Clinical Relevance of Lipoprotein(a) in Young Acute Myocardial Infarction: STEMI vs. NSTEMI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions and Measurements
2.3. Objectives
2.4. Statistical Analysis
2.5. Data Management
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lp(A) | Lipoprotein(A) |
| LDL-C | Low density lipoprotein cholesterol |
| HDL-C | High density lipoprotein cholesterol |
| HR | Hazard ration |
| STEMI | ST-elevation myocardial infarction |
| NSTEMI | Non-ST-elevation myocardial infarction |
| RR | Relative risk |
| BMI | Body mass index |
| CI | Confidence interval |
| p | p-value |
References
- Zaheen, M.; Pender, P.; Dang, Q.M.; Sinha, E.; Chong, J.J.H.; Chow, C.K.; Zaman, S. Myocardial Infarction in the Young: Aetiology, Emerging Risk Factors, and the Role of Novel Biomarkers. J. Cardiovasc. Dev. Dis. 2025, 12, 148. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. Available online: https://pubmed.ncbi.nlm.nih.gov/28183512/ (accessed on 11 September 2024). [CrossRef]
- Meyers, H.P.; Bracey, A.; Lee, D.; Lichtenheld, A.; Li, W.J.; Singer, D.D.; Kane, J.A.; Dodd, K.W.; Meyers, K.E.; Thode, H.C.; et al. Comparison of the ST-Elevation Myocardial Infarction (STEMI) vs. NSTEMI and Occlusion MI (OMI) vs. NOMI Paradigms of Acute MI. J. Emerg. Med. 2021, 60, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Buciu, I.C.; Tieranu, E.N.; Pircalabu, A.S.; Istratoaie, O.; Zlatian, O.M.; Cioboata, R.; Donoiu, I.; Militaru, C.; Militaru, S.; Militaru, C. Exploring the Relationship Between Lipoprotein (a) Level and Myocardial Infarction Risk: An Observational Study. Medicina 2024, 60, 1878. [Google Scholar] [CrossRef]
- Kronenberg, F. Lipoprotein(a). In Prevention and Treatment of Atherosclerosis Improving State-of-the-Art Management and Search for Novel Targets; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2022; Volume 270, pp. 201–232. Available online: https://pubmed.ncbi.nlm.nih.gov/36122123/ (accessed on 13 September 2024).
