Sepsis-Induced Cardiomyopathy and Cardiac Arrhythmias: Pathophysiology and Implications for Novel Therapeutic Approaches
Abstract
1. Introduction
2. Methods
3. Sepsis-Induced Cardiomyopathy
3.1. Epidemiology and Definition
3.2. Pathophysiological Mechanisms of SICM
3.2.1. Myocardial Depression Due to Inflammation
3.2.2. Membrane Dysfunction and Attenuated β-Adrenergic Response
3.2.3. Autonomic Nervous System (ANS) Imbalance
3.2.4. Dysregulated Calcium Handling
3.2.5. Mitochondrial Dysfunction
3.2.6. Metabolic Reprogramming
3.2.7. Impaired Endothelial Function and Microcirculatory Failure
4. Sepsis-Induced Cardiac Arrhythmias
4.1. Inflammation
4.2. Electrolyte Abnormalities
4.3. Myocardial Ischemia
4.4. QT Prolongation and Dispersion
4.5. Fever-Induced Arrhythmias in Brugada Patients
5. SICM Management and Novel Therapeutic Agents
- Vitamin C has been explored given its antioxidant and anti-inflammatory effect [230,231]; however, most clinical trials had negative results [232]. In contrast, only one study of 127 patients has provided evidence that septic patients presenting with an overt inflammatory response might benefit from vitamin C [233]. Additionally, a propensity score-matched analysis of 166 patients reported that vitamin C was associated with reduced use of vasopressors and improvement of clinical and laboratory markers [257]. Importantly, the time to therapy initiation was a significant effect modifier, since early administration (within 2 h) was associated with greater vasopressor weaning and lower mortality. However, given the retrospective design of both studies and the small sample sizes, these results should be interpreted with caution.
- Melatonin has been tested in several animal models of SICM. Melatonin exerts its beneficial effects mainly through the regulation of mitochondrial homeostasis. Macrophage-stimulating 1 (Mst1) overexpression has been associated with mitochondrial apoptosis, while melatonin reduces Mst1 expression in mice with SICM [234]. Melatonin regulates the JAK2/STAT3 pathway [235,236,237] and leads to elevated inducible NO synthase activity [238] providing vasodilatory effects. Furthermore, melatonin exerts several anti-inflammatory properties via the suppression of the hypoxia-inducible factor and the nuclear factor erythroid-2 related factor 2, alongside activation of the phosphatidylinositol 3–kinase (PI3K)/Akt signaling pathway [239,258,259], which could counteract myocardial depression due to inflammation. Collectively, all these effects coupled with a favorable safety profile render melatonin an attractive therapy in SICM. Nevertheless, clinical data is not available, and its efficacy in humans remains unknown.
- Engineered exosomes provide vehicles able to transfer specific molecules to targeted sites, acting via the three main mechanisms. (a) Direct and targeted drug delivery in specific tissues [260], including microRNAs [261], for example, delivery of MiR21-loaded exosomes to cardiomyocytes, produced significant anti-apoptotic effects and reduction of myocardial inflammation in a murine model of reperfusion injury [240], while exosomes containing miR-126 were associated with reduced expression of adhesion molecules in septic mice [262]. (b) Modulation of the inflammatory response [263] is based on evidence that exosomes can attenuate the TNF-a and IL-6 pathways even further when compared to established anti-inflammatory treatments [241]. (c) Enhancement of protective and reparatory pathways maintains cell survival [242,243]. It should be noted, though, that most evidence for exosomes arises from preclinical animal studies in MI without representation of SICM models. Small studies in humans have also been performed in various clinical settings [244,245,246] with promising results thus far. Hence, preclinical SICM models and large-scale human studies are lacking.
- Schistosoma japonicum-produced cystatin (Sj-Cys) is a cystatin originating from the trematode Schistosoma japonicum. During SICM, its use in a mouse model of cecal ligation and puncture (CLP)-induced sepsis was associated with several improvements in biomarkers and histological evidence of inflammation [247]. Sj-Cys-treated mice demonstrated reduced levels of cardiac troponin and natriuretic peptides as well reduced infiltration of inflammatory cells within the heart. These beneficial actions were exerted through the downregulation of pro-inflammatory cytokines (mainly TNF-α and IL-6) and the upregulation of anti-inflammatory cytokines (mainly IL-10 and TGF-β) via inhibition of the LPS-MyD88 pathway. However, this was a small-scale monocentric study of 24 mice, and these results have not been further reproduced yet.
- Τ3 and Τ4 significantly regulate tissue development, angiogenesis, and mitochondrial biogenesis, partly via facilitation of tissue adaptation to hypoxia through the p38 MAPK and Akt [248] pathways. Of note, initial low T3 levels are frequent [264] and have been associated with worse outcomes in sepsis [265]. In a mouse model of CLP-induced peritonitis, early T3 administration was associated with reduced lactate and attenuated hypoxia in heart and liver specimens [250]. Furthermore, T3 was recently reported to be beneficial in a murine SICM model via improved calcium homeostasis through phospholamban downregulation [249]. Interestingly, these promising preclinical findings were also translated into a double-blind RCT including 95 severely ill patients with septic shock. In patients with low T3 and T4, oral T3 at high doses for 4 days was associated with reduced mortality, shorter time on mechanical ventilation, and attenuated inflammatory response [251]. It should be highlighted, however, that patients with isolated low T3 presented higher mortality rates. Positive results have also been reported in a small RCT of 52 patients with acute MI, where T3 improved myocardial systolic function and post-infarction remodeling [266]. Nonetheless, both RCTs were exploratory phase II studies with small samples, and large-scale confirmatory studies are needed.
- Ginsenoside Rc (ginseng isolate) was also recently investigated in mice with SICM [252], where it attenuated myocardial injury via inhibition of macrophage activation. The authors found that this anti-inflammatory action was exhibited via downregulation of the Signal transducer and activator of transcription 3 (STAT3)/forkhead box O 3a (FoxO3a) pathway and upregulation of Sirtuin1 (Sirt1). Nonetheless, these results arise from only 15 mice, and echocardiography was performed in the first 24 h with no follow-up measurements. Externally validated and large-scale animal studies with longer follow-ups are needed.
- α-Ketoglutarate was also associated with improved histological markers in a small-scale study of 32 male mice with SICM [253] via improvement of mitochondrial function (increased mitophagy and mitochondrial fission) and reduced myocardial apoptosis. The main limitation of the present study was the limited follow-up time and the inability to elucidate the molecular pathways involved in these beneficial effects.
- Mei et al. tested the gasmerdin-D inhibitor Y2 (GI-Y2) in mice with CLP- or LPS-induced sepsis [254]. In this SICM model, GI-Y2 attenuated myocardial injury via direct binding to gasmerdin-D, leading to reduced production of cytokines and adhesion molecules as well as attenuation of the macrophage pyroptosis by LPS/nigericin. Additionally, gasmerdin-D blockage inside the macrophages’ mitochondria reduced mitochondrial damage and improved mitochondrial function. Nonetheless, the direct effect of GI-Y2 was only tested in macrophages with unclear actions in cardiomyocytes. Furthermore, the interactions between macrophages and cardiomyocyte were studied in vitro, outside the complex in vivo environment.
- Previous reports have suggested the cardioprotective effects of sodium octanoate in mice after ΜΙ through the expression of antioxidants in genes and inhibition of myocardial apoptosis [267]. Based on these results, Lin et al. used sodium octanoate in a murine model of LPS-induced sepsis [255]. Interestingly, the authors found that it exhibited beneficial actions through the inhibition of G protein-coupled receptor 84 (GPR84), leading to antioxidant and anti-inflammatory effects. This was also coupled with improved energy metabolism via increased acetyl-CoA synthesis and upregulation of gene expression related to fatty acid oxidation. Potential limitations were that the mice used to study GPR84 presented with global and not heart-specific GPR84 deficiency, that the improvements in energy metabolism were indirectly evaluated, and that several observed epigenetic modifications were not further explored.
