Amnestic Mild Cognitive Impairment Does Not Alter Cerebrocortical Oxygenation Dynamics During Acute Hypoxia–Reoxygenation in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Protocol
2.3. Measurements
2.4. Data Analysis and Statistics
3. Results
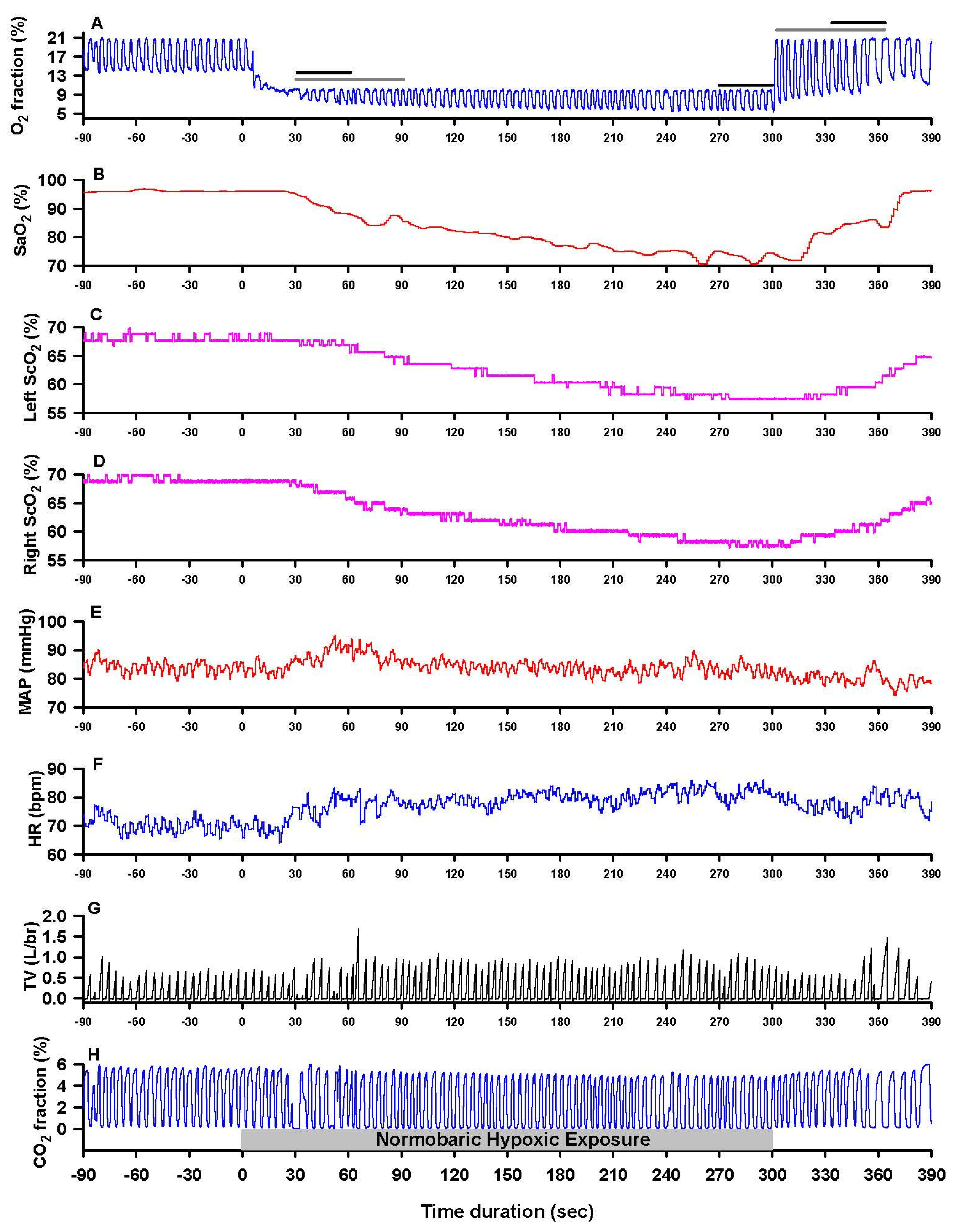
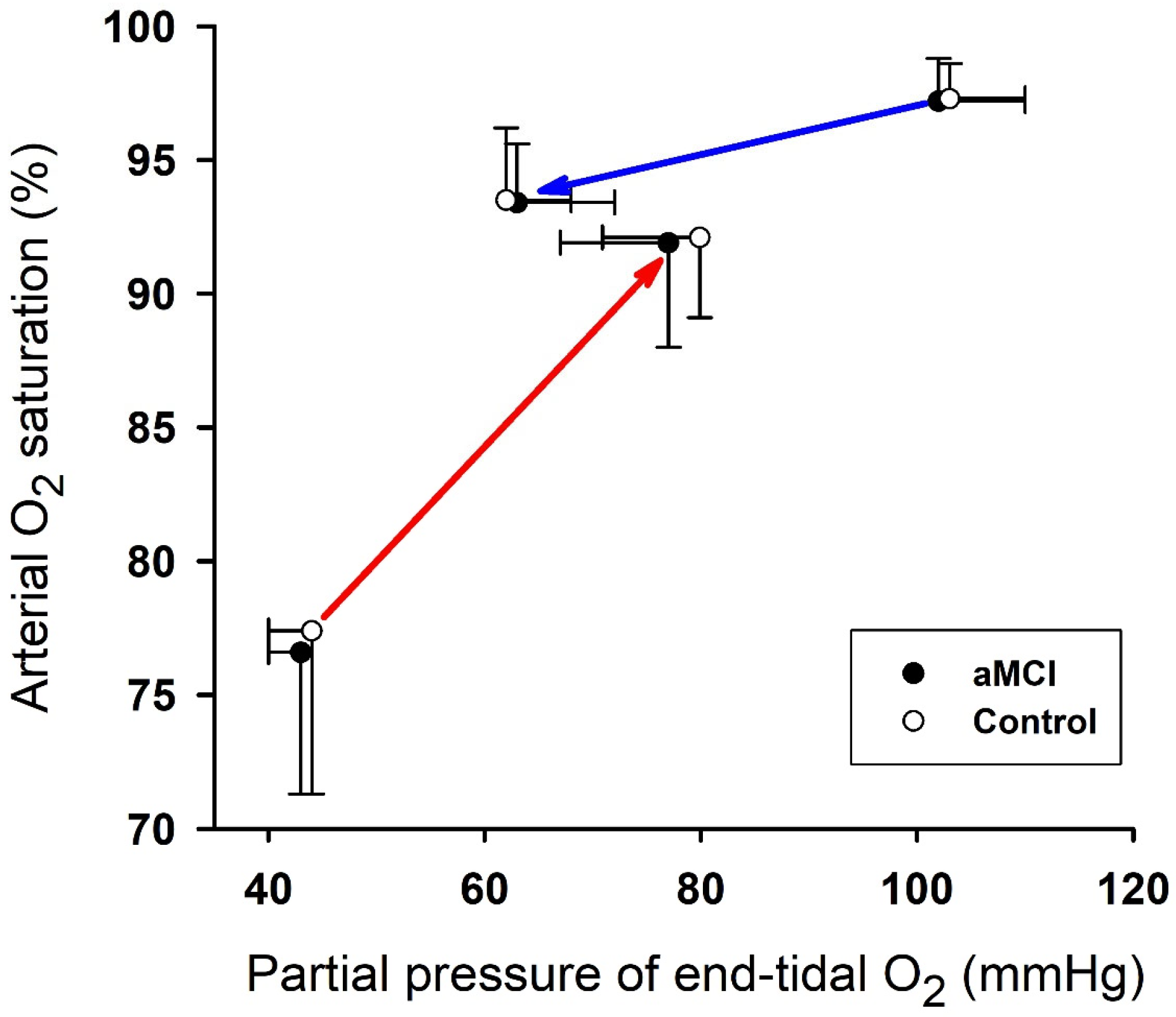
3.1. Cardiopulmonary Responses to Hypoxia and Reoxygenation
3.2. Cerebrocortical Oxygenation During Hypoxia and Reoxygenation
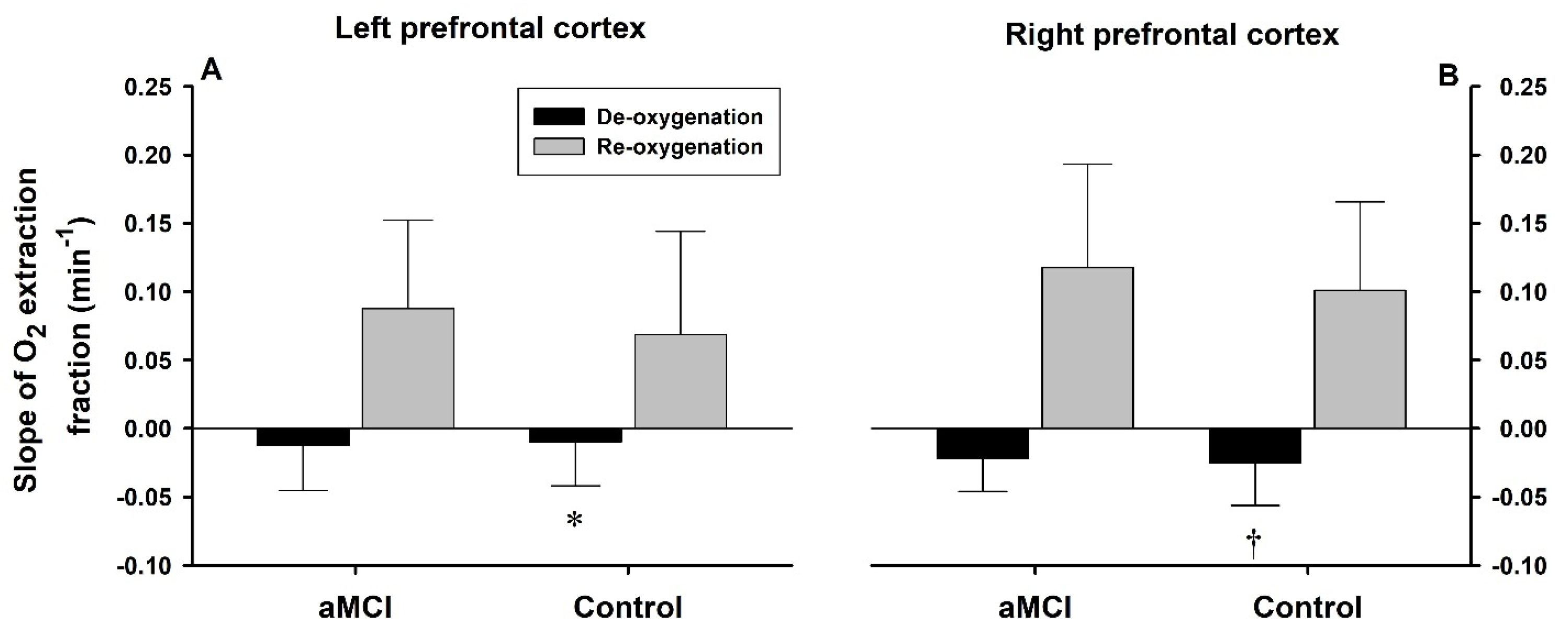
3.3. Rates of De- and Reoxygenation
4. Discussion
4.1. Hypoxemia and Cerebral Tissue Hypoxia During Hypoxia vs. Reoxygenation
4.2. Impact of MCI on Cerebral O2 Dynamics During Hypoxia–Reoxygenation
4.3. Study Limitations and Perspectives
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulin, M.J.; Liang, P.J.; Robbins, P.A. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J. Appl. Physiol. 1996, 81, 1084–1095. [Google Scholar] [CrossRef]
- Harris, A.D.; Murphy, K.; Diaz, C.M.; Saxena, N.; Hall, J.E.; Liu, T.T.; Wise, R.G. Cerebral blood flow response to acute hypoxic hypoxia. NMR Biomed. 2013, 26, 1844–1852. [Google Scholar] [CrossRef]
- Kety, S.S.; Schmidt, C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Investig. 1948, 27, 484–492. [Google Scholar] [CrossRef]
- Xu, F.; Liu, P.; Pascual, J.M.; Xiao, G.; Lu, H. Effect of Hypoxia and Hyperoxia on Cerebral Blood Flow, Blood Oxygenation, and Oxidative Metabolism. J. Cereb. Blood Flow Metab. 2012, 32, 1909–1918. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Shaw, A.D.; Smith, K.J.; Willie, C.K.; Ikeda, K.; Graham, J.; Macleod, D.B. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin. Sci. 2014, 126, 661–670. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Lindberg, U.; Aachmann-Andersen, N.J.; Lisbjerg, K.; Christensen, S.J.; Law, I.; Rasmussen, P.; Olsen, N.V.; Larsson, H.B.W. Acute hypoxia increases the cerebral metabolic rate—A magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 2016, 36, 1046–1058. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Ghanizada, H.; Lindberg, U.; Arngrim, N.; Paulson, O.B.; Gjedde, A.; Ashina, M.; Larsson, H.B.W. Human Cerebral Perfusion, Oxygen Consumption, and Lactate Production in Response to Hypoxic Exposure. Cereb. Cortex 2022, 32, 1295–1306. [Google Scholar] [CrossRef]
- Johnson, N.A.; Jahng, G.H.; Weiner, M.W.; Miller, B.L.; Chui, H.C.; Jagust, W.J.; Gorno-Tempini, M.L.; Schuff, N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. Radiology 2005, 234, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L.; Pa, J.; Duarte, A.; Schuff, N.; Weiner, M.W.; Kramer, J.H.; Miller, B.L.; Freeman, K.M.; Johnson, J.K. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis. Assoc. Disord. 2009, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; RAlvarado, L.; Stephens, K.; Wey, H.Y.; Wang, D.J.J.; Leritz, E.C.; Salat, D.H. Associations between cerebral blood flow and structural and functional brain imaging measures in individuals with neuropsychologically defined mild cognitive impairment. Neurobiol. Aging 2020, 86, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Dunsky, D.I.; Khan, M.A.; Liu, J.; Hill, C.; Armstrong, K.; Martin-Cook, K.; Cullum, C.M.; Zhang, R. Dynamic cerebral autoregulation and tissue oxygenation in amnestic mild cognitive impairment. J. Alzheimers Dis. 2014, 41, 765–778. [Google Scholar] [CrossRef]
- Murayama, Y.; Sato, Y.; Hu, L.; Brugnera, A.; Compare, A.; Sakatani, K. Relation Between Cognitive Function and Baseline Concentrations of Hemoglobin in Prefrontal Cortex of Elderly People Measured by Time-Resolved Near-Infrared Spectroscopy. Adv. Exp. Med. Biol. 2017, 977, 269–276. [Google Scholar] [PubMed]
