In Vitro Investigation of the Effects of Octenidine Dihydrochloride on Nasal Septum Squamous Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Viability Analysis
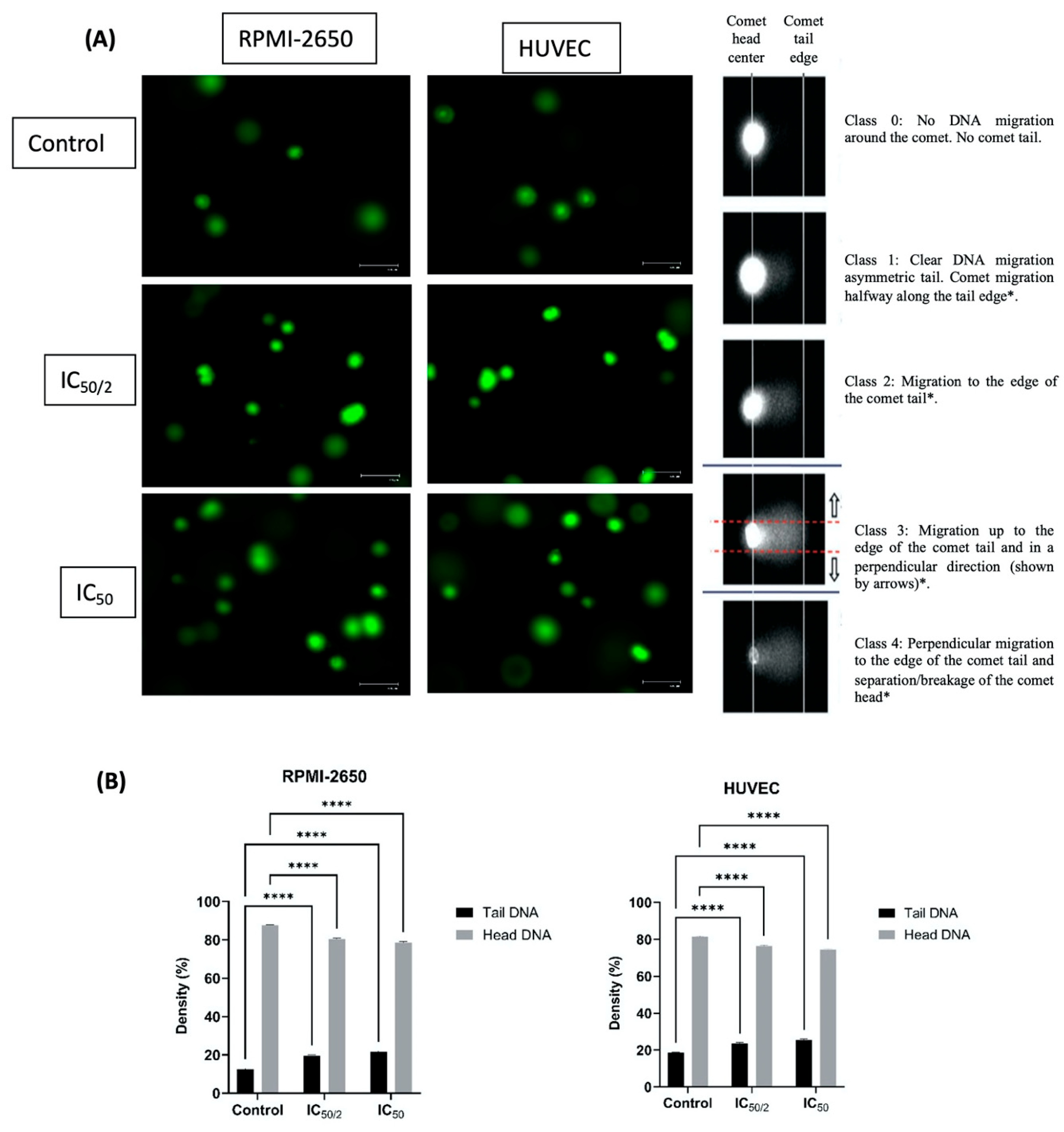
2.3. Single Cell Gel Electrophoresis (Comet Assay)
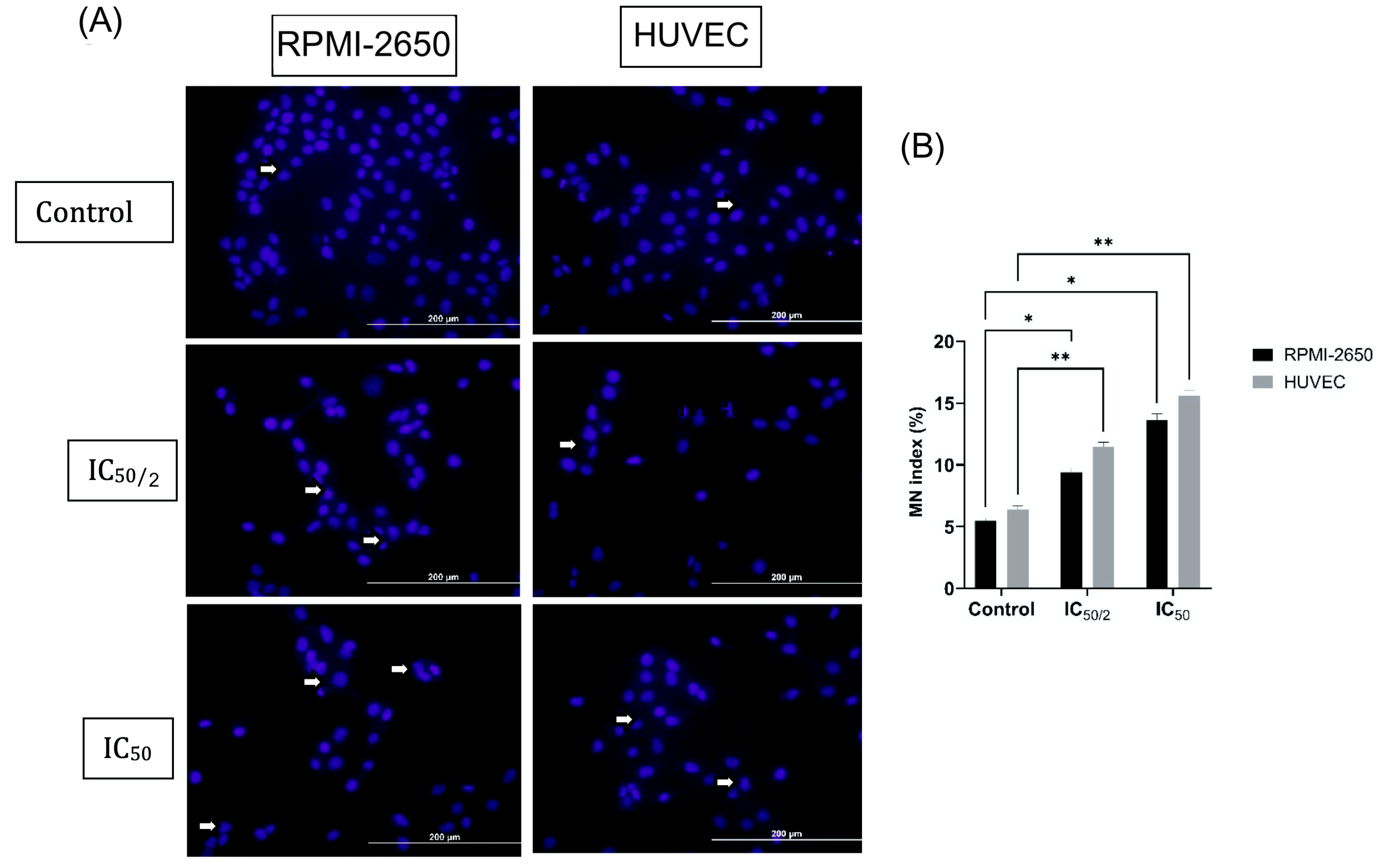
2.4. Cytokinesis-Blocked Micronucleus (CBMN) Analysis
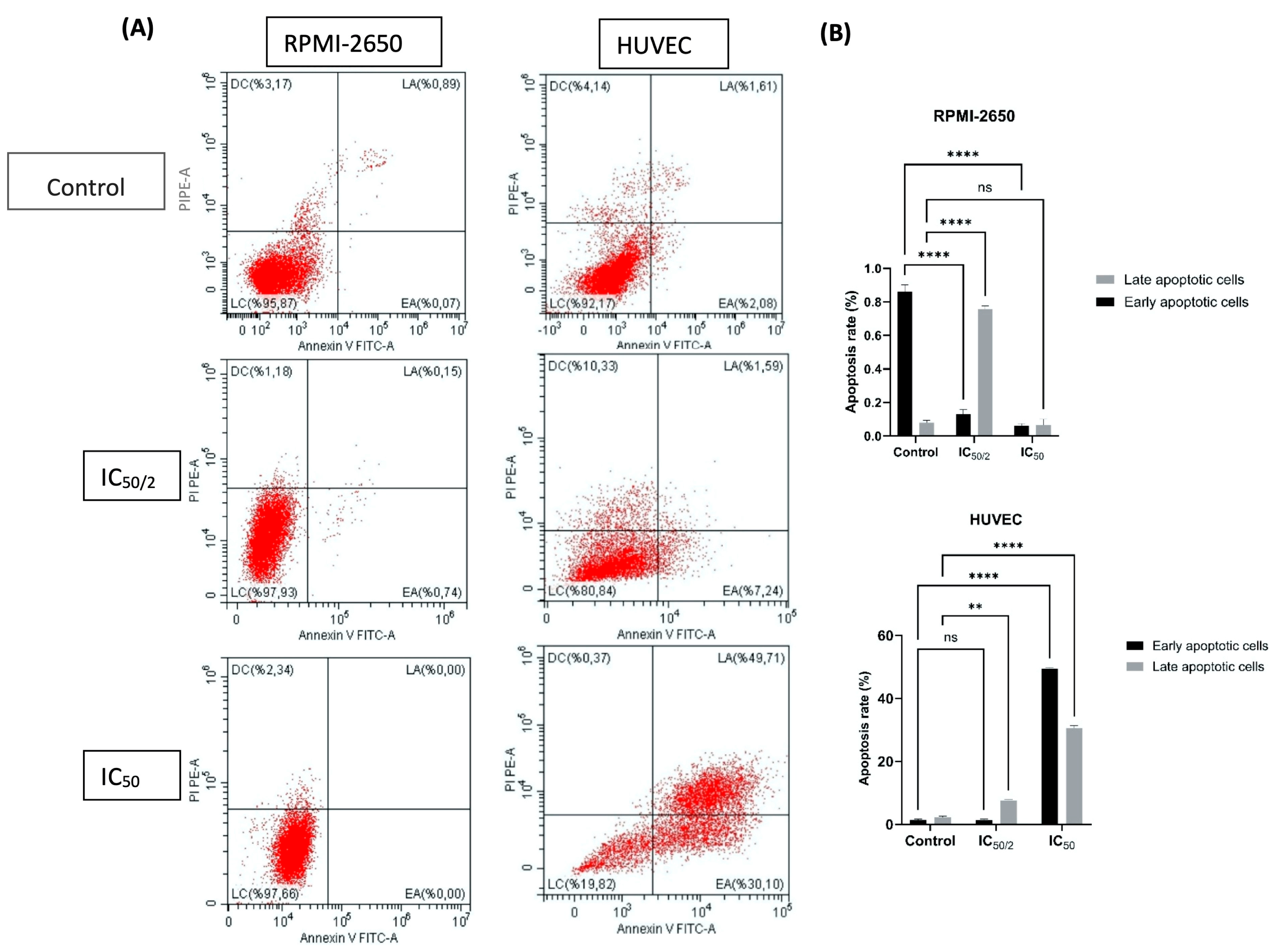
2.5. Apoptosis and Reactive Oxygen Species (ROS) Flow Cytometry Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of OCT-D on Cell Viability
3.2. Effect of OCT-D on DNA Damage
3.3. The Effect of OCT-D on MN Formation
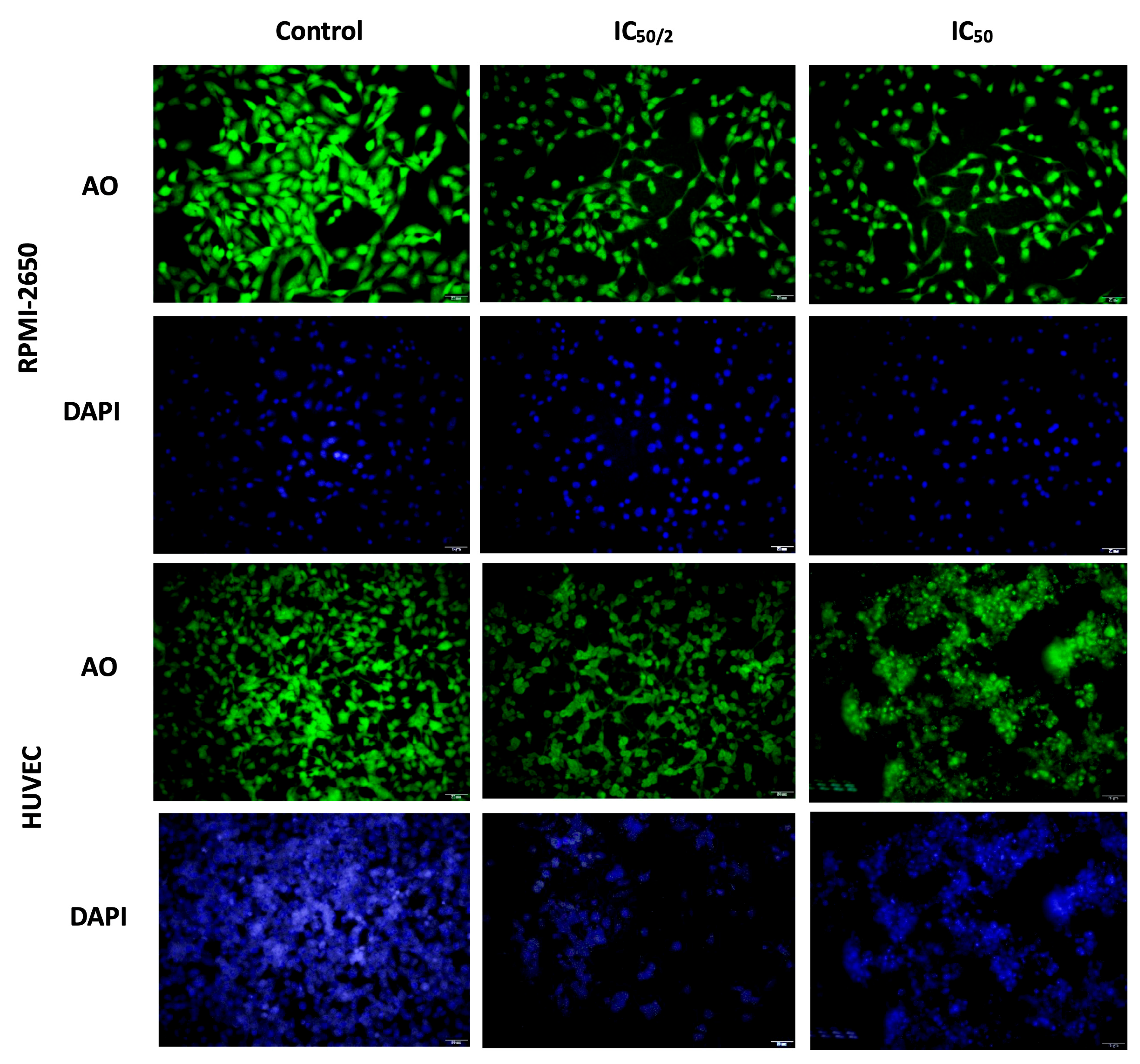
3.4. Effect of OCT-D on Apoptotic Cell Percentage, Cell, and Nuclear Morphology
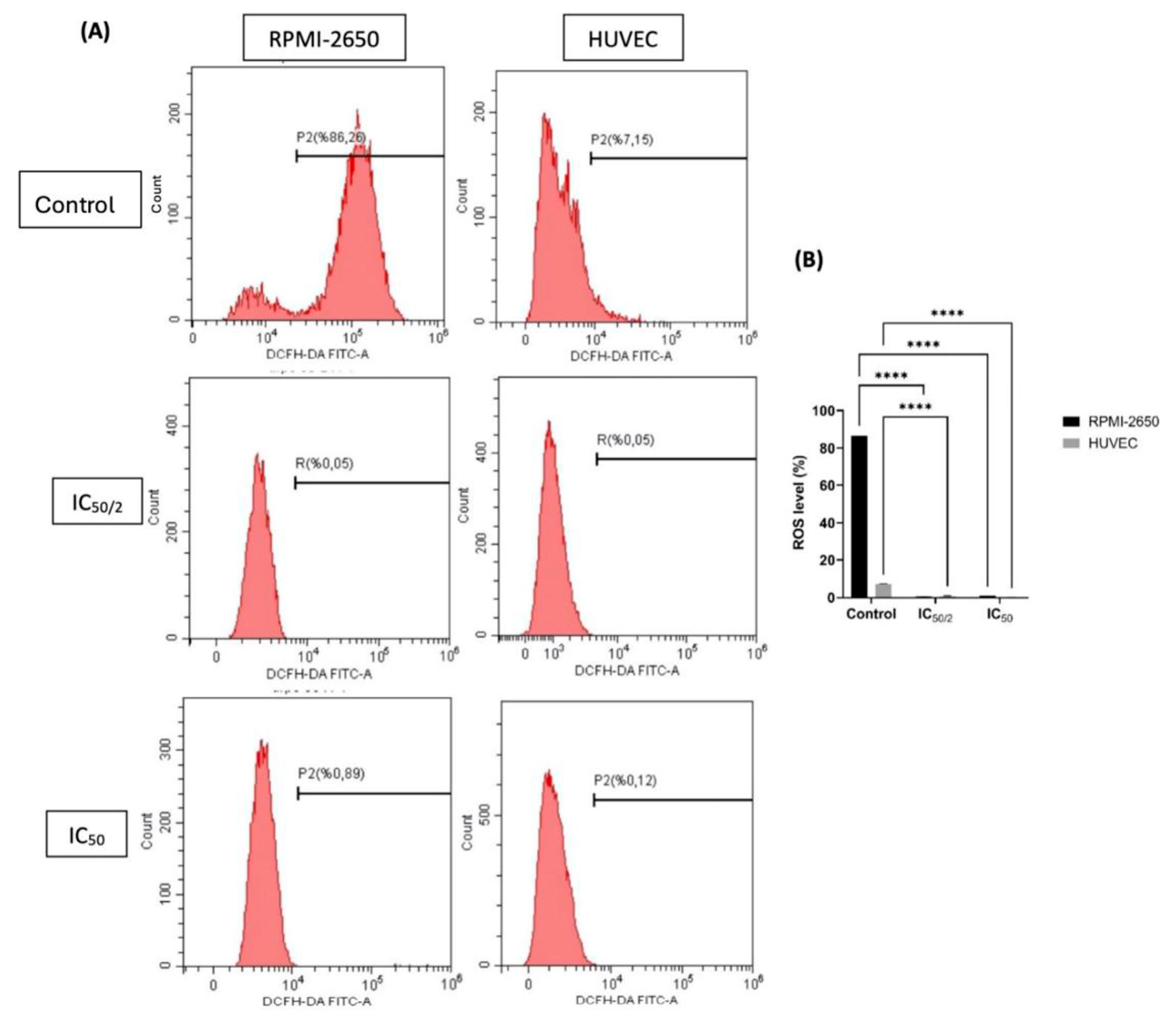
3.5. The Effect of OCT-D on ROS Quantification
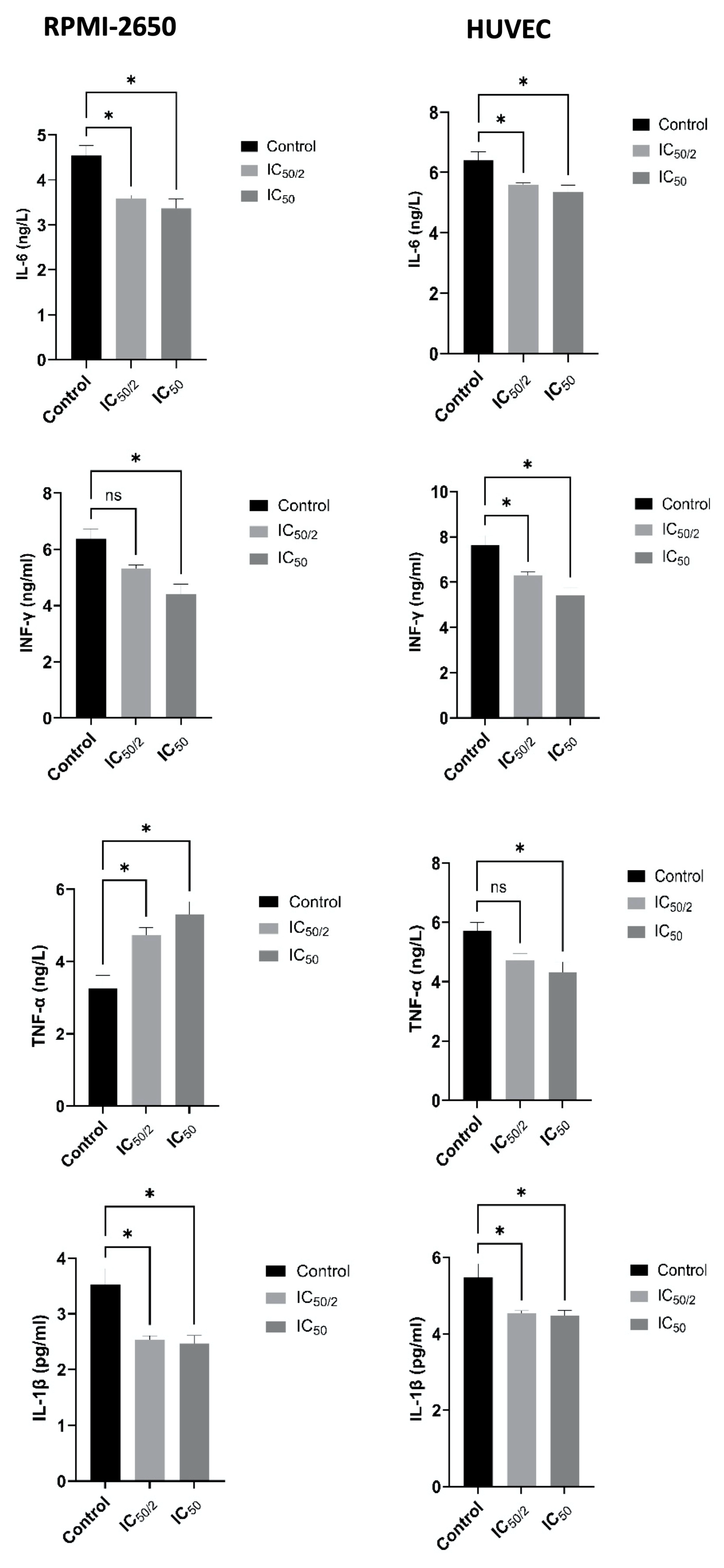
3.6. Effect of OCT-D on Inflammatory Markers
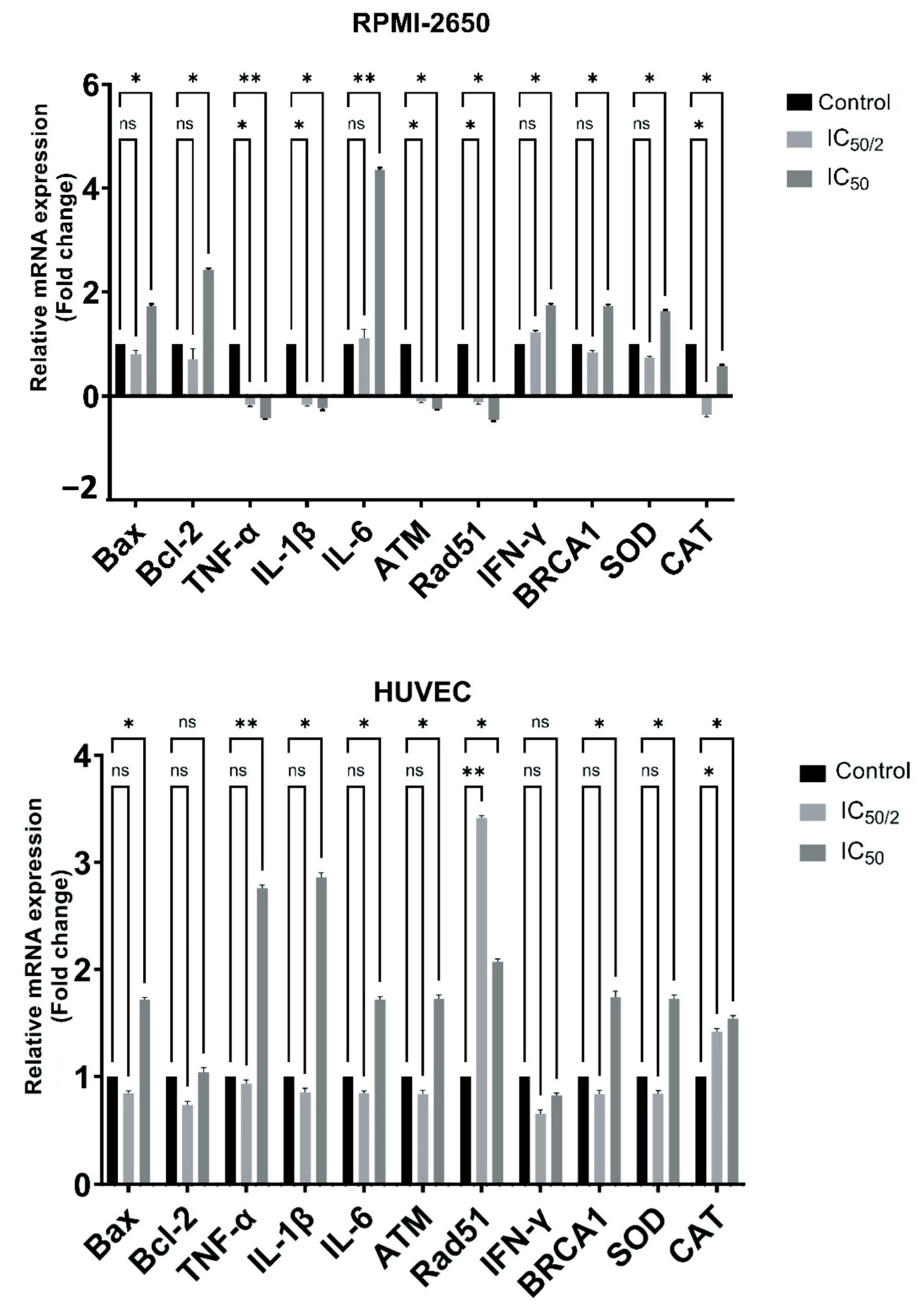
3.7. Effect of OCT-D on mRNA Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bührer, C.; Bahr, S.; Siebert, J.; Wettstein, R.; Geffers, C.; Obladen, M. Use of 2% 2-phenoxyethanol and 0.1% octenidine as antiseptic in premature newborn infants of 23–26 weeks gestation. J. Hosp. Infect. 2002, 51, 305–307. [Google Scholar] [CrossRef]