- Gene therapies are also being explored to facilitate targeted drug delivery. In a recent study of LPS-induced sepsis in mice, four hub genes (Itgb1, Il1b, Rac2, Vegfa) were identified as candidate therapeutic targets [256]. Based on these results, the authors performed an additional investigatory analysis using the Connectivity Map database, where they identified KU-0063794 and dasatinib as candidate compounds, with several other miRNAs serving as potential therapeutic and/or diagnostic targets. Nonetheless, this was just a hypothesis-generating study with the limitation of inadequate experimental verification of identified genes, whose mechanism should be elucidated in future research.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadri, S.S.; Rhee, C.; Strich, J.R.; Morales, M.K.; Hohmann, S.; Menchaca, J.; Suffredini, A.F.; Danner, R.L.; Klompas, M. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest 2017, 151, 278–285. Available online: https://pubmed.ncbi.nlm.nih.gov/27452768/ (accessed on 7 August 2024). [CrossRef] [PubMed]
- Aissaoui, N.; Boissier, F.; Chew, M.; Singer, M.; Vignon, P. Sepsis-induced cardiomyopathy. Eur. Heart J. 2025, 46, 3339–3353. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. Available online: https://link.springer.com/article/10.1007/s11886-020-01277-2 (accessed on 7 August 2024). [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. Available online: https://journals.lww.com/ccmjournal/fulltext/2018/04000/septic_cardiomyopathy.20.aspx (accessed on 28 July 2024). [CrossRef]
- Ehrman, R.R.; Sullivan, A.N.; Favot, M.J.; Sherwin, R.L.; Reynolds, C.A.; Abidov, A.; Levy, P.D. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: A review of the literature. Crit. Care 2018, 22, 112. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-018-2043-8 (accessed on 7 August 2024). [CrossRef]
- Walkey, A.J.; Wiener, R.S.; Ghobrial, J.M.; Curtis, L.H.; Benjamin, E.J. Incident Stroke and Mortality Associated With New-Onset Atrial Fibrillation in Patients Hospitalized with Severe Sepsis. JAMA 2011, 306, 2248–2254. Available online: https://jamanetwork.com/journals/jama/fullarticle/1104649 (accessed on 27 June 2025). [CrossRef]
- Boissier, F.; Razazi, K.; Seemann, A.; Bedet, A.; Thille, A.W.; de Prost, N.; Lim, P.; Brun-Buisson, C.; Dessap, A.M. Left ventricular systolic dysfunction during septic shock: The role of loading conditions. Intensive Care Med. 2017, 43, 633–642. Available online: https://link.springer.com/article/10.1007/s00134-017-4698-z (accessed on 7 August 2024). [CrossRef]
- Vieillard-Baron, A.; Caille, V.; Charron, C.; Belliard, G.; Page, B.; Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 2008, 36, 1701–1706. Available online: https://journals.lww.com/ccmjournal/fulltext/2008/06000/actual_incidence_of_global_left_ventricular.1.aspx (accessed on 7 August 2024). [CrossRef]
- PiCCO Pulmonary Edema Study Group; Endo, T.; Kushimoto, S.; Yamanouchi, S.; Sakamoto, T.; Ishikura, H.; Kitazawa, Y.; Taira, Y.; Okuchi, K.; Tagami, T.; et al. Limitations of global end-diastolic volume index as a parameter of cardiac preload in the early phase of severe sepsis: A subgroup analysis of a multicenter, prospective observational study. J. Intensive Care 2013, 1, 11. Available online: https://pubmed.ncbi.nlm.nih.gov/25705404/ (accessed on 7 September 2025). [CrossRef]
- Orde, S.R.; Pulido, J.N.; Masaki, M.; Gillespie, S.; Spoon, J.N.; Kane, G.C.; Oh, J.K. Outcome prediction in sepsis: Speckle tracking echocardiography based assessment of myocardial function. Crit. Care 2014, 18, R149. Available online: https://pubmed.ncbi.nlm.nih.gov/25015102/ (accessed on 7 September 2025). [CrossRef]
- Lanspa, M.J.; Cirulis, M.M.; Wiley, B.M.; Olsen, T.D.; Wilson, E.L.; Beesley, S.J.; Brown, S.M.; Hirshberg, E.L.; Grissom, C.K. Right Ventricular Dysfunction in Early Sepsis and Septic Shock. Chest 2021, 159, 1055–1063. Available online: http://journal.chestnet.org/article/S001236922034900X/fulltext (accessed on 7 August 2024). [CrossRef]
- De Geer, L.; Engvall, J.; Oscarsson, A. Strain echocardiography in septic shock—A comparison with systolic and diastolic function parameters, cardiac biomarkers and outcome. Crit. Care 2015, 19, 122. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-015-0857-1 (accessed on 8 August 2024). [CrossRef]
- Dalla, K.; Hallman, C.; Bech-Hanssen, O.; Haney, M.; Ricksten, S.E. Strain echocardiography identifies impaired longitudinal systolic function in patients with septic shock and preserved ejection fraction. Cardiovasc. Ultrasound 2015, 13, 30. Available online: https://cardiovascularultrasound.biomedcentral.com/articles/10.1186/s12947-015-0025-4 (accessed on 7 August 2024). [CrossRef] [PubMed]
- Sato, R.; Kuriyama, A.; Takada, T.; Nasu, M.; Luthe, S.K. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine 2016, 95, e5031. Available online: https://pubmed.ncbi.nlm.nih.gov/27684877/ (accessed on 26 August 2024). [CrossRef] [PubMed]
- Jayaprakash, N.; Gajic, O.; Frank, R.D.; Smischney, N. Elevated modified shock index in early sepsis is associated with myocardial dysfunction and mortality. J. Crit. Care 2018, 43, 30–35. Available online: https://pubmed.ncbi.nlm.nih.gov/28843067/ (accessed on 7 September 2025). [CrossRef]
- Jeong, H.S.; Lee, T.H.; Bang, C.H.; Kim, J.H.; Hong, S.J. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock. Medicine 2018, 97, e0263. Available online: https://pubmed.ncbi.nlm.nih.gov/29595686/ (accessed on 7 September 2025). [CrossRef] [PubMed]
- Narváez, I.; Canabal, A.; Martín, C.; Sánchez, M.; Moron, A.; Alcalá, J.; Giacoman, S.; Magro, M. Incidence and evolution of sepsis-induced cardiomyopathy in a cohort of patients with sepsis and septic shock. Med. Intensiv. 2018, 42, 283–291. Available online: https://pubmed.ncbi.nlm.nih.gov/29100618/ (accessed on 7 September 2025). [CrossRef]
- Cheng, M.M.W.; Long, Y.; Wang, H.; Han, M.M.W.; Zhang, J.; Cui, N. Role of the mTOR Signalling Pathway in Human Sepsis-Induced Myocardial Dysfunction. Can. J. Cardiol. 2019, 35, 875–883. Available online: https://pubmed.ncbi.nlm.nih.gov/31292086/ (accessed on 7 September 2025). [CrossRef]
- Lu, N.-F.; Jiang, L.; Zhu, B.; Yang, D.-G.; Zheng, R.-Q.; Shao, J.; Yuan, J.; Xi, X.-M. Elevated plasma histone h4 levels are an important risk factor in the development of septic cardiomyopathy. Balk. Med. J. 2020, 37, 72–78. Available online: https://pubmed.ncbi.nlm.nih.gov/31674172/ (accessed on 7 September 2025). [CrossRef]
- Chen, F.C.; Xu, Y.C.; Zhang, Z.C. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J. Zhejiang Univ. Sci. B 2020, 21, 537–548. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7383321/ (accessed on 7 September 2025). [CrossRef]
- Wang, L.; Xie, W.; Li, G.; Hu, B.; Wu, W.; Zhan, L.; Zou, H. Lipocalin 10 as a New Prognostic Biomarker in Sepsis-Induced Myocardial Dysfunction and Mortality: A Pilot Study. Mediat. Inflamm. 2021, 2021, 6616270. Available online: https://pubmed.ncbi.nlm.nih.gov/34121925/ (accessed on 7 September 2025). [CrossRef]
- Tucker, R.V.; Williams, K.; Theyyunni, N.; Fung, C.M. Sepsis-Induced Cardiomyopathy Detected With Focused Cardiac Ultrasound in the Emergency Department. J. Emerg. Med. 2022, 63, e91–e99. Available online: https://pubmed.ncbi.nlm.nih.gov/36229320/ (accessed on 7 September 2025). [CrossRef] [PubMed]
- Cutuli, S.L.; Carelli, S.; Cascarano, L.; Cicconi, S.; Silvestri, D.; Cicetti, M.; Vallecoccia, M.S.; Pintaudi, G.; Tanzarella, E.S.; Grieco, D.L.; et al. Clinical implications of endotoxin activity and Polymyxin-B hemoperfusion in critically ill patients with septic cardiomyopathy: A single-center, retrospective, observational study. Artif. Organs 2023, 47, 1865–1873. Available online: https://pubmed.ncbi.nlm.nih.gov/37737449/ (accessed on 7 September 2025). [CrossRef]
- Zhang, J.; Zhu, J.; Xie, T.; Sun, F.; Wang, N.; Guo, F.M. Quantitative evaluation of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography in septic patients. BMC Anesth. 2023, 23, 271. Available online: https://pubmed.ncbi.nlm.nih.gov/37568093/ (accessed on 7 September 2025). [CrossRef] [PubMed]
- Cutuli, S.L.; Carelli, S.; Cascarano, L.; Cicconi, S.; Silvestri, D.; Cicetti, M.; Vallecoccia, M.S.; Pintaudi, G.; Tanzarella, E.S.; Grieco, D.L.; et al. Identifying predictors and determining mortality rates of septic cardiomyopathy and sepsis-related cardiogenic shock: A retrospective, observational study. PLoS ONE 2024, 19, e0299876. Available online: https://pubmed.ncbi.nlm.nih.gov/38662672/ (accessed on 7 September 2025).