- Elbanna, S.; Cortez, C.; Smith, E.; Rattanavong, J.; Ross, S.; Kline, G.; Wiechmann, A.; Dyson, H.; Mallet, R.T.; Shi, X. Enhanced cerebral oxygenation during mental and physical activity in older adults is unaltered by amnestic mild cognitive impairment. Front. Physiol. 2025, 16, 1535045. [Google Scholar] [CrossRef] [PubMed]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Intermittent hypoxic-hyperoxic training on cognitive performance in geriatric patients. Alzheimers Dement. 2017, 3, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Schenck, H.; Hall, J.R.; Ross, S.E.; Kline, G.P.; Chen, S.; Mallet, R.T.; Chen, P. Intermittent Hypoxia Training for Treating Mild Cognitive Impairment: A Pilot Study. Am. J. Alzheimers Dis. Other Dement. 2020, 35, 1533317519896725. [Google Scholar] [CrossRef]
- Zhu, X.H.; Yan, H.C.; Zhang, J.; Qu, H.D.; Qiu, X.S.; Chen, L.; Li, S.J.; Cao, X.; Bean, J.C.; Chen, L.H.; et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J. Neurosci. 2010, 30, 12653–12663. [Google Scholar] [CrossRef]
- Ryou, M.G.; Chen, X.; Cai, M.; Wang, H.; Jung, M.E.; Metzger, D.B.; Mallet, R.T.; Shi, X. Intermittent Hypoxia Training Prevents Deficient Learning-Memory Behavior in Mice Modeling Alzheimer’s Disease: A Pilot Study. Front. Aging Neurosci. 2021, 13, 674688. [Google Scholar] [CrossRef]
- Yue, X.; Zhou, Y.; Qiao, M.; Zhao, X.; Huang, X.; Zhao, T.; Cheng, X.; Fan, M.; Zhao, Y.; Chen, R.; et al. Intermittent hypoxia treatment alleviates memory impairment in the 6-month-old APPswe/PS1dE9 mice and reduces amyloid beta accumulation and inflammation in the brain. Alzheimers Res. Ther. 2021, 13, 194. [Google Scholar] [CrossRef]
- Serebrovska, Z.; Xi, L.; Fedoriuk, M.; Dosenko, V.; Shysh, A.; Khetsuriani, M.; Porkhalo, D.; Savchenko, A.; Goncharov, S.; Utko, N.; et al. Intermittent hypoxia-hyperoxia training ameliorates cognitive impairment and neuroinflammation in a rat model of Alzheimer’s disease. Brain Res. 2025, 1847, 149301. [Google Scholar] [CrossRef]