- Leistner, D.M.; Laguna-Fernandez, A.; Haghikia, A.; Abdelwahed, Y.S.; Schatz, A.S.; Erbay, A.; Roehle, R.; Fonseca, A.F.; Ferber, P.; Landmesser, U. Impact of elevated lipoprotein(a) on coronary artery disease phenotype and severity. Eur. J. Prev. Cardiol. 2024, 31, 856–865, Erratum in Eur. J. Prev. Cardiol. 2024, 31, 915. https://doi.org/10.1093/eurjpc/zwae104. [Google Scholar] [CrossRef] [PubMed]
- Usalp, S.; Altuntaş, E.; Bağırtan, B.; Karabay, K.Ö. Comparison of serum lipoprotein(a) levels in young and middle-aged patients presenting for the first time with ST-elevation myocardial infarction: A single-centre study. Cardiovasc. J. Afr. 2023, 34, 1–5, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Arshdeep Singh, S.; Imran, A. Serum Lipoprotein(a) and Angiographic Severity of Coronary Aartery Disease in Asian Indians. Res. Cardiovasc. Med. 2024, 13, 42–47. [Google Scholar] [CrossRef]
- Țieranu, E.N.; Cureraru, S.I.; Târtea, G.C.; Vlăduțu, V.-C.; Cojocaru, P.A.; Piorescu, M.T.L.; Dincă, D.; Popescu, R.; Militaru, C.; Donoiu, I.; et al. Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 4304. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, A.; Zavaleanu, A.D.; Călina, D.C.; Gheonea, D.I.; Osiac, E.; Boboc, I.K.S.; Militaru, C.; Militaru, S.; Buciu, I.C.; Țieranu, E.N.; et al. Different Age Related Neurological and Cardiac Effects of Verapamil on a Transgenic Mouse Model of Alzheimer’s Disease. Curr. Health Sci. J. 2021, 47, 263–269. [Google Scholar] [CrossRef]
- Volgman, A.S.; Koschinsky, M.L.; Mehta, A.; Rosenson, R.S. Genetics and Pathophysiological Mechanisms of Lipoprotein(a)-Associated Cardiovascular Risk. J. Am. Heart. Assoc. 2024, 13, 33654. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Maggioni, A.P.; Scicchitano, P.; Zuin, M.; D’Elia, E.; Colivicchi, F. Lipoprotein (a), Inflammation, and Atherosclerosis. J. Clin. Med. 2023, 12, 2529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.Q.; Liu, H.X.; Yu, X.Q.; Tong, L.; Chen, X.; Qi, L.Y.; Cui, C.Y.; Cheng, L.C.; Cai, L. Elevated lipoprotein(a) levels as an independent predictor of long-term recurrent events in patients with acute coronary syndrome: An observational, retrospective cohort study. Coron. Artery Dis. 2022, 33, 385–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borzillo, I.; Ascenzo, F.D.; Ravetti, E.; Balducci, M.; Pilia, R.; Michelone, M.; Annoni, G.; Toscano, A.; Giannino, G.; De Ferrari, G.M.; et al. Lipoprotein(a) in youth and childhood as a marker of cardiovascular risk stratification: A meta-analysis. J. Cardiovasc. Med. 2025, 26, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stürzebecher, P.E.; Uttinger, K.L.; Vogel, M.; Schlingmann, M.; Ceglarek, U.; Isermann, B.; Kiess, W.; Körner, A.; Laufs, U. Lipoprotein(a) serum concentrations in children in relation to body mass index, age and sex. Pediatr. Res. 2024, 96, 177–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Młynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction—The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef]
- Baumann, A.A.W.; Tavella, R.; Air, T.M.; Mishra, A.; Montarello, N.J.; Arstall, M.; Zeitz, C.; Worthley, M.I.; Beltrame, J.F.; Psaltis, P.J. Prevalence and real-world management of NSTEMI with multivessel disease. Cardiovasc. Diagn. Ther. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Cheng, W.; Liu, D.; Liu, G.; Cui, C.; Feng, C.; Wang, X. NLRP3-Mediated Inflammation in Atherosclerosis and Associated Therapeutics. Front. Cell Dev. Biol. 2022, 10, 823387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Wang, L.; Wang, H.; Hao, X.; Du, Z.; Li, C.; Hou, X. The Association of Lipoprotein(a) with Major Adverse Cardiovascular Events after Acute Myocardial Infarction: A Meta-Analysis of Cohort Studies. Rev. Cardiovasc. Med. 2025, 26, 27376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Assini, J.M.; Boffa, M.B.; Koschinsky, M.L. The complex pro-atherosclerotic role of lipoprotein(a): A multiplicity of cellular targets. Curr. Opin. Lipidol. 2025, 36, 268–275. [Google Scholar] [CrossRef]