- Chang, X.; Guo, Y.; Wang, J.; Liu, J.; Ma, Y.; Lu, Q.; Han, Y. Heart-type fatty acid binding protein (H-FABP) as an early biomarker in sepsis-induced cardiomyopathy: A prospective observational study. Lipids Health Dis. 2024, 23, 283. Available online: https://pubmed.ncbi.nlm.nih.gov/39232765/ (accessed on 7 September 2025). [CrossRef] [PubMed]
- Yang, X.; Sun, W.; Chen, K.; Wang, X. Establishment and validation of a critical care echocardiography-based predictive model for sepsis-induced cardiomyopathy: A prospective cohort study. J. Crit. Care 2025, 88, 155066. Available online: https://pubmed.ncbi.nlm.nih.gov/40132344/ (accessed on 7 September 2025). [CrossRef]
- Zhou, Y.T.; Wang, G.S.; Gao, X.C.; Wang, S.D.; Wang, S.W.; Tong, D.M. Sepsis-associated myocardial injury: Incidence and mortality. Medicine 2025, 104, e42513. Available online: https://pubmed.ncbi.nlm.nih.gov/40550027/ (accessed on 7 September 2025). [CrossRef]
- Poelaert, J.; Declerck, C.; Vogelaers, D.; Colardyn, F.; Visser, C.A. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997, 23, 553–560. Available online: https://pubmed.ncbi.nlm.nih.gov/9201528/ (accessed on 28 July 2024). [CrossRef]
- Sevilla Berrios, R.A.; O’Horo, J.C.; Velagapudi, V.; Pulido, J.N. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2014, 29, 495–499. Available online: https://pubmed.ncbi.nlm.nih.gov/24746109/ (accessed on 28 July 2024). [CrossRef]
- Huang, S.J.; Nalos, M.; McLean, A.S. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit. Care 2013, 17, R96. Available online: https://ccforum.biomedcentral.com/articles/10.1186/cc12741 (accessed on 28 July 2024). [CrossRef] [PubMed]
- Munt, B.; Jue, J.; Gin, K.; Fenwick, J.; Tweeddale, M. Diastolic filling in human severe sepsis: An echocardiographic study. Crit. Care Med. 1998, 26, 1829–1833. Available online: https://pubmed.ncbi.nlm.nih.gov/9824075/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Jafri, S.M.; Lavine, S.; Field, B.E.; Bahorozian, M.T.; Carlson, R.W. Left ventricular diastolic function in sepsis. Crit. Care Med. 1990, 18, 709–714. Available online: https://pubmed.ncbi.nlm.nih.gov/2364710/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Arcadipane, A.; Landesberg, G.; Vieillard-Baron, A.; Cecconi, M.; Fletcher, N. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: A systematic review and meta-analysis. Br. J. Anaesth. 2017, 119, 583–594. Available online: https://pubmed.ncbi.nlm.nih.gov/29121301/ (accessed on 28 July 2024). [CrossRef]
- Brown, S.M.; E Pittman, J.; Hirshberg, E.L.; Jones, J.P.; Lanspa, M.J.; Kuttler, K.G.; E Litwin, S.; Grissom, C.K. Diastolic dysfunction and mortality in early severe sepsis and septic shock: A prospective, observational echocardiography study. Crit. Ultrasound J. 2012, 4, 8. Available online: https://pubmed.ncbi.nlm.nih.gov/22870900/ (accessed on 28 July 2024). [CrossRef]
- Dhainaut, J.F.; Lanore, J.J.; de Gournay, J.M.; Huyghebaert, M.F.; Brunet, F.; Villemant, D.; Monsallier, J.F. Right ventricular dysfunction in patients with septic shock. Intensive Care Med. 1988, 14 (Suppl. 1), 488–491. Available online: https://link.springer.com/article/10.1007/BF00256967 (accessed on 28 July 2024). [CrossRef]
- Winkelhorst, J.C.; Bootsma, I.T.; Koetsier, P.M.; De Lange, F.; Boerma, E.C. Right Ventricular Function and Long-Term Outcome in Sepsis: A Retrospective Cohort Study. Shock 2020, 53, 537–543. Available online: https://pubmed.ncbi.nlm.nih.gov/31318835/ (accessed on 28 July 2024). [CrossRef]
- Vallabhajosyula, S.; Kumar, M.; Pandompatam, G.; Sakhuja, A.; Kashyap, R.; Kashani, K.; Gajic, O.; Geske, J.B.; Jentzer, J.C. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: An 8-year historical cohort study. Ann. Intensive Care 2017, 7, 94. Available online: https://pubmed.ncbi.nlm.nih.gov/28884343/ (accessed on 28 July 2024). [CrossRef]
- Vieillard-Baron, A.; Prigent, A.; Repessé, X.; Goudelin, M.; Prat, G.; Evrard, B.; Charron, C.; Vignon, P.; Geri, G. Right ventricular failure in septic shock: Characterization, incidence and impact on fluid responsiveness. Crit. Care 2020, 24, 630. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03345-z (accessed on 7 August 2024). [CrossRef]
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. Available online: https://www.nature.com/articles/s41569-020-00492-2 (accessed on 28 July 2024). [CrossRef]
- Malomo, S.; Oswald, T.; Alway, T.; Hadjivassilev, S.; Coombs, S.; Ellery, S.; Lee, J.; Phillips, C.; Philips, B.; James, R.; et al. Characterization of Coronary Artery Disease in Sepsis Survivors. Biomedicines 2025, 13, 1181. [Google Scholar] [CrossRef]
- Macido, A. A Brief Overview of Sepsis Induced Cardiomyopathy. J. Crit. Care Emerg. Med. 2024, 3, 48. [Google Scholar] [CrossRef]
- Sato, R.; Nasu, M. A review of sepsis-induced cardiomyopathy. J. Intensive Care 2015, 3, 48. Available online: https://jintensivecare.biomedcentral.com/articles/10.1186/s40560-015-0112-5 (accessed on 7 August 2025). [CrossRef]
- Bouferrache, K.; Vieillard-Baron, A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr. Opin. Crit. Care 2011, 17, 30–35. Available online: https://pubmed.ncbi.nlm.nih.gov/21157319/ (accessed on 28 July 2024). [CrossRef]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Landesberg, G.; Benedetto, U.; Foex, P.; Cecconi, M. Diastolic dysfunction and mortality in septic patients: A systematic review and meta-analysis. Intensive Care Med. 2015, 41, 1004–1013. Available online: https://pubmed.ncbi.nlm.nih.gov/25800584/ (accessed on 28 July 2024). [CrossRef]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. Available online: https://pubmed.ncbi.nlm.nih.gov/6703540/ (accessed on 8 August 2024). [CrossRef] [PubMed]
- Parker, M.M.; Suffredini, A.F.; Natanson, C.; Ognibene, F.P.; Shelhamer, J.H.; Parrillo, J.E. Responses of left ventricular function in survivors and nonsurvivors of septic shock. J. Crit. Care. 1989, 4, 19–25. [Google Scholar] [CrossRef]
- Chotalia, M.B.; Ali, M.M.; Hebballi, R.F.; Singh, H.F.; Parekh, D.; Bangash, M.N.; Patel, J.M. Hyperdynamic Left Ventricular Ejection Fraction in ICU Patients with Sepsis. Crit. Care Med. 2022, 50, 770–779. Available online: https://journals.lww.com/ccmjournal/fulltext/2022/05000/hyperdynamic_left_ventricular_ejection_fraction_in.6.aspx (accessed on 8 August 2024). [CrossRef]
- Chang, W.-T.; Lee, W.-H.; Lee, W.-T.; Chen, P.-S.; Su, Y.-R.; Liu, P.-Y.; Liu, Y.-W.; Tsai, W.-C. Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Med. 2015, 41, 1791–1799. Available online: https://link.springer.com/article/10.1007/s00134-015-3970-3 (accessed on 7 August 2024). [CrossRef]
- Hai, P.D.; Binh, N.T.; Hien, N.V.Q.; Hoang, N.H.; Hoan, V.N.; Son, P.N.; Hoa, L.T.V. Prognostic Role of Left Ventricular Systolic Function Measured by Speckle Tracking Echocardiography in Septic Shock. Biomed. Res. Int. 2020, 2020, 7927353. Available online: https://pubmed.ncbi.nlm.nih.gov/33150180/ (accessed on 7 August 2024). [CrossRef] [PubMed]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Tritapepe, L.; Lorini, F.L.; Arcadipane, A.; Vieillard-Baron, A.; Cecconi, M. Left ventricular systolic function evaluated by strain echocardiography and relationship with mortality in patients with severe sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2018, 22, 183. Available online: https://pubmed.ncbi.nlm.nih.gov/30075792/ (accessed on 7 August 2024). [CrossRef] [PubMed]
- Palmieri, V.; Innocenti, F.; Guzzo, A.; Guerrini, E.; Vignaroli, D.; Pini, R. Left Ventricular Systolic Longitudinal Function as Predictor of Outcome in Patients with Sepsis. Circ Cardiovasc. Imaging 2015, 8, e003865. Available online: https://pubmed.ncbi.nlm.nih.gov/26546483/ (accessed on 8 August 2024). [CrossRef] [PubMed]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2016, 37, 1642–1650. Available online: https://pubmed.ncbi.nlm.nih.gov/26417058/ (accessed on 8 August 2024). [CrossRef]
- Sanfilippo, F.; Huang, S.; Messina, A.; Franchi, F.; Oliveri, F.; Vieillard-Baron, A.; Cecconi, M.; Astuto, M. Systolic dysfunction as evaluated by tissue Doppler imaging echocardiography and mortality in septic patients: A systematic review and meta-analysis. J. Crit. Care. 2021, 62, 256–264. [Google Scholar] [CrossRef]
- Sanderson, T.; Samuels, T. A cohort study evaluating myocardial work and right ventricle strain in sepsis in critical care. Sci. Rep. 2025, 15, 16606. Available online: https://www.nature.com/articles/s41598-025-94909-y (accessed on 20 August 2025). [CrossRef]
- Furian, T.; Aguiar, C.; Prado, K.; Ribeiro, R.V.P.; Becker, L.; Martinelli, N.; Clausell, N.; Rohde, L.E.; Biolo, A. Ventricular dysfunction and dilation in severe sepsis and septic shock: Relation to endothelial function and mortality. J. Crit. Care. 2012, 27, 319.e9–319.e15. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.J.; Kim, M.; Ryoo, S.M.; Kim, W.Y. Association between right ventricle dysfunction and poor outcome in patients with septic shock. Heart 2020, 106, 1665–1671. Available online: https://heart.bmj.com/content/106/21/1665 (accessed on 7 August 2024). [CrossRef]
- Innocenti, F.; Palmieri, V.; Stefanone, V.T.; Donnini, C.; D’aRgenzio, F.; Cigana, M.; Tassinari, I.; Pini, R. Epidemiology of right ventricular systolic dysfunction in patients with sepsis and septic shock in the emergency department. Intern. Emerg. Med. 2020, 15, 1281–1289. Available online: https://link.springer.com/article/10.1007/s11739-020-02325-z (accessed on 7 August 2024). [CrossRef]
- Chen, H.; Huang, L.; Xing, B.; Gao, Y.; Zhang, J.; Zhang, B. Prognostic value of right ventricular free wall strain in patients with sepsis. Front. Cardiovasc. Med. 2024, 11, 1334759. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, W.; Zhu, R.; Long, T.; Zhang, Z.; Song, Z.; Mu, S.; Wang, S.; Wang, H.; Lei, J.; et al. Testosterone and soluble ST2 as mortality predictive biomarkers in male patients with sepsis-induced cardiomyopathy. Front. Med. 2023, 10, 1278879. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10801257/ (accessed on 20 August 2025). [CrossRef]
- Ye, X.; Wang, J.; Hu, L.; Zhang, Y.; Li, Y.; Xuan, J.; Han, S.; Qu, Y.; Yang, L.; Yang, J.; et al. The diagnostic and prognostic value of soluble ST2 in Sepsis. Front. Med. 2024, 11, 1487443. [Google Scholar] [CrossRef]
- Davini, F.; Fogolari, M.; D’avanzo, G.; Ristori, M.V.; Nucciarelli, S.; Bani, L.; Cristiano, A.; De Cesaris, M.; Spoto, S.; Angeletti, S. Soluble Suppression of Tumorigenicity 2 (sST2) as a Diagnostic and Prognostic Marker in Acute Heart Failure and Sepsis: A Comparative Analysis. Diagnostics 2025, 15, 1010. [Google Scholar] [CrossRef]
- Karabacak, P. Serum galectin-3 levels predict poor prognosis in sepsis and septic shock patients. Rev. Assoc. Med. Bras. 2023, 69, e20220940. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10443910/ (accessed on 20 August 2025). [CrossRef]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; Von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: Implications for its use as a biomarker. Circ. Heart Fail. 2017, 10, e003804. Available online: https://pubmed.ncbi.nlm.nih.gov/28288987/ (accessed on 20 August 2025). [CrossRef] [PubMed]
- Rutai, A.; Fejes, R.; Juhász, L.; Tallósy, S.P.; Poles, M.Z.; Földesi, I.; Mészáros, A.T.; Szabó, A.; Boros, M.; Kaszaki, J. Endothelin a and b receptors: Potential targets for microcirculatory-mitochondrial therapy in experimental sepsis. Shock 2020, 54, 87–95. Available online: https://pubmed.ncbi.nlm.nih.gov/31318833/ (accessed on 20 August 2025). [CrossRef] [PubMed]
- Kowalczyk, A.; Kleniewska, P.; Kolodziejczyk, M.; Skibska, B.; Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. Available online: https://pubmed.ncbi.nlm.nih.gov/25288367/ (accessed on 20 August 2025). [CrossRef]
- Rossi, M.A.; Celes, M.R.N.; Prado, C.M.; Saggioro, F.P. Myocardial structural changes in long-term human severe sepsis/septic shock may be responsible for cardiac dysfunction. Shock 2007, 27, 10–18. Available online: https://pubmed.ncbi.nlm.nih.gov/17172974/ (accessed on 28 July 2024). [CrossRef]
- Schmittinger, C.A.; Dünser, M.W.; Torgersen, C.; Luckner, G.; Lorenz, I.; Schmid, S.; Joannidis, M.; Moser, P.; Hasibeder, W.R.; Halabi, M.; et al. Histologic pathologies of the myocardium in septic shock: A prospective observational study. Shock 2013, 39, 329–335. Available online: https://journals.lww.com/shockjournal/fulltext/2013/04000/histologic_pathologies_of_the_myocardium_in_septic.2.aspx (accessed on 7 August 2024). [CrossRef]
- Conway-Morris, A.; Wilson, J.; Shankar-Hari, M. Immune Activation in Sepsis. 2018. Available online: https://www.repository.cam.ac.uk/handle/1810/278217 (accessed on 24 July 2025).