- Smith, E.; Cortez, C.; Wiechmann, A.; Davis, S.; Dyson, H.; Kucharski, K.; Ross, S.; Kline, G.; Mallet, R.T.; Shi, X. Preserved learning despite impaired short-term memory in older adults with mild cognitive impairment. Front. Aging Neurosci. 2025, 17, 1560791. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Rocca, W.A. Prevalence of mild cognitive impairment is higher in men. Neurology 2010, 75, 889–897. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Hall, J.R.; Ross, S.; Chen, S.; Liu, H.; Mallet, R.T.; Shi, X. Enhanced cerebral perfusion during brief exposures to cyclic intermittent hypoxemia. J. Appl. Physiol. 2017, 123, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Kline, G.; Ross, S.E.; Hall, J.R.; Ding, Y.; Mallet, R.T.; Shi, X. Reduced cerebrovascular and cardioventilatory responses to intermittent hypoxia in elderly. Respir. Physiol. Neurobiol. 2020, 271, 103306. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, Y.; Kline, G.P.; Zhou, Z.; Mallet, R.T.; Shi, X. Hypoxic breathing produces more intense hypoxemia in elderly women than in elderly men. Front. Physiol. 2022, 13, 989635. [Google Scholar] [CrossRef]
- Zohdi, H.; Scholkmann, F.; Wolf, U. Frontal cerebral oxygenation asymmetry: Intersubject variability and dependence on systemic physiology, season, and time of day. Neurophotonics 2020, 7, 025006. [Google Scholar] [CrossRef] [PubMed]
- Olopade, C.O.; Mensah, E.; Gupta, R.; Huo, D.; Picchietti, D.L.; Gratton, E.; Michalos, A. Noninvasive determination of brain tissue oxygenation during sleep in obstructive sleep apnea: A near-infrared spectroscopic approach. Sleep 2007, 30, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Ide, K. Acute hypoxia elicits prefrontal oxygenation asymmetry in young adults. Neurophotonics 2023, 10, 045002. [Google Scholar] [CrossRef]
- Matsukawa, K.; Asahara, R.; Uzumaki, M.; Hashiguchi, Y.; Ishii, K.; Wang, J.; Smith, S.A. Central command-related increases in blood velocity of anterior cerebral artery and prefrontal oxygenation at the onset of voluntary tapping. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H518–H531. [Google Scholar] [CrossRef]
