Abstract
Objectives: This in silico study sought to identify specific biomarkers for mild traumatic brain injury (mTBI) through the analysis of publicly available gene and miRNA databases, hypothesizing their influence on neuronal structure, axonal integrity, and regeneration. Methods: This study implemented a three-step process: (1) data searching for mTBI-related genes in Gene and MalaCard databases and literature review, (2) data analysis involved performing functional annotation through GO and KEGG, identifying hub genes using Cytoscape, mapping protein–protein interactions via DAVID and STRING, and predicting miRNA targets using miRSystem, miRWalk2.0, and mirDIP, and (3) RNA-sequencing analysis applied to the mTBI dataset GSE123336. Results: Eleven candidate hub genes associated with mTBI outcome were identified: APOE, S100B, GFAP, BDNF, AQP4, COMT, MBP, UCHL1, DRD2, ASIC1, and CACNA1A. Enrichment analysis linked these genes to neuron projection regeneration and synaptic plasticity. miRNAs linked to the mTBI candidate genes were hsa-miR-9-5p, hsa-miR-204-5p, hsa-miR-1908-5p, hsa-miR-16-5p, hsa-miR-10a-5p, has-miR-218-5p, has-miR-34a-5p, and has-miR-199b-5p. The RNA sequencing revealed 2664 differentially expressed miRNAs post-mTBI, with 17 showing significant changes at the time of injury and 48 h post-injury. Two miRNAs were positively correlated with direct head hits. Conclusions: Our bioinformatic analysis suggests that specific genes and miRNAs, particularly hsa-miR-10a-5p, may be involved in molecular pathways influencing mTBI outcomes. Our research may guide future mTBI diagnostics, emphasizing the need to measure and track these specific genes and miRNAs in diverse cohorts.
1. Introduction
A traumatic brain injury (TBI) is a neurological injury that can lead to debilitating cognitive, emotional, and physical symptoms in the acute and chronic stages of recovery [1,2]. The majority (~90%) of TBIs are classified as mild (i.e., mTBI) [3]; however, the subjectivity surrounding grading injury severity and underreporting of head injuries remain substantial challenges [4,5,6]. Most adults recover within 10–14 days [1,7,8]; however, approximately 20% of adults who suffer an mTBI have symptoms lasting more than one month [1,9,10]. It has been shown that older individuals with a history of brain injury may be at higher risk of developing neurodegenerative diseases, including Alzheimer’s disease [11,12,13].
The stress and strain from mechanical forces applied during an mTBI event can cause shearing and compression of brain structures [14]. Those biomechanical forces initiate the primary injury phase, characterized by loss of axonal and blood–brain barrier (BBB) integrity, which then progresses into a secondary phase as ion imbalances and neuroinflammation gradually become exacerbated [15,16,17,18]. The secondary injury phase involves a neurometabolic cascade that affects brain structure and function on a molecular level that can develop into excitotoxicity, oxidative stress, and cell death [18,19,20,21]. Due to the unique nature of each mTBI, highly sensitive and objective diagnostic tools are eminently required to aid in personalized assessments and treatments for the diverse clinical presentations associated with these injuries.
Numerous genes and molecular factors, such as APOE (neuronal repair), BDNF (synaptic plasticity), IL-6, IL-10, TNFα (neuroinflammation), ENO2, UCHL1 (neuronal injury), GFAP, and S100B (glial activation, blood–brain barrier integrity), are implicated in mTBI [22,23,24,25,26,27]. These genes play important roles in critical processes such as inflammation, neuronal injury, repair, and cognitive dysfunction, influencing both short- and long-term outcomes. Short-term brain health following head trauma could be related to genetic variants that affect the severity of axonal damage, BBB disruption, inflammation, neuronal survival, and cognitive dysfunction [28,29]. Furthermore, long-term outcome may be determined by genes involved in neuroplasticity and neuronal regeneration [29]. Research highlights the significance of immune-related gene expression changes in concussion. Simpson et al. (2024) examined peripheral blood transcriptomes in concussed athletes, revealing an initial activation of the immune response post-injury, which was later followed by cytokine downregulation [30]. As a result, identifying and characterizing the role of specific genes and micro ribonucleic acids (miRNAs) associated with mTBI could lead to the development of targeted interventions based on injury specific diagnostic biomarkers.
miRNAs are small non-coding regulatory RNAs that directly regulate gene expression by preventing or increasing the translation of target messenger RNA (mRNA) [31,32,33]. The substantial influence of miRNAs on brain development and function makes them potentially useful biomarkers with high specificity for mTBI severity and prognosis [34,35,36]. In a healthy person, the BBB protects the brain by providing highly specific transportation of small molecules and macromolecules such as proteins [37]. However, direct head injury can cause damage that allows brain-specific miRNAs to cross the BBB through microvesicles, exosomes, and lipoprotein carriers, leading to their abnormal presence in peripheral circulation [36,38]. Recent studies emphasize the role of miRNAs, particularly exosomal miRNAs, as non-invasive biomarkers for diagnosing and understanding TBI [39]. Yang et al. (2024) identified miR-206 and miR-549a-3p as key markers for tracking neuronal damage and recovery, supporting the importance of circulating miRNAs in brain injury response [39]. Feng et al. (2024) found that hsa-miR-122-5p and hsa-miR-193b-3p were notably elevated in the blood of TBI patients after treatment, showing a correlation with injury severity and microglial activation [40]. These results underscore the value of miRNA profiling in tracking brain injury progression and understanding its mechanisms.
In the current study, we used a bioinformatics workflow to systematically identify genes and their regulating miRNAs specifically associated with mTBI (Figure 1). Bioinformatics analysis tools have been widely used to detect genes, miRNAs, and functional pathways involved in the pathogenesis and progression of TBI [41,42,43,44]. In silico studies (i.e., which rely on databases and pathway analysis software) provide valuable information about direct and indirect gene interactions to identify alterations present in specific genes and miRNAs. However, mTBI is a heterogeneous condition that affects numerous neurological processes, making it challenging to identify accurate and specific biomarkers. Currently, there are no meaningful and specific biomarkers that can be utilized to predict the clinical outcome of mTBI [42].
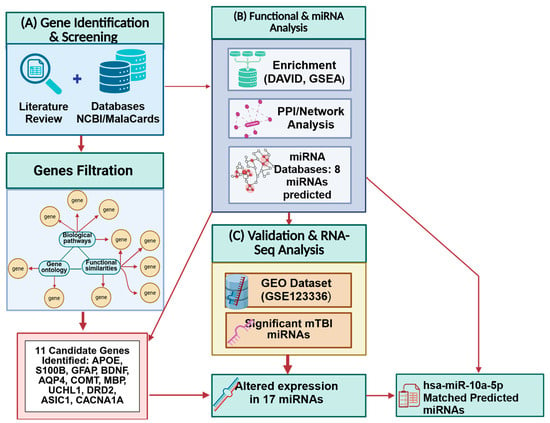
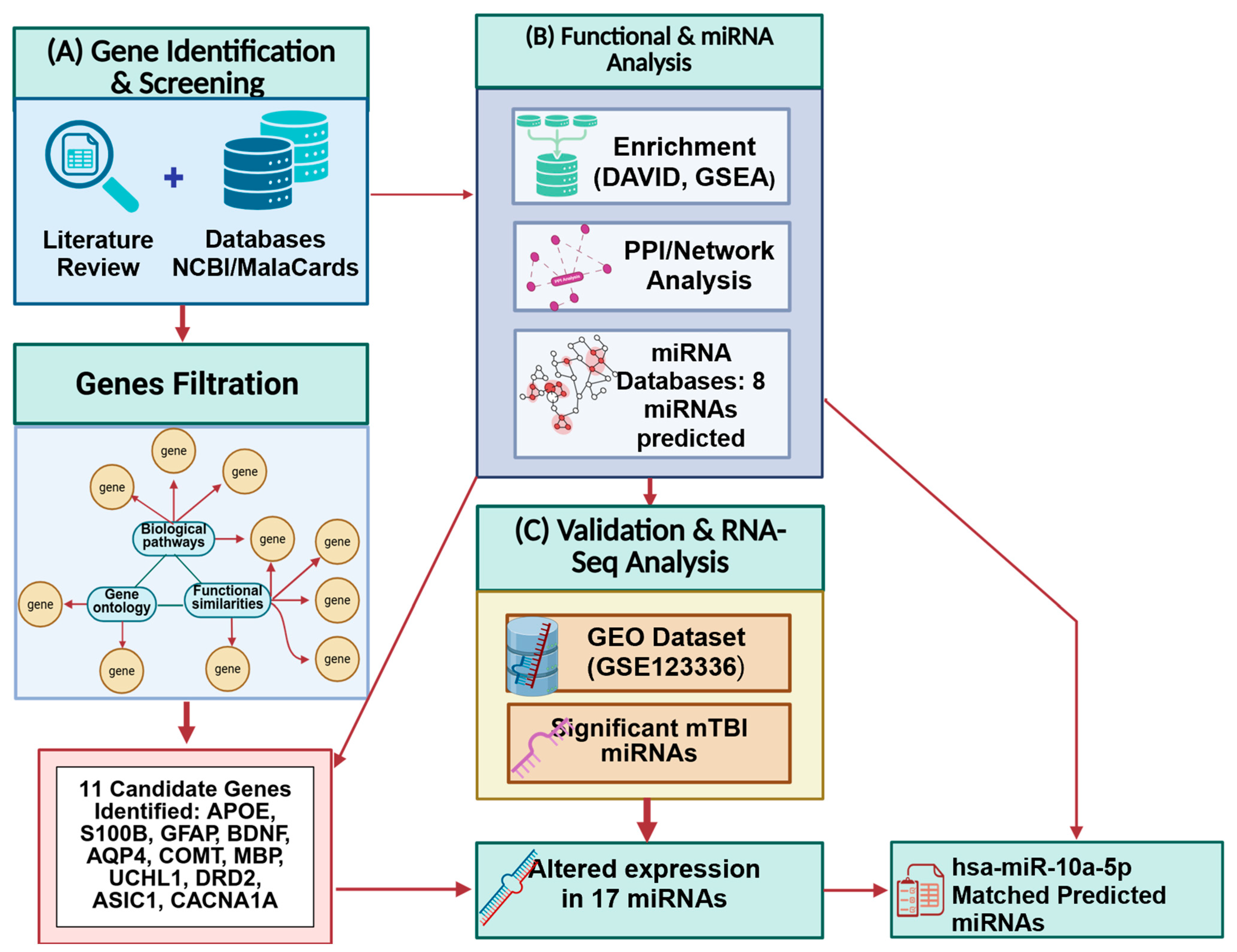
Figure 1.
Integrated methodological workflow summarizing the study design. (A) Literature and database screening. (B) Functional enrichment and network analyses. (C) RNA-seq dataset (GSE123336) analysis. Created in BioRender. Tajik, M. (2025) https://BioRender.com/1lsg3r7 (accessed on 20 October 2025).
Taking all of the above into account, the purpose of our study was to identify potential hub genes and biological pathways associated with mTBI neurological sequelae using multiple online databases and pathway analyses. Online miRNA bioinformatics tools were then used to predict target miRNAs correlated with hub genes and the pathophysiological processes associated with mTBI. Additionally, we searched the Gene Expression Omnibus or GEO database for RNA-sequencing studies on acute mTBI [45] to analyze freely accessible data and compare it with our miRNA predictions. Our goal was to determine how acute mTBI affects the temporal expression of circulating miRNAs and to survey the biological and neuronal pathways related to these miRNAs. It was hypothesized that several genes related to neuronal regeneration and healthy cognition would be identified as hub genes related to mTBIs, and that several miRNAs would be identified as potentially useful biomarkers.
2. Methods
Our previously published literature review article [46] focused on estimating how many genes are associated with neurological structural and functional changes related to mTBIs, and which genes are more significantly altered post-injury (Figure 2). These genes were chosen by functional similarity, biological pathways, gene ontology, and phenotype related to mTBI outcome (Figure 1). We reviewed the physiological, molecular, and omics changes that are present following a brain injury, particularly mTBI, as well as earlier research on gene and miRNA changes, polymorphism, and the effect of single-nucleotide polymorphisms (SNPs) on mTBI patient recovery [46]. We also reviewed epigenetic mechanisms that follow head trauma and their impacts on gene expression and neurological dysfunction. We included studies on human and mammalian animal models that examined changes in blood biomarker levels following mTBI and their correlation with neurological symptoms. The search was performed using primary databases such as PubMed. Search terms included combinations of ‘mild traumatic brain injury’ or ‘mTBI’ and ‘genes’ and ‘miRNA’. We focused on experimental studies that explored the genetic and molecular factors contributing to the pathophysiology and recovery of mTBI [43]. Inclusion criteria for gene selection were based on the following: (1) functional similarity to established mTBI-related pathways (such as neuroinflammation, neuronal repair, and synaptic plasticity); (2) involvement in pertinent biological processes (identified through gene ontology terms associated with inflammation, glial activity, cognition, and axonal repair); and (3) their purported influence on mTBI outcomes (such as functional recovery, cognitive impairment, and long-term neurological effects). Gene ontology, biological pathways, functional similarities, and symptoms associated with mTBI outcomes were taken into consideration while choosing our gene set. Based on our initial search, inclusion criteria, prevalence of genes in the literature, their involvement in key biological pathways, and their relevance to mTBI-related symptoms, 30 genes were identified as potentially playing a role in mTBI neuronal health and function and selected for further analysis in our study (the methodology workflow is shown in Figure 1).