- Rosengart, M.R.; Nathens, A.B.; Arbabi, S.; Neff, M.J.; Garcia, I.; Martin, T.R.; Maier, R.V. Mitogen-activated protein kinases in the intensive care unit: Prognostic potential. Ann. Surg. 2003, 237, 94–100. Available online: https://pubmed.ncbi.nlm.nih.gov/12496535/ (accessed on 24 July 2025). [CrossRef]
- Odeh, M. Tumor necrosis factor-α as a myocardial depressant substance. Int. J. Cardiol. 1993, 42, 231–238. Available online: http://www.internationaljournalofcardiology.com/article/016752739390053J/fulltext (accessed on 28 July 2024). [CrossRef] [PubMed]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. Available online: https://pubmed.ncbi.nlm.nih.gov/8642298/ (accessed on 28 July 2024). [CrossRef]
- Wang, H.; Ward, M.F.; Sama, A.E. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 2009, 32, 348–357. Available online: https://journals.lww.com/shockjournal/fulltext/2009/10000/novel_hmgb1_inhibiting_therapeutic_agents_for.2.aspx (accessed on 7 August 2024). [CrossRef]
- Razazi, K.; Boissier, F.; Surenaud, M.; Bedet, A.; Seemann, A.; Carteaux, G.; de Prost, N.; Brun-Buisson, C.; Hue, S.; Dessap, A.M. A multiplex analysis of sepsis mediators during human septic shock: A preliminary study on myocardial depression and organ failures. Ann. Intensive Care 2019, 9, 64. Available online: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-019-0538-3 (accessed on 7 August 2024). [CrossRef]
- Nemoto, S.; Vallejo, J.G.; Knuefermann, P.; Misra, A.; Defreitas, G.; Carabello, B.A.; Mann, D.L. Escherichia coli LPS-induced LV dysfunction: Role of toll-like receptor-4 in the adult heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2316–H2323. Available online: https://pubmed.ncbi.nlm.nih.gov/12003842/ (accessed on 8 August 2024). [CrossRef] [PubMed]
- Cain, B.S.; Meldrum, D.R.; Dinarello, C.A.; Meng, X.; Joo, K.S.; Banerjee, A.; Harken, A.H. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit. Care Med. 1999, 27, 1309–1318. Available online: https://pubmed.ncbi.nlm.nih.gov/10446825/ (accessed on 8 August 2024). [CrossRef]
- Sharma, A.C.; Motew, S.J.; Farias, S.; Alden, K.J.; Bosmann, H.; Law, W.R.; Ferguson, J.L. Sepsis Alters Myocardial and Plasma Concentrations of Endothelin and Nitric Oxide in Rats. J. Mol. Cell. Cardiol. 1997, 29, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Sharma, A.C. Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction. Life Sci. 2007, 81, 306–316. [Google Scholar] [CrossRef][Green Version]
- Natanson, C.; Eichenholz, P.W.; Danner, R.L.; Eichacker, P.Q.; Hoffman, W.D.; Kuo, G.C.; Banks, S.M.; MacVittie, T.J.; E Parrillo, J. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J. Exp. Med. 1989, 169, 823–832. Available online: https://pubmed.ncbi.nlm.nih.gov/2647895/ (accessed on 6 July 2025). [CrossRef] [PubMed]
- Pagani, F.D.; Baker, L.S.; Hsi, C.; Knox, M.; Fink, M.P.; Visnert, M.S. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-α in conscious dogs. J. Clin. Investig. 1992, 90, 389–398. Available online: https://pubmed.ncbi.nlm.nih.gov/1644912/ (accessed on 6 July 2025). [CrossRef]
- Mester, P.; Utrata, A.; Schmidtner, N.; Birner, C.; Schmid, S.; Müller, M.; Pavel, V.; Buechler, C. Lower Plasma IL-32 Levels Linked to Better Survival in Sepsis. Biomedicines 2025, 13, 750. [Google Scholar] [CrossRef]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.-C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta—Mol. Basis Dis. 2015, 1852, 2362–2371. Available online: https://pubmed.ncbi.nlm.nih.gov/26300484/ (accessed on 24 July 2025). [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Gluvic, Z.; Banjac, K.; Rizzo, M.; Isenovic, E.R. The Na+/K+-ATPase: A potential therapeutic target in cardiometabolic diseases. Front. Endocrinol. 2023, 14, 1150171. [Google Scholar] [CrossRef]
- Celes, M.R.N.; Torres-Dueñas, D.; Prado, C.M.; Campos, E.C.; Moreira, J.E.; Cunha, F.Q.; Rossi, M.A. Increased sarcolemmal permeability as an early event in experimental septic cardiomyopathy: A potential role for oxidative damage to lipids and proteins. Shock 2010, 33, 322–331. Available online: https://journals.lww.com/shockjournal/fulltext/2010/03000/increased_sarcolemmal_permeability_as_an_early.15.aspx (accessed on 6 June 2025). [CrossRef]
- Haileselassie, B.; Su, E.; Pozios, I.; Niño, D.F.; Liu, H.; Lu, D.-Y.; Ventoulis, I.; Fulton, W.B.; Sodhi, C.P.; Hackam, D.; et al. Myocardial oxidative stress correlates with left ventricular dysfunction on strain echocardiography in a rodent model of sepsis. Intensive Care Med. Exp. 2017, 5, 21. [Google Scholar] [CrossRef]
- Hofmaenner, D.A.; Kleyman, A.; Press, A.; Bauer, M.; Singer, M. The Many Roles of Cholesterol in Sepsis A Review. Am. J. Respir. Crit. Care Med. 2022, 205, 388–396. Available online: https://pubmed.ncbi.nlm.nih.gov/34715007/ (accessed on 6 June 2025). [CrossRef]
- Tang, C.; Liu, M.S. Initial externalization followed by internalization of β-adrenergic receptors in rat heart during sepsis. Am. J. Physiol—Regul. Integr. Comp. Physiol. 1996, 270, R254–R263. Available online: https://pubmed.ncbi.nlm.nih.gov/8769809/ (accessed on 3 June 2025). [CrossRef]
- Rudiger, A.; Dyson, A.; Felsmann, K.; Carré, J.E.; Taylor, V.; Hughes, S.; Clatworthy, I.; Protti, A.; Pellerin, D.; Lemm, J.; et al. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin. Sci. 2013, 124, 391–401. Available online: https://pubmed.ncbi.nlm.nih.gov/22988837/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Kawaguchi, S.; Okada, M.; Ijiri, E.; Koga, D.; Watanabe, T.; Hayashi, K.; Kashiwagi, Y.; Fujita, S.; Hasebe, N. β3-Adrenergic receptor blockade reduces mortality in endotoxin-induced heart failure by suppressing induced nitric oxide synthase and saving cardiac metabolism. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H283–H294. Available online: https://pubmed.ncbi.nlm.nih.gov/31834837/ (accessed on 3 June 2025). [CrossRef]
- Lyon, A.R.; Rees, P.S.C.; Prasad, S.; Poole-Wilson, P.A.; Harding, S.E. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 22–29. Available online: https://pubmed.ncbi.nlm.nih.gov/18094670/ (accessed on 7 August 2024).
