Progress in the Application of Nanomaterials in Tumor Treatment
Abstract
1. Introduction
2. Common Types of Nanomaterials and Their Characteristics
2.1. Organic Nanomaterials
2.1.1. Liposomes
2.1.2. High Polymer Nanoparticles
2.1.3. Peptide Nanoparticles
2.2. Inorganic Nanomaterials
2.2.1. Gold Nanoparticles
2.2.2. Silica Nanoparticles
2.2.3. Magnetic Nanoparticles
2.2.4. Novel Metal Nanoparticles
2.3. Carbon-Based Nanomaterials
2.3.1. Carbon Nanotubes
2.3.2. Graphene Oxide
3. Application of Nanomaterials in Tumor Treatment
3.1. Targeted Drug Delivery System
3.2. Photothermal Therapy and Photodynamic Therapy
3.3. Nano-Immunotherapy
3.4. Multimodal Combined Therapy
4. Challenges and Issues of Nanomaterials in Tumor Therapy
4.1. Drug Delivery and Biodistribution
4.2. Toxicology and Biocompatibility
4.2.1. Toxicity Induced by Particle Size and Surface Properties
4.2.2. Oxidative Stress and Inflammatory Response
4.2.3. Immune Response and Immune Escape
4.2.4. Long-Term Residence and Chronic Toxicity
4.3. Scalable Production and Quality Consistency
4.4. Multidimensional Analysis of Key Bottlenecks in Nanomedicine Clinical Translation
5. Future Development and Prospects of Nanomaterials in Tumor Treatment
5.1. Safe and Controllable Material Design Strategy
5.2. Multifunctional Integrated Intelligent Nano-Platform
5.3. Green and Sustainable Manufacturing and Scale-Up
5.4. Big Data and Artificial Intelligence-Assisted Design
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| Au-MSN NPs | gold nanorods and mesoporous silica nanoparticles |
| Bi | bismuth |
| BSA | bovine serum albumin |
| CCNs | cubic carbon nanostructures |
| CDP | cyclodextrin polymer |
| Ce6 | chlorin e6 |
| CNTs | Carbon nanotubes |
| CPPs | cell-penetrating peptides |
| CPT | camptothecin |
| CSM | cutoff scanning matrix |
| CSMNs | clover-shaped magnetic nanoclusters |
| CTCs | circulating tumor cells |
| CV | coefficient of variation |
| DNA | deoxyribonucleic acid |
| EMA | the European Medicines Agency |
| EPR | enhanced permeability and retention |
| FA | folic acid |
| FDA | the U.S. Food and Drug Administration |
| Gd-DTPA | gadopentetate dimeglumine |
| ICD | immunogenic cell death |
| ICIs | immune checkpoint inhibitors |
| MHT | magnetic hyperthermia therapy |
| MOFs | metal–organic frameworks |
| MoS2 | molybdenum disulfide |
| MPS | mononuclear phagocyte system |
| MSNs | mesoporous silica nanoparticles |
| MWCNTs | multi-walled carbon nanotubes |
| NGO | Nanoparticles graphene oxide |
| NIR | near-infrared |
| NMEs | New Molecular Entities |
| PDA | polydopamine |
| PDT | photodynamic therapy |
| PDX | patient-derived xenograft |
| PEG | polyethylene glycol |
| PLGA | Polylactic-co-glycolic acid |
| PPy | polypyrrole |
| PSMA | prostate-specific membrane antigen |
| Pt II | platinum II |
| Pt IV | platinum IV |
| PTT | photothermal therapy |
| PTX | paclitaxel |
| RES | reticuloendothelial system |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RT | radiotherapy |
| Se | selenium |
| SeNPs | selenium nanoparticles |
| Si-motor | silicon-based oxygen-driven nanomotor |
| siRNA | small interfering RNA |
| SWCNTs | single-walled carbon nanotubes |
| TDDS | Targeted drug delivery systems |
| Te | tellurium |
| TeNPs | tellurium nanoparticles |
| TMDs | transition metal dichalcogenides |
| TME | tumor microenvironment |
References
- Gao, S.; Lin, H.; Zhang, H.; Yao, H.; Chen, Y.; Shi, J. Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv. Sci. 2019, 6, 1801733. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; Shi, J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018, 47, 6930–6946. [Google Scholar] [CrossRef]
- Xiong, J.; Zheng, T.J.; Shi, Y.; Wei, F.; Ma, S.C.; He, L.; Wang, S.C.; Liu, X.S. Analysis of the fingerprint profile of bioactive constituents of traditional Chinese medicinal materials derived from animal bile using the HPLC-ELSD and chemometric methods: An application of a reference scaleplate. J. Pharm. Biomed. Anal. 2019, 174, 50–56. [Google Scholar] [CrossRef]
- Xue, C.; Sutrisno, L.; Li, M.; Zhu, W.; Fei, Y.; Liu, C.; Wang, X.; Cai, K.; Hu, Y.; Luo, Z. Implantable multifunctional black phosphorus nanoformulation-deposited biodegradable scaffold for combinational photothermal/chemotherapy and wound healing. Biomaterials 2021, 269, 120623. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, B.; Pascual, L.; Bernardos, A.; Marcos, M.D.; Jeppesen, J.O.; Salinas, Y.; Martínez-Máñez, R.; Sancenón, F. Pseudorotaxane capped mesoporous silica nanoparticles for 3,4-methylenedioxymethamphetamine (MDMA) detection in water. Chem. Commun. 2017, 53, 3559–3562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, Y.; Wu, L.; Zhang, S.; Su, Q.; Yang, Q. Deguelin and paclitaxel loaded PEG-PCL nano-micelles for suppressing the proliferation and inducing apoptosis of breast cancer cells. Front. Biosci. 2024, 29, 90. [Google Scholar] [CrossRef] [PubMed]
- Vasanthakumar, G.; Ahmed, N.K. Uptake and metabolism of daunorubicin by human myelocytic cells. Cancer Chemother. Pharmacol. 1985, 15, 35–39. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Weiss, L.; Grundmann, E.; Torhorst, J.; Hartveit, F.; Moberg, I.; Eder, M.; Fenoglio-Preiser, C.M.; Napier, J.; Horne, C.H.; Lopez, M.J.; et al. Haematogenous metastatic patterns in colonic carcinoma: An analysis of 1541 necropsies. J. Pathol. 1986, 150, 195–203. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Chen, K.; Kang, M.; Zhang, H.; Jin, X.; Lin, L.; Chen, J. Relationship between postoperative complications and the prognosis of gastric carcinoma patients who underwent surgical resection: A systematic review and meta-analysis. Cancer Control 2021, 28, 10732748211011955. [Google Scholar] [CrossRef]
- Feler, J.; Sun, F.; Bajaj, A.; Hagan, M.; Kanekar, S.; Sullivan, P.L.Z.; Fridley, J.S.; Gokaslan, Z.L. Complication avoidance in surgical management of vertebral column tumors. Curr. Oncol. 2022, 29, 1442–1454. [Google Scholar] [CrossRef]
- Ohashi, K.; Suzuki, H.; Sata, Y.; Tanaka, K.; Yamamoto, T.; Sakairi, Y.; Wada, H.; Nakajima, T.; Nozaki-Taguchi, N.; Isono, S.; et al. Postoperative pain and quality of life after lung cancer surgery: A prospective observational study. Ann. Palliat. Med. 2023, 12, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and copper-based nanoparticles in medicine-perspectives and challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Gohain, M.B.; Yadav, D.; Ingole, P.G. Nanocomposite and bio-nanocomposite polymeric materials/membranes development in energy and medical sector: A review. Int. J. Biol. Macromol. 2021, 193, 2121–2139. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Bocancia-Mateescu, L.A.; Stan, D.; Mirica, A.C.; Ghita, M.G.; Stan, D.; Ruta, L.L. Nanobodies as diagnostic and therapeutic tools for cardiovascular diseases (CVDs). Pharmaceuticals 2023, 16, 863. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Aminolroayaei, F.; Mehri, A.; Shahbazi-Gahrouei, D.; Rostami, M. Polyoxometalates as next-generation of theragnostic gadgets in cancer. Rev. Inorg. Chem. 2024, 44, 271–287. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Ong, Y.S.; Bañobre-López, M.; Costa Lima, S.A.; Reis, S. A multifunctional nanomedicine platform for co-delivery of methotrexate and mild hyperthermia towards breast cancer therapy. Mater. Sci. Eng. C 2020, 116, 111255. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Rodrigues, B.; Banerjee, A.; Kanekiyo, T.; Singh, J. Functionalized liposomal nanoparticles for efficient gene delivery system to neuronal cell transfection. Int. J. Pharm. 2019, 566, 717–730. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Marqués-Gallego, P.; de Kroon, A.I. Ligation strategies for targeting liposomal nanocarriers. BioMed Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, L.W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef]