- Masamoto, K.; Tanishita, K. Oxygen transport in brain tissue. J. Biomech. Eng. 2009, 131, 074002. [Google Scholar] [CrossRef]
- Xu, K.; Lamanna, J.C. Chronic hypoxia and the cerebral circulation. J. Appl. Physiol. 2006, 100, 725–730. [Google Scholar] [CrossRef] [PubMed]


| aMCI (n = 32) | Control (n = 35) | t | p | |
|---|---|---|---|---|
| Men, Women | 8, 24 | 7, 28 | − | 0.771 |
| Age (year) | 71.1 ± 5.7 | 71.2 ± 5.8 | −0.06 | 0.955 |
| Weight (kg) | 72.8 ± 17.1 | 76.4 ± 14.4 | −0.92 | 0.361 |
| Height (m) | 1.66 ± 0.09 | 1.67 ± 0.10 | −0.58 | 0.568 |
| Education (year) | 16.2 ± 1.3 | 15.9 ± 1.8 | 0.86 | 0.396 |
| Geriatric Depression Scale (score) | 1.1 ± 1.2 | 1.0 ± 1.2 | 0.22 | 0.830 |
| Clinical Dementia Rating (point) | 0.48 ± 0.09 | 0.33 ± 0.24 | 3.57 | 0.001 |
| Mini-mental State Examination (point) | 27.7 ± 1.4 | 28.7 ± 1.1 | −3.16 | 0.003 |
| Trail Making Test–version A (sec) | 44 ± 17 | 29 ± 7 | 4.67 | <0.001 |
| Trail Making Test–version B (sec) | 128 ± 61 | 71 ± 18 | 5.05 | <0.001 |
| Verbal Memory (word) | 5.8 ± 1.9 | 7.6 ± 1.8 | −3.65 | 0.001 |
| Visuospatial Memory (point) | 5.3 ± 2.6 | 8.6 ± 1.8 | −5.91 | <0.001 |
| Right vs. Left Handedness | 27 vs. 5 | 30 vs. 5 | − | 1.000 |
| Duration of Hypoxic Exposure (sec) | 273 ± 43 | 271 ± 37 | 0.22 | 0.825 |
| Number (%) of subjects with prescribed medications | ||||
| Category of Medication | aMCI | Control | Total | p |
| Hypertension/Coronary Arterial Disease | 17 (53.1) | 12 (34.3) | 29 (43.3) | 0.309 |
| Hyper-cholesterol/Hyper-lipidemia | 12 (37.5) | 11 (31.4) | 23 (34.3) | 0.618 |
| Hyperglycemia | 7 (21.9) | 3 (8.6) | 10 (14.9) | 0.175 |
| Anxiety/Depression | 15 (46.9) | 9 (25.7) | 24 (36.8) | 0.081 |
| Reflux/Gastric Acid | 9 (28.1) | 10 (28.6) | 19 (28.4) | 1.000 |
| Hypothyroid/Hyperthyroid | 6 (18.8) | 11 (31.4) | 17 (25.4) | 0.384 |
| Allergy | 9 (28.1) | 8 (22.9) | 17 (25.4) | 0.780 |
| Sleep Aid | 7 (21.9) | 3 (8.6) | 10 (14.9) | 0.175 |
| Hormone Replacement | 6 (18.8) | 4 (11.4) | 10 (14.9) | 0.501 |