- Hollenberg, S.M. Understanding stress cardiomyopathy. Intensive Care Med. 2016, 42, 432–435. Available online: https://pubmed.ncbi.nlm.nih.gov/26271909/ (accessed on 7 August 2024). [CrossRef] [PubMed]
- Borodzicz-Jażdżyk, S.; Kołodzińska, A.; Czarzasta, K.; Wojciechowska, M.; Główczyńska, R.; Szczepankiewicz, B.; Puchalska, L.; Opolski, G.; Cudnoch-Jędrzejewska, A. Inflammatory Forms of Cardiomyocyte Cell Death in the Rat Model of Isoprenaline-Induced Takotsubo Syndrome. Biomedicines 2023, 11, 2060. [Google Scholar] [CrossRef]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. Available online: https://pubmed.ncbi.nlm.nih.gov/33602474/ (accessed on 22 July 2025). [CrossRef]
- Celes, M.R.N.; Malvestio, L.M.; Suadicani, S.O.; Prado, C.M.; Figueiredo, M.J.; Campos, E.C.; Freitas, A.C.; Spray, D.C.; Tanowitz, H.B.; da Silva, J.S.; et al. Disruption of Calcium Homeostasis in Cardiomyocytes Underlies Cardiac Structural and Functional Changes in Severe Sepsis. PLoS ONE 2013, 8, e68809. Available online: https://pubmed.ncbi.nlm.nih.gov/23935889/ (accessed on 6 June 2025). [CrossRef] [PubMed]
- Macarthur, H.; Westfall, T.C.; Riley, D.P.; Misko, T.P.; Salvemini, D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc. Natl. Acad. Sci. USA 2000, 97, 9753. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC16937/ (accessed on 24 July 2025). [CrossRef] [PubMed]
- Kalbitz, M.; Fattahi, F.; Herron, T.J.; Grailer, J.J.; Jajou, L.; Lu, H.; Huber-Lang, M.; Zetoune, F.S.; Sarma, J.V.; Day, S.M.; et al. Complement Destabilizes Cardiomyocyte Function in Vivo after Polymicrobial Sepsis and In Vitro. J. Immunol. 2016, 197, 2353. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4988523/ (accessed on 24 July 2025). [CrossRef]
- Aldhoon, B.; Melenovský, V.; Peichl, P.; Kautzner, J. New insights into mechanisms of atrial fibrillation. Physiol. Res. 2010, 59, 1–12. Available online: https://pubmed.ncbi.nlm.nih.gov/19249911/ (accessed on 27 June 2025). [CrossRef] [PubMed]
- Hobai, I.A.; Edgecomb, J.; LaBarge, K.; Colucci, W.S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 2015, 43, 3–15. Available online: https://journals.lww.com/shockjournal/fulltext/2015/01000/dysregulation_of_intracellular_calcium.2.aspx (accessed on 7 August 2024). [CrossRef]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.-X.; Wronska, A.; Marks, A.R. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2012, 111, 708–717. Available online: https://pubmed.ncbi.nlm.nih.gov/22828895/ (accessed on 8 August 2024). [CrossRef]
- Zhang, C.; Mo, M.; Ding, W.; Liu, W.; Yan, D.; Deng, J.; Luo, X.; Liu, J. High-mobility group box 1 (HMGB1) impaired cardiac excitation-contraction coupling by enhancing the sarcoplasmic reticulum (SR) Ca(2+) leak through TLR4-ROS signaling in cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 74, 260–273. Available online: https://pubmed.ncbi.nlm.nih.gov/24937603/ (accessed on 28 July 2024). [CrossRef]
- Pinto, B.B.; Dyson, A.; Umbrello, M.; Carré, J.E.; Ritter, C.; Clatworthy, I.; Duchen, M.R.; Singer, M. Improved Survival in a Long-Term Rat Model of Sepsis Is Associated with Reduced Mitochondrial Calcium Uptake Despite Increased Energetic Demand. Crit. Care Med. 2017, 45, e840–e848. Available online: https://pubmed.ncbi.nlm.nih.gov/28410346/ (accessed on 28 July 2024). [CrossRef]
- Takeuchi, K.; Del Nido, P.J.; Ibrahim, A.E.; Poutias, D.N.; Glynn, P.; Cao-Danh, H.; Cowan, D.B.; McGowan, F.X., Jr. Increased myocardial calcium cycling and reduced myofilament calcium sensitivity in early endotoxemia. Surgery 1999, 126, 231–238. Available online: https://www.surgjournal.com/action/showFullText?pii=S0039606099701601 (accessed on 6 June 2025). [CrossRef] [PubMed]
- Zhong, J.; Hwang, T.C.; Adams, H.R.; Rubin, L.J. Reduced L-type calcium current in ventricular myocytes from endotoxemic guinea pigs. Am. J. Physiol.—Heart Circ. Physiol. 1997, 273, H2312–H2324. Available online: https://pubmed.ncbi.nlm.nih.gov/9374768/ (accessed on 6 June 2025). [CrossRef]
- Kao, Y.H.; Chen, Y.C.; Cheng, C.C.; Lee, T.I.; Chen, Y.J.; Chen, S.A. Tumor necrosis factor-α decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit. Care Med. 2010, 38, 217–222. Available online: https://pubmed.ncbi.nlm.nih.gov/19730253/ (accessed on 24 July 2025). [CrossRef]
- Arina, P.; Sorge, M.; Gallo, A.; Di Mauro, V.; Vitale, N.; Cappello, P.; Brazzi, L.; Barandalla-Sobrados, M.; Cimino, J.; Ranieri, V.M.; et al. Modulation of LTCC Pathways by a Melusin Mimetic Increases Ventricular Contractility During LPS-Induced Cardiomyopathy. Shock 2022, 57, 318–325. Available online: https://journals.lww.com/shockjournal/fulltext/2022/06000/modulation_of_ltcc_pathways_by_a_melusin_mimetic.18.aspx (accessed on 6 June 2025). [CrossRef]
- Martin, L.; Horst, K.; Chiazza, F.; Oggero, S.; Collino, M.; Brandenburg, K.; Hildebrand, F.; Marx, G.; Thiemermann, C.; Schuerholz, T. The synthetic antimicrobial peptide 19-2.5 attenuates septic cardiomyopathy and prevents down-regulation of SERCA2 in polymicrobial sepsis. Sci. Rep. 2016, 6, 37277. Available online: https://www.nature.com/articles/srep37277 (accessed on 24 July 2025). [CrossRef]
- Morse, J.C.; Huang, J.; Khona, N.; Miller, E.J.; Siwik, D.A.; Colucci, W.S.; Hobai, I.A. Up-regulation of intracellular calcium handling underlies the recovery of endotoxemic cardiomyopathy in mice. Anesthesiology 2017, 126, 1125–1138. Available online: https://journals.lww.com/anesthesiology/fulltext/2017/06000/up_regulation_of_intracellular_calcium_handling.25.aspx (accessed on 4 July 2025). [CrossRef]
- Dobrev, D.; Wehrens, X.H.T. Calcium-mediated cellular triggered activity in atrial fibrillation. J. Physiol. 2017, 595, 4001. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5471363/ (accessed on 24 July 2025). [CrossRef]
- Durand, A.; Duburcq, T.; Dekeyser, T.; Neviere, R.; Howsam, M.; Favory, R.; Preau, S. Involvement of Mitochondrial Disorders in Septic Cardiomyopathy. Oxid. Med. Cell Longev. 2017, 2017, 4076348. Available online: https://onlinelibrary.wiley.com/doi/full/10.1155/2017/4076348 (accessed on 7 August 2024). [CrossRef] [PubMed]
- Stanzani, G.; Duchen, M.R.; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 759–773. Available online: https://pubmed.ncbi.nlm.nih.gov/30342158/ (accessed on 6 June 2025). [CrossRef] [PubMed]
- Carré, J.E.; Orban, J.-C.; Re, L.; Felsmann, K.; Iffert, W.; Bauer, M.; Suliman, H.B.; Piantadosi, C.A.; Mayhew, T.M.; Breen, P.; et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am. J. Respir. Crit. Care Med. 2010, 182, 745–751. Available online: https://www.atsjournals.org/doi/pdf/10.1164/rccm.201003-0326OC?download=true (accessed on 24 July 2025). [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. Available online: https://pubmed.ncbi.nlm.nih.gov/12133657/ (accessed on 28 July 2024). [CrossRef]
- Brealey, D.; Karyampudi, S.; Jacques, T.S.; Novelli, M.; Stidwill, R.; Taylor, V.; Smolenski, R.T.; Singer, M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R491–R497. Available online: https://pubmed.ncbi.nlm.nih.gov/14604843/ (accessed on 28 July 2024). [CrossRef]
- Shimada, B.K.; Boyman, L.; Huang, W.; Zhu, J.; Yang, Y.; Chen, F.; Kane, M.A.; Yadava, N.; Zou, L.; Lederer, W.J.; et al. Pyruvate-Driven Oxidative Phosphorylation is Downregulated in Sepsis-Induced Cardiomyopathy: A Study of Mitochondrial Proteome. Shock 2021, 57, 553. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8904652/ (accessed on 23 August 2025). [CrossRef] [PubMed]
- Yap, J.Q.; Nikouee, A.; Kim, M.; Cao, Q.; Rademacher, D.J.; Lau, J.E.; Arora, A.; Zou, L.Y.; Sun, Y.; Szweda, L.; et al. Myocardial pyruvate dehydrogenase kinase 4 drives sex-specific cardiac responses to endotoxemia. JCI Insight 2025, 10, e191649. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC12288905/ (accessed on 23 August 2025). [CrossRef]
- Levy, R.J.; Piel, D.A.; Acton, P.D.; Zhou, R.; Ferrari, V.A.; Karp, J.S.; Deutschman, C.S. Evidence of myocardial hibernation in the septic heart. Crit. Care Med. 2005, 33, 2752–2756. Available online: https://pubmed.ncbi.nlm.nih.gov/16352955/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Hobai, I.A. Cardiomyocyte Reprogramming in Animal Models of Septic Shock. Shock 2023, 59, 200–213. Available online: https://journals.lww.com/shockjournal/fulltext/2023/02000/cardiomyocyte_reprogramming_in_animal_models_of.9.aspx (accessed on 6 June 2025). [CrossRef] [PubMed]