- Park, J.W.; Hong, K.; Kirpotin, D.B.; Colbern, G.; Shalaby, R.; Baselga, J.; Shao, Y.; Nielsen, U.B.; Marks, J.D.; Moore, D.; et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin. Cancer Res. 2002, 8, 1172–1181. [Google Scholar] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [PubMed]
- Huwyler, J.; Drewe, J.; Krähenbuhl, S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008, 3, 21–29. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Misran, M.; Kalantari, K.; Webster, T.J.; Kia, P.; Basrowi, N.A.; Rasouli, E.; Shameli, K. Advancements in liposomal nanomedicines: Innovative formulations, therapeutic applications, and future directions in precision medicine. Int. J. Nanomed. 2025, 20, 1213–1262. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., II; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Sun, L.; Li, Q.; Li, Z.; Yong, H.; Che, D.; Yan, C.; Geng, S.; Wang, W.; et al. Cyclic poly(β-amino ester)s with enhanced gene transfection activity synthesized through intra-molecular cyclization. Chem. Commun. 2022, 58, 2136–2139. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc. Natl. Acad. Sci. USA 1987, 84, 7851–7855. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Araújo, R.S.; Mosqueira, V.C.F. PEGylated and functionalized polylactide-based nanocapsules: An overview. Int. J. Pharm. 2023, 636, 122760. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, Y.M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.H.; Kim, B.G.; Kim, S.C.; Ryu, H.S.; et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- López-Rios de Castro, R.; Ziolek, R.M.; Ulmschneider, M.B.; Lorenz, C.D. Therapeutic peptides are preferentially solubilized in specific microenvironments within PEG-PLGA polymer nanoparticles. Nano Lett. 2024, 24, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.; Nazim, U.; Sharma, A.; Manuel, V.; Elnaggar, M.G.; Taye, A.; Nasr, N.E.H.; Hofni, A.; Abdel Hakiem, A.F. Selective targeting of the novel CK-10 nanoparticles to the MDA-MB-231 breast cancer cells. J. Pharm. Sci. 2022, 111, 1197–1207. [Google Scholar] [CrossRef]
- Yang, S.; Leong, J.; Wang, Y.; Sim, R.; Tan, K.H.; Chua, Y.H.; Tan, N.; Lee, A.L.Z.; Tay, J.; Yang, Y.Y. Drug-free neutrally charged polypeptide nanoparticles as anticancer agents. J. Control. Release 2022, 345, 464–474. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Shi, Q.; Zhao, Q.; Ma, H. Tumor microenvironment-responsive polypeptide nanogels for controlled antitumor drug delivery. Front. Pharmacol. 2021, 12, 748102. [Google Scholar] [CrossRef]
- Biel, N.M.; Siemann, D.W. Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016, 380, 525–533. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Z.; Xu, B. Assemblies of peptides in a complex environment and their applications. Angew. Chem. Int. Ed. 2019, 58, 10423–10432. [Google Scholar] [CrossRef]
- Trac, N.T.; Chung, E.J. Peptide-based targeting of immunosuppressive cells in cancer. Bioact. Mater. 2020, 5, 92–101. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wang, Z.; Gao, L.; Shen, J. Peptide-modified nanoparticles for tumor targeting and molecular imaging. Curr. Med. Chem. 2021, 28, 6411–6436. [Google Scholar] [CrossRef]
- Yao, Y.; Saw, P.E.; Nie, Y.; Wong, P.P.; Jiang, L.; Ye, X.; Chen, J.; Ding, T.; Xu, L.; Yao, H.; et al. Multifunctional sharp pH-responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J. Mater. Chem. B 2019, 7, 576–585. [Google Scholar] [CrossRef]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Ding, X.; Moser, T.; Gao, Q.; Shokuhfar, T.; Heiden, P.A. Peptide-directed self-assembly of functionalized polymeric nanoparticles. Part II: Effects of nanoparticle composition on assembly behavior and multiple drug loading ability. Macromol. Biosci. 2015, 15, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Bailey, L.O.; Wooley, K.L. Peptide-derivatized shell-cross-linked nanoparticles. 2. Biocompatibility evaluation. Bioconjug. Chem. 2004, 15, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Doll, T.A.; Dey, R.; Burkhard, P. Design and optimization of peptide nanoparticles. J. Nanobiotechnol. 2015, 13, 73. [Google Scholar] [CrossRef]
- Song, H.; Huang, P.; Niu, J.; Shi, G.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials 2018, 159, 119–129. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Z.; Chen, D.; Zhang, C.; Zhang, Q.; Cai, B.; Qin, Y.; Wang, K.; Shang, F.; Wan, J. Formulating spray-dried albumin-modified lipid nanoparticles encapsulating acyclovir for enhanced pulmonary drug delivery. Front. Biosci. 2024, 29, 363. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Yang, J.; Wang, J.; Wang, J.; Zhu, G.; Li, D.; Ding, J.; Sun, T. A Tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv. Mater. 2022, 34, e2200449. [Google Scholar] [CrossRef]
- Asadi, K.; Samiraninezhad, N.; Akbarizadeh, A.R.; Amini, A.; Gholami, A. Stimuli-responsive hydrogel based on natural polymers for breast cancer. Front. Chem. 2024, 12, 1325204. [Google Scholar] [CrossRef]
- Guo, H.; Xu, W.; Chen, J.; Yan, L.; Ding, J.; Hou, Y.; Chen, X. Positively charged polypeptide nanogel enhances mucoadhesion and penetrability of 10-hydroxycamptothecin in orthotopic bladder carcinoma. J. Control. Release 2017, 259, 136–148. [Google Scholar] [CrossRef]
- Huang, K.; Shi, B.; Xu, W.; Ding, J.; Yang, Y.; Liu, H.; Zhuang, X.; Chen, X. Reduction-responsive polypeptide nanogel delivers antitumor drug for improved efficacy and safety. Acta Biomater. 2015, 27, 179–193. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble metal nanomaterials for nir-triggered photothermal therapy in cancer. Adv. Healthc. Mater. 2021, 10, e2001806. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Rao, Y.; Sun, Q.; Li, D.; Cheng, Y.; Dong, J.; Qian, W. Shape-controlled synthesis of gold nanoplates and their self-assembly by repulsive electrostatic interactions. J. Nanosci. Nanotechnol. 2012, 12, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.H.; Hickman, Z.N.; Govorov, A.O.; Thomas, A.C.; Zhang, W.; Kordesch, M.E. Thermooptical properties of gold nanoparticles embedded in ice: Characterization of heat generation and melting. Nano Lett. 2006, 6, 783–788. [Google Scholar] [CrossRef]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef]
- Dam, D.H.; Culver, K.S.; Kandela, I.; Lee, R.C.; Chandra, K.; Lee, H.; Mantis, C.; Ugolkov, A.; Mazar, A.P.; Odom, T.W. Biodistribution and in vivo toxicity of aptamer-loaded gold nanostars. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 671–679. [Google Scholar] [CrossRef]
- Raeesi, V.; Chan, W.C. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale 2016, 8, 12524–12530. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Deng, W.W.; Zan, M.; Rao, L.; Yu, G.T.; Zhu, D.M.; Wu, W.T.; Chen, B.; Ji, L.W.; Chen, L.; et al. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano 2019, 13, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic acid (PLA) biocomposite: Processing, additive manufacturing and advanced applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Palanki, R.; Peranteau, W.H.; Mitchell, M.J. Delivery technologies for in utero gene therapy. Adv. Drug Deliv. Rev. 2021, 169, 51–62. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Meng, F.; Zhong, Z. Apolipoprotein E Peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano 2018, 12, 11070–11079. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet. Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Tivnan, A.; Orr, W.S.; Gubala, V.; Nooney, R.; Williams, D.E.; McDonagh, C.; Prenter, S.; Harvey, H.; Domingo-Fernández, R.; Bray, I.M.; et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS ONE 2012, 7, e38129. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M.Z. Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Tishin, A.M.; Shtil, A.A.; Pyatakov, A.P.; Zverev, V.I. Developing antitumor magnetic hyperthermia: Principles, materials and devices. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Dehghankhold, M.; Ahmadi, F.; Nezafat, N.; Abedi, M.; Iranpour, P.; Dehghanian, A.; Koohi-Hosseinabadi, O.; Akbarizadeh, A.R.; Sobhani, Z. A versatile theranostic magnetic polydopamine iron oxide NIR laser-responsive nanosystem containing doxorubicin for chemo-photothermal therapy of melanoma. Biomater. Adv. 2024, 159, 213797. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and biomedical applications of multifunctional nanoparticles. Adv. Mater. 2018, 30, e1802309. [Google Scholar] [CrossRef] [PubMed]