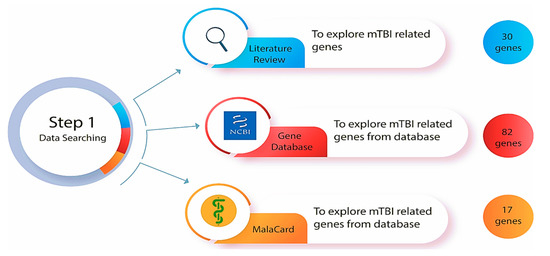
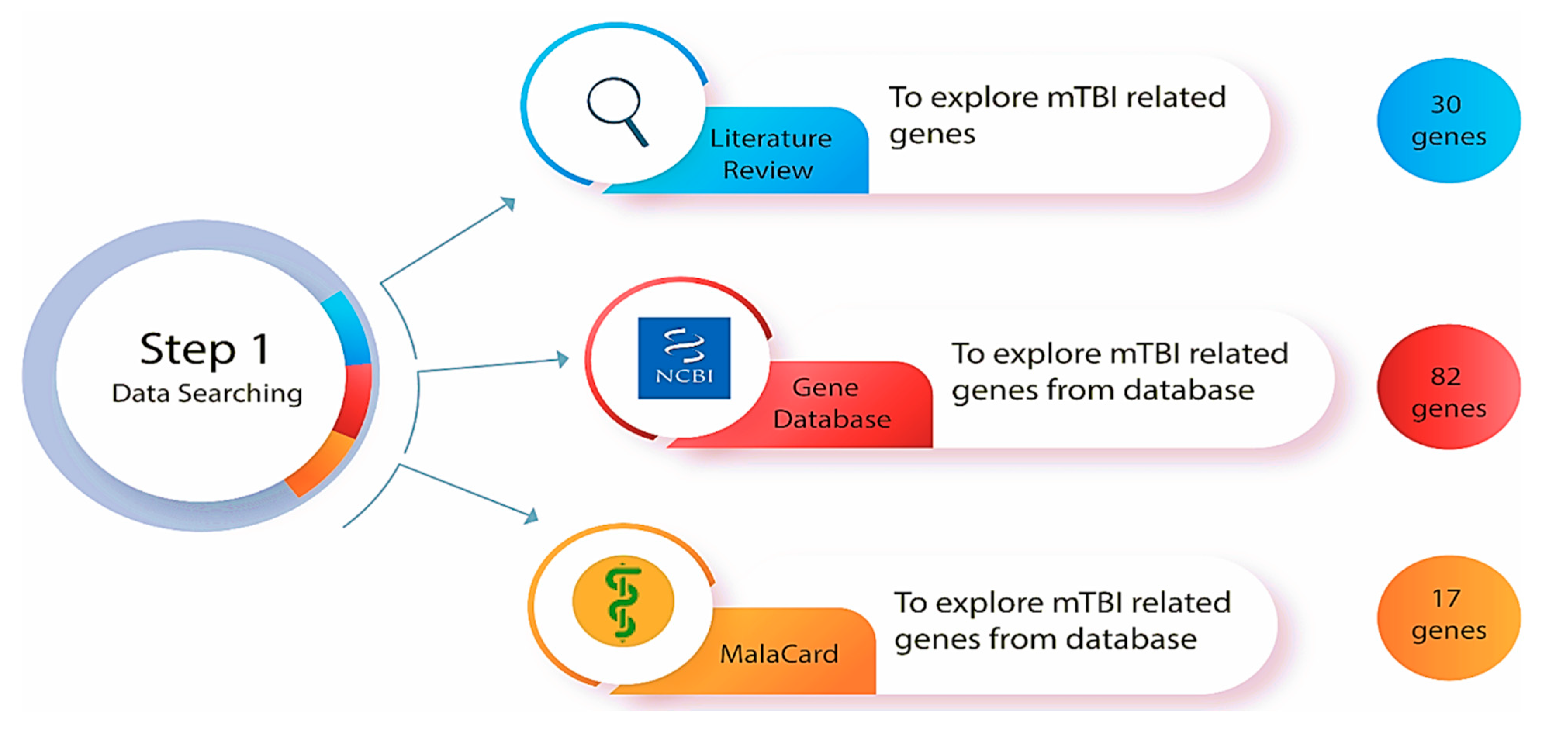
Figure 2.
Step one of the methodologies was to explore mTBI-related genes in the databases (Gene database from National Center for Biotechnology Information (NCBI) and MalaCard) and a comprehensive literature review of genetically focused mTBI research.
2.1. Bioinformatic Databases
A comprehensive and thorough search of online bioinformatics databases was conducted using the gene databases from the National Center for Biotechnology Information (NCBI) [47] and the human disease database MalaCards [48] to determine how many genes have been correlated with mTBI (Figure 2). This search involved the utilization of specific search terms (such as ‘gene database’, ‘disease database’ and ‘miRNA prediction database’), filters, and criteria to ensure the retrieval of relevant and up-to-date information. Gene databases supply gene-specific connections in the nexus of map, sequence, expression, structure, function, citation, and homology data, and the identity of a gene can be determined by specifying the sequence, map position, or phenotypic characteristics [47]. These gene identification numbers are utilized across all NCBI datasets and kept up to date via annotation changes [47]. This database incorporates data and linkages to the Online Mendelian Inheritance in Man (OMIM) database (https://www.omim.org/) (accessed on 18 October 2025), which is a continuously updated catalog of human genes, genetic disorders, and traits. “Mild traumatic brain injury” was the key phrase used to search in the gene database, and the results were automatically displayed in tabular format. The results were sorted by relevance to the condition and gene weight, and humans were selected as the primary organism. As determined by the gene dataset, 82 genes and 5 miRNAs are known to be involved with mTBI in humans (a list of affiliated genes with mTBI was determined based on a Gene Weight calculation, which included multiple lines of evidence such as gene expression, protein clusters, and OMIM entries [47].
MalaCards is a comprehensive database of diseases and their annotations that creates an electronic card for each of the 16,919 human disorders by combining and mining 44 data sources [48]. MalaCards contains disease-specific prioritized annotations and inter-disease connections based on GeneCards, GeneDecks, and their search capabilities [48]. We performed a MalaCards search to determine how many genes are associated with TBI, and 17 genes were identified as key. The relevance score for each was calculated by considering the significance of the many resources linking the gene to the condition [48] (https://www.malacards.org/) (accessed on 18 October 2025).
2.2. Data Analysis: Functional Enrichment and Pathway Analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID, version 6.8) was used to address functional annotation, visualization, and biological meanings behind the mTBI-associated genes with specific Gene Ontology (GO) [49,50] (https://davidbioinformatics.nih.gov/) (accessed on 20 October 2025). The DAVID bioinformatics resources include a combined biological pool of knowledge and algorithms for systematically deriving the biological role of genes from an extensive list of genes and proteins [50]. GO defines the interaction across genes by annotating and categorizing the molecular function (MF), biological process (BP), and cellular components (CCs) linked with a gene product [51]. This process allowed enrichment analysis of a gene collection [51], which was carried out in this study to reveal which MF, BP, and CC were disproportionately represented in our list of human genes related to mTBIs. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was then used to explore molecular interactions and to create a network of relationships between our candidate genes [52] (https://www.genome.jp/kegg/pathway.html) (accessed on 20 October 2025). To better understand the biological significance of mTBI candidate genes, our gene list was uploaded into DAVID for enrichment analysis related to GO and KEGG terms. A filter of 0.05 was used as the significance cut-off value for fold enrichment analysis in the DAVID database. A Gene Set Enrichment Analysis (GSEA) (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp) (accessed on 20 October 2025) was performed that focused on gene groups that shared a similar biological function, regulation, and chromosomal location [53]. GSEA analysis was performed on our candidate genes to identify biological processes or pathways that may cause neurological perturbation following mTBI (Figure 3).
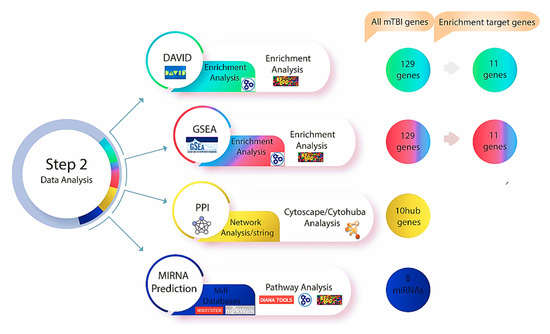
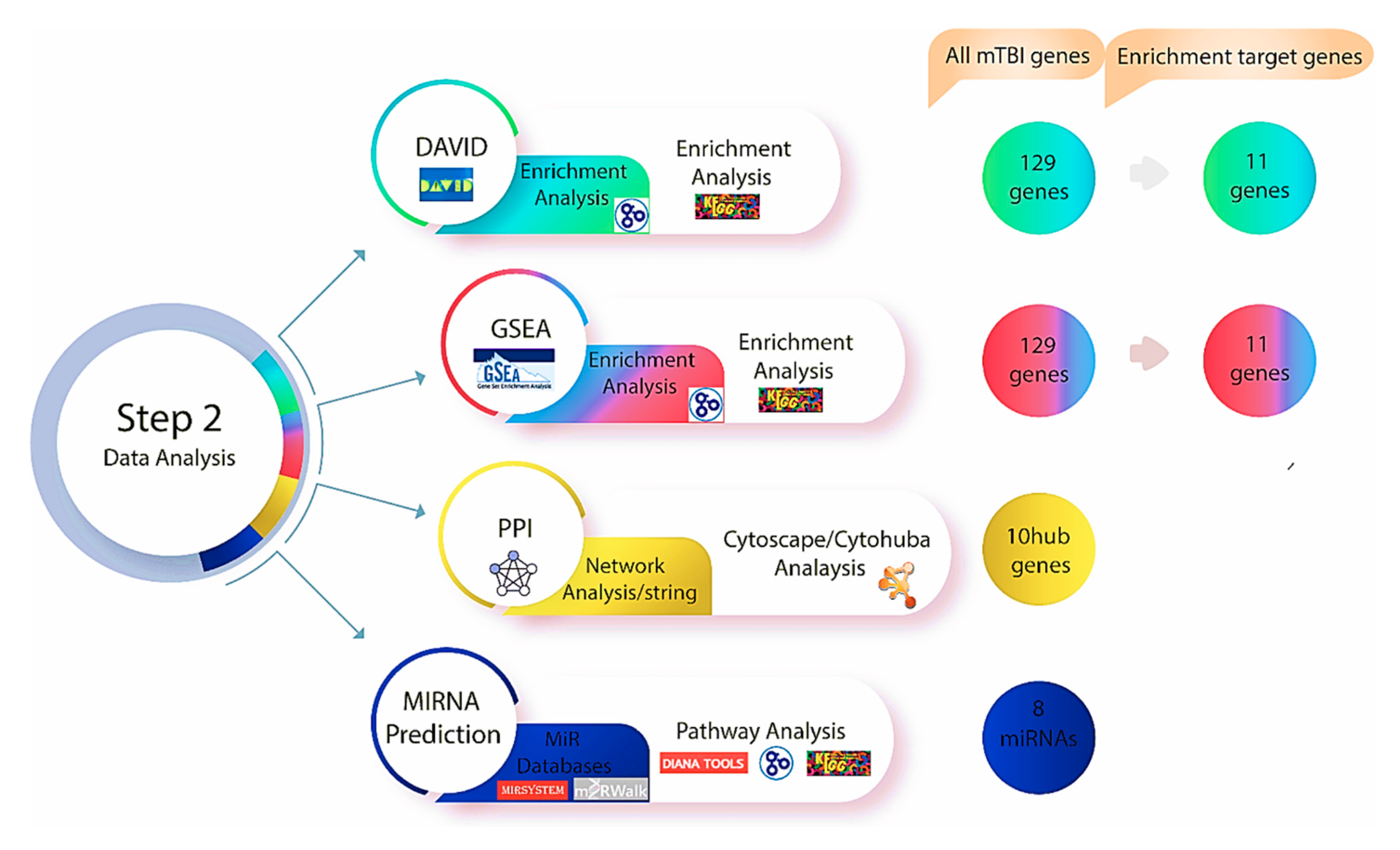
Figure 3.
Step two of the methodology was performed after investigating genes using Gene Database and Malacards, the DAVID and GSEA databases were used to find relevant genes and biological pathways. Based on the enrichment analysis, 11 genes out of 129 exhibited a significant relationship with mTBI outcomes in both datasets. Protein–protein interaction and network analyse were performed utilizing string databases, while 10 Hub genes were identified using Cytohubba. Multiple miRNA databases were used to predict miRNAs linked to mTBI candidate genes and key pathways tied to mTBI changes.
2.3. Protein–Protein Interaction (PPI) Network and Hub Genes
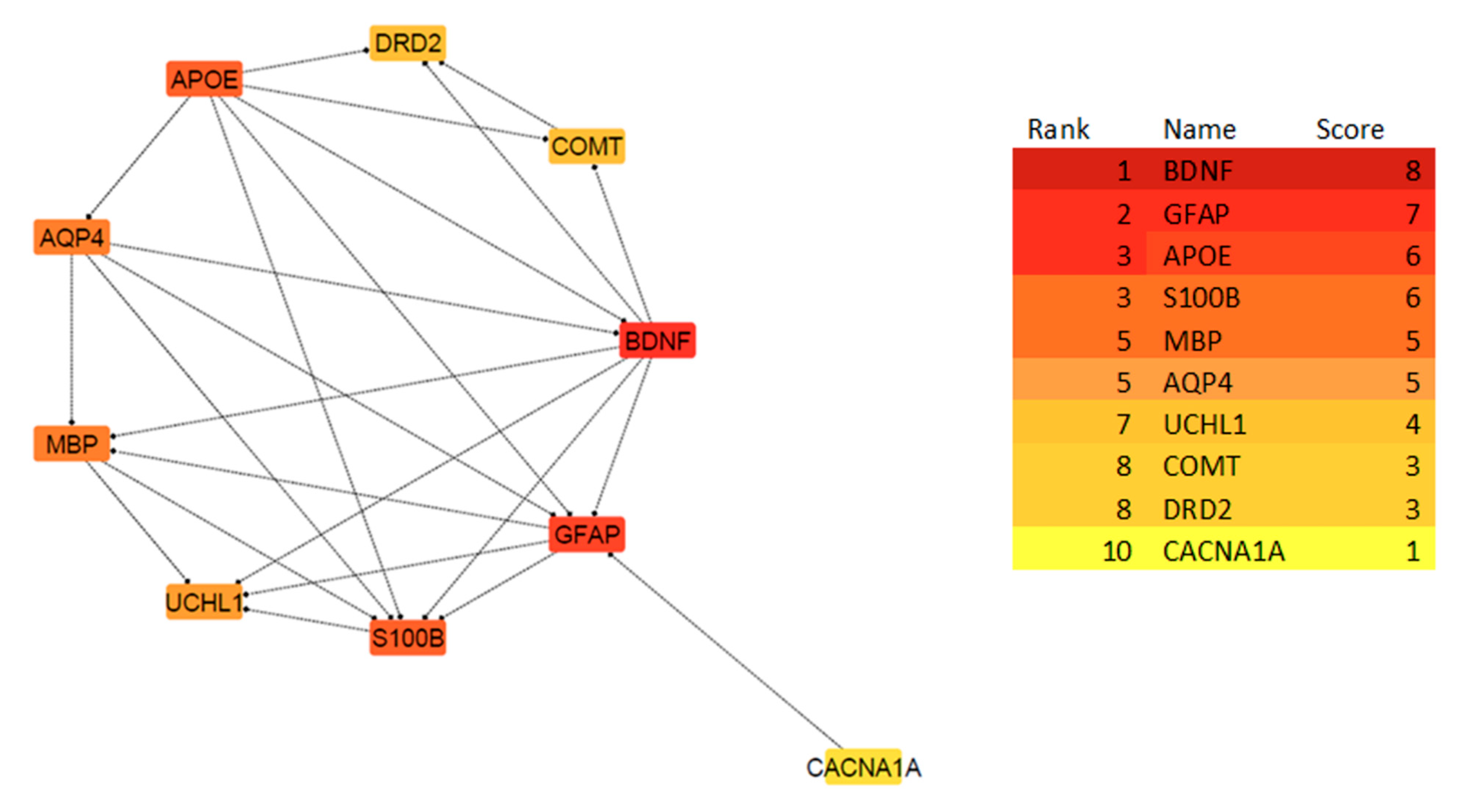
The Search Tool for the Retrieval of Interacting Genes (STRING, version 11.0) is an online database that offers access to experimentally determined and predicted protein–protein interaction (PPI) data [54] (https://string-db.org) (accessed on 18 October 2025). PPI networks were built using STRING to investigate the functional interactions between the mTBI candidate genes and proteins that we identified using DAVID and GSEA. The parameter was set to have a medium confidence score of >0.4. To visualize the network, Cytoscape (version 3.8.2) [55] with the CytoHubba plug-in [56] was used to perform a network analysis on the top ten candidate genes to indicate interactions based on 11 scoring or topological approaches [56] (Figure 3). We used the degree of interaction as the main measure of relevance in this study and identified the top ten mTBI genes that were used moving forward in this study.