- Gritte, R.B.; Souza-Siqueira, T.; Curi, R.; Machado, M.C.C.; Soriano, F.G. Why Septic Patients Remain Sick After Hospital Discharge? Front. Immunol 2021, 11, 605666. Available online: https://pubmed.ncbi.nlm.nih.gov/33658992/ (accessed on 28 July 2024). [CrossRef]
- Cavaillon, J.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. Available online: https://www.embopress.org/doi/full/10.15252/emmm.201810128 (accessed on 28 July 2024). [CrossRef]
- Singer, P.M.; De Santis, V.; Vitale, D.; Jeffcoate, W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inaflammation. Lancet 2004, 364, 545–548. Available online: https://www.thelancet.com/action/showFullText?pii=S0140673604168153 (accessed on 6 June 2025). [CrossRef]
- Fekete, C.; Lechan, R.M. Central Regulation of Hypothalamic-Pituitary-Thyroid Axis Under Physiological and Pathophysiological Conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Lourbopoulos, A.I.; Mourouzis, I.S.; Trikas, A.G.; Tseti, I.K.; Pantos, C.I. Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy. J. Clin. Med. 2021, 10, 5855. [Google Scholar] [CrossRef]
- Kim, J.; Arnaout, L.; Remick, D. Hydrocortisone, Ascorbic Acid, and Thiamine (HAT) Therapy Decreases Oxidative Stress, Improves Cardiovascular Function, and Improves Survival in Murine Sepsis. Shock 2020, 53, 460–467. Available online: https://pubmed.ncbi.nlm.nih.gov/31169765/ (accessed on 28 July 2024). [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-018-2292-6 (accessed on 28 July 2024). [CrossRef] [PubMed]
- Vellinga, N.A.R.; Boerma, E.C.; Koopmans, M.; Donati, A.; Dubin, A.; Shapiro, N.I.; Pearse, R.M.; Machado, F.R.; Fries, M.; Akarsu-Ayazoglu, T. International study on microcirculatory shock occurrence in acutely ill patients. Crit. Care Med. 2015, 43, 48–56. Available online: https://pubmed.ncbi.nlm.nih.gov/25126880/ (accessed on 28 July 2024). [CrossRef]
- Hollenberg, S.M. Think locally: Evaluation of the microcirculation in sepsis. Intensive Care Med. 2010, 36, 1807–1809. Available online: https://pubmed.ncbi.nlm.nih.gov/20725822/ (accessed on 28 July 2024). [CrossRef][Green Version]
- Cusack, R.; Leone, M.; Rodriguez, A.H.; Martin-Loeches, I. Endothelial Damage and the Microcirculation in Critical Illness. Biomedical 2022, 10, 3150. [Google Scholar] [CrossRef]
- Farquhar, I.; Martin, C.M.; Lam, C.; Potter, R.; Ellis, C.G.; Sibbald, W.J. Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J. Surg. Res. 1996, 61, 190–196. Available online: http://www.journalofsurgicalresearch.com/article/S0022480496901031/fulltext (accessed on 9 August 2024). [CrossRef]
- De Backer, D.; Creteur, J.; Preiser, J.C.; Dubois, M.J.; Vincent, J.L. Microvascular blood flow is altered in patients with sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 98–104. Available online: https://pubmed.ncbi.nlm.nih.gov/12091178/ (accessed on 8 August 2024). [CrossRef] [PubMed]
- Höcherl, K.; Schmidt, C.; Kurt, B.; Bucher, M. Activation of the PGI(2)/IP system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension 2008, 52, 330–335. Available online: https://pubmed.ncbi.nlm.nih.gov/18606903/ (accessed on 8 August 2024). [CrossRef]
- Landry, D.W.; Oliver, J.A. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J. Clin. Investig. 1992, 89, 2071–2074. Available online: https://pubmed.ncbi.nlm.nih.gov/1602014/ (accessed on 8 August 2024). [CrossRef] [PubMed]
- Croner, R.S.; Hoerer, E.; Kulu, Y.; Hackert, T.; Gebhard, M.M.; Herfarth, C.; Klar, E. Hepatic platelet and leukocyte adherence during endotoxemia. Crit. Care 2006, 10, R15. Available online: https://pubmed.ncbi.nlm.nih.gov/16420661/ (accessed on 8 August 2024). [CrossRef]
- Trzeciak, S.; Dellinger, R.P.; Parrillo, J.E.; Guglielmi, M.; Bajaj, J.; Abate, N.L.; Arnold, R.C.; Colilla, S.; Zanotti, S.; Hollenberg, S.M. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann. Emerg. Med. 2007, 49, 88–98.e2. Available online: http://www.annemergmed.com/article/S019606440602141X/fulltext (accessed on 9 August 2024). [CrossRef]
- Sakr, Y.; Dubois, M.J.; De Backer, D.; Creteur, J.; Vincent, J.L. Persistent-microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004, 32, 1825–1831. Available online: https://journals.lww.com/ccmjournal/fulltext/2004/09000/persistent_microcirculatory_alterations_are.2.aspx (accessed on 9 August 2024). [CrossRef]
- Kanoore Edul, V.S.; Ince, C.; Vazquez, A.R.; Rubatto, P.N.; Valenzuela Espinoza, E.D.; Welsh, S.; Enrico, C.; Dubin, A. Similar microcirculatory alterations in patients with normodynamic and hyperdynamic septic shock. Ann. Am. Thorac. Soc. 2015, 13, 240–247. Available online: https://www.atsjournals.org/doi/10.1513/AnnalsATS.201509-606OC (accessed on 9 August 2024). [CrossRef] [PubMed]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.L. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. Available online: https://journals.lww.com/ccmjournal/fulltext/2013/03000/microcirculatory_alterations_in_patients_with.11.aspx (accessed on 9 August 2024). [CrossRef] [PubMed]
- Beurskens, D.M.H.; Bol, M.E.; Delhaas, T.; van de Poll, M.C.G.; Reutelingsperger, C.P.M.; Nicolaes, G.A.F.; Sels, J.E. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth. Intensive Care 2020, 48, 221–228. Available online: https://sage.cnpereading.com/paragraph/article/?doi=10.1177/0310057X20916471 (accessed on 9 August 2024). [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, E1063–E1143. Available online: https://journals.lww.com/ccmjournal/fulltext/2021/11000/surviving_sepsis_campaign__international.21.aspx (accessed on 28 July 2024). [CrossRef]
- Østergaard, L.; Granfeldt, A.; Secher, N.; Tietze, A.; Iversen, N.K.; Jensen, M.S.; Andersen, K.K.; Nagenthiraja, K.; Gutiérrez-Lizardi, P.; Mouridsen, K.; et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol. Scand. 2015, 59, 1246–1259. Available online: https://pubmed.ncbi.nlm.nih.gov/26149711/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Vincent, J.L.; De Backer, D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit. Care 2005, 9 (Suppl. 4), S9–S12. Available online: https://pubmed.ncbi.nlm.nih.gov/16168075/ (accessed on 28 July 2024). [CrossRef]
- De Backer, D.; Creteur, J.; Dubois, M.J.; Sakr, Y.; Koch, M.; Verdant, C.; Vincent, J.L. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit. Care Med. 2006, 34, 403–408. Available online: https://journals.lww.com/ccmjournal/fulltext/2006/02000/the_effects_of_dobutamine_on_microcirculatory.18.aspx (accessed on 9 August 2024). [CrossRef]
- Pottecher, J.; Deruddre, S.; Teboul, J.L.; Georger, J.F.; Laplace, C.; Benhamou, D.; Vicaut, E.; Duranteau, J. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010, 36, 1867–1874. Available online: https://link.springer.com/article/10.1007/s00134-010-1966-6 (accessed on 9 August 2024). [CrossRef]
- Pranskunas, A.; Koopmans, M.; Koetsier, P.M.; Pilvinis, V.; Boerma, E.C. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013, 39, 612–619. Available online: https://link.springer.com/article/10.1007/s00134-012-2793-8 (accessed on 9 August 2024). [CrossRef]
- Potter, E.K.; Hodgson, L.; Creagh-Brown, B.; Forni, L.G. Manipulating the Microcirculation in Sepsis—The Impact of Vasoactive Medications on Microcirculatory Blood Flow: A Systematic Review. Shock 2019, 52, 5–12. Available online: https://journals.lww.com/shockjournal/fulltext/2019/07000/manipulating_the_microcirculation_in_sepsis___the.2.aspx (accessed on 9 August 2024). [CrossRef] [PubMed]
- Joffre, J.; Hellman, J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Singh, J.; Lee, Y.; Kellum, J.A. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit. Care 2022, 26, 246. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-022-04075-0 (accessed on 8 August 2024). [CrossRef] [PubMed]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial responses in sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. Available online: https://www.atsjournals.org/doi/10.1164/rccm.201910-1911TR (accessed on 9 August 2024). [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The endothelium in sepsis. Shock 2016, 45, 259–270. Available online: https://journals.lww.com/shockjournal/fulltext/2016/03000/the_endothelium_in_sepsis.5.aspx (accessed on 26 August 2024). [CrossRef]
- Smart, L.; Bosio, E.; Macdonald, S.P.J.; Dull, R.; Fatovich, D.M.; Neil, C.; Arendts, G. Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J. Crit. Care 2018, 47, 93–98. Available online: https://pubmed.ncbi.nlm.nih.gov/29936329/ (accessed on 9 August 2024). [CrossRef] [PubMed]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. Available online: https://pubmed.ncbi.nlm.nih.gov/32437596/ (accessed on 9 August 2024). [CrossRef]