- Cova, M.; Oliveira-Silva, R.; Ferreira, J.A.; Ferreira, R.; Amado, F.; Daniel-da-Silva, A.L.; Vitorino, R. Glycoprotein enrichment method using a selective magnetic nano-probe platform (MNP) functionalized with lectins. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1243, pp. 83–100. [Google Scholar] [CrossRef]
- Dowaidar, M.; Abdelhamid, H.N.; Hällbrink, M.; Freimann, K.; Kurrikoff, K.; Zou, X.; Langel, Ü. Magnetic nanoparticle assisted self-assembly of cell penetrating peptides-oligonucleotides complexes for gene delivery. Sci. Rep. 2017, 7, 9159. [Google Scholar] [CrossRef]
- Obayemi, J.D.; Dozie-Nwachukwu, S.; Danyuo, Y.; Odusanya, O.S.; Anuku, N.; Malatesta, K.; Soboyejo, W.O. Biosynthesis and the conjugation of magnetite nanoparticles with luteinizing hormone releasing hormone (LHRH). Mater. Sci. Eng. C 2015, 46, 482–496. [Google Scholar] [CrossRef]
- Liang, H.; Liu, B.; Yuan, Q.; Liu, J. Magnetic iron oxide nanoparticle seeded growth of nucleotide coordinated polymers. ACS Appl. Mater. Interfaces 2016, 8, 15615–15622. [Google Scholar] [CrossRef]
- Wang, W.; Jing, Y.; He, S.; Wang, J.P.; Zhai, J.P. Surface modification and bioconjugation of FeCo magnetic nanoparticles with proteins. Colloids Surf. B Biointerfaces 2014, 117, 449–456. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy. Colloids Surf. B Biointerfaces 2018, 170, 529–537. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Wang, Q.; Lin, H.; Tong, R.; An, N.; Qu, F. The synthesis of LA-Fe3O4@PDA-PEG-DOX for photothermal therapy-chemotherapy. Dalton Trans. 2018, 47, 2435–2443. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, Y. Multifunctional platinum-based nanoparticles for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1410. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, Y.; Kang, X.; Li, C.; Huang, S.; Lian, H.; Hou, Z.; Ma, P.; Lin, J. Gelatin-encapsulated iron oxide nanoparticles for platinum (IV) prodrug delivery, enzyme-stimulated release and MRI. Biomaterials 2014, 35, 6359–6368. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Babincová, N.; Sourivong, P.; Babinec, P.; Bergemann, C.; Babincová, M.; Durdík, Š. Applications of magnetoliposomes with encapsulated doxorubicin for integrated chemotherapy and hyperthermia of rat C6 glioma. Z. Naturforschung C J. Biosci. 2018, 73, 265–271. [Google Scholar] [CrossRef]
- He, S.; Zhang, H.; Liu, Y.; Sun, F.; Yu, X.; Li, X.; Zhang, L.; Wang, L.; Mao, K.; Wang, G.; et al. Maximizing specific loss power for magnetic hyperthermia by hard-soft mixed ferrites. Small 2018, 14, e1800135. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.C.; Ficai, A. Smart magnetic drug delivery systems for the treatment of cancer. Nanomaterials 2023, 13, 876. [Google Scholar] [CrossRef]
- Baldea, I.; Petran, A.; Florea, A.; Sevastre-Berghian, A.; Nenu, I.; Filip, G.A.; Cenariu, M.; Radu, M.T.; Iacovita, C. Magnetic Nanoclusters stabilized with poly[3,4-dihydroxybenzhydrazide] as efficient therapeutic agents for cancer cells destruction. Nanomaterials 2023, 13, 933. [Google Scholar] [CrossRef]
- Saladino, G.M.; Mangarova, D.B.; Nernekli, K.; Wang, J.; Annio, G.; Varniab, Z.S.; Khatoon, Z.; Ribeiro Morais, G.; Shi, Y.; Chang, E.; et al. Multimodal imaging approach to track theranostic nanoparticle accumulation in glioblastoma with magnetic resonance imaging and intravital microscopy. Nanoscale 2025, 17, 9986–9995. [Google Scholar] [CrossRef]
- Singh, P.; Duraisamy, K.; Raitmayr, C.; Sharma, K.S.; Korzun, T.; Singh, K.; Moses, A.S.; Yamada, K.; Grigoriev, V.; Demessie, A.A.; et al. Precision-engineered cobalt-doped iron oxide nanoparticles: From octahedron seeds to cubical bipyramids for enhanced magnetic hyperthermia. Adv. Funct. Mater. 2025, 35, 2414719. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, A.; Yu, N.; Xia, J.; Li, J. Dual-Targeting biomimetic semiconducting polymer nanocomposites for amplified theranostics of bone metastasis. Angew. Chem. Int. Ed. 2024, 63, e202310252. [Google Scholar] [CrossRef]
- Brtko, J.; Podoba, J.; Macejova, D. Selenium—Its role in physiology and endocrinology and as organoselenium compounds in oncology: A minireview. Endocr. Regul. 2024, 58, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Lv, C.; Qi, L.; Wang, Y.; Hao, S.; Li, G.; Sun, H.; Du, L.; Li, J.; Wang, C.; et al. Sodium selenite inhibits cervical cancer progression via ROS-mediated suppression of glucose metabolic reprogramming. Life Sci. 2024, 357, 123109. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G. Selenium-containing compounds, selenium nanoparticles and selenoproteins in the prevention and treatment of lung cancer. J. Trace Elem. Med. Biol. 2025, 88, 127620. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T.R.; Moran, A.E.; Garg, R. HEARTS in the Americas: Saving lives from the world’s deadliest disease. Rev. Panam. Salud Publica 2022, 46, e171. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. Ferroptosis: When metabolism meets cell death. Physiol. Rev. 2025, 105, 651–706. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Shahverdi, F.; Faghfuri, E.; Reza Khoshayand, M.; Mavandadnejad, F.; Yazdi, M.H.; Amini, M. Characterization of folic acid surface-coated selenium nanoparticles and corresponding in vitro and in vivo effects against breast cancer. Arch. Med. Res. 2018, 49, 10–17. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Pu, K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef]
- Cheng, H.; Huo, D.; Zhu, C.; Shen, S.; Wang, W.; Li, H.; Zhu, Z.; Xia, Y. Combination cancer treatment through photothermally controlled release of selenous acid from gold nanocages. Biomaterials 2018, 178, 517–526. [Google Scholar] [CrossRef]
- Parvinen, T. Flow rate, pH, and lactobacillus and yeast counts of stimulated whole saliva in adults. Proc. Finn. Dent. Soc. 1985, 81, 113–116. [Google Scholar] [PubMed]
- Ma, C.; Yan, J.; Huang, Y.; Wang, C.; Yang, G. The optical duality of tellurium nanoparticles for broadband solar energy harvesting and efficient photothermal conversion. Sci. Adv. 2018, 4, eaas9894. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Cheng, X.; Wang, D.; Guo, S.; Jia, M.; Ma, K.; Cui, C.; Wang, L.; Zhou, H. Versatile biomimetic cantharidin-tellurium nanoparticles enhance photothermal therapy by inhibiting the heat shock response for combined tumor therapy. Acta Biomater. 2020, 110, 208–220. [Google Scholar] [CrossRef]
- Mishra, V.; Baranwal, V.; Mishra, R.K.; Sharma, S.; Paul, B.; Pandey, A.C. Immunotoxicological impact and biodistribution assessment of bismuth selenide (Bi2Se3) nanoparticles following intratracheal instillation in mice. Sci. Rep. 2017, 7, 18032. [Google Scholar] [CrossRef]
- Haute, D.V.; Berlin, J.M. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21rm02. [Google Scholar] [CrossRef] [PubMed]