2.4. miRNA-Target Gene Regulatory Network
The role of miRNAs is to control gene expression by interacting with their target genes during the post-transcriptional phase. In our study, online tools were used to first identify miRNAs that regulate mTBI candidate genes and then, secondly, we constructed miRNA-target gene regulatory networks using Cytoscape software (Figure 3). To predict which miRNAs regulate mTBI candidate genes, various prediction tools were used, including miRSystem, miRWalk2.0, and mirDIP. The miRSystem [57] (http://mirsystem.cgm.ntu.edu.tw/) (accessed on 18 October 2025). integrates with seven well-known miRNA target gene prediction programs, and miRNAs were selected based on whether the total hit value was greater than or equal to one. The miRWalk2.0 (http://mirwalk.umm.uni-heidelberg.de/) (accessed on 18 October 2025) [58] interacts with 12 different online databases to predict miRNA. With this approach, miRNAs were chosen if they appeared in at least three out of the 12 miRWalk databases (miRMap (https://mirmap.ezlab.org/) (accessed on 18 October 2025) [59], Targetscan (http://www.targetscan.org/) (accessed on 20 October 2025) [60], and miRDB (http://www.mirdb.org) (accessed on 20 October 2025) [61]). The miRDIP, another comprehensive database for predicting miRNAs, was used to identify miRNAs with very high scores that were directly linked to identified mTBI-related genes (http://ophid.utoronto.ca/mirDIP/) (accessed on 18 October 2025) [62]. The DIANA mirPath (v.3) (http://diana.imis.athena-innovation.gr/DianaTools/index.php) (accessed on 18 October 2025) [63] and the miRNA pathway dictionary (miRPathDB version 2.0) [64] (https://mpd.bioinf.uni-sb.de/) (accessed on 20 October 2025) were also utilized to more precisely identify miRNAs associated with the mTBI candidate genes based on biological pathways and gene ontology enrichment. The only variations between these prediction tools are the algorithms and statistical analyses that each database employs to categorize miRNAs, but by employing several prediction tools, the consistent miRNA across multiple sources allowed for corroboration of results.
2.5. RNA-Sequencing Analysis: Analysis of a mTBI Dataset
Given the recent evidence of miRNA responses in brain injury [65], we performed miRNA sequencing analysis to explore differential expression patterns and their association with injury severity and recovery. A small RNA sequencing study of human serum and saliva prior to, during and after amateur mixed martial arts (MMA) competitions (dataset name: GSE123336) [45] was downloaded from the GEO repository to apply the result of our miRNA predictions to a human mTBI dataset (http://www.ncbi.nlm.nih.gov/geo) (accessed on 20 October 2025) [66] as of March 2023 (Figure 4). Institutional ethics approval was not required at our site for this study, as data was downloaded from a publicly available dataset and participants from the original dataset provided informed consent at the time of their data collection [42]. GEO is a global public database that stores and openly disseminates high-throughput functional genomics data from microarray, next-generation sequencing, and other sources that are contributed by the science community [66]. The dataset was based on Illumina NextSeq 500 (Homo sapiens) and consists of a total of 218 samples (131 serum and 87 saliva samples) [45]. In that study, raw data was obtained from serum samples that were collected at different time points in relation to the acute mTBI (1-week pre-injury = 7, 0 days pre-injury = 52 samples, 0 days post-injury = 52 samples, 2–3 days post-injury = 17 samples, 1-week post-injury = 3) [45]. The original paper did not provide a clear explanation for the decrease in sample numbers after the immediate post-injury time points. However, this reduction could likely be due to common issues in longitudinal studies, such as participant dropout, logistical challenges, or the unavailability of participants for follow-up sample collection. The fastq files belonging to 131 serum samples were directly downloaded from the European Nucleotide Archive (ENA) database.

Figure 4.
Step three of the methodology was to investigate the GEO repository and download RNA-seq data associated with mTBI for mapping and differential expression analysis.
2.6. Data Processing
The original fastq files related to the GSE123336 data were downloaded from the European Nucleotide Archive (ENA) (https://www.ebi.ac.uk/ena/browser/home) (accessed on 19 October 2025). As a first step, fastq data were checked using the FastQC tool (Babraham Bioinformatics) (Figure 4). The fastq files contained 3′ Illumina adapters that were trimmed using Trimmomatic software Version 0.39 [67] (http://www.usadellab.org/cms/?page=trimmomatic) (accessed on 19 October 2025). The data utilized for the analysis were single-end reads (i.e., the sequencer reads DNA fragments from one end to the other). Command-line-based scripts were used to specify the trimming stages to remove the adapters from sequences. Subsequently, two rounds of alignment were carried out using the Burrows–Wheeler Alignment (BWA) software 0.7.19 (r1273), one against the reference genome »hg38« and the other against the mature miRNA sequences (https://bio-bwa.sourceforge.net) (accessed on 19 October 2025) [68]. Aligning to the reference genome is a crucial supplementary measure, as it can further enhance the count matrix generated from miRBase alignment [69]. In cases where reads did not align with miRBase, they were aligned with the genomic coordinates of mature miRNA present in the reference genome [69].
The BWA index function was used to build a full index for the genome reference »hg38«. A mature Fasta miRNA sequence, downloaded from miRBase database version 22 [70,71], then the BWA aln algorithm were used to align short read sequences with the indexes. Samtools view and Samtools sort were used to create BAM files and sorted BAM files, respectively [72]. FeatureCounts were conducted for counting reads and building a count matrix and comparing aligned reads with miRNA transcripts downloaded from the miRBase database (has.gff 3) (https://www.mirbase.org/) (accessed on 19 October 2025) [70,71]. As a final step, R software (version 4.5.1) and differential gene expression analysis, based on the negative binomial distribution (DESeq2) package, were employed to identify differential miRNA expressions following an acute mTBI at the various time points and relative to the numbers of hits to the head the athlete sustained. Comparisons were made between each post-injury time point (0 days post-injury, 2–3 days post-injury, and 1-week post-injury) and the pre-injury baseline (0 days pre-injury and 1 week pre-injury). The pre-injury samples of each participant were compared to their equivalent post-injury samples, rather than being combined across time points. This ensured that each subject served as their own control, reducing variability due to inter-individual differences. Statistical analysis was used to identify miRNAs with significantly altered levels, based on a P-adjusted value threshold of ≤0.05 [73]. A Bonferroni correction was used to adjust for the total number of miRNAs analyzed across different time points. All analyses are available in supplemental files (All the analysis are provided in Supplementary File SA).
3. Results
3.1. Identifying Candidate Genes Using Databases and Literature
According to published research, neurotrophic, inflammatory and catecholamine genes are more often altered after brain trauma and serve as reliable predictors of injury severity and recovery [28]. These genes have a substantial influence on biological processes associated with acute mTBI outcomes. To identify genes involved with mTBI, peer-reviewed journal articles [46] and a search of two databases (Gene and MalaCards) were conducted. From these searches, 129 genes were significantly correlated with mTBI (82 genes from Gene database, 17 genes from MalaCards, and 30 genes were identified from experimental studies). For a gene to meet study inclusion criteria, function, expression in the blood circulation system, and the most direct relationship to mTBI neuronal dysfunction and recovery were considered. Genes that are expressed only in brain tissue, inflammatory genes, and cytokines were excluded. We exclude genes exclusively expressed in brain tissue because the goal of our research was to identify circulating genes, particularly those involved in blood circulation, which could potentially act as in vivo and non-invasive biomarkers of mTBI-related dysfunction. However, we acknowledge that excluding brain-specific genes could limit the scope of our findings, particularly in relation to their direct involvement in brain pathology and recovery after mTBI. Inflammatory genes and cytokines, although crucial in the context of mTBI, were excluded due to their typical association with general inflammatory responses and immune system regulation. Given that inflammation engages diverse systemic and glial responses that extend beyond neuron-specific injury mechanisms, we limited our analysis to genes more directly implicated in neuronal dysfunction, repair, and recovery to maintain biomarker specificity. Based on these criteria (i.e., neurological symptoms, cognitive impairment, recovery timeline and hub gene analysis), we were able to focus the mTBI list from 129 to 11 genes: APOE (Apolipoprotein E), S100B (s100 calcium binding protein B-), GFAP (Glial fibrillary acidic protein), BDNF (Brain-derived neurotrophic factor), AQP4 (Aquaporin-4), COMT (Catechol-O-methyltransferase), MBP (Myelin basic protein), UCHL1 (Ubiquitin C-terminal hydrolase L1), DRD2 (Dopamine receptor D2), ASIC1 (Acid-sensing ion channel 1), and CACNA1A (Calcium voltage-gated channel subunit alpha 1 A). These genes were determined based on their functional similarities, involvement in biological pathways, phenotypes, protein interactions, and expression related to mTBI outcome. Previous studies have demonstrated that many of these 11 candidate genes exhibit deregulated expression following mild traumatic brain injury. GFAP and S100B are consistently upregulated in serum and cerebrospinal fluid and correlate with neuronal and axonal injury severity [74,75,76]. APOE genotype variants influence post-injury cognitive outcome and repair capacity [77], while BDNF levels fluctuate with synaptic plasticity during recovery [78]. COMT [79] and DRD2 [80,81] are involved in dopaminergic transmission, which is altered after head trauma and contributes to changes in cognition and behavior [82]. Clinical biomarker studies report that UCH-L1 is significantly elevated in peripheral blood early after mild TBI, and in combination with GFAP can achieve >80% sensitivity with high negative predictive value for intracranial lesions, supporting its utility in triage and monitoring [82,83]. Together, these reported alterations confirm that the genes identified through literature, NCBI, and MalaCards not only meet our bioinformatic inclusion criteria but also display experimentally observed deregulation in mTBI contexts, reinforcing their biological relevance to injury severity and recovery (additional details are provided in Supplementary File SB).
3.2. Data Analysis: Functional and Pathway Enrichment Analyses
Functional enrichment analysis was performed to identify GO terms and KEGG pathways for the 11 mTBI candidate genes (Figure 5). The top five enriched GO terms and two significant KEGG pathways for our candidate genes based on fold enrichment analysis shown in Table 1. The biological processes connected with mTBI genes were considerably rich in neuronal processes, and more specifically, neuron projection regeneration, which is related to the regrowth of axons or dendrites in response to their loss or damage (fold enrichment = 381.6, p = 4.80 × 103). The neuronal cell body has the highest concentration of cellular components associated with mTBI genes, and tau protein binding was found to be the most enriched molecular function associated with mTBI genes (Fold enrichment = 279, p = 6.50 × 103). Based on fold enrichment analysis, the only two significantly enriched KEGG pathways were cocaine addiction (fold-enrichment = 35.1; p = 4.90 × 10−2) and dopaminergic synapse pathways (fold-enrichment = 20.2; p = 6.80 × 10−3). Furthermore, GSEA analysis showed the majority of mTBI candidate genes were involved in cognition, synaptic signaling, memory, and nervous system processes. According to the GSEA phenotype analysis, APOE, BDNF, CACNA1A, COMT and UCHL1 were identified as the main genes associated with cognitive impairment (FDR q-value < 0.05) (Table 2).
