cancer. Cancer Immunol. Immunother. 2024, 74, 7. [Google Scholar] [CrossRef]
- Liang, B.; Xing, X.; Storts, H.; Ye, Z.; Claybon, H.; Austin, R.; Ding, R.; Liu, B.; Wen, H.; Miles, W.O.; et al. Antagonistic Roles of cGAS/STING Signaling in Colorectal Cancer Chemotherapy. Front. Oncol. 2024, 14, 1441935. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zheng, Z.; Zhang, H.; Wu, Y.; Shen, Y.; Chen, Q. cGAS-STING Pathway Mediates Activation of Dendritic Cell Sensing of Immunogenic Tumors. Cell. Mol. Life Sci. 2024, 81, 149. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ji, C.; Lu, X.; Cao, H.; Ling, Y.; Wu, Y.; Qian, L.; He, Y.; Song, B.; Wang, H. DNA Origami Plasmonic Nanoantenna for Programmable Biosensing of Multiple Cytokines in Cancer Immunotherapy. Anal. Chem. 2024, 96, 9684–9692. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, G.; Torres, K.; Stavros, F.; Ahmed, A.; Miller, J.; Zhao, T.; Gao, J.; Han, R. Abstract LB245: ONM-501, a Dual-Activating Polyvalent STING Agonist, Enhances Tumor Retention and Demonstrates Favorable Preclinical Safety Profile. Cancer Res. 2023, 83, LB245. [Google Scholar] [CrossRef]
- A First-in-Human Study of CDK-002 (exoSTING) in Subjects with Advanced/Metastatic, Recurrent, Injectable Solid Tumors, with Emphasis on Squamous Cell Carcinoma of the Head and Neck, Triple Negative Breast Cancer, Anaplastic Thyroid Carcinoma, and Cutaneous Squamous Cell Carcinoma. Available online: https://clinicaltrials.gov/study/NCT04592484 (accessed on 10 May 2025).
- Moser, J.C.; Alistar, A.; Cohen, E.; Garmey, E.; Kazmi, S.; Mooneyham, T.; Sun, L.; Yap, T.; Mahalingam, D. 618 Phase 1 Clinical Trial Evaluating the Safety, Biologic and Anti-Tumor Activity of the Novel STING Agonist IMSA101 Administered Both as Monotherapy and in Combination with PD-(L)1 Checkpoint Inhibitors. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Calvo, E.; Garralda, E.; Alonso, G.; Gambardella, V.; Parkes, E.E.; Thompson, J.; Latek, R.; Sikken, P.; Schmohl, M.; Harrington, K.J. 1030P Phase I, First-in-Human Trial Evaluating the STING Agonist BI 1387446 Alone and in Combination with Ezabenlimab in Solid Tumors. Ann. Oncol. 2023, 34, S626. [Google Scholar] [CrossRef]
- Luke, J.J.; Pinato, D.J.; Juric, D.; LoRusso, P.; Hosein, P.J.; Desai, A.M.; Haddad, R.; de Miguel, M.; Cervantes, A.; Kim, W.S.; et al. Phase I Dose-Escalation and Pharmacodynamic Study of STING Agonist E7766 in Advanced Solid Tumors. J. Immunother. Cancer 2025, 13, e010511. [Google Scholar] [CrossRef]
- Harrington, K.J.; Champiat, S.; Brody, J.D.; Cho, B.C.; Romano, E.; Golan, T.; Hyngstrom, J.R.; Strauss, J.; Oh, D.Y.; Popovtzer, A.; et al. Phase I and II Clinical Studies of the STING Agonist Ulevostinag with and without Pembrolizumab in Participants with Advanced or Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res. 2025, 31, 3400–3411. [Google Scholar] [CrossRef]
- Jacoby, J.; Mahalingam, D.; Alistar, A.; Garmey, E.; Kazmi, S.; Mooneyham, T.; Sun, L.; Yap, T.A.; Vu, P.; Moser, J. Phase 1 First-in-Human Dose-Escalation Study of IMSA101, a Novel Cyclic Di-Nucleotide STING Agonist, for Patients with Advanced Solid Tumor Malignancies. J. Immunother. Cancer 2025, 13, e011572. [Google Scholar] [CrossRef] [PubMed]
- STING Agonist and Personalized Ultra-Fractionated Stereotactic Adaptive Radiotherapy in Combination with Checkpoint Inhibition for Patients with Metastatic Kidney Cancer (SPARK). Available online: https://clinicaltrials.gov/ (accessed on 10 May 2025).
- An Open-Label, Dose Escalation and Expansion, Phase 1/2 Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Antitumor Activity of TAK-500, a Novel Stimulator of Interferon Genes Agonist, as a Single Agent and in Combination with Pembrolizumab in Adult Patients with Select Locally Advanced or Metastatic Solid Tumors. Available online: https://www.dana-farber.org/clinical-trials/22-038 (accessed on 10 May 2025).