- Alvarez-Marin, R.; Aires-de-Sousa, M.; Nordmann, P.; Kieffer, N.; Poirel, L. Antimicrobial Activity of Octenidine against Multidrug-Resistant Gram-Negative Pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2379–2383. [Google Scholar] [CrossRef]
- Heckel, M.; Geißdörfer, W.; Herbst, F.A.; Stiel, S.; Ostgathe, C.; Bogdan, C. Nasal Carriage of Methicillin-Resistant Staphylococcus aureus (MRSA) at a Palliative Care Unit: A Prospective Single Service Analysis. PLoS ONE 2017, 12, e0188940. [Google Scholar] [CrossRef]
- Allport, J.; Choudhury, R.; Bruce-Wootton, P.; Reed, M.; Tate, D.; Malviya, A. Efficacy of Mupirocin, Neomycin and Octenidine for Nasal Staphylococcus aureus Decolonisation: A Retrospective Cohort Study. Antimicrob. Resist. Infect. Control. 2022, 11, 5. [Google Scholar] [CrossRef]
- Sipahi, O.R. Economics of Antibiotic Resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 523–539. [Google Scholar] [CrossRef]
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel Insights into the Detailed Killing Mechanism of Gram-Negative Bacteria at a Cellular and Molecular Level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef] [PubMed]
- Mert, G.; Kilic, A.; Bedir, O.; Basustaoglu, A.C. Clinical Significance and Staphylococcal Cassette Chromosome mec (SCCmec) Characterization of Coagulase-Negative Staphylococci Isolated from Blood Cultures. Turk. J. Med. Sci. 2011, 41, 859–865. [Google Scholar] [CrossRef]