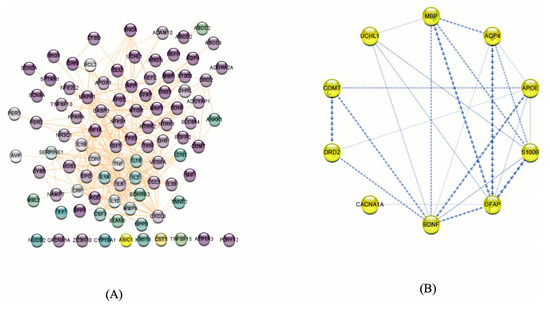
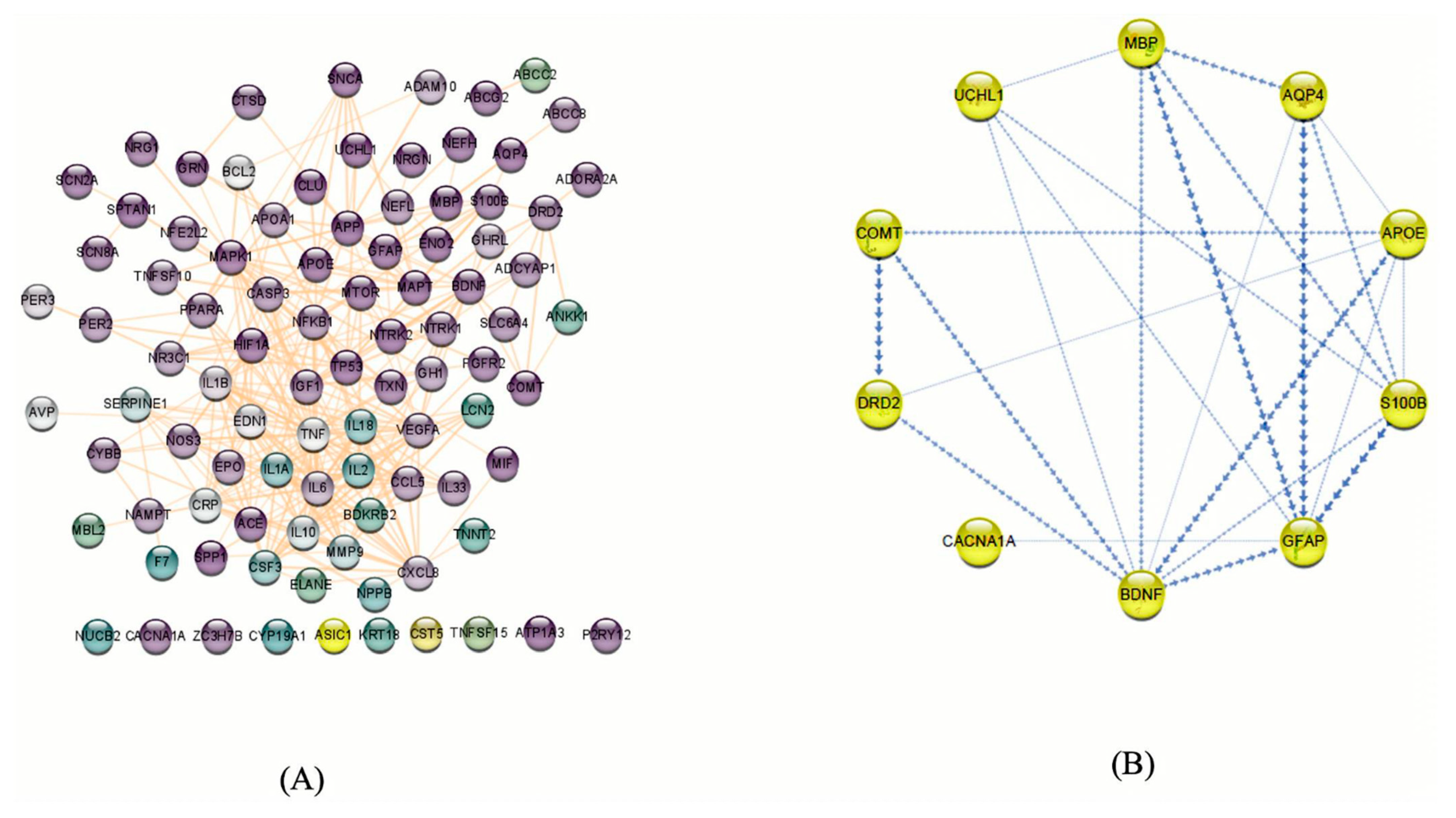
Figure 5.
(A) Potential genes related to mTBIs in humans based on our literature review and database searches. These genes were filtered based on neural functions, and purple nodes show the genes that have more neurological functions. The protein–protein interaction network was visualized using Cytoscape v.3.8.2. (92 nodes and 327 edges). (B) mTBI candidate genes according to the enrichment analysis and PPI and co-expression between them indicated by Cytoscape v.3.8.2. The boldness of the arrows represents the strength of the interaction between candidate genes.

Table 1.
DAVID functional analysis for the 11 mTBI genes. The highest fold enrichment analysis represents GO terms that are strongly related to candidate genes (abbreviations: biological process (BP), cellular component (CC), molecular function (MF)).

Table 2.
Gene set enrichment analysis for mTBI candidate genes. A GSEA analysis revealed that most mTBI genes involved cognitive, synaptic signaling, memory, and nervous system functions. According to this analysis, APOE, BDNF, CACNA1A, COMT, and UCHL1 are the major genes associated with cognitive impairment (p-value = 3.40 × 107) (abbreviations: biological process (BP), cellular component (CC), human phenotype (HP)).
3.3. Protein–Protein Interaction (PPI) Network and Hub Genes Analysis
By submitting the candidate genes into STRING, PPIs associated with the mTBI candidate genes were obtained (Figure 5). The hub genes with CytoHubba, and their degree of interaction, display 10 nodes and 24 edges (Figure 6). The interaction network revealed that most candidate genes exhibited strong interactions with each other, and enrichment analysis outcomes for hub genes showed different neurological tasks and neurological pathways related to the mTBI hub genes. For instance, ASIC1, APOE, S100B, COMT and DRD2 play essential roles in memory function; APOE, UCHL1, S100B, DRD2, BDNF and GFAP play key roles in neuron projection development; UCHL1, S100B, COMT, DRD2 and APOE are essential genes related to behavior; and APOE, S100B, DRD2 and BDNF play a significant role in the regulation of cell death (these processes were selected based on their higher node degree and FDR q-value) (Figure 6).
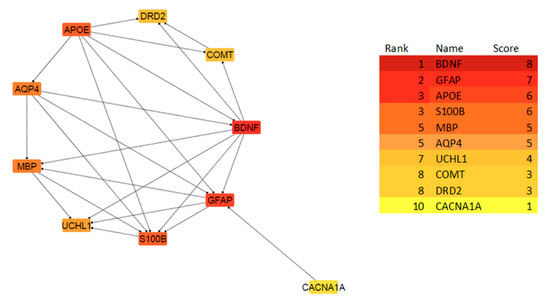
Figure 6.
The PPI network of the 10 Hub genes is clustered by CytoHubba in cytoscape software based on the degree of interactions. The degree is indicated with the color of nodes, where darker colors represent a higher degree of interaction, and lighter colors indicate a lower degree of interaction. Based on the CytoHubba analysis, BDNF showed the highest degree of interaction, while CACNA1A showed a lower degree of interaction.
3.4. miRNA—Target Analysis
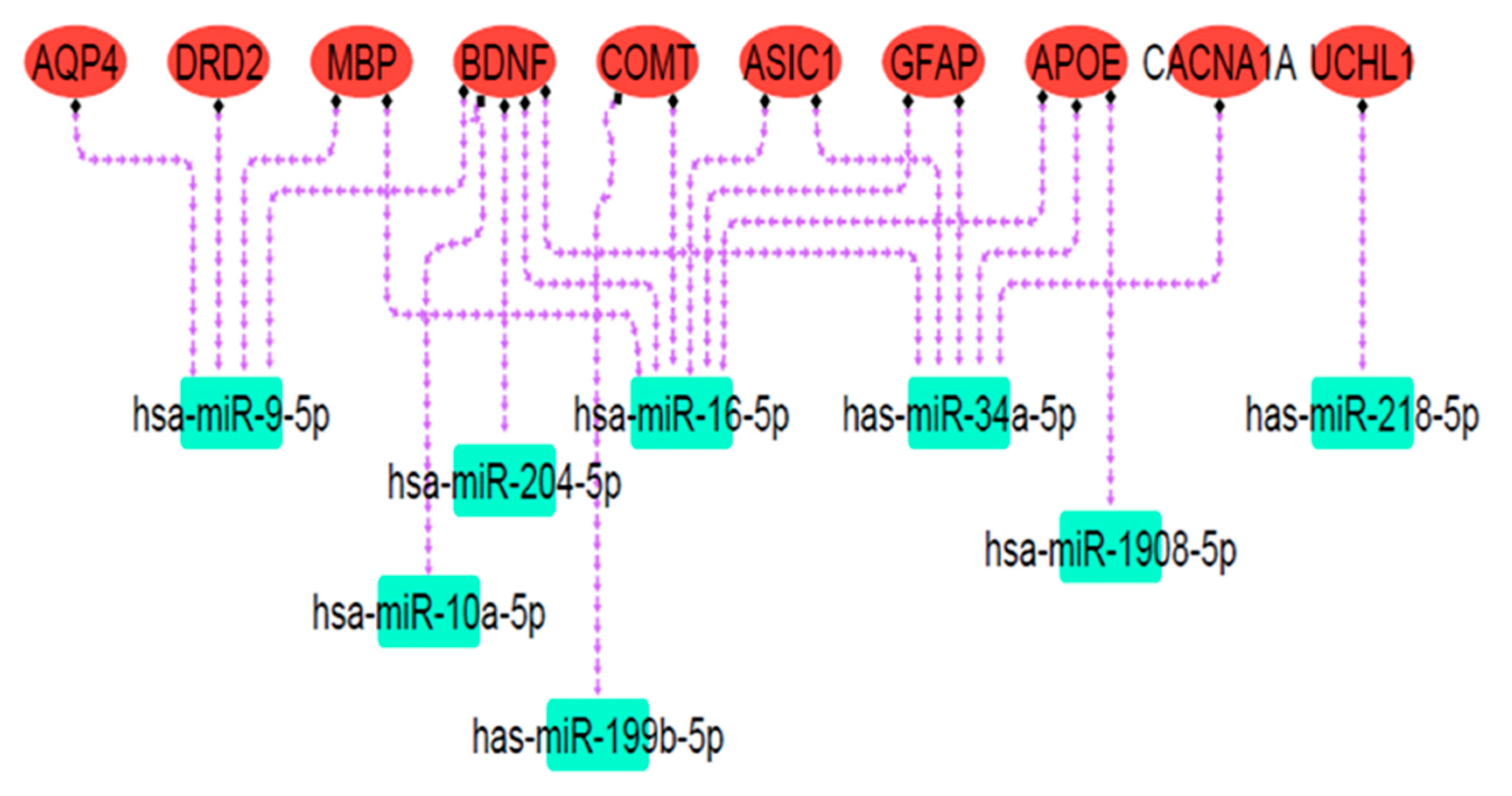
Online databases were utilized to predict target miRNAs connected to the mTBI candidate genes. Over 100 miRNAs were associated with each gene according to miRSystem, miRwalk 2.0, and miRDip. To identify significant miRNAs closely linked to mTBI genes, the DIANA mirPath v3.0 bioinformatic tool and miRpathDB v2.0 database were used. DIANA miRPath obtained miRNA gene targets and KEGG pathways related to gene function, while miRpathDB used GO to display molecular function, cellular component, and biological process associated with mTBI-selected miRNAs and their related genes. The results showed that miRNAs of hsa-miR-9-5p, hsa-miR-204-5p, hsa-miR-1908-5p, hsa-miR-16-5p, hsa-miR-10a-5p, has-miR-218-5p, has-miR-34a-5p, and has-miR-199b-5p were related to the mTBI candidate genes and exhibited overlap in databases, thus possibly indicating dysfunction following mTBI. Pathway analysis revealed that predicted miRNA targets were mainly engaged in nervous system signaling, neuron projection, and cell differentiation (Figure 7; Table 3). Because neuronal signal transmission is governed by transmembrane voltage differences [84], an ion imbalance following brain injury may affect neuronal communication and resultant neurological impairment. In the cortex, projection neuron extension or process axons to distant intracortical, subcortical, and sub-cerebral targets regulate sensory input, motor functions, and cognitive abilities [85], all of which could be disrupted following mTBI.
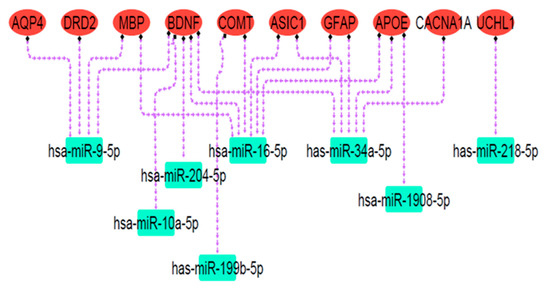
Figure 7.
The miRNA targets’ regulatory network. Red ovals represent mTBI candidate genes, and green rectangles indicate predicted miRNA targets. Dashed purple arrows show the regulatory relationships between miRNAs and their target genes. Pathway analysis revealed that these miRNA targets are primarily involved in nervous system signaling, neuron projection, and cell differentiation.

Table 3.
Target miRNAs related to mTBI candidate genes. Canonical pathways selected by p-value < 0.05 as the significance cut-off value for miRNA pathway dictionary (miRPathDB version 2.0).
3.5. Identification of Differential Expression miRNAs in GSE123336
In the GSE123336 study, samples were also taken based on the frequency of blows to the head, in addition to times before and after MMA competition. In light of this, we established conditions for each status (time points and number of hits) and analyzed the data based on each condition. The DESeq2 package of RStudio version 4.5.1 was used to identify differentially expressed miRNAs after acute mTBI, and the results showed that the expression profile of 2664 miRNAs had changed. In the first condition, we used the P-adjusted (Padj) value (i.e., Bonferroni corrected) to compare 0-d post-mTBI with normal (0-d pre & 1-week preinjury) to identify significant miRNAs (Padj ≤ 0.05). We employed a specific significance threshold of Padj ≤ 0.05 after applying the Bonferroni Correction, which effectively controlled the issue of multiple comparisons while maintaining transparency in our analysis. We found that expression levels of 17 miRNAs were considerably altered immediately after injury (i.e., day 0, post), with 14 miRNAs showing upregulation and three displaying downregulation (Table 4; Figure 8). First, hsa-miR-10a-5p was overexpressed in serum immediately after injury (Padj = 9.48 × 106). After 2days post-mTBI, similar miRNAs also displayed differential expression (17 miRNAs were found to have changed, with 14 increasing and 3 showing decreasing). Based on the analysis, there were no discernible changes in miRNA levels one week after injury compared to controls.

Table 4.
The level of miRNAs that were differentially expressed at zero days and 2–3 days after injury. The miRwalk and mirsystem databases were used to investigate predicted targets, while mirpathDB was used to analyze pathways. All of these are significant based on Padj ≤ 0.05, some with very high significance.