- A Phase I Trial of CRD3874-SI, a STING Agonist, in Patients with Advanced/Metastatic Malignant Solid Tumors. Available online: https://www.mskcc.org/cancer-care/clinical-trials/23-169 (accessed on 10 May 2025).
- Chen, X.; Xu, Z.; Li, T.; Thakur, A.; Wen, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Liu, W.; Qin, J.-J.; et al. Nanomaterial-Encapsulated STING Agonists for Immune Modulation in Cancer Therapy. Biomark. Res. 2024, 12, 2. [Google Scholar] [CrossRef] [PubMed]
| Additional Therapy | Mechanism of Action (Component 3) | Examples of Nanoparticles | Cancer Model | Main Result |
|---|---|---|---|---|
| Chemotherapy | Cytotoxic DNA damage in cancer cells | PhenPT NPs | A549 in vitro | Induction of apoptosis occurs by DNA damage, both with PhenPt NPs and with cisplatin. Cisplatin activates the canonical cGAS-STING pathway. |
| LLC1 in vivo | PhenPT NPs show stronger cytotoxicity than cisplatin. PhenPt NPs in combination with anti-PD-L1 therapy induce a strong systemic immune response and inhibit the growth of tumor metastases. | |||
| NP2 (Pt(IV) + MK1775) | Biu87, UMUC3, T24, Mb49 in vitro | NP2 showed a significantly higher cytotoxic effect and apoptosis rate than the other molecules compared. The best result was achieved in the T24 and Mb49 models. | ||
| Biu87, Mb49 in vivo | NP2 demonstrates efficacy in vivo. The use of anti-PD-L1 therapy further enhanced the anti-tumor efficacy. | |||
| STACI | LLC-JSP in vivo | Administration of volasertib, a STING agonist, and an anti-PD-L1 antibody in 3 doses increased median survival from 11 days to 24 days. | ||
| G-M NPs | 4T1 in vivo | The use of nanoparticles alone with the STING agonist and gemcitabine resulted in an 87.1% inhibition of tumor growth. Administration of an additional anti-PD-L1 antibody enhanced this effect, with a final inhibition of 98.0% of the primary tumor. | ||
| RMP@Cap | 4T1 in vivo | Administration of RMP@Cap induced tumor cell pyroptosis, which enhanced STING activation. Introduction of anti-PD-L1 into the nanocapsule attenuated the inhibitory effect of tumor cells on the recruitment of cytotoxic T cells. The erythrocyte membrane coating enabled a longer half-life and better accumulation of the drug in the tumor. | ||
| PMDDH | MCF7, MDA-MB-231, 4T1 in vitro and in vivo | The use of the nanoplatform increased the therapeutic efficacy of doxorubicin and reduced its cardiotoxicity. In addition, the use of metformin resulted in activation of the AMPK pathway, decreased PD-L1 expression, and promoted ICD. | ||
| Radiotherapy | Induction of DNA breaks and ROS by radiation | IRIN-silicasomes | MC38 in vitro and in vivo | IRIN-silicasomes in combination with radiotherapy showed higher efficacy than irinotecan (IRIN) alone in combination with radiotherapy. Activation of the immune response and potentiation by anti-PD-1 therapy were confirmed. |
| Photodynamic Therapy (PDT) | Generation of ROS by photosensitizer (Ce6, BMA) leading to mitochondrial damage | TPP-Ce6@siPD-L1 | 4T1 in vitro and in vivo | The therapeutic potential of nanoparticles was demonstrated. The addition of photodynamic therapy as a third treatment component accelerated DC maturation, increased T-lymphocyte infiltration, enhancing the immune response. The biosafety profile was assessed as favorable. |
| TCe6@Cu/TP5 | GL261, U87 in vitro and in vivo | We combined therapy with STING agonists, PDT, and copper ions, activating cuproptosis, encapsulated in a single nanoparticle. Activation of the systemic immune response, the ability to cross the blood–brain barrier, and benefits in immunotherapy for glioblastoma were demonstrated. | ||
| Sonodynamic Therapy (SDT) | Ultrasonic activation of sensitive substances (Ce6, Mn2+ ions), causing ICD | Ce6/PTX Nbs | 4T1 in vitro and in vivo | Inhibition of tumor growth and tumor metastasis formation was observed. The approach offers both imaging and therapeutic potential. |
| Nucleic Acids | Delivery of siRNA/miRNA or DNAzyme for PD-L1 silencing or immunostimulant production | AHA@MnP/siPD-L1 | 4T1 in vitro and in vivo | A strong anti-tumor effect and a high level of safety have been demonstrated. |
| TT-LDCP | HCA-1, Hep3B, JHH-7 in vitro and in vivo | The need for an effective delivery system was highlighted. The designed system effectively inhibited the immune checkpoint and delivered the immunostimulatory cytokine. | ||