- Reines, H.D.; Cook, J.A.; Halushka, P.V.; Wise, W.C.; Rambo, W. Plasma thromboxane concentrations are raised in patients dying with septic shock. Lancet 1982, 2, 174–175. Available online: https://pubmed.ncbi.nlm.nih.gov/6123885/ (accessed on 26 August 2024). [CrossRef]
- Yang, L.L.; Gros, R.; Kabir, M.G.; Sadi, A.; Gotlieb, A.I.; Husain, M.; Stewart, D.J. Conditional Cardiac Overexpression of Endothelin-1 Induces Inflammation and Dilated Cardiomyopathy in Mice. Circulation 2004, 109, 255–261. Available online: https://www.ahajournals.org/doi/10.1161/01.CIR.0000105701.98663.D4 (accessed on 26 August 2024). [CrossRef]
- Rinaldi, I.; Sudaryo, M.K.; Prihartono, N.A. Disseminated Intravascular Coagulation in Sepsis and Associated Factors. J. Clin. Med. 2022, 11, 6480. [Google Scholar] [CrossRef]
- Velissaris, D.; Karamouzos, V.; Paraskevas, T.; Velissari, E.K.; Pierrakos, C.; Michailides, C. Neutrophil Extracellular Traps in the Prognosis of Sepsis: A Current Update. Medicine. 2025, 61, 1145. [Google Scholar] [CrossRef]
- Zeng, M.; Niu, Y.; Huang, J.; Deng, L. Advances in neutrophil extracellular traps and ferroptosis in sepsis-induced cardiomyopathy. Front. Immunol. 2025, 16, 1590313. Available online: https://pubmed.ncbi.nlm.nih.gov/40356926/ (accessed on 22 July 2025). [CrossRef]
- Krüttgen, A.; Rose-John, S. Interleukin-6 in sepsis and capillary leakage syndrome. J. Interf. Cytokine Res. 2012, 32, 60–65. Available online: https://pubmed.ncbi.nlm.nih.gov/22136372/ (accessed on 24 July 2025). [CrossRef]
- Vasques-Nóvoa, F.; Laundos, T.L.; Madureira, A.; Bettencourt, N.; Nunes, J.P.; Carneiro, F.; Paiva, J.A.; Pinto-Do-Ó, P.; Nascimento, D.S.; Leite-Moreira, A.F.; et al. Myocardial edema: An overlooked mechanism of septic cardiomyopathy? Shock 2020, 53, 616–619. Available online: https://journals.lww.com/shockjournal/fulltext/2020/05000/myocardial_edema__an_overlooked_mechanism_of.13.aspx (accessed on 7 August 2024). [CrossRef] [PubMed]
- Miyamoto, M.; McClure, D.E.; Schertel, E.R.; Andrews, P.J.; Jones, G.A.; Pratt, J.W.; Ross, P.; Myerowitz, P.D. Effects of hypoproteinemia-induced myocardial edema on left ventricular function. Am. J. Physiol—Heart Circ. Physiol. 1998, 274, H937–H944. Available online: https://journals.physiology.org/doi/pdf/10.1152/ajpheart.1998.274.3.H937 (accessed on 24 July 2025). [CrossRef] [PubMed]
- Pogátsa, G.; Dubecz, E.; Gábor, G. The role of myocardial edema in the left ventricular diastolic stiffness. Basic Res. Cardiol. 1976, 71, 263–269. Available online: https://pubmed.ncbi.nlm.nih.gov/938438/ (accessed on 24 July 2025). [CrossRef]
- Weng, Z.-C.; Nicolosi, A.C.; Detwiler, P.W.; Hsu, D.T.; Schierman, S.W.; Goldstein, A.H.; Spotnitz, H.M. Effects of crystalloid, blood, and University of Wisconsin perfusates on weight, water content, and left ventricular compliance in an edema-prone, isolated porcine heart model. J. Thorac. Cardiovasc. Surg. 1992, 103, 504–513. Available online: https://www.sciencedirect.com/science/article/pii/S002252231934992X (accessed on 24 July 2025). [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. [Google Scholar] [CrossRef]
- Pierucci, N.; Marianim, V.; Iannetti, G.; Maffei, L.; Coluccio, A.; Laviola, D.; Palombi, M.; Trivigno, S.; Spadafora, L.; Chourda, E.; et al. Atrial cardiomyopathy: New pathophysiological and clinical aspects. Minerva. Cardiol. Angiol. 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/40548651/ (accessed on 20 August 2025).
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial cardiomyopathy revisited—Evolution of a concept: A clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). EP Eur. 2024, 26, euae204. [Google Scholar] [CrossRef]
- Desai, R.; Hanna, B.; Singh, S.; Omar, A.; Deshmukh, A.; Kumar, G.; Foreman, M.G.; Sachdeva, R. Trends and Outcomes in Sepsis Hospitalizations With and Without Atrial Fibrillation: A Nationwide Inpatient Analysis. Crit. Care Med. 2019, 47, E630–E638. Available online: https://journals.lww.com/ccmjournal/fulltext/2019/08000/trends_and_outcomes_in_sepsis_hospitalizations.29.aspx (accessed on 27 June 2025). [CrossRef]
- Meierhenrich, R.; Steinhilber, E.; Eggermann, C.; Weiss, M.; Voglic, S.; Bögelein, D.; Gauss, A.; Georgieff, M.; Stahl, W. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: A prospective observational study. Crit. Care 2010, 14, R108. Available online: https://ccforum.biomedcentral.com/articles/10.1186/cc9057 (accessed on 27 June 2025). [CrossRef]
- Klouwenberg, P.M.C.K.; Frencken, J.F.; Kuipers, S.; Ong, D.S.Y.; Peelen, L.M.; van Vught, L.A.; Schultz, M.J.; van der Poll, T.; Bonten, M.J.; Cremer, O.L. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis a cohort study. Am. J. Respir. Crit. Care Med. 2017, 195, 205–211. Available online: https://pubmed.ncbi.nlm.nih.gov/27467907/ (accessed on 27 June 2025). [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Karakasis, P.; Tzeis, S.; Pamporis, K.; Schuermans, A.; Theofilis, P.; Milaras, N.; Tsiachris, D.; Efremidis, M.; Antoniadis, A.P.; Fragakis, N. Impact of catheter ablation timing according to duration of atrial fibrillation history on arrhythmia recurrences and clinical outcomes: A meta-analysis. EP Eur. 2025, 27, 110. [Google Scholar] [CrossRef]
- Kariki, O.; Saplaouras, A.; Mililis, P.; Pamporis, K.; Efremidis, T.; Dragasis, S.; Martinos, A.; Miliopoulos, D.; Letsas, K.P.; Efremidis, M. Atrial Fibrillation in the Young: Catheter Ablation Efficacy Investigated in a Propensity-Score Matched Cohort. J. Cardiovasc. Electrophysiol. 2025, 36, 1752–1761. Available online: https://pubmed.ncbi.nlm.nih.gov/40457951/ (accessed on 20 August 2025). [CrossRef] [PubMed]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of asymptomatic atrial fibrillation and risk factors associated with asymptomatic status: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2025, zwaf138. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major clinical outcomes in symptomatic vs. asymptomatic atrial fibrillation: A meta-analysis. Eur. Heart J. 2024, 46, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Sagris, M.; Pamporis, K.; Stachteas, P.; Sidiropoulos, G.; Vlachakis, P.K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Artificial Intelligence in Atrial Fibrillation: From Early Detection to Precision Therapy. J. Clin. Med. 2025, 14, 2627. [Google Scholar] [CrossRef] [PubMed]
- Leoni, D.; Rello, J. Cardiac arrest among patients with infections: Causes, clinical practice and research implications. Clin. Microbiol. Infect. 2017, 23, 730–735. Available online: https://pubmed.ncbi.nlm.nih.gov/27903458/ (accessed on 22 July 2025). [CrossRef] [PubMed]
- Lazzerini, P.E.; Acampa, M.; Laghi-Pasini, F.; Bertolozzi, I.; Finizola, F.; Vanni, F.; Natale, M.; Bisogno, S.; Cevenini, G.; Cartocci, A.; et al. Cardiac Arrest Risk During Acute Infections: Systemic Inflammation Directly Prolongs QTc Interval via Cytokine-Mediated Effects on Potassium Channel Expression. Circ. Arrhythmia Electrophysiol. 2020, 13, E008627. Available online: https://pubmed.ncbi.nlm.nih.gov/32654514/ (accessed on 27 June 2025). [CrossRef]
- Engelmann, M.D.M.; Svendsen, J.H. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur. Heart J. 2005, 26, 2083–2092. [Google Scholar] [CrossRef]
- Haj Ali, L.; Suhov, L.; Apostol, A.; Dăniluc, L.; Sandu, O.; Bogdan, C.; Ivan, V.M. The Risk of Arrhythmias in Patients with COVID-19. Biomedical 2025, 13, 1368. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. Available online: https://pubmed.ncbi.nlm.nih.gov/9971870/ (accessed on 6 July 2025). [CrossRef]
- Fabre, A.; Sheppard, M.N. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 2006, 92, 316–320. Available online: https://pubmed.ncbi.nlm.nih.gov/15923280/ (accessed on 22 July 2025). [CrossRef]
- Karakasis, P.; Pamporis, K.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Grigoriou, K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N. Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. Int. J. Mol. Sci. 2025, 26, 5954. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. Available online: https://pubmed.ncbi.nlm.nih.gov/20303873/ (accessed on 24 July 2025). [CrossRef]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. Available online: https://pubmed.ncbi.nlm.nih.gov/29802206/ (accessed on 24 July 2025). [CrossRef] [PubMed]