- Hheidari, A.; Mohammadi, J.; Ghodousi, M.; Mahmoodi, M.; Ebrahimi, S.; Pishbin, E.; Rahdar, A. Metal-based nanoparticle in cancer treatment: Lessons learned and challenges. Front. Bioeng. Biotechnol. 2024, 12, 1436297. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.K.; Karthikeyan, C.; Ashby, C.R., Jr.; Neupane, R.; Singh, V.; Babu, R.J.; Narayana Moorthy, N.S.H.; Tiwari, A.K. Ligand-conjugated multiwalled carbon nanotubes for cancer targeted drug delivery. Front. Pharmacol. 2024, 15, 1417399. [Google Scholar] [CrossRef]
- Chen, S.; Hu, M.; Liu, L.; Pan, Y.; Li, P.; He, J.; Ding, J. Covalent three-dimensional carbon nanotube and derived B-C-N polymorphs with superhardness and zero Poisson’s ratio. iScience 2022, 25, 105563. [Google Scholar] [CrossRef]
- Alawdi, S.H.; Eidi, H.; Safar, M.M.; Abdel-Wahhab, M.A. Loading amlodipine on diamond nanoparticles: A novel drug delivery system. Nanotechnol. Sci. Appl. 2019, 12, 47–53. [Google Scholar] [CrossRef]
- Abdou, R.; Mojally, M.; Attia, H.G.; Dawoud, M. Cubic nanoparticles as potential carriers for a natural anticancer drug: Development, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2023, 13, 2463–2474. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Gomes, E.; Carroll, D.; D’Agostino, R., Jr.; Olson, J.; Guthold, M.; Gmeiner, W.H. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano 2009, 3, 2667–2673. [Google Scholar] [CrossRef]
- Jeyamohan, P.; Hasumura, T.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Accelerated killing of cancer cells using a multifunctional single-walled carbon nanotube-based system for targeted drug delivery in combination with photothermal therapy. Int. J. Nanomed. 2013, 8, 2653–2667. [Google Scholar] [CrossRef]
- Elhaj Baddar, Z.; Gurusamy, D.; Laisney, J.; Tripathi, P.; Palli, S.R.; Unrine, J.M. Polymer-coated hydroxyapatite nanocarrier for double-stranded RNA delivery. J. Agric. Food Chem. 2020, 68, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Abergel, D.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T.J.A.i.P. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Yoon, H.W.; Cho, Y.H.; Park, H.B. Graphene-based membranes: Status and prospects. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150024. [Google Scholar] [CrossRef]
- Luo, J.; Cote, L.J.; Tung, V.C.; Tan, A.T.; Goins, P.E.; Wu, J.; Huang, J. Graphene oxide nanocolloids. J. Am. Chem. Soc. 2010, 132, 17667–17669. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Khan, H.A.; Lee, Y.K.; Shaik, M.R.; Siddiqi, N.J.; Siddiqui, M.R.; Alrashood, S.T.; Alharbi, A.S.; Ekhzaimy, A.A. Hybrid nanoparticles of manganese oxide and highly reduced graphene oxide for photodynamic Therapy. Front. Biosci. 2023, 28, 19. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Yang, H.W.; Lu, Y.J.; Lin, K.J.; Hsu, S.C.; Huang, C.Y.; She, S.H.; Liu, H.L.; Lin, C.W.; Xiao, M.C.; Wey, S.P.; et al. EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials 2013, 34, 7204–7214. [Google Scholar] [CrossRef]
- Xiao, Y.; Pang, Y.X.; Yan, Y.; Qian, P.; Zhao, H.; Manickam, S.; Wu, T.; Pang, C.H. Synthesis and functionalization of graphene materials for biomedical applications: Recent advances, challenges, and perspectives. Adv. Sci. 2023, 10, e2205292. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Sharma, A.; Narang, R.K.; Rawal, R.K. Recent nanocarrier approaches for targeted drug delivery in cancer therapy. Curr. Mol. Pharmacol. 2021, 14, 350–366. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Buabeid, M.; Ibrahim, N.A.; Kharaba, Z.J.; Ijaz, M.; Noreen, S.; Murtaza, G. Potential of nanocarrier-based drug delivery systems for brain targeting: A current review of literature. Int. J. Nanomed. 2021, 16, 7517–7533. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Kesharwani, P.; Majeed, M.; Teng, Y.; Sahebkar, A. Recent advances in nanogold as a promising nanocarrier for curcumin delivery. Colloids Surf. B Biointerfaces 2022, 215, 112481. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M. Tumor-Targeting Theranostic Polymers. Langmuir 2025, 41, 7928–7945. [Google Scholar] [CrossRef]
- Handa, M.; Singh, A.; Flora, S.J.S.; Shukla, R. Stimuli-responsive polymeric nanosystems for therapeutic applications. Curr. Pharm. Des. 2022, 28, 910–921. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.D.; Heidari, G.; Zare, E.N.; Djatoubai, E.; Paiva-Santos, A.C.; Bertani, F.R.; Wu, A. Carbohydrate polymer-based nanocomposites for breast cancer treatment. Carbohydr. Polym. 2023, 304, 120510. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, D.; Zhu, H.; Bao, L.; Chang, R.; Gao, X.; Yin, J. Unstructured polypeptides as a versatile drug delivery technology. Acta Biomater. 2023, 164, 74–93. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, S.; Arora, S.; Samsonraj, R.; Govindaiah, P.; Vuree, S. Lipid-based nanoparticles for treatment of cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, S.; Hu, Y.; Zhao, X.; Lee, R.J. Application of lipid-based nanoparticles in cancer immunotherapy. Front. Immunol. 2022, 13, 967505. [Google Scholar] [CrossRef]
- Mahmoud, K.; Swidan, S.; El-Nabarawi, M.; Teaima, M. Lipid based nanoparticles as a novel treatment modality for hepatocellular carcinoma: A comprehensive review on targeting and recent advances. J. Nanobiotechnol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharmacal Res. 2014, 37, 43–52. [Google Scholar] [CrossRef]
- Safarkhani, M.; Moghaddam, S.S.; Taghavimandi, F.; Bagherzadeh, M.; Fatahi, Y.; Park, U.; Radmanesh, F.; Huh, Y.S.; Rabiee, N. Bioengineered smart nanocarriers for breast cancer treatment: Adorned carbon-based nanocomposites with silver and palladium complexes for efficient drug delivery. ACS Omega 2024, 9, 1183–1195. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-based nanomaterials for targeted cancer nanotherapy: Recent trends and future prospects. J. Drug Target. 2021, 29, 716–741. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mohammadnejad, J.; Najafi-Taher, R.; Zadeh, Z.B.; Tanhaei, M.; Ramakrishna, S. Multifunctional carbon-based nanoparticles: Theranostic applications in cancer therapy and diagnosis. ACS Appl. Bio Mater. 2023, 6, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, C.; Qiu, Y.; Xu, C.; Yao, J. Paying attention to tumor blood vessels: Cancer phototherapy assisted with nano delivery strategies. Biomaterials 2021, 268, 120562. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, P.; Zhang, X.; Lin, J.; Yang, S.; Liu, B.; Gao, F.; Xi, P.; Ren, Q.; Cui, D. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol. Pharm. 2010, 7, 94–104. [Google Scholar] [CrossRef]
- Yang, P.; Xu, Y.; Zhi, X.; Li, R.; Wang, B.; Liu, R.; Dai, Z.; Qian, L. Photodynamically tumor vessel destruction amplified tumor targeting of nanoparticles for efficient chemotherapy. ACS Nano 2024, 18, 12933–12944. [Google Scholar] [CrossRef]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor microenvironment targeted nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Kim, E.H.; Park, W.; Jang, H.; Jang, Y.; Chi, S.G.; Kweon, D.H.; Lee, K.; Kim, S.H.; et al. Design of PD-L1-targeted lipid nanoparticles to turn on PTEN for efficient cancer therapy. Adv. Sci. 2024, 11, e2309917. [Google Scholar] [CrossRef]
- Qu, F.; Wang, P.; Zhang, K.; Shi, Y.; Li, Y.; Li, C.; Lu, J.; Liu, Q.; Wang, X. Manipulation of Mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy 2020, 16, 1413–1435. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Li, R.; Xie, Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release 2017, 251, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tsukigawa, K.; Fang, J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: Next-generation chemotherapeutics and photodynamic therapy—Problems, solutions, and prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, J.; Yang, W. Stimuli-responsive nanocarriers for therapeutic applications in cancer. Cancer Biol. Med. 2021, 18, 319–335. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef]