- Seah, C.; Alexander, D.C.; Louie, L.; Simor, A.; Low, D.E.; Longtin, J.; Melano, R.G. MupB, a New High-Level Mupirocin Resistance Mechanism in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 1916–1920. [Google Scholar] [CrossRef]
- Telchik, C.; Jinadatha, C.; Cadnum, J.; Donskey, C.; Chatterjee, P.; Dhar, S.; Kaye, K.; Yakubik, T.; Hwang, M.; Grimbergen, A. Assessing Chlorhexidine Resistance in MRSA Isolates from Hospitals in Cleveland, OH and Detroit, MI. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, s114. [Google Scholar] [CrossRef]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine Dihydrochloride, a Modern Antiseptic for Skin, Mucous Membranes and Wounds. Skin Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef]
- Schmidt, J.; Zyba, V.; Jung, K.; Rinke, S.; Haak, R.; Mausberg, R.F.; Ziebolz, D. Cytotoxic Effects of Octenidine Mouth Rinse on Human Fibroblasts and Epithelial Cells—An In Vitro Study. Drug Chem. Toxicol. 2016, 39, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, P.S. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650). Exp. Cell Res. 1965, 39, 190–196. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 Cell Models for Nasal Drug Permeability Prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef]
- Mercier, C.; Perek, N.; Delavenne, X. Is RPMI 2650 a Suitable In Vitro Nasal Model for Drug Transport Studies? Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 13–24. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Hayashi, M. The Micronucleus Test—Most Widely Used in Vivo Genotoxicity Test. Genes Environ. 2016, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB Staining to Detect Apoptosis in Osteosarcoma Cells Compared with Flow Cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.H.; Park, J.H.; Kim, H.S.; Lee, H.M.; Kim, S.D.; Mun, S.J.; Cho, K.S. Inflammatory Effects of Particulate Matter Exposure on the Nasal and Paranasal Sinus Mucosa in Rats. Int. J. Mol. Sci. 2025, 26, 5885. [Google Scholar] [CrossRef]
- Mitupatum, T.; Aree, K.; Kittisenachai, S.; Roytrakul, S.; Puthong, S.; Kangsadalampai, S.; Rojpibulstit, P. mRNA Expression of Bax, Bcl-2, p53, Cathepsin B, Caspase-3 and Caspase-9 in the HepG2 Cell Line Following Induction by a Novel Monoclonal Ab Hep88 mAb: Cross-Talk for Paraptosis and Apoptosis. Asian Pac. J. Cancer Prev. 2016, 17, 703–712. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Wang, T.; Song, J.; Zhou, Z. Effects of Apoptosis-Related Proteins Caspase-3, Bax and Bcl-2 on Cerebral Ischemia Rats. Biomed. Rep. 2013, 1, 861–867. [Google Scholar] [CrossRef]