| cGAMP-siPDL1@GalNPs | B16F10, 4T1 in vitro and in vivo | The anti-tumor effect of the primary tumor and distant tumors was demonstrated. Additional synergism with PDT has been demonstrated. | ||
| dsDNA@DMONs | MC38, 4T1, Panc02, MDA-MB-231, A375, B16-F10 in vitro and in vivo | A dsDNA system was developed to induce IFN-I production inside the tumor, which indirectly activates the STING pathway. High therapeutic efficacy was demonstrated, with 51.0% inhibition of melanoma growth. In addition, the combination with anti-PD-L1 antibodies increased efficacy up to 96.7% regression. | ||
| Cuproptosis | Cu2+ release (+/− Zn2+) → mitochondrial stress → mtDNA → stronger cGAS-STING activation | CZP NPs | 4T1 in vitro and in vivo | A combination of cuprotosis therapy, cGAS-STING activation, photothermal therapy, and immunotherapy was used. CZP NPs also increased the sensitivity of tumor cells to anti-PD-L1 treatment. |
| CLDCu | B16F10 in vitro and in vivo | Inhaled nanoparticles have been developed to treat cancer metastases to the lungs. | ||
| Nanovaccines | Simultaneous delivery of cGAMP and antigen → presentation of neoantigen and activation of the immune system | LDH-cGAMP (RFA) | Hepa1-6 in vitro and in vivo | The inhibition of cancer growth and the establishment of long-term immunity have been observed. |
| Acid-reactive polymer LNPs | B16-OVA, 4T1 | Nanovaccines accumulated in lymph nodes and caused dendritic cell uptake and neoantigen release from the cytosol. The STING agonist activated the STING pathway in dendritic cells. |
| Nanoparticle | Doses | Dosages (Days) |
|---|---|---|
| STING NPs | 10 µg cGAMP i.t. | 14, 17, 20 |
| TPP-MMONs | 10 mg/kg i.v. | 0, 2, 4 |
| NP2 (Pt(IV) + MK1775) | 3 mg Pt kg−1, i.v. | 0, 2, 4, 6, 8 |
| G-M NPs | 10 mg/kg intraperitoneal | 1, 3, 5, 7, 9 |
| cGAMP-siPDL1@GalNPs | 25 μL; 15 μg of cGAMP per mouse and 15 μg of siPDL1/siNC per mouse i.t. | 0 |
| dsDNA@DMONs | 25 µg/dose i.t. | 3, 5, 7, 9 |
| CZP NPs | 10/20/30/40/50 mg/kg i.t. | 2, 4, 6 |
| CLDCu | 10 mg/kg inhaled | 3 times every 7 days |
| Agonist/Platform | Formulation (NP/Free) | Trial/Status | Cancer Type | Combination | Key Note | Source |
|---|---|---|---|---|---|---|
| exoSTING (CDK-002) | Exosome-based NP | NCT04592484 Phase I/II Completed | HNSCC, TNBC, ATC, cSCC | Monotherapy | Focused on safety and pharmacodynamics; no extensive published results yet. | [144] |
| IMSA101 | Free | NCT04020185 Phase I/IIa Completed | Different Solid Tumors | ±ICI | Overall favorable safety profile in Phase I; transient cytokine-release-like events; RP2D established during escalation. Minimal sign of anticancer activity. | [145,149] |
| IMSA101 | Free | NCT06601296 Phase II Recruiting | Metastatic Kidney Cancer | +nivolumab + PULSAR | Estimated study completion: 2028-10. | [150] |
| BI 1387446 | Free | NCT04147234 Phase I Completed | Different Solid Tumors | ±ezabenlimab (anti-PD-1) | Early data: good tolerability in phase I; biomarker analyses ongoing. | [146] |
| E7766 | Free | NCT04144140 Phase I/Ib Terminated | Different Solid Tumors or Lymphomas | Monotherapy | Strong immune activation in phase I/Ib; AEs consistent with immune activation (fever, cytokine release). | [147] |
| Ulevostinag | Free | NCT03010176 Phase I/II Completed | HNSCC, TNBC | ±pembrolizumab | In a small cohort, activity signal observed; most common AE: fever. | [148] |
| TAK-500 | Free | NCT05070247 Phase I/II Terminated | Different Solid Tumors | ±pembrolizumab | Clinical futility of TAK 500 met. | [151] |
| CRD3874-SI | Free | NCT06021626 Phase I Recruiting | Different Solid Tumors | Monotherapy | Estimated study completion: 2029-08. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy. Biomedicines 2025, 13, 2160. https://doi.org/10.3390/biomedicines13092160
Bartusik-Aebisher D, Rogóż K, Aebisher D. STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy. Biomedicines. 2025; 13(9):2160. https://doi.org/10.3390/biomedicines13092160
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Kacper Rogóż, and David Aebisher. 2025. "STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy" Biomedicines 13, no. 9: 2160. https://doi.org/10.3390/biomedicines13092160
APA StyleBartusik-Aebisher, D., Rogóż, K., & Aebisher, D. (2025). STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy. Biomedicines, 13(9), 2160. https://doi.org/10.3390/biomedicines13092160