- Joya, J.E.; Tsuji, T.; Jacalne, A.V.; Arita, M.; Tsukamoto, T.; Honda, T.; Miwatani, T. Demonstration of enterotoxigenic Escherichia coli in diarrheic broiler chicks. Eur. J. Epidemiol. 1990, 6, 88–90. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Kuang, Y.; Li, Q.; Zheng, D.W.; Song, Q.; Chen, H.; Chen, X.; Xu, Y.; Li, C.; et al. Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int. J. Biol. Macromol. 2017, 98, 691–700. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- He, C.F.; Wang, S.H.; Yu, Y.J.; Shen, H.Y.; Zhao, Y.; Gao, H.L.; Wang, H.; Li, L.L.; Liu, H.Y. Advances in biodegradable nanomaterials for photothermal therapy of cancer. Cancer Biol. Med. 2016, 13, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Osuchowski, M.; Adamczyk, M.; Stopa, J.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Advancements in photodynamic therapy of esophageal cancer. Front. Oncol. 2022, 12, 1024576. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, M.; Zhou, C.; Yin, F.; Wang, Z.; Hua, Y.; Cai, Z. Hematoporphyrin monomethyl ether-mediated photodynamic therapy selectively kills sarcomas by inducing apoptosis. PLoS ONE 2013, 8, e77727. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Selvan, S.T.; Yang, Y.; Kim, M.J.; Yi, D.K.; Kwon, I.C.; Kim, K. Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials 2018, 178, 597–607. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Fan, Z.; Zhuang, C.; Wang, S.; Zhang, Y. Photodynamic and photothermal therapy of hepatocellular carcinoma. Front. Oncol. 2021, 11, 787780. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Parveen, S.; Konde, D.V.; Paikray, S.K.; Tripathy, N.S.; Sahoo, L.; Samal, H.B.; Dilnawaz, F. Nanoimmunotherapy: The smart trooper for cancer therapy. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002308. [Google Scholar] [CrossRef]
- Dhas, N.; Kudarha, R.; Kulkarni, S.; Soman, S.; Navti, P.D.; Kulkarni, J.; Roy, A.A.; Colaco, V.; Raychaudhuri, R.; Gupta, A.; et al. Nanoengineered platform-based microenvironment-triggered immunotherapy in cancer treatment. Front. Biosci. 2024, 29, 349. [Google Scholar] [CrossRef]
- Hamouda, A.E.I.; Filtjens, J.; Brabants, E.; Kancheva, D.; Debraekeleer, A.; Brughmans, J.; Jacobs, L.; Bardet, P.M.R.; Knetemann, E.; Lefesvre, P.; et al. Intratumoral delivery of lipid nanoparticle-formulated mRNA encoding IL-21, IL-7, and 4-1BBL induces systemic anti-tumor immunity. Nat. Commun. 2024, 15, 10635. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, J.; Li, Y.; Yang, C.; Hou, Y.; Tang, W.; McHugh, K.J.; Jing, L. Nanotechnology-enhanced immunotherapy for metastatic cancer. Innovation 2021, 2, 100174. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, C.; Li, Y.; Wang, H.; Liu, L.; Ye, Y.; Gao, J.; Tian, H.; Peng, F.; Tu, Y.; et al. Bottle nanomotors amplify tumor oxidative stress for enhanced calcium overload/chemodynamic therapy. Small 2024, 20, e2404402. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261. [Google Scholar] [CrossRef]
- Thiruppathi, J.; Vijayan, V.; Park, I.K.; Lee, S.E.; Rhee, J.H. Enhancing cancer immunotherapy with photodynamic therapy and nanoparticle: Making tumor microenvironment hotter to make immunotherapeutic work better. Front. Immunol. 2024, 15, 1375767. [Google Scholar] [CrossRef]
- Du, W.; Chen, C.; Sun, P.; Zhang, S.; Zhang, J.; Zhang, X.; Liu, Y.; Zhang, R.; Yan, C.; Fan, C.; et al. Eliciting an immune hot tumor niche with biomimetic drug-based multi-functional nanohybrids augments immune checkpoint blockade-based breast cancer therapy. Nanoscale 2020, 12, 3317–3329. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Du, Y.; Lin, M.; Li, J.; Gao, D.; Yang, Z.; Wang, W.; Zhou, Y.; Li, X.; et al. Hierarchical self-recognition and response in CSC and non-CSC micro-niches for cancer therapy. Biomaterials 2024, 308, 122581. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Danaeifar, M.; Negahdari, B.; Eslam, H.M.; Zare, H.; Ghanaat, M.; Koushali, S.S.; Malekshahi, Z.V. Polymeric nanoparticles for DNA vaccine-based cancer immunotherapy: A review. Biotechnol. Lett. 2023, 45, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, M.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. CpG-based nanovaccines for cancer immunotherapy. Int. J. Nanomed. 2021, 16, 5281–5299. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bai, L.; Wu, H.; Tian, F.; Guo, G. Radiosensitization of paclitaxel, etanidazole and paclitaxel+etanidazole nanoparticles on hypoxic human tumor cells in vitro. Biomaterials 2007, 28, 3724–3730. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, K.; Ma, S.; Yuan, D.; Bai, Y. Topotecan-loaded mesoporous silica nanoparticles for reversing multi-drug resistance by synergetic chemoradiotherapy. Chem. Asian J. 2015, 10, 344–348. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Gu, Z.; Zhao, Y. Recent advances in upconversion nanoparticles-based multifunctional nanocomposites for combined cancer therapy. Adv. Mater. 2015, 27, 7692–7712. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shi, J. On the latest three-stage development of nanomedicines based on upconversion nanoparticles. Adv. Mater. 2016, 28, 3987–4011. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Transforming weakness into strength: Photothermal-therapy-induced inflammation enhanced cytopharmaceutical chemotherapy as a combination anticancer treatment. Adv. Mater. 2019, 31, e1805936. [Google Scholar] [CrossRef]
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Peng, W.; Xiao, Q.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging. Biomaterials 2014, 35, 8992–9002. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H.; et al. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Chou, S.W.; Shyue, J.J.; Lin, S.Y.; Hsiao, J.K.; Chou, P.T. A Versatile theranostic delivery platform integrating magnetic resonance imaging/computed tomography, pH/cis-diol controlled release, and targeted therapy. ACS Nano 2016, 10, 5809–5822. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Mei, Q.; Wang, P.; Miao, P.; Dong, W.F.; Li, L. Light-triggered multifunctional nanoplatform for efficient cancer photo-immunotherapy. J. Nanobiotechnol. 2022, 20, 181. [Google Scholar] [CrossRef]
- Niu, G.; Gao, F.; Wang, Y.; Zhang, J.; Zhao, L.; Jiang, Y. Bimetallic nanomaterials: A promising nanoplatform for multimodal cancer therapy. Molecules 2022, 27, 8712. [Google Scholar] [CrossRef]
- Corradetti, B.; Pisano, S.; Conlan, R.S.; Ferrari, M. Nanotechnology and immunotherapy in ovarian cancer: Tracing New Landscapes. J. Pharmacol. Exp. Ther. 2019, 370, 636–646. [Google Scholar] [CrossRef]
- Yang, J.; Dai, D.; Zhang, X.; Teng, L.; Ma, L.; Yang, Y.W. Multifunctional metal-organic framework (MOF)-based nanoplatforms for cancer therapy: From single to combination therapy. Theranostics 2023, 13, 295–323. [Google Scholar] [CrossRef]
- Passeri, G.; Northcote-Smith, J.; Suntharalingam, K. Delivery of an immunogenic cell death-inducing copper complex to cancer stem cells using polymeric nanoparticles. RSC Adv. 2022, 12, 5290–5299. [Google Scholar] [CrossRef]