- Nakano, T.; Takahashi, T.; Yamamoto, C.; Yoshida, E.; Kaji, T.; Fujiwara, Y. Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells. Int. J. Mol. Sci. 2021, 22, 739. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Azqueta, A.; Sanz-Serrano, J.; Bakuradze, T.; Richling, E.; Eyluel Bankoglu, E.; Stopper, H.; Claudino Bastos, V.; Langie, S.A.S.; Jensen, A.; et al. Visual comet scoring revisited: A guide to scoring comet assay slides and obtaining reliable results. Mutagenesis 2023, 38, 253–263. [Google Scholar] [CrossRef]
- Chum, J.D.; Lim, D.J.Z.; Sheriff, S.O.; Pulikkotil, S.J.; Suresh, A.; Davamani, F. In Vitro Evaluation of Octenidine as an Antimicrobial Agent against Staphylococcus epidermidis in Disinfecting the Root Canal System. Restor. Dent. Endod. 2019, 44, e8. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Baradaran, B.; Orangi, M.; Zamani, F. In Vitro Cytotoxic Activity of Four Plants Used in Persian Traditional Medicine. Adv. Pharm. Bull. 2013, 3, 453–455. [Google Scholar] [CrossRef]
- Ozkiriş, M.; Akbulut, S.; Aydın, E.; Unver, S. Squamous Cell Carcinoma Originating from the Nasal Septum: A Case Report. J. Ear Nose Throat 2006, 16, 91–93. [Google Scholar]