| aMCI Subjects | Control Subjects | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | H-1 | H-2 | Rec | Base | H-1 | H-2 | Rec | Factor | F | p | |
| HR (bpm) | 67 ± 10 | 70 ± 10 | 77 ± 11 * | 71 ± 10 | 69 ± 10 | 71 ± 11 | 78 ± 11 * | 72 ± 10 | Group Time | 0.83 9.26 | 0.3636 <0.0001 |
| MAP (mmHg) | 95 ± 10 | 97 ± 9 | 94 ± 10 | 92 ± 9 | 96 ± 9 | 96 ± 10 | 93 ± 10 | 91 ± 10 | Group Time | 0.29 2.95 | 0.593 0.033 |
| TV (L) | 0.80 ± 0.32 | 0.88 ± 0.30 | 0.99 ± 0.45 | 0.93 ± 0.45 | 0.88 ± 0.37 | 1.01 ± 0.40 | 1.12 ± 0.49 | 0.92 ± 0.49 | Group Time | 2.56 2.86 | 0.1107 0.0374 |
| fBr (c/m) | 12.6 ± 3.9 | 13.0 ± 4.5 | 12.9 ± 5.6 | 11.2 ± 5.1 | 11.0 ± 4.4 | 11.3 ± 4.6 | 11.5 ± 4.4 | 9.4 ± 4.0 | Group Time | 8.42 2.57 | 0.0040 0.0549 |
| Vent (L/m) | 9.5 ± 3.7 | 10.7 ± 3.1 | 11.4 ± 4.1 | 9.3 ± 4.9 | 8.8 ± 2.4 | 10.6 ± 3.6 | 12.0 ± 5.0 * | 7.8 ± 3.2 * | Group Time | 0.77 8.79 | 0.3824 <0.0001 |
| PETCO2 (mmHg) | 43 ± 3 | 41 ± 4 | 38 ± 4 § | 41 ± 4 | 43 ± 3 | 42 ± 3 | 39 ± 3 § | 42 ± 3 | Group Time | 0.80 19.99 | 0.3711 <0.0001 |
| PETO2 (mmHg) | 102 ± 8 | 63 ± 9 § | 43 ± 3 § | 77 ± 10 § | 103 ± 7 | 62 ± 6 § | 44 ± 4 § | 79 ± 9 § | Group Time | 0.64 772.9 | 0.425 <0.0001 |
| SaO2 (%) | 97.2 ± 1.6 | 93.4 ± 2.2 * | 76.6 ± 5.3 § | 91.9 ± 3.9 * | 97.3 ± 1.3 | 93.5 ± 2.7 * | 77.4 ± 6.1 § | 92.1 ± 3.0 * | Group Time | 0.33 399.3 | 0.564 <0.0001 |
| L-ScO2 (%) | 67.0 ± 4.7 | 64.0 ± 4.3 | 54.0 ± 4.9 § | 60.5 ± 4.5 * | 69.6 ± 4.5 † | 66.6 ± 4.4 † | 55.2 ± 6.4 § | 62.1 ± 5.7 * | Group Time Side | 31.31 175.2 6.01 | <0.0001 <0.0001 0.015 |
| R-ScO2 (%) | 66.8 ± 4.6 | 64.4 ± 4.5 | 56.0 ± 4.3 § | 61.4 ± 4.5 * | 69.4 ± 4.1 † | 67.0 ± 3.9 † | 58.2 ± 4.4 §†‡ | 63.8 ± 4.5 *† | |||
| L-O2EF | 0.31 ± 0.05 | 0.31 ± 0.05 | 0.29 ± 0.06 | 0.34 ± 0.05 | 0.28 ± 0.05 | 0.29 ± 0.04 | 0.29 ± 0.08 | 0.33 ± 0.06 | Group Time Side | 24.90 22.90 7.56 | <0.0001 <0.0001 0.007 |
| R-O2EF | 0.31 ± 0.05 | 0.31 ± 0.05 | 0.27 ± 0.06 * | 0.33 ± 0.05 § | 0.29 ± 0.04 † | 0.28 ± 0.04 † | 0.25 ± 0.05 *‡ | 0.31 ± 0.05 § | |||