- Werner, M.E.; Copp, J.A.; Karve, S.; Cummings, N.D.; Sukumar, R.; Li, C.; Napier, M.E.; Chen, R.C.; Cox, A.D.; Wang, A.Z. Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano 2011, 5, 8990–8998. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Ma, T.; Zhu, D.; Liu, T.; Lv, F. Tumor targeted combination therapy mediated by functional macrophages under fluorescence imaging guidance. J. Control. Release 2020, 328, 127–140. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, T.; Gao, J.; Ren, Y.; Ding, Y.; Liu, S.; Liu, L.; Huang, H.; Wang, H.; Wang, C.; et al. Nanoplatform for synergistic therapy constructed via the co-assembly of a reduction-responsive cholesterol-based block copolymer and a photothermal amphiphile. Mater. Today Bio 2024, 29, 101355. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Christensen, T.; Wahl, A.; Smedshammer, L. Effects of haematoporphyrin derivative and light in combination with hyperthermia on cells in culture. Br. J. Cancer 1984, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.S. Combination studies of hyperthermia induced by the neodymium: Yttrium-aluminum-garnet (Nd:YAG) laser as an adjuvant to photodynamic therapy. Lasers Surg. Med. 1990, 10, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in cancer therapeutics: Conventional thermal therapy to nanotechnology-based photothermal therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Hong, S.; Wang, Q.; Li, L.; Eltayeb, O.; Dong, C.; Shuang, S. MnO2 nanosheets anchored with polypyrrole nanoparticles as a multifunctional platform for combined photothermal/photodynamic therapy of tumors. Food Funct. 2021, 12, 6334–6347. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Cui, W.; Gong, H.; Liang, C.; Shi, X.; Li, Z.; Sun, B.; Liu, Z. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale 2014, 6, 11219–11225. [Google Scholar] [CrossRef]
- Guo, S.; Song, Z.; Ji, D.K.; Reina, G.; Fauny, J.D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Combined photothermal and photodynamic therapy for cancer treatment using a multifunctional graphene oxide. Pharmaceutics 2022, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Patri, S.; Thanh, N.T.K.; Kamaly, N. Magnetic iron oxide nanogels for combined hyperthermia and drug delivery for cancer treatment. Nanoscale 2024, 16, 15446–15464. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced tumor synergistic therapy by injectable magnetic hydrogel mediated generation of hyperthermia and highly toxic reactive oxygen species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, P.; Guo, Y.; Hao, J.; Ni, D.; Xu, Y.; Bao, Q.; Yao, H.; Wei, C.; Wu, Q.; et al. Combined magnetic hyperthermia and immune therapy for primary and metastatic tumor treatments. ACS Nano 2020, 14, 1033–1044. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.; Peng, B.; Zhang, S.; Ma, J.; Deng, G.; Zou, P.; Liu, J.; Chen, A.T.; Li, D.; et al. Vessel-targeting nanoclovers enable noninvasive delivery of magnetic hyperthermia-chemotherapy combination for brain cancer treatment. Nano Lett. 2021, 21, 8111–8118. [Google Scholar] [CrossRef]
- Qi, J.; Jin, F.; Xu, X.; Du, Y. Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int. J. Nanomed. 2021, 16, 1435–1456. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Lu, J. Nanotechnology-enabled immunogenic cell death for improved cancer immunotherapy. Int. J. Pharm. 2023, 634, 122655. [Google Scholar] [CrossRef]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Tumor Microenvironment-Enabled Nanotherapy. Adv. Healthc. Mater. 2018, 7, e1701156. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Wang, X.; Feng, Y.; Qian, Y.; Ma, Q.; Li, X.; Chen, Y.; Chen, K. Joining forces: The combined application of therapeutic viruses and nanomaterials in cancer therapy. Molecules 2023, 28, 7679. [Google Scholar] [CrossRef]
- Mehrotra, N.; Pal, K. Tumor targeted nanohybrid for dual stimuli responsive and NIR amplified photothermal/photo-induced thermodynamic/chemodynamic combination therapy. Biomed. Mater. 2024, 19, 035019. [Google Scholar] [CrossRef]
- Fang, L.; Chen, Z.; Dai, J.; Pan, Y.; Tu, Y.; Meng, Q.; Diao, Y.; Yang, S.; Guo, W.; Li, L.; et al. Recent advances in strategies to enhance photodynamic and photothermal therapy performance of single-component organic phototherapeutic agents. Adv. Sci. 2025, 12, e2409157. [Google Scholar] [CrossRef]
- Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Guo, Q.; Zhang, L.; Pang, X.; Ou-Yang, W.; Zhao, K.; et al. Redox-sensitive folate-conjugated polymeric nanoparticles for combined chemotherapy and photothermal therapy against breast cancer. J. Biomed. Nanotechnol. 2018, 14, 2018–2030. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.; Wang, X.; Cai, J.; Huang, H.; Tang, S.; He, D. Cisplatin-based combination therapy for enhanced cancer treatment. Curr. Drug Targets 2024, 25, 473–491. [Google Scholar] [CrossRef]
- Benson, R.C., Jr. Treatment of diffuse transitional cell carcinoma in situ by whole bladder hematoporphyrin derivative photodynamic therapy. J. Urol. 1985, 134, 675–678. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Chen, M.; Hu, A.; Wei, B.; Yang, Z. Nanomaterials mediated multimodal combined treatment for cancer. Mini-Rev. Med. Chem. 2023, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, L.; Luo, C.; Jiang, M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019, 454, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring immunotherapy in colorectal cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-immunotherapy: A new trend in cancer treatment. Cancers 2023, 15, 2912. [Google Scholar] [CrossRef]
- Adnane, F.; El-Zayat, E.; Fahmy, H.M. The combinational application of photodynamic therapy and nanotechnology in skin cancer treatment: A review. Tissue Cell 2022, 77, 101856. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Yan, S.; Zhong, J.; Du, L.; Yang, C.; Pu, Y.; Li, Y.; Lin, J.; Zeng, M.; et al. MRI-guided photothermal/photodynamic immune activation combined with PD-1 inhibitor for the multimodal combination therapy of melanoma and metastases. Regen. Biomater. 2024, 11, rbae019. [Google Scholar] [CrossRef]
- Kong, C.; Xu, B.; Qiu, G.; Wei, M.; Zhang, M.; Bao, S.; Tang, J.; Li, L.; Liu, J. Multifunctional nanoparticles-mediated PTT/PDT synergistic immune activation and antitumor activity combined with anti-PD-L1 immunotherapy for breast cancer treatment. Int. J. Nanomed. 2022, 17, 5391–5411. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Nie, G. Intelligent nanomaterials for cancer therapy: Recent progresses and future possibilities. Med. Rev. 2023, 3, 321–342. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, L.; Chou, W.C.; Cheng, Y.H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. Meta-analysis of nanoparticle distribution in tumors and major organs in tumor-bearing mice. ACS Nano 2023, 17, 19810–19831. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel nano-drug delivery system for brain tumor treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Ren, Y.; Li, S.; Fu, J.; Shi, J. Study on biomimetic nano tumor targeted delivery system for chemotherapy-laser immunotherapy. Eur. J. Pharm. Biopharm. 2022, 176, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Zheng, D.; Wan, C.; Yang, H.; Xu, L.; Dong, Q.; Du, C.; Du, J.; Li, F. Her2-targeted multifunctional nano-theranostic platform mediates tumor microenvironment remodeling and immune activation for breast cancer treatment. Int. J. Nanomed. 2020, 15, 10007–10028. [Google Scholar] [CrossRef]
- Ranga, V.; Dakal, T.C.; Maurya, P.K.; Johnson, M.S.; Sharma, N.K.; Kumar, A. Role of RGD-binding Integrins in ovarian cancer progression, metastasis and response to therapy. Integr. Biol. 2025, 17, zyaf003. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Long, J.; Ding, Y.; Zou, X.; Liao, G.; Cao, Y. A comparative study of toxicity of TiO2, ZnO, and Ag nanoparticles to human aortic smooth-muscle cells. Int. J. Nanomed. 2018, 13, 8037–8049. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, Y.; Dong, L. A comparison of TiO2 and ZnO nanoparticles as photosensitizers in photodynamic therapy for cancer. J. Biomed. Nanotechnol. 2014, 10, 1450–1457. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Karimi-Maleh, H.; Taheriazam, A.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Makvandi, P.; Nazarzadeh Zare, E.; Sharifi, E.; et al. (Nano)platforms in bladder cancer therapy: Challenges and opportunities. Bioeng. Transl. Med. 2023, 8, e10353. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 671–686. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Yan, Y.; Wang, Y.; Zhao, H.; Wang, X.; Chang, S.; Li, S. Charge-reversal nano-drug delivery systems in the tumor microenvironment: Mechanisms, challenges, and therapeutic applications. Int. J. Mol. Sci. 2024, 25, 9779. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012, 12, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Demetzos, C. Advanced drug delivery nanosystems: Perspectives and regulatory issues. Adv. Exp. Med. Biol. 2015, 822, 195–198. [Google Scholar] [CrossRef]