- Seiser, S.; Janker, L.; Zila, N.; Mildner, M.; Rakita, A.; Matiasek, J.; Bileck, A.; Gerner, C.; Paulitschke, V.; Elbe-Bürger, A. Octenidine-Based Hydrogel Shows Anti-Inflammatory and Protease-Inhibitory Capacities in Wounded Human Skin. Sci. Rep. 2021, 11, 32. [Google Scholar] [CrossRef]
- Cory, S.; Huang, D.C.S.; Adams, J.M. The Bcl-2 Family: Roles in Cell Survival and Oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell Death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Z.; Yao, L.; Sun, Y.; Dian, Y.; Zhao, D.; Ke, Y.; Zeng, F.; Zhang, C.; Deng, G.; et al. Targeting Oxidative Stress-Mediated Regulated Cell Death as a Vulnerability in Cancer. Redox Biol. 2025, 84, 103686. [Google Scholar] [CrossRef]
- Zeng, Z.; Centner, C.; Gollhofer, A.; König, D. Effects of Dietary Strategies on Exercise-Induced Oxidative Stress: A Narrative Review of Human Studies. Antioxidants 2021, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zou, M.H. Redox Regulation of Endothelial Cell Fate. Cell Mol. Life Sci. 2014, 71, 3219–3239. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Pérez, S.; Toledano, M.B.; Sastre, J. Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation. Antioxid. Redox Signal. 2023, 39, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, J.; Huang, J.; Feng, Y.; Zhao, J.; Zhu, Y.; Deng, X.; Li, X.; Zhang, W.; Wang, C. Cell Death Signaling and Immune Regulation: New Perspectives on Targeted Therapy for Sepsis. Cell Mol. Biol. Lett. 2025, 30, 99. [Google Scholar] [CrossRef]