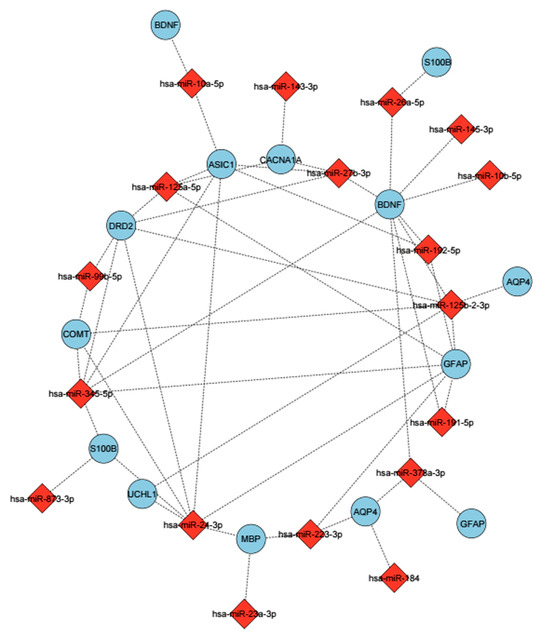
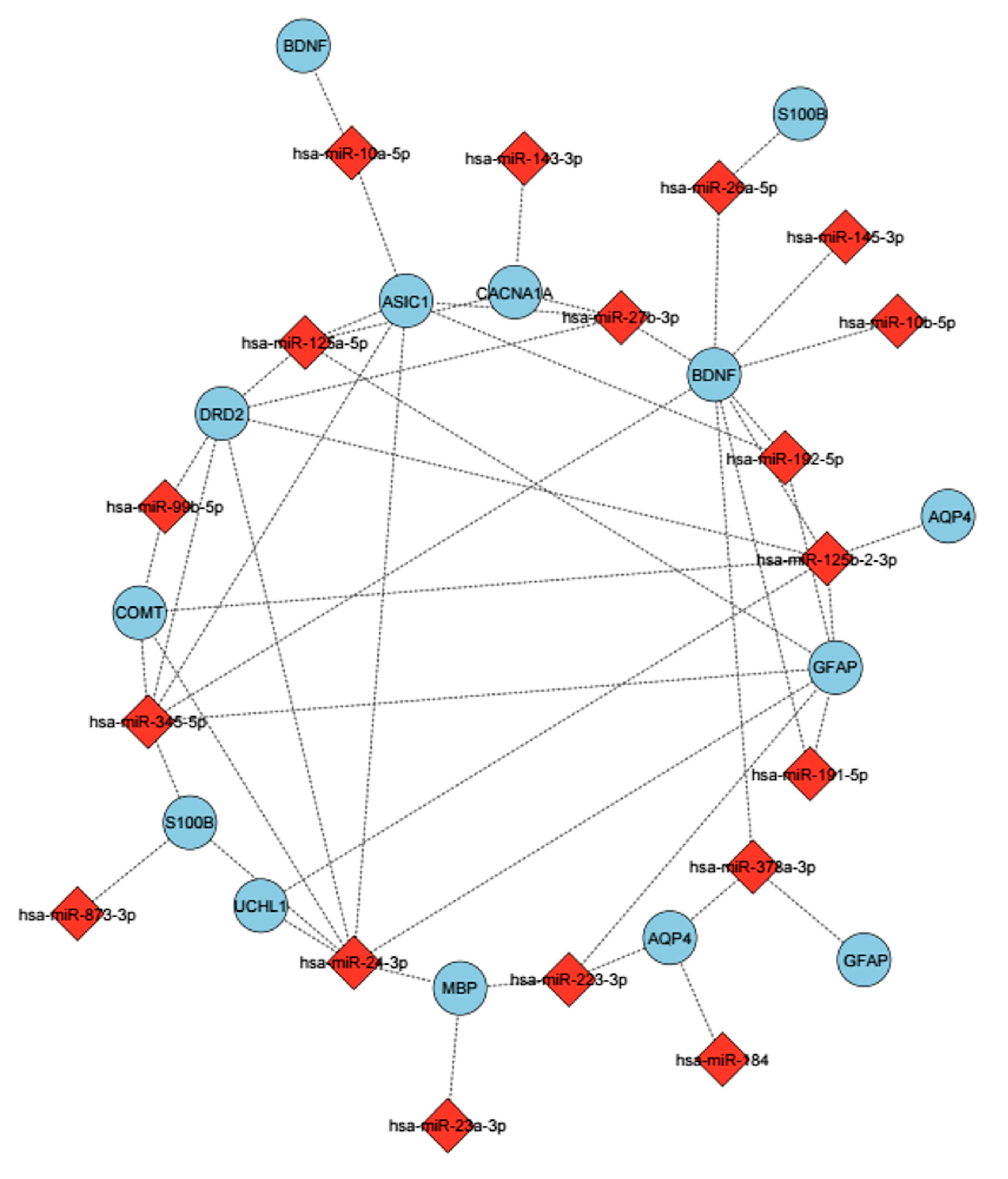
Figure 8.
The miRNAs were determined to be related to mTBIs and their predicted targets. These miRNAs show differential expression based on the BWA analysis. The mirsystem, mirwalk databases were used to predict target genes. miRNAs are shown with a red diamond, and genes are shown with a purple circle. Edges represent predicted regulatory interactions between each miRNA and its corresponding target gene.
The same analysis, based on numbers of head hits, demonstrated that participants who got over 20 hits to the head had altered levels of miRNA expression, which increased the expression of some miRNAs like hsa-miR-10b-5p (Padj = 0.039), and hsa-miR-143-3p (Padj = 0.0082) (Figure 8) In terms of GO and pathway analysis, the majority of miRNAs identified were related to nervous system development, cell projection, neuronal projection, metabolic processes, and neuronal system functions (Table 4).
4. Discussion
Based on our bioinformatic and pathway analyses, 11 candidate genes were identified as being potentially associated with neurological function, and 8 miRNAs were predicted to interact with these genes. The candidate genes were engaged in cell differentiation, nervous system development, synaptic development, the generation of neurons, and cell death. The miRNAs identified as closely linked to mTBI were hsa-miR-9-5p, hsa-miR-204-5p, hsa-miR-1908-5p, hsa-miR-16-5p, hsa-miR-10a-5p, has-miR-218-5p, has-miR-34a-5p, and has-miR-199b-5p. Based on the evidence obtained from the DAVID and GSEA analyses, the mTBI candidate genes were mainly enriched in neuronal projection regeneration, regulation of neuronal synaptic plasticity, cognitive function, memory, and behavior. The identification of specific genes and miRNAs associated with neurological damage provides potential candidates for future biomarker development and may contribute to improving diagnostic and therapeutic strategies for mTBI once experimentally validated. There are numerous complex pathophysiological changes that occur following an mTBI. Therefore, the identification of brain-specific miRNAs can help improve the understanding of molecular alterations associated with the prediction of an individual mTBI patient’s outcome. This study used various bioinformatic analyses to determine hub genes and miRNAs related to brain damage and neuronal dysfunction.
To establish the differential expression of miRNAs after brain damage, we also analyzed the GSE123336 dataset from the GEO repository and compared it with the results of our miRNA predictions. The results indicated significant changes in miRNA expression levels after acute mTBI. The hsa-miR-10a-5p was among the miRNAs that showed an increased expression immediately after injury (0 days post-injury) in the RNA-seq dataset and was the only miRNA that overlapped with our in silico predictions derived from multiple miRNA databases. This overlap may suggest a potential role for hsa-miR-10a-5p in the molecular response to mTBI. Functional enrichment analysis indicated that its predicted targets are associated with BDNF and pathways involved in cell morphogenesis, implying that it could contribute to processes related to early post-injury cellular regulation. Previous studies have indicated the relevance of miR-10a-5p in various brain disorders, such as depressive disorder [86] and Parkinson’s disease (PD) [87]. Nonetheless, there are limited investigations that study the effect of this miRNA on the results of mTBI. Moreover, there was a considerable rise in hsa-miR-10b-5p and hsa-miR-143-3p at 0 days after injury, and there was a positive correlation between these changes and number of hits to the head, which meant that these miRNAs could serve as a useful biomarker to measure the severity of the direct head injury. Although further investigation is necessary to confirm and validate this hypothesis.
Previous research has shown that the majority of mTBI patients will recover from neurological dysfunction. However, as many as 15–30% will experience long-term neurocognitive and behavioral changes [88]. Our findings suggest that mTBI candidate genes such as APOE, S100B, GFAP, BDNF, AQP4, COMT, MBP, UCHL1, DRD2, ASIC1, and CACNA1A may have an essential role in lasting mTBI-related neurological disorders (gene functions detailed in Supplemental Materials). As determined by the GO analysis, GFAP and APOE were the two main genes involved in neuron regrowth, S100B and APOE were involved in the regulation of neuronal synaptic plasticity, CACNA1A, DRD2, and UCHL1 would affect adult walking behaviour, and ASIC1 and DRD2 would contribute to learning. Further research is required to determine the association of these genes with the recovery of neurological dysfunction after mTBI.
Our findings suggest that mTBI-associated miRNAs and genes are involved in critical processes such as neuronal repair and cognitive dysfunction, which are likely to influence both short- and long-term neurological outcomes. These results align with prior research, which identified 21 miRNAs that exhibited notable expression changes after mTBI and were linked to cognitive deficits and balance disturbances (42). They observed that miRNAs were more predictive of mTBI than conventional protein biomarkers like GFAP and UCHL1, which is consistent with our bioinformatics findings. Although we identified these proteins as part of the mTBI response, our focus on miRNA-gene interactions suggests that miRNAs may be more sensitive and specific indicators of injury and recovery.
In addition to the 11 candidate genes, our bioinformatic analysis identified 8 miRNAs that were predicted to be associated with the mTBI candidate genes and pathways related to neurological function. Based on computational analyses, these miRNAs might be involved in affecting mTBI pathophysiology. Previous studies indicated that some of these miRNAs were associated with brain disorders and suggested that they could be used as surrogate biomarkers [89]. Based on predictions made through a bioinformatic study of miRNA with APOE as a regulatory target, miR-1908-5p was found to correlate with genes involved in bipolar disorder [89]. The location of miR-1908-5p is in the first intron of the fatty acid desaturase-1 (FADS1) gene on chromosome 11 [89].
Results from pathway analysis suggested that miR-1908-5p contributes to nervous system development and neuron projection. In the GENFI cohort, miR-204-5p was shown to be significantly lower in symptomatic frontotemporal dementia (FTD) compared to pre-symptomatic mutation carriers [90]. Guedes et al. found that miR-204-5p was upregulated in individuals with post-traumatic stress disorder (PTSD) compared to healthy controls [91]. Moreover, Weisz et al. found a substantial downregulation of miR-204-5p in chronic TBI patients [92]. Surprisingly, miR-204-5p was identified to be abundant in the brain based on tissue expression patterns, and it also showed a dramatic increase 1 h following concussion [93]. These findings suggest that this miRNA could be used as a biomarker for neuropathological disorders. MiR-9 is another interesting miRNA that has attracted attention [94]. It has a unique expression pattern in the brain and implements activities involved in central nervous system development [94,95]. Brain development studies indicated that MiR-9 was related to gene networks that control the proliferation of neural progenitors [95]. Therefore, the presence of this miRNA in neurological diseases is not surprising. For instance, miR-9 was found to be downregulated in Alzheimer’s disease patients [96]. Another significant miRNA we identified was miR-16-5p, which was previously shown as a viable potential biomarker for TBI, with the ability to distinguish between mild and severe cases [43].
Based on GO analysis, miR-16 was involved in a variety of regulatory processes that are triggered by brain injuries, including positive apoptotic regulation via BCL-2 targeting [97]. Sun et al. observed that downregulation of miR-16-5p plays an important role in the faster recovery process in TBI patients via stimulation of osteoblast proliferation and the prevention of apoptosis [98]. Researchers revealed that within the first 24 h after mTBI, the level of miR-16-5p was significantly higher in mTBI patients compared to severe TBI patients [99]. Furthermore, a recent study demonstrated that miR-16-5p and miR-21-5p are significantly upregulated in critical-fatal TBI cases, with miR-21-5p being particularly elevated in short-survival groups [100].
Also, RNA-seq analysis showed that miR-10a-5p, miR-10b-5p, and miR-143-3p levels in serum elevated immediately after mTBI. These results indicate that miR-10a-5p and miR-10b-5p are prominent targets for BDNF. Our analysis also shows a direct correlation between miR-10b-5p and miR-143-3p with the number of hits to the head. Therefore, these miRNAs may be useful candidate biomarkers to determine the severity of brain injury.
Enrichment analysis showed that miR-10a-5p and miR-10b-5p plays a significant role in neuronal processes when it comes to regulating cell morphogenesis and neuron generation, while miR-143-3p plays an active role in cell projection and cell–cell signaling. To our knowledge, there has not been a significant amount of research on miR-10a-5p and brain injury to recommend it as a potential biomarker. Previous studies have established that miR-143-3p is linked to ischemic stroke [101], and that miR-10b-5p is considerably differently expressed in Huntington’s disease [102]. Additionally, bioinformatic analyses revealed that miR-34a-5p miR-218-5p, and miR-199b-5p were targeted for their relationships to mTBI candidate genes, and these miRNAs are involved in pathways such as cell–cell signaling, cell death, regulation of metabolic process, cell differentiation, nervous system development [39]. However, more research is needed to confirm the link between these miRNAs and post-mTBI symptoms.
Within the framework of our bioinformatic study, the 11 candidate genes (APOE, S100B, GFAP, BDNF, AQP4, COMT, MBP, UCHL1, DRD2, ASIC1, CACNA1A) may plausibly map onto recovery—related processes after mTBI. For example, BDNF relates to neuroplasticity [103] and generation of neurons, APOE to lipid transport and synaptic repair [77], MBP to remyelination, UCHL1/GFAP/S100B to neuronal and glial injury responses [104,105], AQP4/ASIC1 to fluid and ionic regulation after injury [106], and DRD2/CACNA1A/COMT to neurotransmission that may affect cognition [46,81,107,108]. The miRNAs predicted in our pipeline (including miR-10a-5p, miR-9-5p, miR-16-5p, miR-204-5p, miR-1908-5p, miR-218-5p, miR-34a-5p, miR-199b-5p) align with pathways for neuron projection, synaptic signaling, and cell morphogenesis observed in our enrichment analyses, suggesting potential regulatory influence on these recovery-related processes. Consistent with the scope of this study, these inferences are computational and should be interpreted as hypotheses to be tested in independent cohorts with longitudinal outcomes.