- Arif, M.; Raza, H.; Akhter, T. Classification, synthesis, characterization, and applications of metal nanoparticle-containing hybrid microgels: A comprehensive review. RSC Adv. 2024, 14, 24604–24630. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef]
- Ma, Z.; Li, B.; Peng, J.; Gao, D. Recent development of drug delivery systems through microfluidics: From synthesis to evaluation. Pharmaceutics 2022, 14, 434. [Google Scholar] [CrossRef]
- Di Cola, E.; Grillo, I.; Ristori, S. Small angle X-ray and neutron scattering: Powerful tools for studying the structure of drug-loaded liposomes. Pharmaceutics 2016, 8, 10. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, Y.; Lu, J.; Chen, X. MicroRNA-targeted nanoparticle delivery systems for cancer therapy: Current status and future prospects. Nanomedicine 2025, 20, 1181–1194. [Google Scholar] [CrossRef]
- Chen, L.J.; Wang, M.Y.; Liu, M.Z. Reproductive toxicity of bimolane in mice and rabbits. Acta Pharmacol. Sin. 1989, 10, 557–560. [Google Scholar] [PubMed]
- Shi, Y.; Li, X.; Li, Z.; Sun, J.; Gao, T.; Wei, G.; Guo, Q. Nano-formulations in disease therapy: Designs, advances, challenges, and future directions. J. Nanobiotechnol. 2025, 23, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, X. Nano and liposome cancer chemotherapy: A review of advances in drug delivery with applications. Int. J. Clin. Pharmacol. Ther. 2025, 63, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Mohammadnejad, J.; Narmani, A.; Jafari, H. Folic acid-chitosan-PLGA nano delivery system against liver cancer cells: In vitro studies. J. Biochem. Mol. Toxicol. 2025, 39, e70478. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bear, A.S.; Young, J.K.; Lewinski, N.A.; Kim, J.; Foster, A.E.; Drezek, R.A. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res. Lett. 2011, 6, 283. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Xiong, Y.; Long, Y.; Shao, Q.; Wu, L.; Wang, J.; Tian, P.; Khan, A.U.; Yang, W.; et al. Carbon micro/nano machining toward miniaturized device: Structural engineering, large-scale fabrication, and performance optimization. Small 2025, 21, e2400179. [Google Scholar] [CrossRef]
- Hu, C.; Qian, A.; Wang, Q.; Xu, F.; He, Y.; Xu, J.; Xia, Y.; Xia, Q. Industrialization of lipid nanoparticles: From laboratory-scale to large-scale production line. Eur. J. Pharm. Biopharm. 2016, 109, 206–213. [Google Scholar] [CrossRef]
- Schuh, L.; Salgado, L.A.; Piau, T.B.; Silveira, A.P.; Leal, C.; Romera, L.F.; Radicchi, M.A.; Santos, M.M.S.; Falcao, L.; Grisolia, C.K.; et al. Integrating natural deep eutectic solvents into nanostructured lipid carriers: An industrial look. Pharmaceuticals 2024, 17, 855. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial lipid-based carrier for non-invasive capsaicin delivery; manufacturing scale-up and human irritation assessment. Molecules 2020, 25, 5575. [Google Scholar] [CrossRef]
- Bosetti, R.; Jones, S.L. Cost-effectiveness of nanomedicine: Estimating the real size of nano-costs. Nanomedicine 2019, 14, 1367–1370. [Google Scholar] [CrossRef]
- Gao, W.; Xiang, B.; Meng, T.T.; Liu, F.; Qi, X.R. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 2013, 34, 4137–4149. [Google Scholar] [CrossRef]
- Xiao, D.; Jia, H.Z.; Zhang, J.; Liu, C.W.; Zhuo, R.X.; Zhang, X.Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small 2014, 10, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Rizzo, L.Y.; Ehling, J.; Kiessling, F.; Lammers, T. The theranostic path to personalized nanomedicine. Clin. Transl. Imaging 2014, 2, 66–76. [Google Scholar] [CrossRef]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized chitosan for cancer nano drug delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Bin, L.; Yang, Y.; Wang, F.; Wang, R.; Fei, H.; Duan, S.; Huang, L.; Liao, N.; Zhao, S.; Ma, X. Biodegradable silk fibroin nanocarriers to modulate hypoxia tumor microenvironment favoring enhanced chemotherapy. Front. Bioeng. Biotechnol. 2022, 10, 960501. [Google Scholar] [CrossRef]
- Li, M.; Li, J.P.; Wang, Y.S.; He, Q. Construction and anti-tumor effect evaluation of a dual-responsive hyaluronic acid carbon quantum dot-gelatin nano-drug delivery system. J. Sichuan Univ. (Med. Sci. Ed.) 2021, 52, 577–584. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Huang, C.; Cho, H.I.; Lloyd, M.; Johnson, J.; Ren, X.; Altiok, S.; Sullivan, D.; Weber, J.; Celis, E.; et al. Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res. 2012, 72, 5483–5493. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y.; Hou, Z.; Lin, J. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y. Magnetically driven microfluidics for isolation of circulating tumor cells. Cancer Med. 2020, 9, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.Y.; Kankala, R.K.; Pan, Y.J.; Yuan, H.; Wang, S.B.; Chen, A.Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018, 13, 4685–4698. [Google Scholar] [CrossRef]
- Mady, M.S.; Sobhy, Y.; Orabi, A.; Sharaky, M.; Mina, S.A.; Abo-Zeid, Y. Preparation and characterization of nano-emulsion formulations of Asparagus densiflorus root and aerial parts extracts: Evaluation of in-vitro antibacterial and anticancer activities of nano-emulsion versus pure plant extract. Drug Dev. Ind. Pharm. 2024, 50, 658–670. [Google Scholar] [CrossRef]
- Wei, M.; Fan, J.; Peng, R.; Ding, X.; Xi, J.; Huang, H. In silico identification of therapeutic targets and novel drug candidates for malignant peripheral nerve sheath tumors. Front. Biosci. 2023, 28, 214. [Google Scholar] [CrossRef]
- Zhu, X.; An, K.; Yan, J.; Xu, P.; Bai, C. In silico optimization of SARS-CoV-2 spike specific nanobodies. Front. Biosci. 2023, 28, 67. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Qi, T.; Qiu, S.; Huang, Y.; Zhao, X.; Sun, Q.; Lin, G. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct. Target. Ther. 2020, 5, 262. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Zhou, C.; Cui, X.; Wang, W.; Wang, J.; Huang, Y.; Jiang, J.; Wang, Z.; Tang, Z.; et al. Application of artificial intelligence in cancer diagnosis and tumor nanomedicine. Nanoscale 2024, 16, 14213–14246. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chou, W.C.; Cheng, Y.H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting nanoparticle delivery to tumors using machine learning and artificial intelligence approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Chen, Q.; Yuan, L.; Cheng, Y.H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Xue, Y.; Zhou, Y.; Han, T.; Ning, B.; Cheng, L.; Xie, H.; Wang, H.; Wang, W.; Meng, Q.; et al. Perovskite probe-based machine learning imaging model for rapid pathologic diagnosis of cancers. ACS Nano 2024, 18, 24295–24305. [Google Scholar] [CrossRef]
- Mi, K.; Chou, W.C.; Chen, Q.; Yuan, L.; Kamineni, V.N.; Kuchimanchi, Y.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. Predicting tissue distribution and tumor delivery of nanoparticles in mice using machine learning models. J. Control. Release 2024, 374, 219–229. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial intelligence and machine learning in computational nanotoxicology: Unlocking and empowering nanomedicine. Adv. Healthc. Mater. 2020, 9, e1901862. [Google Scholar] [CrossRef]