- Patel, D.; Koschinsky, M.L.; Agarwala, A.; Natarajan, P.; Bhatia, H.S.; Mehta, A.; Patel, J.; Peters, M.; Pandya, S.; Sagheer, U.; et al. Role of Lipoprotein(a) in Atherosclerotic Cardiovascular Disease in South Asian Individuals. J. Am. Heart Assoc. 2025, 14, eJAHA2024040361T. [Google Scholar] [CrossRef]
- Ziogos, E.; Vavuranakis, M.A.; Harb, T.; Foran, P.L.; Blaha, M.J.; Jones, S.R.; Lai, S.; Gerstenblith, G.; Leucker, T.M. Lipoprotein(a) concentrations in acute myocardial infarction patients are not indicative of levels at six month follow-up. Eur. Heart J. Open. 2023, 3, oead035. [Google Scholar] [CrossRef]
- Mack, N.; Katwaroo, A.; Maharaj, M.; Ramdin, R.; Ramcharan, P.; Seecheran, V.; Seecheran, R.; Bhagwandass, N.; Seecheran, N. Relapsing Polychondritis-Associated Myocardial Infarction with Non-Obstructive Coronary Arteries. J. Investig. Med. High Impact Case Rep. 2025, 13, 23247096251381593. [Google Scholar] [CrossRef]
- Buciu, I.C.; Tieranu, E.N.; Pircalabu, A.S.; Zlatian, O.M.; Donoiu, I.; Militaru, C.; Militaru, S.; Militaru, C. The Relationship between Lipoprotein A and the Prevalence of Multivessel Coronary Artery Disease in Young Patients with Acute Myocardial Infarction: An Observational Study. Biomedicines 2024, 12, 2159. [Google Scholar] [CrossRef]
- Lopes Almeida Gomes, L.; Forman Faden, D.; Xie, L.; Chambers, S.; Stone, C.; Werth, V.P.; Williams, K.J. Modern therapy of patients with lupus erythematosus must include appropriate management of their heightened rates of atherosclerotic cardiovascular events: A literature update. Lupus Sci. Med. 2025, 12, e001160. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Yeang, C.; Michos, E.D.; Boatwright, W.; Ballantyne, C.M. High lipoprotein(a): Actionable strategies for risk assessment and mitigation. Am. J. Prev. Cardiol. 2024, 18, 100651. [Google Scholar] [CrossRef] [PubMed]