While our study provides valuable insights into gene and miRNA alterations following mTBI, recognition of its limitations is crucial for a more comprehensive interpretation. We used bioinformatics tools and online databases to predict biomarkers that could be altered in mTBI. However, it is important to note that the accuracy and completeness of the available data, as well as the reliability of the databases used, may have introduced potential inaccuracies or variations in our findings. Furthermore, the dynamic nature of bioinformatics databases and tools indicates that changes over time could affect the relevance and accuracy of our results. Brain-specific genes, especially those expressed in neuronal and glial cells, play crucial roles in the underlying mechanisms of traumatic brain injury.
Disruption of the BBB following mTBI could allow some of these brain-specific molecules to circulate in the bloodstream, making them relevant targets for biomarker discovery. As such, we propose that future research should investigate both circulating and brain-specific genes to assess their combined potential in predicting mTBI severity and recovery. Another limitation of our research was the inability to access patients and biological samples, such as blood, to validate the predicted biomarkers. This limitation restricts the generalizability of our findings to patients with truly acute or chronic mTBI. Collecting samples from mTBI patients at various time points would improve our validity. We were unable to include a control group for circulating biomarkers, particularly miRNAs. Introducing controls from patients with extracranial injuries (e.g., orthopedic patients) in future studies could enhance the specificity of the identified biomarkers, such as hsa-miR-10a-5p. This would help determine whether extracranial injuries impact the levels of candidate miRNAs, which could affect their reliability as diagnostic tools for mTBI. The reduced number of samples at 2–3 days post-injury (17 samples) and 1 week post-injury (3 samples) compared to 0 days post-injury (52 samples) was another limitation of our study. This decrease in sample size at later time points may have been due to logistical challenges and participant availability for follow-up assessments, which is common in longitudinal studies. We acknowledge this limitation and recommend that future studies with larger sample sizes across all time points would enable a more comprehensive analysis of time-dependent biomarker trajectories. We were unable to differentiate between acute and chronic mTBI cases. This limitation arose from our reliance on databases and in silico analysis during our research. For future studies, integrating bulk RNA-seq with single-cell transcriptomics could offer a more precise view of neuroplasticity and neuronal repair in mTBI recovery. Specifically, exploring DLK inhibition and ATF3 modulation as therapeutic targets may help mitigate neuronal loss [109]. Lastly, independent validation using additional GEO datasets was not performed because comparable human mTBI datasets with matched sample type, time points, and sequencing platforms are currently unavailable. To avoid potential batch effects and inconsistent variables, we prioritized transparency in our reported analyses and outlined criteria for future validation when suitable datasets become available.
5. Conclusions
Our study identified 11 genes and 8 miRNAs that may be closely associated with mTBI severity and recovery outcomes. They were mainly involved in neurite regeneration, nervous system signaling, neurite outgrowth, and cell differentiation. The results of this study offer a bioinformatic foundation for identifying gene and miRNA candidates that warrant further investigation as potential biomarkers of acute mTBI in experimental and clinical settings. Recent advances in research have expanded the understanding of miRNA biomarkers in mTBI, demonstrating their potential for diagnosis, prognosis, and treatment development [35,40,65,110]. By integrating these findings, our study suggests that circulating miRNAs, particularly those involved in neuronal repair, could serve as non-invasive biomarkers for classifying mTBI severity and predicting long-term recovery.
Our study predicts Important genes and miRNAs linked to mTBI severity and recovery, highlighting their role in neuronal plasticity and neuronal repair, while Jia et al. demonstrate that microglial activation protects against white matter atrophy, whereas endothelial dysfunction worsens neurodegeneration via BBB disruption [111], underscoring the need for targeted therapies integrating miRNA biomarkers, neuroimaging, and inflammatory markers. We anticipate that once these biomarkers are validated and a standardized analytical protocol is established, patients with mTBI will benefit from more precise assessments, targeted treatment strategies, and improved recovery predictions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13112669/s1, References [22,23,24,27,80,81,104,105,107,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168] are cited in the supplementary materials. Supplementary File SA: RNA-sequencing analysis scripts and output files. This file contains the command-line scripts, processed count matrices, and DESeq2 differential expression results used to identify significantly altered miRNAs in the GSE123336 dataset. Supplementary File SB: Detailed description of the 11 candidate genes associated with mTBI. This file includes gene names, biological functions, related pathways, and literature references supporting their relevance to mild traumatic brain injury severity and recovery.
Author Contributions
M.T.: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization M.D.N.: Conceptualization, Resources, Writing—Review and Editing, Visualization, Supervision, Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was associated with this project.
Institutional Review Board Statement
Not applicable, the study did not require ethical approval.
Informed Consent Statement
Not applicable. This bioinformatic study did not involve the collection of new human data. The RNA-Seq dataset analyzed (GEO accession number GSE123336) was obtained from a previously published study in which ethical approval and informed consent were obtained by the original investigators. For further details, please refer to the primary publication associated with the dataset.
Data Availability Statement
The data that support the findings of this study are openly available in Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) (accessed on 20 October 2025), reference number is GSE123336. The supplementary file regarding the coding and analysis are available in the supplementary information.
Acknowledgments
Figure 1 was created using BioRender (Created in BioRender. Tajik, M. (2025); https://BioRender.com/1lsg3r7).
Conflicts of Interest
Noseworthy is the co-founder and of TBIFinder Inc., a data analytics company focused on MRI data, and this has no relationship to the work in this current study.
Abbreviations
(TBI) Traumatic Brain Injury, (mTBI) Mild Traumatic Brain Injury, (microRNA/ miRNA) Micro Ribonucleic Acid, (RNA-seq) RNA-Sequencing, (BBB) Blood–Brain Barrier, (GO); Gene Ontology, (KEGG) Kyoto Encyclopedia of Genes and Genomes, (PPI) Protein–Protein Interactions, (GEO) Gene Expression Omnibus, (RNA) Ribonucleic acid, (STRING) Search Tool for the Retrieval of Interacting Genes/Proteins, (mRNA) Messenger RNA, (OMIM) Online Mendelian Inheritance in Man, (DAVID) Database for Annotation, Visualization, and Integrated Discovery, (MF) Molecular function, (BP) Biological process, (CC) Cellular components, (GSEA) Gene Set Enrichment Analysis, (BWA) Burrows–Wheeler Alignment, (MMA) Mixed Martial Arts, (hg38) Human genom38, (FDR) False Discovery Rate, (Padj) P. adjusted, (DAergic) Dopaminergic, (PTSD) Post-traumatic stress disorder, (UPP) Ubiquitin-proteasome pathway, (FHM) Familial Hemiplegic Migraine, (FTD) Frontotemporal dementia, (FADS1) Fatty Acid Desaturase 1, (SNP) Single Nucleotide Polymorphism, (CSF) Cerebrospinal Fluid, (ENA) European Nucleotide Archive, (FastQC) Quality-Control Tool for Sequence Reads, (Trimmomatic) Read Trimming Software for Illumina Data, (SAMtools) Sequence Alignment/Map tools, (miRbase) MicroRNA Sequences Database, (DESeq2) Differential Expression Analysis for RNA-seq, (NCBI) National Center for Biotechnology Information, (HP) Human Phenotype.
References
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, A.G.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport–Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Carroll, L.; Peloso, P.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef]
- Kaut, K.P.; DePompei, R.; Kerr, J.; Congeni, J. Reports of Head Injury and Symptom Knowledge Among College Athletes: Implications for Assessment and Educational Intervention. Clin. J. Sport Med. 2003, 13, 213–221. [Google Scholar] [CrossRef]
- Roozenbeek, B.; Maas, A.I.R.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Halstead, M.E.; Walter, K.D.; Moffatt, K. Sport-Related Concussion in Children and Adolescents. Pediatrics 2018, 142, e20183074. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Kriz, P.; Mannix, R.C.; Kirchberg, T.; Master, C.L.; Meehan, W.P. Concussion Symptom Profiles Among Child, Adolescent, and Young Adult Athletes. Clin. J. Sport Med. 2019, 29, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Leung, A.K.; Torres, A.R. Concussion: A Global Perspective. Semin. Pediatr. Neurol. 2019, 30, 117–127. [Google Scholar] [CrossRef]
- Rutherford, W.; Merrett, J.; McDonald, J. Symptoms at one year following concussion from minor head injuries. Injury 1979, 10, 225–230. [Google Scholar] [CrossRef]
- Dischinger, P.; Read, K.; Kerns, T.; Ho, S.; Kufera, J.; Burch, C.; Jawed, N.; Burgess, A.; Bents, F. Causes and Outcomes of Mild Traumatic Brain Injury: An Analysis of Ciren Data. Annu. Proc. Assoc. Adv. Automot. Med. 2003, 47, 577. [Google Scholar] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Baugh, C.M.; Stamm, J.M.; Riley, D.O.; Gavett, B.E.; Shenton, M.E.; Lin, A.; Nowinski, C.J.; Cantu, R.C.; McKee, A.C.; Stern, R.A. Chronic traumatic encephalopathy: Neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012, 6, 244–254. [Google Scholar] [CrossRef]
- Abner, E.L.; Nelson, P.T.; Schmitt, F.A.; Browning, S.R.; Fardo, D.W.; Wan, L.; Jicha, G.A.; Cooper, G.E.; Smith, C.D.; Caban-Holt, A.M.; et al. Self-Reported Head Injury and Risk of Late-Life Impairment and AD Pathology in an AD Center Cohort. Dement. Geriatr. Cogn. Disord. 2014, 37, 294–306. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H. Biomechanics of Concussion. Clin. Sports Med. 2011, 30, 19–31. [Google Scholar] [CrossRef]
- Povlishock, J.T.; Christman, C.W. The Pathobiology of Traumatically Induced Axonal Injury in Animals and Humans: A Review of Current Thoughts. J. Neurotrauma 1995, 12, 555–564. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Dabiri, B.E.; Gabriele, S.; Kerscher, L.; Franck, C.; Goss, J.A.; Alford, P.W.; Parker, K.K. A Possible Role for Integrin Signaling in Diffuse Axonal Injury. PLoS ONE 2011, 6, e22899. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.R.; Valadka, A.; Manley, G.T. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Faden, A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010, 31, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Longhi, L.; Saatman, K.E.; Raghupathi, R.; Laurer, H.L.; Lenzlinger, P.M.; Riess, P.; Neugebauer, E.; Trojanowski, J.Q.; Lee, V.M.-Y.; Grady, M.S.; et al. A Review and Rationale for the Use of Genetically Engineered Animals in the Study of Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2001, 21, 1241–1258. [Google Scholar] [CrossRef]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Johannesson, L.; Nelson, D.; Bellander, B.M. S100B Is an Important Outcome Predictor in Traumatic Brain Injury. J. Neurotrauma 2013, 30, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Brophy, G.M.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Giordano, D.I.M.; et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients with and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016, 73, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Padgett, C.R.; Summers, M.J.; Vickers, J.C.; McCormack, G.H.; Skilbeck, C.E. Exploring the effect of the apolipoprotein E (APOE) gene on executive function, working memory, and processing speed during the early recovery period following traumatic brain injury. J. Clin. Exp. Neuropsychol. 2016, 38, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Cusimano, M.D.; Bendena, W.G. Post-traumatic brain injury: Genetic susceptibility to outcome. Neuroscientist 2015, 21, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Panenka, W.J.; Gardner, A.J.; Dretsch, M.N.; Crynen, G.C.; Crawford, F.C.; Iverson, G.L. Systematic Review of Genetic Risk Factors for Sustaining a Mild Traumatic Brain Injury. J. Neurotrauma 2017, 34, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.; Klang, A.; Thams, S.; Rostami, E. The Role of BDNF in Experimental and Clinical Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 3582. [Google Scholar] [CrossRef]
- McAllister, T.W. Genetic Factors Modulating Outcome After Neurotrauma. PM&R 2010, 2, S241–S252. [Google Scholar] [CrossRef]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Primers 2016, 2, 16084. [Google Scholar] [CrossRef]