- Gao, F.; Xu, T.; Zang, F.; Luo, Y.; Pan, D. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms, Clinical Management and Innovative Treatment. Drug Des. Dev. Ther. 2024, 18, 4089–4116. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox Regulation: Mechanisms, Biology and Therapeutic Targets in Diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Maître, B.; Jornot, L.; Junod, A.F. Effects of Inhibition of Catalase and Superoxide Dismutase Activity on Antioxidant Enzyme mRNA Levels. Am. J. Physiol. 1993, 265, L636–L643. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Denkel, L.; Feßler, A.T.; Eicker, R.; Mellmann, A.; Schwarz, S.; Geffers, C.; Hübner, N.-O.; Leistner, R. Clinical Evidence for the Use of Octenidine Dihydrochloride to Prevent Healthcare-Associated Infections and Decrease Staphylococcus aureus Carriage or Transmission—A Review. Pathogens 2023, 12, 612. [Google Scholar] [CrossRef]
- Unterfrauner, I.; Bragatto-Hess, N.; Studhalter, T.; Farshad, M.; Uçkay, I. General Skin and Nasal Decolonization with Octenisan® Set before and after Elective Orthopedic Surgery in Selected Patients at Elevated Risk for Revision Surgery and Surgical Site Infections—A Single-Center, Unblinded, Superiority, Randomized Controlled Trial (BALGDEC Trial). Trials 2024, 25, 461. [Google Scholar] [CrossRef]