- Shiyovich, A.; Berman, A.N.; Besser, S.A.; Biery, D.W.; Kaur, G.; Divakaran, S.; Singh, A.; Huck, D.M.; Weber, B.; Plutzky, J.; et al. Association of Lipoprotein (a) and Standard Modifiable Cardiovascular Risk Factors with Incident Myocardial Infarction: The Mass General Brigham Lp(a) Registry. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2024, 13, e034493. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.L.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef]
- Cojocaru, P.A.; Țieranu, M.L.; Piorescu, M.T.L.; Buciu, I.C.; Belu, A.M.; Cureraru, S.I.; Țieranu, E.N.; Moise, G.C.; Istratoaie, O. Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways. Medicina 2025, 61, 1615. [Google Scholar] [CrossRef]
- Donoiu, I.; Târtea, G.; Sfredel, V.; Raicea, V.; Țucă, A.M.; Preda, A.N.; Cozma, D.; Vătășescu, R. Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice. Biomedicines 2023, 11, 3324. [Google Scholar] [CrossRef] [PubMed]
- Dhankhar, S.; Chauhan, S.; Mehta, D.K.; Nitika; Saini, K.; Saini, M.; Das, R.; Gupta, S.; Gautam, V. Novel targets for potential therapeutic use in Diabetes mellitus. Diabetol. Metab. Syndr. 2023, 15, 17. [Google Scholar] [CrossRef]

| Parameter | STEMI n = 88 No. (%) Median (IQR) | NSTEMI n = 63 No. (%) Median (IQR) | CONTROL n = 40 No. (%) Median (IQR) | p | |
|---|---|---|---|---|---|
| Gender | Men | 72 (81.8%) | 48 (76.2%) | 20 (50%) | <0.001 |
| Women | 16 (18.2%) | 15 (23.8%) | 20 (50%) | <0.001 | |
| AGE | 48.0 (43.8–54.2) | 50.0 (46.0–53.0) | 34.0 (29.0–40.0) | <0.001 | |
| BMI | Normalweight | 27 (30.7%) | 12 (19.0%) | 20 (64.3%) | <0.001 |
| Overweight | 16 (18.2%) | 42 (66.7%) | 10 (23.8%) | <0.001 | |
| Obesity | 45 (51.1%) | 9 (14.3%) | 10 (23.8%) | <0.001 | |
| Smoking status | Smoker | 67 (76.1%) | 33 (52.4%) | 20 (50.0%) | 0.002 |
| Non-smoker | 21 (23.9%) | 30 (47.6%) | 20 (50.0%) | ||