| aMCI (n = 32) | Control (n = 35) | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Hypoxia | Recovery | Hypoxia | Recovery | Factor | F | p | |
| PETO2/time (mmHg/s) | −0.37 ± 0.22 | 0.83 ± 0.21 * | −0.35 ± 0.19 | 0.81 ± 0.21 * | Group Phase | 0.33 157.85 | 0.568 <0.0001 |
| SaO2/time (%/s) | −0.16 ± 0.06 | 0.40 ± 0.13 * | −0.17 ± 0.05 | 0.37 ± 0.12 * | Group Phase | 0.38 179.10 | 0.539 <0.0001 |
| L-ScO2/time (%/s) | −0.09 ± 0.04 | 0.16 ± 0.07 * | −0.11 ± 0.04 | 0.17 ± 0.06 * | Group Phase Hemisphere | 2.80 75.94 17.42 | 0.096 <0.0001 <0.0001 |
| R-ScO2/time (%/s) | −0.08 ± 0.03 | 0.12 ± 0.05 *‡ | −0.09 ± 0.03 ‡ | 0.13 ± 0.06 *‡ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez, C.; Rattanavong, J.; Dyson, H.; Ross, S.; Mallet, R.T.; Shi, X. Amnestic Mild Cognitive Impairment Does Not Alter Cerebrocortical Oxygenation Dynamics During Acute Hypoxia–Reoxygenation in Older Adults. Biomedicines 2025, 13, 2661. https://doi.org/10.3390/biomedicines13112661
Cortez C, Rattanavong J, Dyson H, Ross S, Mallet RT, Shi X. Amnestic Mild Cognitive Impairment Does Not Alter Cerebrocortical Oxygenation Dynamics During Acute Hypoxia–Reoxygenation in Older Adults. Biomedicines. 2025; 13(11):2661. https://doi.org/10.3390/biomedicines13112661
Chicago/Turabian StyleCortez, Christopher, Jewelia Rattanavong, Hannah Dyson, Sarah Ross, Robert T. Mallet, and Xiangrong Shi. 2025. "Amnestic Mild Cognitive Impairment Does Not Alter Cerebrocortical Oxygenation Dynamics During Acute Hypoxia–Reoxygenation in Older Adults" Biomedicines 13, no. 11: 2661. https://doi.org/10.3390/biomedicines13112661
APA StyleCortez, C., Rattanavong, J., Dyson, H., Ross, S., Mallet, R. T., & Shi, X. (2025). Amnestic Mild Cognitive Impairment Does Not Alter Cerebrocortical Oxygenation Dynamics During Acute Hypoxia–Reoxygenation in Older Adults. Biomedicines, 13(11), 2661. https://doi.org/10.3390/biomedicines13112661