- Simpson, E.; Reiter, J.L.; Ren, J.; Zhang, Z.; Nudelman, K.N.; Riggen, L.D.; Menser, M.D.; Harezlak, J.; Foroud, T.M.; Saykin, A.J.; et al. Gene Expression Alterations in Peripheral Blood Following Sport-Related Concussion in a Prospective Cohort of Collegiate Athletes: A Concussion Assessment, Research and Education (CARE) Consortium Study. Sports Med. 2024, 54, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, F.; Guan, Y.; Meng, F.; Zhao, Z.; Su, Q.; Bao, W.; Wang, X.; Zhao, J.; Huo, Z.; et al. The Biogenesis of miRNAs and Their Role in the Development of Amyotrophic Lateral Sclerosis. Cells 2022, 11, 572. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Rau, T.F.; Surendran, N.; Brennan, J.H.; Thaveenthiran, P.; Sorich, E.; Fitzgerald, M.C.; Rosenfeld, J.V.; Patel, S.A. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: A pilot study. J. Clin. Neurosci. 2017, 38, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.C.; Smothers, C.G.; Winkelman, C. Bioinformatic analysis of brain-specific miRNAs for identification of candidate traumatic brain injury blood biomarkers. Brain Inj. 2020, 34, 965–974. [Google Scholar] [CrossRef]
- Marchi, N.; Bazarian, J.J.; Puvenna, V.; Janigro, M.; Ghosh, C.; Zhong, J.; Zhu, T.; Blackman, E.; Stewart, D.; Ellis, J.; et al. Consequences of Repeated Blood-Brain Barrier Disruption in Football Players. PLoS ONE 2013, 8, e56805. [Google Scholar] [CrossRef] [PubMed]
- Scott, H. Extracellular microRNAs as messengers in the central and peripheral nervous system. Neuronal Signal. 2017, 1, NS20170112. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, P.; Bai, F.; Liu, C.; Huang, X. Serum exosomes miR-206 and miR-549a-3p as potential biomarkers of traumatic brain injury. Sci. Rep. 2024, 14, 10082. [Google Scholar] [CrossRef]
- Feng, S.; Wu, Z.; Zheng, X.; Shao, Z.; Lin, Q.; Sun, S. Abnormal levels of expression of microRNAs in peripheral blood of patients with traumatic brain injury are induced by microglial activation and correlated with severity of injury. Eur. J. Med. Res. 2024, 29, 188. [Google Scholar] [CrossRef] [PubMed]
- White, T.E.; Ford, G.D.; Surles-Zeigler, M.C.; Gates, A.S.; LaPlaca, M.C.; Ford, B.D. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genom. 2013, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.B.; Byers, D.M.; Irwin, L.N. Gene expression following traumatic brain injury in humans: Analysis by microarray. J. Clin. Neurosci. 2005, 12, 284–290. [Google Scholar] [CrossRef]
- Di Pietro, V.; Yakoub, K.M.; Scarpa, U.; Di Pietro, C.; Belli, A. MicroRNA Signature of Traumatic Brain Injury: From the Biomarker Discovery to the Point-of-Care. Front. Neurol. 2018, 9, 429. [Google Scholar] [CrossRef]
- Xiao, X.; Bai, P.; Cao, S.; Jiang, Y.; Liang, W.; Wang, T.; Luo, X.; Guan, Q.; Gao, L.; Zhang, L. Integrated Bioinformatics Analysis for the Identification of Key Molecules and Pathways in the Hippocampus of Rats After Traumatic Brain Injury. Neurochem. Res. 2020, 45, 928–939. [Google Scholar] [CrossRef]
- LaRocca, D.; Barns, S.; Hicks, S.D.; Brindle, A.; Williams, J.; Uhlig, R.; Johnson, P.; Neville, C.; Middleton, F.A. Comparison of serum and saliva miRNAs for identification and characterization of mTBI in adult mixed martial arts fighters. PLoS ONE 2019, 14, e0207785. [Google Scholar] [CrossRef]
- Tajik, M.; Danielli, E.; Sharma, B.; Noseworthy, M.D. A review of molecular and genetic factors for determining mild traumatic brain injury severity and recovery. Brain Disord. 2022, 8, 100058. [Google Scholar] [CrossRef]
- Murphy, M.; Brown, G.; Wallin, C. Gene Help: Integrated Access to Genes of Genomes in the Reference Sequence Collection; National Center for Biotechnology Information: Bethesda, MD, USA, 2006. [Google Scholar]
- Rappaport, N.; Nativ, N.; Stelzer, G.; Twik, M.; Guan-Golan, Y.; Stein, T.I.; Bahir, I.; Belinky, F.; Morrey, C.P.; Safran, M.; et al. MalaCards: An integrated compendium for diseases and their annotation. Database 2013, 2013, bat018. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8--a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Lu, T.-P.; Lee, C.-Y.; Tsai, M.-H.; Chiu, Y.-C.; Hsiao, C.K.; Lai, L.-C.; Chuang, E.Y. miRSystem: An Integrated System for Characterizing Enriched Functions and Pathways of MicroRNA Targets. PLoS ONE 2012, 7, e42390. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Vejnar, C.E.; Blum, M.; Zdobnov, E.M. miRmap web: Comprehensive microRNA target prediction online. Nucleic Acids Res. 2013, 41, W165–W168. [Google Scholar] [CrossRef]
- Grimson, A.; Farh, K.K.-H.; Johnston, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA Targeting Specificity in Mammals: Determinants beyond Seed Pairing. Mol. Cell. 2007, 27, 91–105. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Tokar, T.; Pastrello, C.; Rossos, A.E.M.; Abovsky, M.; Hauschild, A.-C.; Tsay, M.; Lu, R.; Jurisica, I. mirDIP 4.1—Integrative database of human microRNA target predictions. Nucleic Acids Res. 2018, 46, D360–D370. [Google Scholar] [CrossRef]
- Papadopoulos, G.L.; Alexiou, P.; Maragkakis, M.; Reczko, M.; Hatzigeorgiou, A.G. DIANA-mirPath: Integrating human and mouse microRNAs in pathways. Bioinformatics 2009, 25, 1991–1993. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Kehl, T.; Stöckel, D.; Fehlmann, T.; Schneider, L.; Meese, E.; Lenhof, H.-P.; Keller, A. miRPathDB: A new dictionary on microRNAs and target pathways. Nucleic Acids Res. 2017, 45, D90–D96. [Google Scholar] [CrossRef] [PubMed]
- Tomé, G.A. Investigating miRNA Expression in Athletes with Sport-Related Concussion. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2024. [Google Scholar]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Potla, P.; Ali, S.A.; Kapoor, M. A bioinformatics approach to microRNA-sequencing analysis. Osteoarthr. Cartil. Open 2021, 3, 100131. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; McKinley, W.I.; Valadka, A.B.; Newman, Z.C.; Nordgren, R.K.; Pramuka, P.E.; Barbosa, C.E.; Brito, A.M.P.; Loss, L.J.; Tinoco-Garcia, L.; et al. Diagnostic Performance of GFAP, UCH-L1, and MAP-2 Within 30 and 60 Minutes of Traumatic Brain Injury. JAMA Netw. Open 2024, 7, e2431115. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Spitz, G.; Major, B.P.; O’Brien, W.T.; Giesler, L.P.; Bain, J.W.P.; Xie, B.; Rosenfeld, J.V.; Law, M.; Ponsford, J.L.; et al. Utility of Acute and Subacute Blood Biomarkers to Assist Diagnosis in CT-Negative Isolated Mild Traumatic Brain Injury. Neurology 2023, 101, e1992–e2004. [Google Scholar] [CrossRef] [PubMed]
- Liu Thelin, E.P.; Nelson, D.W.; Bellander, B.M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2016, 159, 209. [Google Scholar] [CrossRef] [PubMed]
- Mannix, R.; Meehan, W.P., III. Evaluating the Effects of APOE4 after Mild Traumatic Brain Injury in Experimental Models. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; CRC Press: Boca Raton, FL, USA, 2015; pp. 91–96. [Google Scholar] [CrossRef] [PubMed]
- Da Giarratana, A.O.; Teng, S.; Reddi, S.; Zheng, C.; Adler, D.; Thakker-Varia, S.; Alder, J. BDNF Val66Met Genetic Polymorphism Results in Poor Recovery Following Repeated Mild Traumatic Brain Injury in a Mouse Model and Treatment With AAV-BDNF Improves Outcomes. Front. Neurol. 2019, 10, 1175. [Google Scholar] [CrossRef]
- Antrobus, M.R.; Brazier, J.; Callus, P.; Herbert, A.J.; Stebbings, G.K.; Day, S.H.; Kilduff, L.P.; Bennett, M.A.; Erskine, R.M.; Raleigh, S.M.; et al. Concussion-Associated Gene Variant COMT rs4680 Is Associated with Elite Rugby Athlete Status. Clin. J. Sport Med. 2022, 33, e145–e151. [Google Scholar] [CrossRef]
- Sc Bales, J.W.; Wagner, A.K.; Kline, A.E.; Dixon, C.E. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009, 33, 981. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.D.; Myrga, J.M.; Ricker, J.H.; Dixon, C.E.; Conley, Y.P.; Wagner, A.K. Posttraumatic Brain Injury Cognitive Performance Is Moderated by Variation Within ANKK1 and DRD2 Genes. J. Head Trauma Rehabil. 2015, 30, E54–E66. [Google Scholar] [CrossRef] [PubMed]
- We Spaziani, G.; Rozzi, G.; Baroni, S.; Simeoni, B.; Racco, S.; Barone, F.; Fuorlo, M.; Franceschi, F.; Covino, M. From the Emergency Department to Follow-Up: Clinical Utility of Biomarkers in Mild Traumatic Brain Injury. Emerg. Care Med. 2025, 2, 45. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef] [PubMed]
- The principles of nerve cell communication. Alcohol. Health Res. World 1997, 21, 107–108. [PubMed]
- Greig, L.C.; Woodworth, M.B.; Galazo, M.J.; Padmanabhan, H.; Macklis, J.D. Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 2013, 14, 755–769. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.; Zheng, Y.; He, S.; Zhang, T.; Guo, Q.; Xu, H.; Chen, H.; Liu, C.; Yu, S.; et al. The role of circulating blood microRNA-374 and microRNA-10 levels in the pathogenesis and therapeutic mechanisms of major depressive disorder. Neurosci. Lett. 2021, 763, 136184. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef]
- Su Daneshvar, D.H.; Riley, D.O.; Nowinski, C.J.; McKee, A.C.; Stern, R.A.; Cantu, R.C. Long-Term Consequences: Effects on Normal Development Profile After Concussion. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 683–700. [Google Scholar] [CrossRef]
- Forstner, A.J.; Hofmann, A.; Maaser, A.; Sumer, S.; Khudayberdiev, S.; Mühleisen, T.W.; Leber, M.; Schulze, T.G.; Strohmaier, J.; Degenhardt, F.; et al. Genome-wide analysis implicates microRNAs and their target genes in the development of bipolar disorder. Transl. Psychiatry 2015, 5, e678. [Google Scholar] [CrossRef]
- Schneider, R.; McKeever, P.; Kim, T.H.; Graff, C.; van Swieten, J.C.; Karydas, A.; Boxer, A.; Rosen, H.; Miller, B.L.; Laforce, R.; et al. Downregulation of exosomal miR-204-5p and miR-632 as a biomarker for FTD: A GENFI study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Guedes, V.A.; Lai, C.; Devoto, C.; Edwards, K.A.; Mithani, S.; Sass, D.; Vorn, R.; Qu, B.-X.; Rusch, H.L.; Martin, C.A.; et al. Extracellular Vesicle Proteins and MicroRNAs Are Linked to Chronic Post-Traumatic Stress Disorder Symptoms in Service Members and Veterans With Mild Traumatic Brain Injury. Front. Pharmacol. 2021, 12, 745348. [Google Scholar] [CrossRef] [PubMed]
- Weisz, H.A.; Kennedy, D.; Widen, S.; Spratt, H.; Sell, S.L.; Bailey, C.; Sheffield-Moore, M.; DeWitt, D.S.; Prough, D.S.; Levin, H.; et al. MicroRNA sequencing of rat hippocampus and human biofluids identifies acute, chronic, focal and diffuse traumatic brain injuries. Sci. Rep. 2020, 10, 3341. [Google Scholar] [CrossRef] [PubMed]
- Al Sandmo, S.B.; Matyasova, K.; Filipcik, P.; Cente, M.; Koerte, I.K.; Pasternak, O.; Andersen, T.E.; Straume-Næsheim, T.M.; Bahr, R.; Jurisica, I. Changes in circulating microRNAs following head impacts in soccer. Brain Inj. 2022, 36, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.S.; Rajanikant, G.K. miR-9 Upregulation Integrates Post-ischemic Neuronal Survival and Regeneration In Vitro. Cell Mol. Neurobiol. 2019, 39, 223–240. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA Changes in Alzheimer’s Disease Brain and CSF Yields Putative Biomarkers and Insights into Disease Pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, Y.; Yan, C.; Chen, L.; Chen, D.; Mi, B.; Liu, G. Downregulation of microRNA-16-5p accelerates fracture healing by promoting proliferation and inhibiting apoptosis of osteoblasts in patients with traumatic brain injury. Am. J. Transl. Res. 2019, 11, 4746–4760. [Google Scholar] [PubMed]
- Redell, J.B.; Moore, A.N.; Ward, N.H.; Hergenroeder, G.W.; Dash, P.K. Human Traumatic Brain Injury Alters Plasma microRNA Levels. J. Neurotrauma. 2010, 27, 2147–2156. [Google Scholar] [CrossRef]
- Consalvo, F.; Padovano, M.; Scopetti, M.; Morena, D.; Cipolloni, L.; Fineschi, V.; Santurro, A. Analysis of miRNA Expression Profiles in Traumatic Brain Injury (TBI) and Their Correlation with Survival and Severity of Injury. Int. J. Mol. Sci. 2024, 25, 9539. [Google Scholar] [CrossRef]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Dong, X.-Y.; Cong, S.-Y. Bioinformatic analysis of a microRNA regulatory network in Huntington’s disease. J. Integr. Neurosci. 2020, 19, 641. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.H.; Gao, G.D.; Zhou, X.F.; Wang, T.H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef] [PubMed]
- Day, I.N.M.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar] [CrossRef] [PubMed]
- Metting, Z.; Wilczak, N.; Rodiger, L.A.; Schaaf, J.M.; Van Der Naalt, J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 2012, 78, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, R.H.; Sparling, M.B.; Ryan, L.M.; Xu, K.; Salazar, A.M.; Goldman, D.; Warden, D.L. Association of COMT Val158Met Genotype With Executive Functioning Following Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 465–471. [Google Scholar] [CrossRef]
- Winkler, E.A.; Yue, J.K.; McAllister, T.W.; Temkin, N.R.; Oh, S.S.; Burchard, E.G.; Hu, D.; Ferguson, A.R.; Lingsma, H.F.; Burke, J.F.; et al. COMT Val 158 Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics 2016, 17, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Alkaslasi, M.R.; Lloyd, E.Y.H.; Gable, A.S.; Silberberg, H.; Yarur, H.E.; Tsai, V.S.; Sohn, M.; Margolin, G.; Tejeda, H.A.; Le Pichon, C.E. The transcriptional response of cortical neurons to concussion reveals divergent fates after injury. Nat. Commun. 2025, 16, 1097. [Google Scholar] [CrossRef]