| Diabetes mellitus | YES | 24 (27.3%) | 18 (28.6%) | 0 (0%) | <0.001 |
| NO | 64 (72.7%) | 45 (71.4%) | 40 (100%) | ||
| HBP status | YES | 57 (64.8%) | 48 (76.2%) | 0 (0%) | <0.001 |
| NO | 31 (35.2%) | 15 (23.8%) | 40 (100%) | ||
| LDL Cholesterol | 133 (100–168) | 103 (79.0–121) | 110 (90.0–123) | <0.001 | |
| HDL Cholesterol | 39.1 (32.1–44.8) | 41.2 (36.3–51.9) | 55.0 (46.4–60.0) | <0.001 | |
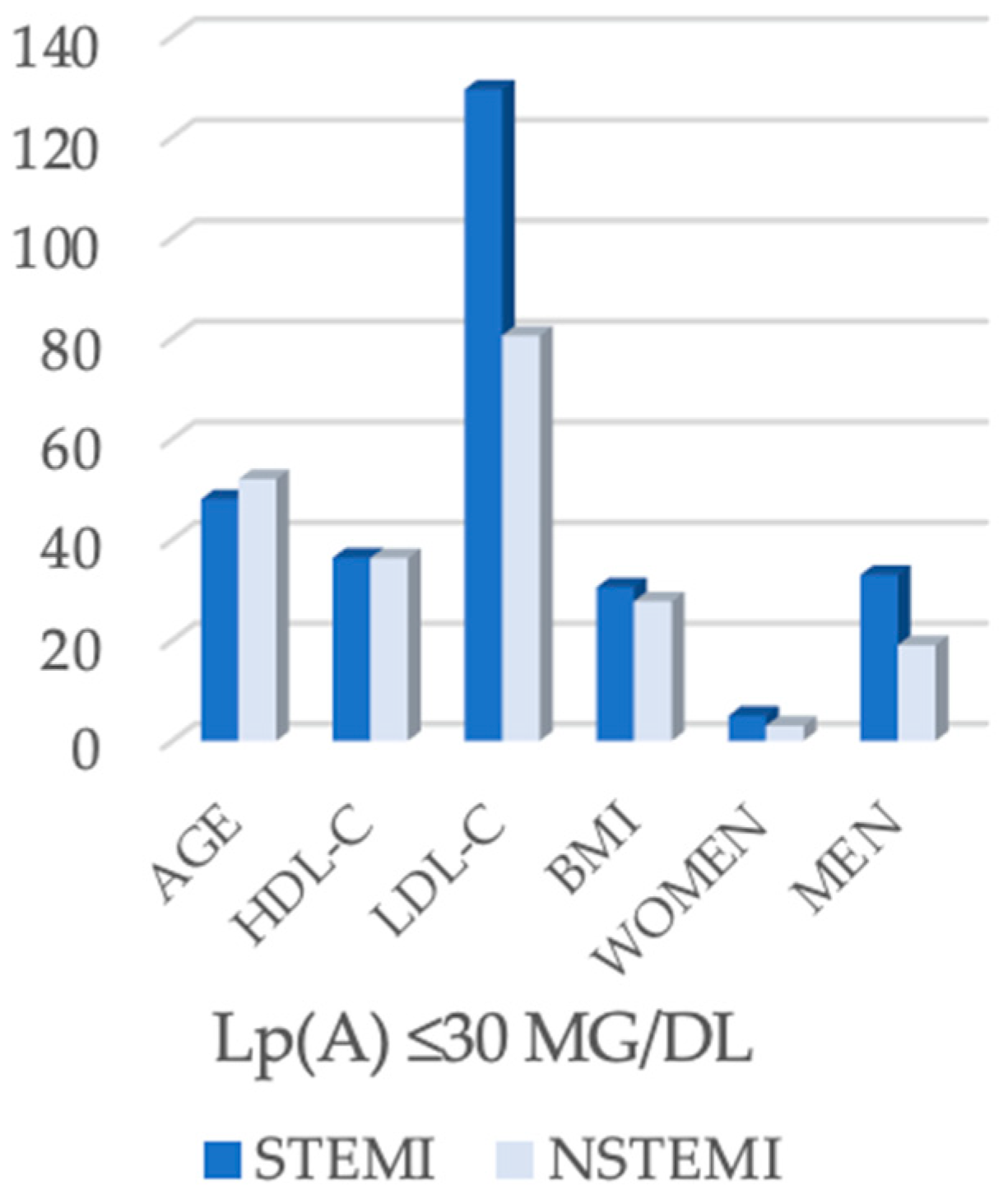
| Lipoprotein(a) level | Lp(a) ≤ 30 mg/dL | 38 (43.2%) | 22 (34.9%) | 30 (66.67%) | 0.002 |
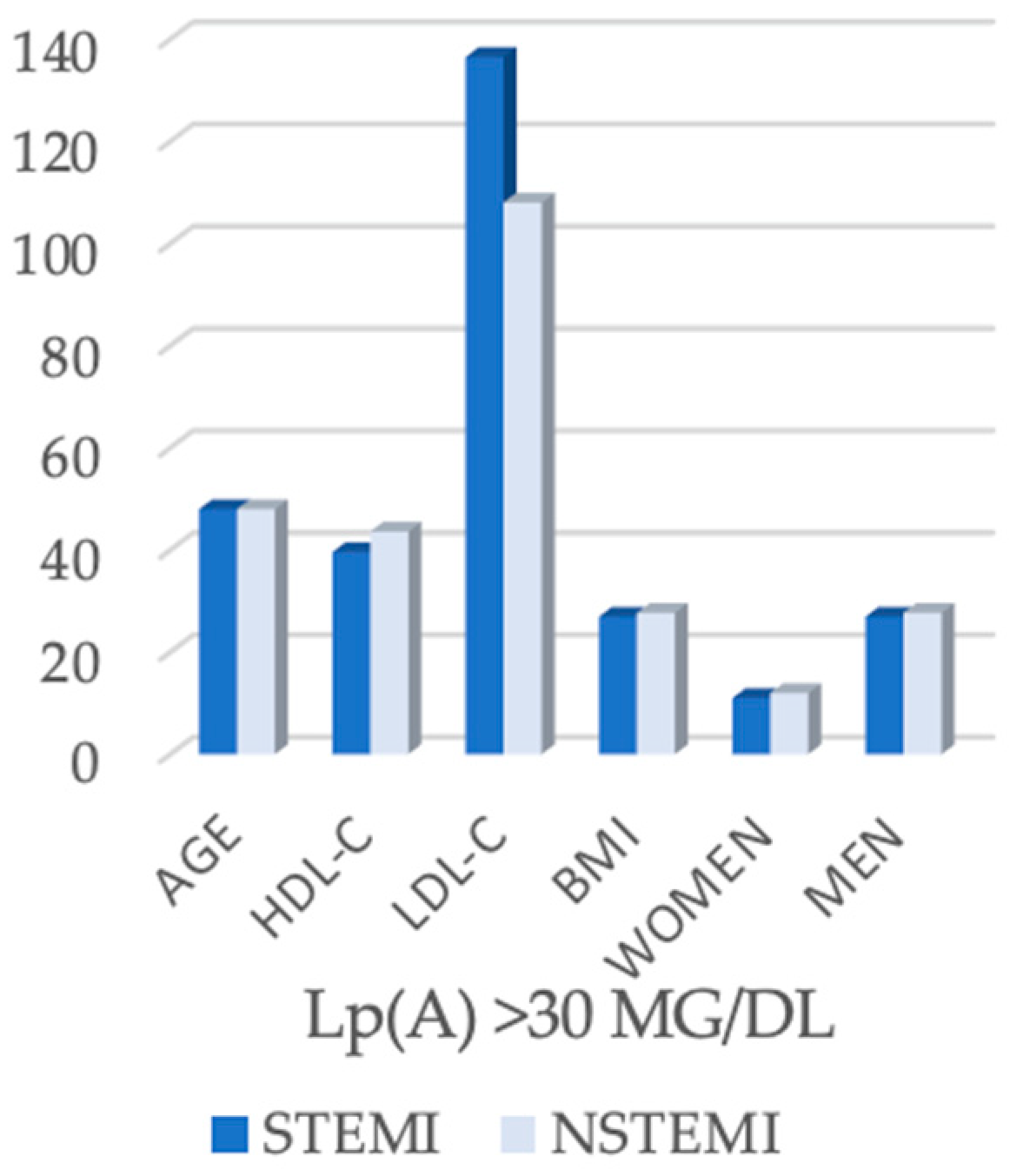
| Lp(a) > 30 mg/dL | 50 (56.8%) | 41 (65.1%) | 10 (33.33%) | ||
| Parameter | STEMI (n = 38) | NSTEMI (n = 22) | p-Value |
|---|---|---|---|
| Age (years) | 48.0 (43.0–54.0) | 52.0 (51.0–54.5) | 0.039 |
| HDL-C (mg/dL) | 36.4 (31.7–44.5) | 36.3 (28.5–53.2) | 0.607 |
| LDL-C (mg/dL) | 129.4 (89.2–157.0) | 80.5 (67.9–113.2) | <0.001 |
| BMI (kg/m2) | 30.5 (26.4–34.4) | 27.7 (26.4–28.6) | 0.021 |
| Men | 33 (86.8%) | 19 (86.4%) | 1.000 |
| Women | 5 (13.2%) | 3 (13.6%) | |
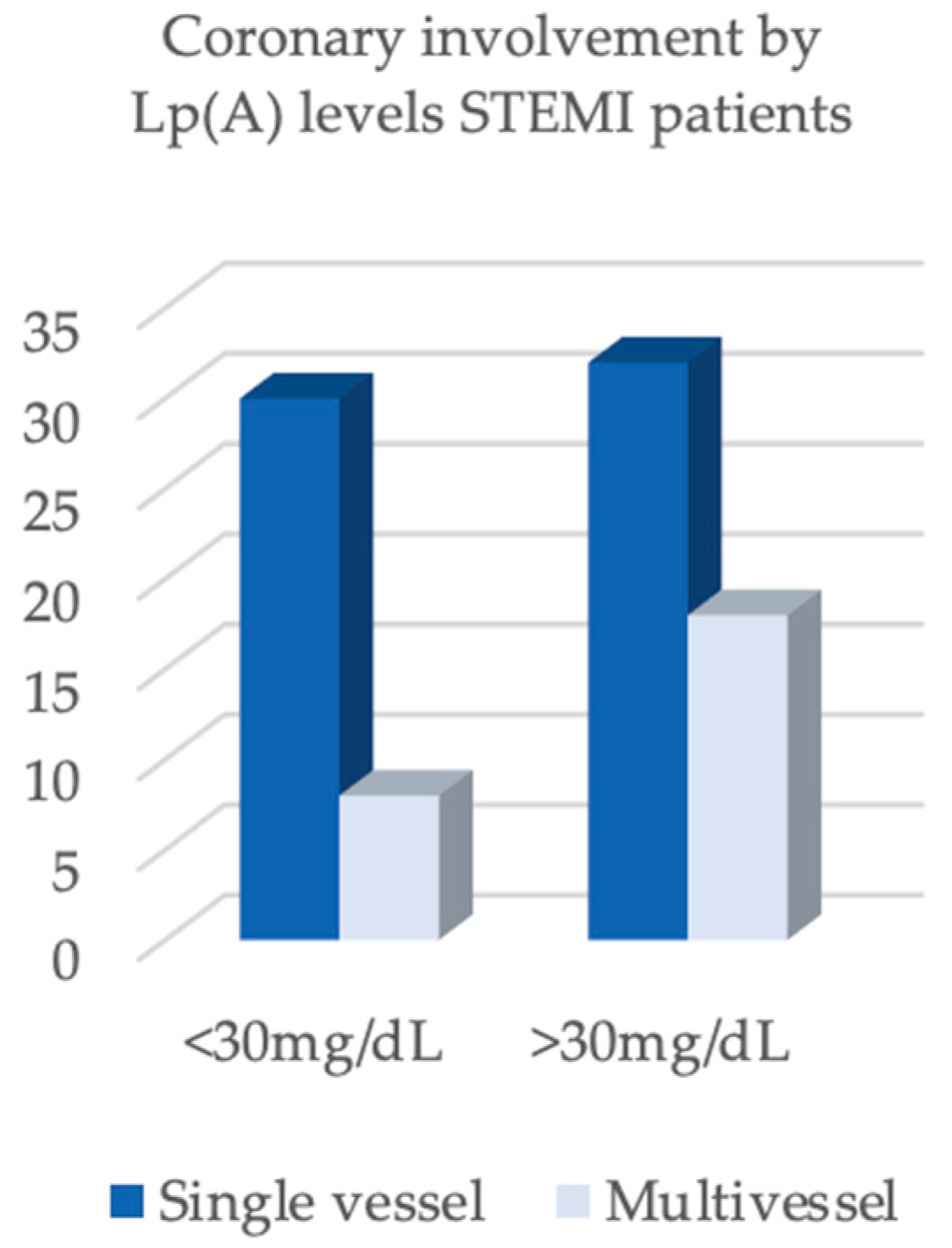
| Single-Vessel Disease | 30 (78.9%) | 18 (81.8%) | 1.000 |
| Multivessel Disease | 8 (21.1%) | 4 (18.2%) |
| Parameter | STEMI (n = 50) | NSTEMI (n = 41) | p-Value |
|---|---|---|---|
| Age (years) | 48.0 (45.0–54.8) | 48.0 (43.0–50.0) | 0.251 |
| HDL-C (mg/dL) | 39.6 (33.1–49.9) | 43.6 (38.8–44.9) | 0.089 |
| LDL-C (mg/dL) | 137.5 (108.5–180.6) | 108.0 (93.0–145.0) | 0.004 |
| BMI (kg/m2) | 26.9 (24.2–32.1) | 27.7 (24.9–29.4) | 0.808 |
| Men | 39 (78.0%) | 29 (70.7%) | 0.474 |
| Women | 11 (22.0%) | 12 (29.3%) | |
| Single-vessel disease | 32 (64.0%) | 11 (26.8%) | <0.001 |
| Multivessel disease | 18 (36.0%) | 30 (73.2%) |
| Predictor | RR | CI | p |
|---|---|---|---|
| Lp(a) ≥ 30 mg/dL (vs. <30) | 4.25 | 1.73–10.45 | 0.0016 |
| Gender (men vs. women) | 1.54 | 0.88–2.68 | 0.130 |
| Diabetes mellitus (yes or no) | 1.29 | 0.81–2.05 | 0.285 |