- Singh, A.V.; Varma, M.; Laux, P.; Choudhary, S.; Datusalia, A.K.; Gupta, N.; Luch, A.; Gandhi, A.; Kulkarni, P.; Nath, B. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: A comprehensive review. Arch. Toxicol. 2023, 97, 963–979. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Pires, D.E.V.; Ascher, D.B. dendPoint: A web resource for dendrimer pharmacokinetics investigation and prediction. Sci. Rep. 2019, 9, 15465. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Mostovenko, E.; Young, T.; Muldoon, P.P.; Bishop, L.; Canal, C.G.; Vucetic, A.; Zeidler-Erdely, P.C.; Erdely, A.; Campen, M.J.; Ottens, A.K. Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part. Fibre Toxicol. 2019, 16, 20. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Ranzenigo, A.; Fayad, Z.A.; Teunissen, A.J.P. Nanotherapeutic heterogeneity: Sources, effects, and solutions. Small 2024, 20, e2307502. [Google Scholar] [CrossRef]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming hurdles in nanoparticle clinical translation: The influence of experimental design and surface modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Nieh, M.P.; Li, Y. Decorating nanoparticle surface for targeted drug delivery: Opportunities and challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Li, S.; Chen, X.; Deng, Z.; Huang, Y. Nanoparticles for cancer therapy: A review of influencing factors and evaluation methods for biosafety. Clin. Transl. Oncol. 2023, 25, 2043–2055. [Google Scholar] [CrossRef]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]

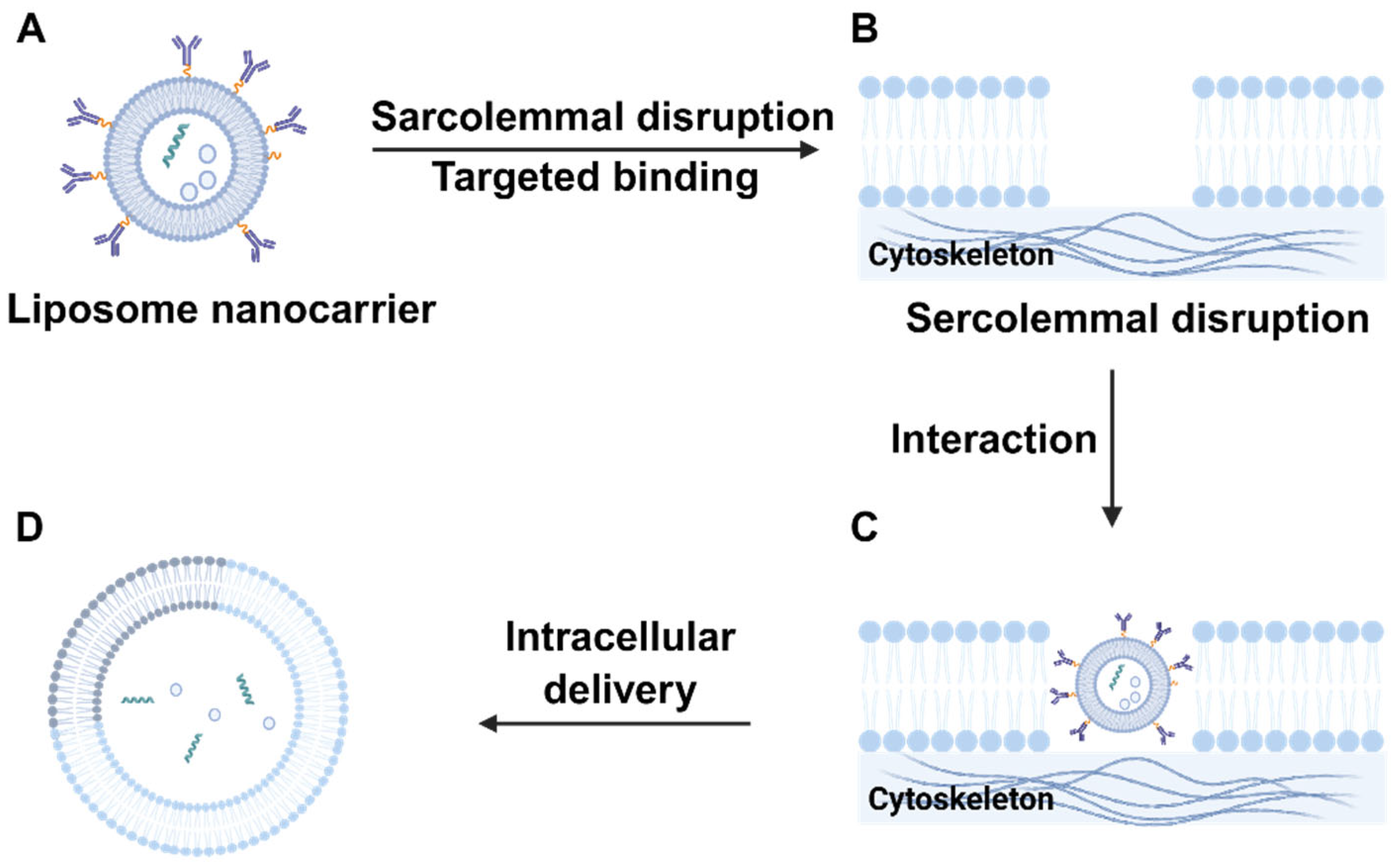
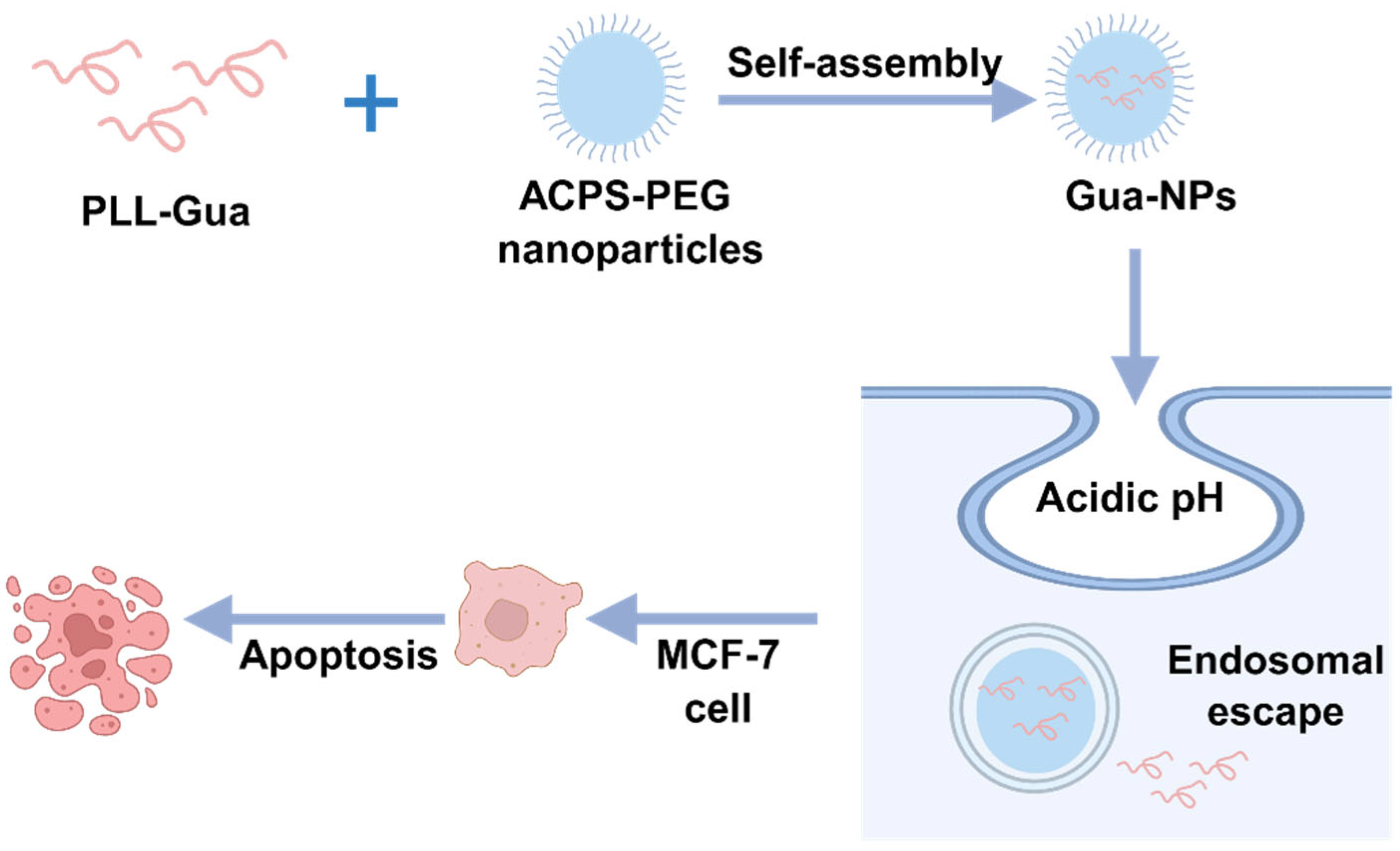
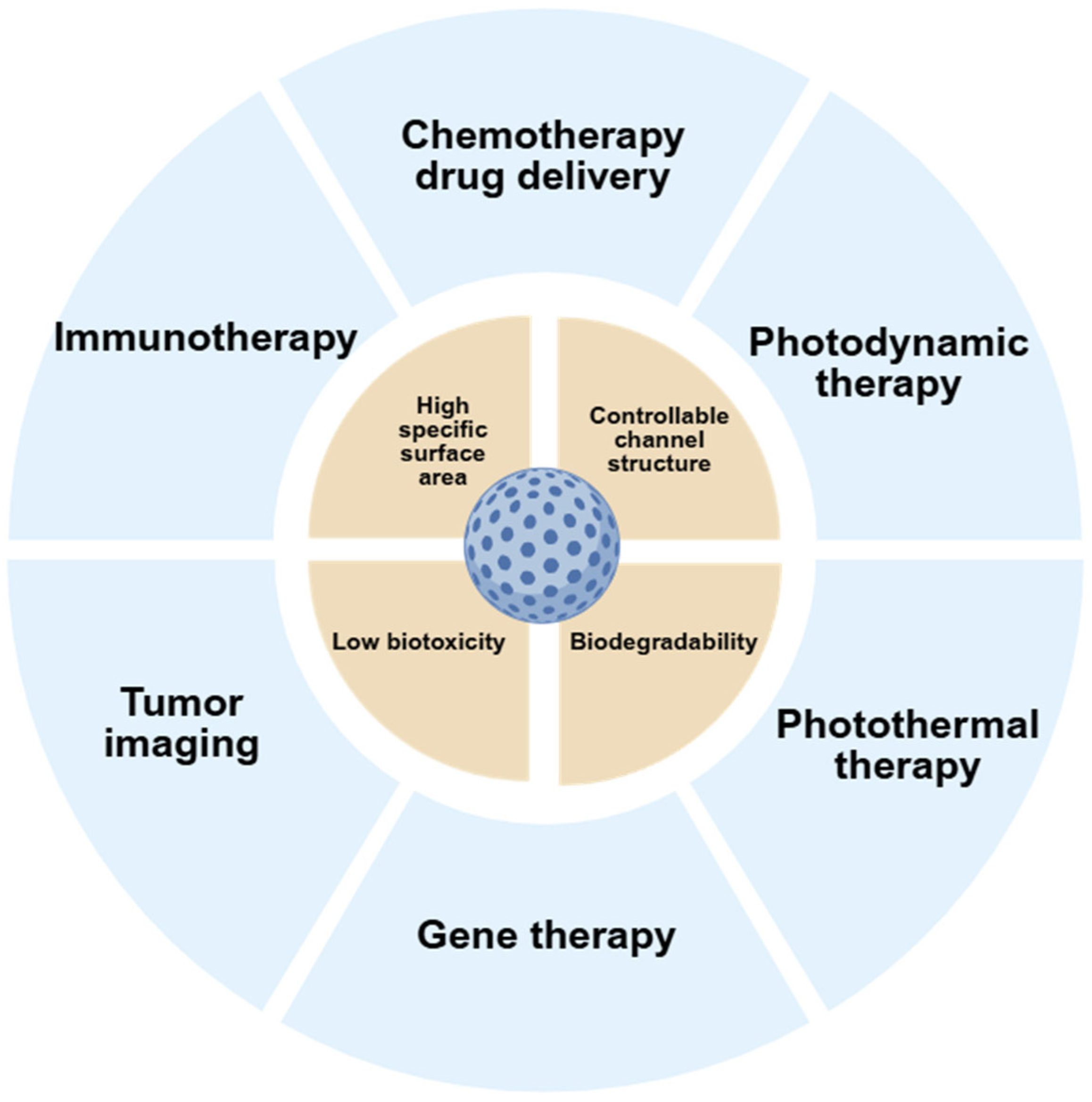
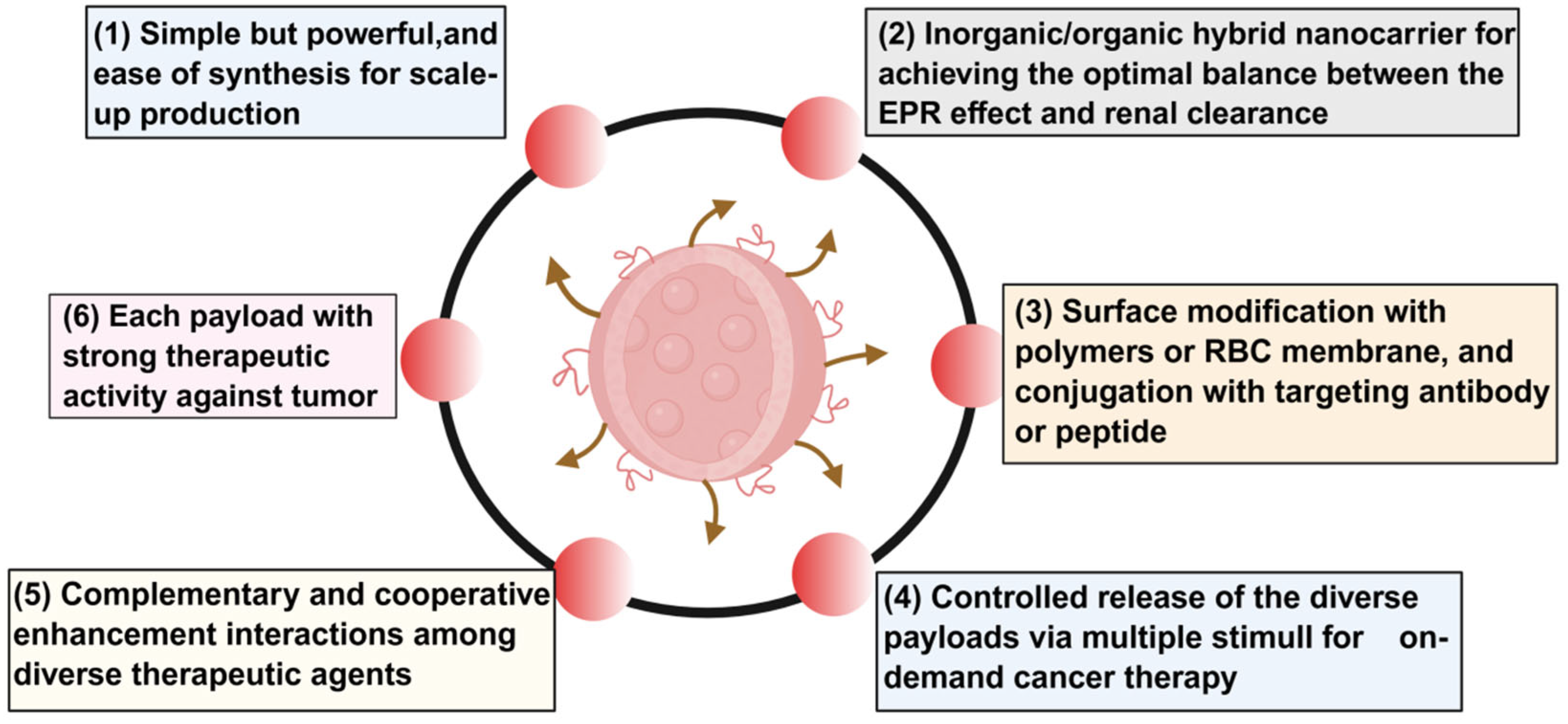
| Types of Nanocarriers | Core Components/ Structure | Key Advantages | Key Features | Typical Application Scenarios | Reference |
|---|---|---|---|---|---|
| Metal Nanocarriers | Gold, silver, iron oxide, titanium dioxide, and other metals/metal oxides; predominantly spherical, cage-like, or core–shell structures. |
|
|
| [159,160,161] |
| Polymeric nanocarriers | Synthetic polymers or natural polymers, typically in the form of micelles, microspheres, or nanoparticles. |
|
|
| [162,163,164,165,166] |
| Lipid-based nanocarriers | Phospholipids, cholesterol, ionizable lipids; primarily liposomes, lipid nanoparticles, micelles |
|
|
| [167,168,169,170] |
| Carbon-based nanocarriers | Graphene, carbon nanotubes, mesoporous carbon, fullerenes; predominantly sheet-like, tubular, or porous structures. |
|
|
| [171,172,173,174] |
| Combination Therapy | Core Principle | Selection of Nanomaterial Carriers | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Chemotherapy + Photothermal Therapy | Nanocarriers encapsulate chemotherapy drugs; the carriers themselves or loaded with photothermal agents generate heat upon near-infrared irradiation to directly destroy tumor cells; the thermal effect enhances cell membrane permeability, promotes endocytosis of chemotherapy drugs, and reverses drug resistance. | Gold nanocages, PLGA-PEG nanoparticles, graphene quantum dots | Synergistically enhances lethal effects; Light-controlled delivery enables precise spatiotemporal release, minimizing exposure of normal tissues. | The penetration depth of photothermal therapy is limited, rendering it ineffective for deep-seated tumors; high temperatures (>45 °C) may damage surrounding healthy tissues; prolonged photothermal exposure may induce thermal tolerance in tumor cells. | [261,262,263] |
| Chemotherapy + PDT | Nanocarriers co-load chemotherapy drugs and photosensitizers; light of specific wavelengths excites the photosensitizer to generate singlet oxygen, oxidatively damaging tumor cells; chemotherapy drugs induce apoptosis, synergizing with the oxidative stress induced by PDT. | Liposomes, mesoporous silica nanoparticles | Dual-mechanism killing reduces drug resistance risk; photosensitizer targets tumor enrichment to minimize systemic phototoxicity; fluorescence imaging enables real-time monitoring of photosensitizer distribution. | Photosensitizers require an oxygen-rich environment for activation, limiting their efficacy in hypoxic tumors; insufficient light penetration depth necessitates fiber-optic intervention; strict photoprotection is required to prevent skin phototoxicity. | [264,265,266] |
| Chemotherapy + Immunotherapy | Nanocarriers encapsulate chemotherapy drugs and immunomodulators; chemotherapy induces immunogenic cell death, releasing tumor-associated antigens; immunomodulators activate dendritic cells and cytotoxic T cells, suppressing immune escape. | Polyethylene glycol–polycaprolactone micelles, dendritic polymerization | Chemotherapy transforms “cold tumors” into “hot tumors,” enhancing immunotherapy response; co-delivery reduces immunosuppressant dosage and alleviates systemic toxicity; long-term immune memory effect lowers recurrence rates. | Immune-related adverse reactions may still occur; Tumor microenvironment immunosuppression may diminish efficacy; Individual immune heterogeneity leads to significant response variability. | [267,268,269,270] |
| PDT + Immunotherapy | Nano-photothermal agents generate heat upon laser irradiation, directly destroying tumors while releasing tumor-associated antigens TAAs. Heat shock proteins assist antigen presentation, and when combined with immune checkpoint inhibitors or CAR-T cells, they activate systemic antitumor immunity. | Gold Nanoparticles, Black Phosphorus Quantum Dots | Photothermal therapy exhibits no drug resistance and synergizes with the immune system to achieve “local ablation + systemic tumor control”; the thermal effect promotes immune cell infiltration; it avoids the damage chemotherapy inflicts on immune cells. | The photothermal range is limited, requiring precise tumor localization; high temperatures may disrupt antigen structures, weakening immunogenicity; photothermal therapy alone struggles to activate effective immunity against immune-cold tumors. | [261,271,272,273] |
| Photothermal + Photodynamic + Chemotherapy Triple Therapy | The nanocarrier integrates photothermal agents, photosensitizers, and chemotherapeutic drugs; near-infrared light excites photothermal heating and singlet oxygen production while simultaneously releasing chemotherapeutic drugs. This triple-mechanism synergy targets both primary tumors and micrometastases. | Mesoporous carbon nanospheres | Multiple mechanisms work together to minimize the risk of drug resistance; A single light source triggers multiple treatments for simplified operation; Suitable for refractory tumors. | The carrier synthesis is complex, with poor batch-to-batch consistency; multiple therapeutic units may interfere with each other; adverse effects may accumulate, necessitating strict dose control. | [274,275] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wang, L.; Zhang, T.; Lu, T. Progress in the Application of Nanomaterials in Tumor Treatment. Biomedicines 2025, 13, 2666. https://doi.org/10.3390/biomedicines13112666
He X, Wang L, Zhang T, Lu T. Progress in the Application of Nanomaterials in Tumor Treatment. Biomedicines. 2025; 13(11):2666. https://doi.org/10.3390/biomedicines13112666
Chicago/Turabian StyleHe, Xingyu, Lilin Wang, Tongtong Zhang, and Tianqi Lu. 2025. "Progress in the Application of Nanomaterials in Tumor Treatment" Biomedicines 13, no. 11: 2666. https://doi.org/10.3390/biomedicines13112666
APA StyleHe, X., Wang, L., Zhang, T., & Lu, T. (2025). Progress in the Application of Nanomaterials in Tumor Treatment. Biomedicines, 13(11), 2666. https://doi.org/10.3390/biomedicines13112666