- Di Pietro, V.; Porto, E.; Ragusa, M.; Barbagallo, C.; Davies, D.; Forcione, M.; Logan, A.; Di Pietro, C.; Purrello, M.; Grey, M.; et al. Salivary MicroRNAs: Diagnostic Markers of Mild Traumatic Brain Injury in Contact-Sport. Front. Mol. Neurosci. 2018, 11, 395270. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, W.; Zhang, H.; Zhang, X.; Ji, Q.; Li, X.; Pan, Y.; Jiang, X.; Zhang, J.; Bai, L. Cell-Specific Gene Expressions Underlie Selective White Matter Loss Vulnerability in Mild Traumatic Brain Injury. J. Neurotrauma 2024, 42, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.; Pueyo, R.; Matarín, M.D.M.; Junqué, C.; Mataró, M.; Clemente, I.; Moral, P.; Poca, M.A.; Garnacho, Á.; Sahuquillo, J. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1191. [Google Scholar] [CrossRef] [PubMed]
- Nathan, B.P.; Bellosta, S.; Sanan, D.A.; Weisgraber, K.H.; Mahley, R.W.; Pitas, R.E. Differential Effects of Apolipoproteins E3 and E4 on Neuronal Growth in Vitro. Science 1994, 264, 850–852. [Google Scholar] [CrossRef]
- Veinbergs, I.; Mante, M.; Jung, M.W.; van Uden, E.; Masliah, E. Synaptotagmin and synaptic transmission alterations in apolipoprotein E-deficient mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Nathan, B.P.; Pitas, R.E.; Apolipoprotein, E. Structure, Function, and Possible Roles in Alzheimer’s Diseasea. Ann. N. Y Acad. Sci. 1996, 777, 139–145. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Comper, P.; Hutchison, M.G.; Sharma, B. The role of apolipoprotein E episilon (ɛ)-4 allele on outcome following traumatic brain injury: A systematic review. Brain Inj. 2015, 29, 1018–1031. [Google Scholar] [CrossRef]
- Chiang, M.F.; Chang, J.G.; Hu, C.J.; Dunn, L.; Nicoll, J.A.R. Association between apolipoprotein E genotype and outcome of traumatic brain injury. Acta Neurochirurgica 2003, 145, 649–654. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Role of Lipids in Brain Injury and Diseases. Future Lipidol. 2007, 2, 403–422. [Google Scholar] [CrossRef]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. GFAP Versus S100B in Serum after Traumatic Brain Injury: Relationship to Brain Damage and Outcome. J. Neurotrauma. 2004, 21, 1553–1561. [Google Scholar] [CrossRef]
- Ribotta, M.G.; Menet, V.; Privat, A. Glial scar and axonal regeneration in the CNS: Lessons from GFAP and vimentin transgenic mice. In Mechanisms of Secondary Brain Damage from Trauma and Ischemia; Springer: Vienna, Austria, 2004; pp. 87–92. [Google Scholar]
- Honda, M.; Tsuruta, R.; Kaneko, T.; Kasaoka, S.; Yagi, T.; Todani, M.; Fujita, M.; Izumi, T.; Maekawa, T. Serum Glial Fibrillary Acidic Protein Is a Highly Specific Biomarker for Traumatic Brain Injury in Humans Compared with S-100B and Neuron-Specific Enolase. J. Trauma Inj. Infect. Crit. Care 2010, 69, 104–109. [Google Scholar] [CrossRef]
- Kim, H.J.; Tsao, J.W.; Stanfill, A.G. The current state of biomarkers of mild traumatic brain injury. JCI Insight 2018, 3, e97105. [Google Scholar] [CrossRef]
- Pelinka, L.E.; Kroepfl, A.; Schmidhammer, R.; Krenn, M.; Buchinger, W.; Redl, H.; Raabe, A. Glial Fibrillary Acidic Protein in Serum After Traumatic Brain Injury and Multiple Trauma. J. Trauma Inj. Infect. Crit. Care 2004, 57, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, R.H.; Marini, A.M. Brain-Derived Neurotrophic Factor in Neuronal Survival and Behavior-Related Plasticity. Ann. N. Y. Acad. Sci. 2007, 1122, 130–143. [Google Scholar] [CrossRef]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Persson, L.; Hårdemark, H.G.; Gustafsson, J.; Rundström, G.; Mendel-Hartvig, I.; Esscher, T.; Påhlman, S. S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: Markers of cell damage in human central nervous system. Stroke 1987, 18, 911–918. [Google Scholar] [CrossRef]
- Olsson, B.; Zetterberg, H.; Hampel, H.; Blennow, K. Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 2011, 95, 520–534. [Google Scholar] [CrossRef]
- Kempuraj, D. Current Trends in Biomarkers for Traumatic Brain Injury. Open Access J. Neurol. Neurosurg. 2020, 12, 86–94. [Google Scholar] [CrossRef]
- Blyth, B.J.; Farahvar, A.; He, H.; Nayak, A.; Yang, C.; Shaw, G.; Bazarian, J.J. Elevated Serum Ubiquitin Carboxy-Terminal Hydrolase L1 Is Associated with Abnormal Blood–Brain Barrier Function after Traumatic Brain Injury. J. Neurotrauma. 2011, 28, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Flöel, A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 2012, 88, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S.; Cohen, J.D. On the Control of Control: The Role of Dopamine in Regulating Prefrontal Function and Working Memory. Atten. Perform. 2000, 18, 712–737. [Google Scholar]
- Aalto, S.; Brück, A.; Laine, M.; Någren, K.; Rinne, J.O. Frontal and Temporal Dopamine Release during Working Memory and Attention Tasks in Healthy Humans: A Positron Emission Tomography Study Using the High-Affinity Dopamine D2 Receptor Ligand [11C]FLB 457. J. Neurosci. 2005, 25, 2471. [Google Scholar] [CrossRef]
- McAllister, T.W.; Rhodes, C.H.; Flashman, L.A.; McDonald, B.C.; Belloni, D.; Saykin, A.J. Effect of the dopamine D2 receptor T allele on response latency after mild traumatic brain injury. Am. J. Psychiatry 2005, 162, 1749–1751. [Google Scholar] [CrossRef]
- McAllister, T.W.; Flashman, L.A.; Harker Rhodes, C.; Tyler, A.L.; Moore, J.H.; Saykin, A.J.; McDonald, B.C.; Tosteson, T.D.; Tsongalis, G.J. Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: A replication and extension study. Brain Inj. 2008, 22, 705–714. [Google Scholar] [CrossRef]
- Elwood, R.W. The California Verbal Learning Test: Psychometric characteristics and clinical application. Neuropsychol. Rev. 1995, 5, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Kors, E.E.; Terwindt, G.M.; Vermeulen, F.L.M.G.; Fitzsimons, R.B.; Jardine, P.E.; Heywood, P.; Love, S.; Maagdenberg, A.M.V.D.; Haan, J.; Frants, R.R.; et al. Delayed cerebral edema and fatal coma after minor head trauma: Role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann. Neurol. 2001, 49, 753–760. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W. Genetic Factors in Traumatic Brain Injury; Elsevier: Amsterdam, The Netherlands, 2015; pp. 723–739. [Google Scholar]
- Stam, A.H.; Luijckx, G.J.; Poll-The, B.T.; Ginjaar, I.B.; Frants, R.R.; Haan, J.; Ferrari, M.D.; Terwindt, G.M.; Maagdenberg, A.M.J.M.v.D. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Paterakis, K.; Tsivgoulis, G.; Tsintou, M.; Hadjigeorgiou, G.F.; Dardioti, M.; Grigoriadis, S.; Simeonidou, C.; Komnos, A.; Kapsalaki, E.; et al. AQP4 Tag Single Nucleotide Polymorphisms in Patients with Traumatic Brain Injury. J. Neurotrauma. 2014, 31, 1920–1926. [Google Scholar] [CrossRef]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in Brain: Distribution, Physiology, and Pathophysiology. J. Cereb. Blood Flow. Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef]
- Aoki, K.; Uchihara, T.; Tsuchiya, K.; Nakamura, A.; Ikeda, K.; Wakayama, Y. Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol. 2003, 106, 121–124. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Potential utility of aquaporin modulators for therapy of brain disorders. Prog. Brain Res. 2008, 170, 589–601. [Google Scholar]
- Sorani, M.D.; Zador, Z.; Hurowitz, E.; Yan, D.; Giacomini, K.M.; Manley, G.T. Novel variants in human Aquaporin-4 reduce cellular water permeability. Hum. Mol. Genet. 2008, 17, 2379–2389. [Google Scholar] [CrossRef]
- Donkin, J.J.; Vink, R. Mechanisms of cerebral edema in traumatic brain injury: Therapeutic developments. Curr. Opin. Neurol. 2010, 23, 293–299. Available online: https://journals.lww.com/co-neurology/Fulltext/2010/06000/Mechanisms_of_cerebral_edema_in_traumatic_brain.16.aspx (accessed on 23 October 2025). [CrossRef]
- Ghabriel, M.N.; Thomas, A.; Vink, R. Magnesium restores altered aquaporin-4 immunoreactivity following traumatic brain injury to a pre-injury state. Acta Neurochir. Suppl. 2006, 96, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Shahjouei, S.; Sadeghi-Naini, M.; Yang, Z.; Kobeissy, F.; Rathore, D.; Shokraneh, F.; Blackburn, S.; Manley, G.T.; Wang, K.K. The diagnostic values of UCH-L1 in traumatic brain injury: A meta-analysis. Brain Inj. 2017, 32, 1–17. [Google Scholar] [CrossRef]
- Mondello, S.; Shear, D.A.; Bramlett, H.M.; Dixon, C.E.; Schmid, K.E.; Dietrich, W.D.; Wang, K.K.W.; Hayes, R.L.; Glushakova, O.; Catania, M.; et al. Insight into Pre-Clinical Models of Traumatic Brain Injury Using Circulating Brain Damage Biomarkers: Operation Brain Trauma Therapy. J. Neurotrauma 2016, 33, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.; Blackburn-Munro, G.; Garry, E.M.; Blakemore, J.A.; Dickinson, T.; Rosie, R.; Mitchell, R.; Fleetwood-Walker, S.M. A role of the ubiquitin-proteasome system in neuropathic pain. J. Neurosci. 2002, 22, 1363–1372. Available online: https://pubmed.ncbi.nlm.nih.gov/11850463/ (accessed on 23 October 2025). [CrossRef]
- Setsuie, R.; Wada, K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem. Int. 2007, 51, 105–111. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Thompson, R.J. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J. Neurol. Sci. 1981, 49, 429–438. Available online: http://www.jns-journal.com/article/0022510X81900320/fulltext (accessed on 23 October 2025). [CrossRef]
- Todi, S.V.; Paulson, H.L. Balancing act: Deubiquitinating enzymes in the nervous system. Trends Neurosci. 2011, 34, 370–382. [Google Scholar] [CrossRef]
- Liu, M.C.; Akinyi, L.; Scharf, D.; Mo, J.; Larner, S.F.; Muller, U.; Oli, M.W.; Zheng, W.; Kobeissy, F.; Papa, L.; et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010, 31, 722–732. [Google Scholar] [CrossRef]
- Rafter, D.; Li, Z.; Schaaf, T.; Gault, K.; Thorpe, M.; Venkatesh, S.; Edpuganti, R.; Song, T.; Kuang, R.; Samadani, U. Machine Learning with Objective Serum Markers and Algorithmic Deep Learning Computed Tomography Scan Analysis for Classification of Brain Injury. medRxiv 2021. [Google Scholar] [CrossRef]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J.; Oli, M.W.; Scheld, K.; Carson, J.H. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef]
- Barbarese, E.; Barry, C.; Chou, C.J.; Goldstein, D.J.; Nakos, G.A.; Hyde-DeRuyscher, R.; Scheld, K.; Carson, J.H. Expression and Localization of Myelin Basic Protein in Oligodendrocytes and Transfected Fibroblasts. J. Neurochem. 1988, 51, 1737–1745. [Google Scholar] [CrossRef]
- Berger, R.P.; Adelson, P.D.; Pierce, M.C.; Dulani, T.; Cassidy, L.D.; Kochanek, P.M. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. Pediatr. 2005, 103, 61–68. Available online: https://thejns.org/pediatrics/view/journals/j-neurosurg-pediatr/103/1/article-p61.xml (accessed on 23 October 2025). [CrossRef] [PubMed]
- Kochanek, P.M.; Berger, R.P.; Bayr, H.; Wagner, A.K.; Jenkins, L.W.; Clark, R.S. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: Diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care. 2008, 14, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.P.; Bazaco, M.C.; Wagner, A.K.; Kochanek, P.M.; Fabio, A. Trajectory Analysis of Serum Biomarker Concentrations Facilitates Outcome Prediction after Pediatric Traumatic and Hypoxemic Brain Injury. Dev. Neurosci. 2010, 32, 96–405. [Google Scholar] [CrossRef]
- D’Aversa, T.G.; Eugenin, E.A.; Lopez, L.; Berman, J.W. Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood-brain barrier disruption: Implications for the pathogenesis of multiple sclerosis. Neuropathol. Appl. Neurobiol. 2013, 39, 270–283. Available online: https://pubmed.ncbi.nlm.nih.gov/22524708/ (accessed on 23 October 2025). [CrossRef]
- Jeter, C.B.; Hergenroeder, G.W.; Hylin, M.J.; Redell, J.B.; Moore, A.N.; Dash, P.K. Biomarkers for the Diagnosis and Prognosis of Mild Traumatic Brain Injury/Concussion. J Neurotrauma. 2013, 30, 657–670. [Google Scholar] [CrossRef]
- Clausen, T.; Khaldi, A.; Zauner, A.; Reinert, M.; Doppenberg, E.; Menzel, M.; Soukup, J.; Alves, O.L.; Bullock, M.R. Cerebral acid—Base homeostasis after severe traumatic brain injury. J. Neurosurg. 2005, 103, 597–607. [Google Scholar] [CrossRef]
- Yin, T.; Lindley, T.E.; Albert, G.W.; Ahmed, R.; Schmeiser, P.B.; Grady, M.S.; Howard, M.A.; Welsh, M.J. Loss of Acid Sensing Ion Channel-1a and Bicarbonate Administration Attenuate the Severity of Traumatic Brain Injury. PLoS ONE 2013, 8, e72379. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Chen, J.; Askwith, C.C.; Hruska-Hageman, A.M.; Price, M.P.; Nolan, B.C.; Yoder, P.G.; Lamani, E.; Hoshi, T.; Freeman, J.H.; et al. The Acid-Activated Ion Channel ASIC Contributes to Synaptic Plasticity, Learning, and Memory. Neuron. 2002, 34, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.G.; Chu, X.P.; Simon, R.P. Acid sensing ion channels—Novel therapeutic targets for ischemic brain injury. Front. Biosci. 2007, 12, 1376–1386. Available online: https://pubmed.ncbi.nlm.nih.gov/17127388/ (accessed on 23 October 2025). [CrossRef] [PubMed]
- Wemmie, J.A.; Askwith, C.C.; Lamani, E.; Cassell, M.D.; Freeman, J.H.; Welsh, M.J. Acid-Sensing Ion Channel 1 Is Localized in Brain Regions with High Synaptic Density and Contributes to Fear Conditioning. J. Neurosci. 2003, 23, 5496–5502. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).