| Age (+5 years) | 1.05 | 0.86–1.29 | 0.646 |
| LDL-C (+10 mg/dL) | 0.99 | 0.95–1.04 | 0.773 |
| IMC (+5 kg/m2) | 1.55 | 1.01–2.38 | 0.043 |
| Predictor | RR | CI | p |
|---|---|---|---|
| Lp(a) ≥ 30 mg/dL (vs. <30) | 1.78 | 1.10–2.90 | 0.020 |
| Gender (men vs. women) | 1.37 | 0.73–2.56 | 0.329 |
| Diabetes mellitus (yes or no) | 1.71 | 1.07–2.74 | 0.026 |
| Age (+5 years) | 0.98 | 0.81–1.18 | 0.804 |
| LDL-C (+10 mg/dL) | 1.05 | 1.00–1.10 | 0.044 |
| IMC (+5 kg/m2) | 1.07 | 0.90–1.27 | 0.471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cureraru, S.I.; Belu, A.M.; Țieranu, E.N.; Buciu, I.C.; Piorescu, M.T.; Donoiu, I.; Iovănescu, M.; Târtea, G.C.; Militaru, C.; Cojocaru, P.A.; et al. Clinical Relevance of Lipoprotein(a) in Young Acute Myocardial Infarction: STEMI vs. NSTEMI. Biomedicines 2025, 13, 2662. https://doi.org/10.3390/biomedicines13112662
Cureraru SI, Belu AM, Țieranu EN, Buciu IC, Piorescu MT, Donoiu I, Iovănescu M, Târtea GC, Militaru C, Cojocaru PA, et al. Clinical Relevance of Lipoprotein(a) in Young Acute Myocardial Infarction: STEMI vs. NSTEMI. Biomedicines. 2025; 13(11):2662. https://doi.org/10.3390/biomedicines13112662
Chicago/Turabian StyleCureraru, Silvana Isabella, Alexandru Mugurel Belu, Eugen Nicolae Țieranu, Ionuț Cezar Buciu, Mina Teodora Piorescu, Ionuț Donoiu, Maria Iovănescu, Georgică Costinel Târtea, Cristian Militaru, Petre Alexandru Cojocaru, and et al. 2025. "Clinical Relevance of Lipoprotein(a) in Young Acute Myocardial Infarction: STEMI vs. NSTEMI" Biomedicines 13, no. 11: 2662. https://doi.org/10.3390/biomedicines13112662
APA StyleCureraru, S. I., Belu, A. M., Țieranu, E. N., Buciu, I. C., Piorescu, M. T., Donoiu, I., Iovănescu, M., Târtea, G. C., Militaru, C., Cojocaru, P. A., & Istratoaie, O. (2025). Clinical Relevance of Lipoprotein(a) in Young Acute Myocardial Infarction: STEMI vs. NSTEMI. Biomedicines, 13(11), 2662. https://doi.org/10.3390/biomedicines13112662