- Haesler, E. Evidence Summary: Octenidine for Chronic Wounds. Wound Pract. Res. 2020, 28, 42–44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciftci, I.H.; Deveci Ozkan, A.; Erman, G.; Kahraman Kilbas, E.P.; Koroglu, M. In Vitro Investigation of the Effects of Octenidine Dihydrochloride on Nasal Septum Squamous Carcinoma Cells. Biomedicines 2025, 13, 2668. https://doi.org/10.3390/biomedicines13112668
Ciftci IH, Deveci Ozkan A, Erman G, Kahraman Kilbas EP, Koroglu M. In Vitro Investigation of the Effects of Octenidine Dihydrochloride on Nasal Septum Squamous Carcinoma Cells. Biomedicines. 2025; 13(11):2668. https://doi.org/10.3390/biomedicines13112668
Chicago/Turabian StyleCiftci, Ihsan Hakki, Asuman Deveci Ozkan, Gulay Erman, Elmas Pinar Kahraman Kilbas, and Mehmet Koroglu. 2025. "In Vitro Investigation of the Effects of Octenidine Dihydrochloride on Nasal Septum Squamous Carcinoma Cells" Biomedicines 13, no. 11: 2668. https://doi.org/10.3390/biomedicines13112668
APA StyleCiftci, I. H., Deveci Ozkan, A., Erman, G., Kahraman Kilbas, E. P., & Koroglu, M. (2025). In Vitro Investigation of the Effects of Octenidine Dihydrochloride on Nasal Septum Squamous Carcinoma Cells. Biomedicines, 13(11), 2668. https://doi.org/10.3390/biomedicines13112668