- Luan, Y.; Guo, Y.; Li, S.; Yu, B.; Zhu, S.; Li, N.; Tian, Z.; Peng, C.; Cheng, J.; Li, Q.; et al. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace 2010, 12, 1713–1718. Available online: https://pubmed.ncbi.nlm.nih.gov/20833691/ (accessed on 24 July 2025). [CrossRef]
- Gungor, B.; Ekmekci, A.; Arman, A.; Ozcan, K.S.; Ucer, E.; Alper, A.T.; Calik, N.; Yilmaz, H.; Tezel, T.; Coker, A.; et al. Assessment of Interleukin-1 Gene Cluster Polymorphisms in Lone Atrial Fibrillation: New Insight into the Role of Inflammation in Atrial Fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Grigoriou, K.; Karakasis, P.; Pamporis, K.; Theofilis, P.; Patoulias, D.; Karagiannidis, E.; Fyntanidou, B.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrillation in Diabetes: Pathogenesis and Targeted Rhythm Control Strategies. Curr. Issues Mol. Biol. 2025, 47, 559. [Google Scholar] [CrossRef]
- El-Sherif, N.; Turitto, G. Electrolyte disorders and arrhythmogenesis. Cardiol. J. 2011, 18, 233–245. Available online: https://journals.viamedica.pl/cardiology_journal/article/view/21240 (accessed on 22 July 2025). [PubMed]
- Lu, Y.Y.; Cheng, C.C.; Chen, Y.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Electrolyte disturbances differentially regulate sinoatrial node and pulmonary vein electrical activity: A contribution to hypokalemia- or hyponatremia-induced atrial fibrillation. Heart Rhythm. 2016, 13, 781–788. Available online: https://www.heartrhythmjournal.com/action/showFullText?pii=S1547527115015180 (accessed on 27 June 2025). [CrossRef] [PubMed]
- Krijthe, B.P.; Heeringa, J.; Kors, J.A.; Hofman, A.; Franco, O.H.; Witteman, J.C.M.; Stricker, B.H. Serum potassium levels and the risk of atrial fibrillation: The Rotterdam Study. Int. J. Cardiol. 2013, 168, 5411–5415. Available online: https://www.internationaljournalofcardiology.com/action/showFullText?pii=S0167527313015982 (accessed on 27 June 2025). [CrossRef]
- Lee, J.W. Fluid and Electrolyte Disturbances in Critically Ill Patients. Electrolytes Blood Press. E BP 2010, 8, 72. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3043756/ (accessed on 29 July 2025). [CrossRef]
- Misialek, J.R.; Lopez, F.L.; Lutsey, P.L.; Huxley, R.R.; Peacock, J.M.; Chen, L.Y.; Soliman, E.Z.; Agarwal, S.K.; Alonso, A. Serum and Dietary Magnesium and Incidence of Atrial Fibrillation in Whites and in African Americans—Atherosclerosis Risk in Communities (ARIC) Study. Circ. J. 2013, 77, 323–329. Available online: https://pubmed.ncbi.nlm.nih.gov/23047297/ (accessed on 27 June 2025). [CrossRef]
- Cecchi, E.; Grossi, F.; Rossi, M.; Giglioli, C.; Laura De Feo, M. Severe hypocalcemia and life-threatening ventricular arrhytmias: Case report and proposal of a diagnostic and therapeutic algorithm. Clin. Cases Min. Bone Metab. 2015, 12, 265. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4708975/ (accessed on 29 July 2025). [CrossRef]
- Larsson, S.C.; Drca, N.; Michaëlsson, K. Serum Magnesium and Calcium Levels and Risk of Atrial Fibrillation: A Mendelian Randomization Study. Circ. Genomic Precis. Med. 2019, 12, E002349. Available online: https://pubmed.ncbi.nlm.nih.gov/30645173/ (accessed on 29 July 2025). [CrossRef]
- Mihatov, N.; Januzzi, J.L.; Gaggin, H.K. Type 2 myocardial infarction due to supply–demand mismatch. Trends Cardiovasc. Med. 2017, 27, 408–417. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1050173817300427 (accessed on 22 July 2025). [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. Available online: https://www.thelancet.com/action/showFullText?pii=S1473309909703317 (accessed on 22 July 2025). [CrossRef]
- Shah, M.; Patnaik, S.; Maludum, O.; Patel, B.; Tripathi, B.; Agarwal, M.; Garg, L.; Agrawal, S.; Jorde, U.P.; Martinez, M.W. Mortality in sepsis: Comparison of outcomes between patients with demand ischemia, acute myocardial infarction, and neither demand ischemia nor acute myocardial infarction. Clin. Cardiol. 2018, 41, 936. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6489770/ (accessed on 22 July 2025). [CrossRef] [PubMed]
- Roué, M.; Guédon, A.F.; Lapidus, N.; Razazi, K.; Hariri, G.; Morawiec, E.; Desnos, C.; Ederhy, S.; Cohen, A.; Dessap, A.M.; et al. In-hospital outcomes after acute myocardial infarction with obstructive coronary artery disease in critically ill patients hospitalized for non-cardiac disease. Ann. Intensive Care 2023, 13, 87. Available online: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-023-01188-9 (accessed on 4 July 2025). [CrossRef] [PubMed]
- Gorenek, B.; Lundqvist, C.B.; Terradellas, J.B.; Camm, A.J.; Hindricks, G.; Huber, K.; Kirchhof, P.; Kuck, K.-H.; Kudaiberdieva, G.; Lin, T.; et al. Cardiac arrhythmias in acute coronary syndromes: Position paper from the joint EHRA, ACCA, and EAPCI task force. Europace 2014, 16, 1655–1673. Available online: https://pubmed.ncbi.nlm.nih.gov/25172845/ (accessed on 22 July 2025). [CrossRef]
- Janse, M.J.; Wit, A.L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 1989, 69, 1049–1169. Available online: https://pubmed.ncbi.nlm.nih.gov/2678165/ (accessed on 22 July 2025). [CrossRef]
- Said, M.; Becerra, R.; Valverde, C.; Kaetzel, M.; Dedman, J.; Mundiña-Weilenmann, C.; Wehrens, X.; Vittone, L.; Mattiazzi, A. Calcium-calmodulin dependent protein kinase II (CaMKII): A main signal responsible for early reperfusion arrhythmias. J. Mol. Cell. Cardiol. 2011, 51, 936–944. Available online: https://www.jmcc-online.com/action/showFullText?pii=S0022282811003300 (accessed on 22 July 2025). [CrossRef] [PubMed]
- Mitcheson, J.S.; Chen, J.; Lin, M.; Culberson, C.; Sanguinetti, M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 12329–12333. Available online: https://pubmed.ncbi.nlm.nih.gov/11005845/ (accessed on 22 July 2025). [CrossRef]
- Kallergis, E.M.; Goudis, C.A.; Simantirakis, E.N.; Kochiadakis, G.E.; Vardas, P.E. Mechanisms, Risk Factors, and Management of Acquired Long QT Syndrome: A Comprehensive Review. Sci. World J. 2012, 2012, 212178. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3347892/ (accessed on 27 June 2025). [CrossRef]
- Liu, W.; Shao, R.; Zhang, S.; Jin, L.; Liu, R.; Chen, P.; Hu, J.; Ma, H.; Wu, B.; Liang, W.; et al. Characteristics, predictors and outcomes of new-onset QT prolongation in sepsis: A multicenter retrospective study. Crit. Care 2024, 28, 115. Available online: https://ccforum.biomedcentral.com/articles/10.1186/s13054-024-04879-2 (accessed on 27 June 2025). [CrossRef]
- Li, D.; Weng, Y.; Zhen, G.; Jiang, L. Tp-Te Interval and Tp-Te/QT Ratio Are Valuable Tools in Predicting Poor Outcome in Sepsis Patients. Front. Cardiovasc. Med. 2022, 9, 879085. Available online: https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.879085/full (accessed on 4 July 2025). [CrossRef]
- Lackner, I.; Weber, B.; Chakraborty, S.; Braumüller, S.; Huber-Lang, M.; Gebhard, F.; Kalbitz, M. Toll-Like Receptor-Mediated Cardiac Injury during Experimental Sepsis. Mediat. Inflamm. 2020, 2020, 6051983. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7199613/ (accessed on 23 August 2025). [CrossRef]
- Celes, M.R.N.; Torrez-Dueñas, D.; Alves-Filho, J.C.; Duarte, D.B.; Cunha, F.Q.; Rossi, M.A. Reduction of gap junction proteins and intercalated disc structural remodeling in the hearts of mice submitted to sepsis. Crit. Care 2007, 11 (Suppl. 4), P45. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3300685/ (accessed on 23 August 2025). [CrossRef][Green Version]
- Adler, A.; Topaz, G.; Heller, K.; Zeltser, D.; Ohayon, T.; Rozovski, U.; Halkin, A.; Rosso, R.; Ben-Shachar, S.; Antzelevitch, C.; et al. Fever-induced Brugada pattern: How common is it and what does it mean? Heart Rhythm. 2013, 10, 1375–1382. Available online: https://www.heartrhythmjournal.com/action/showFullText?pii=S1547527113007741 (accessed on 4 July 2025). [CrossRef] [PubMed]
- Keller, D.; Rougier, J.; Kucera, J.; Benammar, N.; Fressart, V.; Guicheney, P.; Madle, A.; Fromer, M.; Schlapfer, J.; Abriel, H. Brugada syndrome and fever: Genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc. Res. 2005, 67, 510–519. Available online: https://pubmed.ncbi.nlm.nih.gov/15890323/ (accessed on 22 July 2025). [CrossRef] [PubMed]
- Wilde, A.A.; Postema, P.G.; Di Diego, J.M.; Viskin, S.; Morita, H.; Fish, J.M.; Antzelevitch, C. The pathophysiological mechanism underlying Brugada syndrome. Depolarization versus repolarization. J. Mol. Cell. Cardiol. 2010, 49, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Pamporis, K.; Antoniadis, A.P.; Fragakis, N. Catheter Ablation vs. Standard Implantable Cardioverter Defibrillator Therapy in Symptomatic Brugada Syndrome: A Systematic Review and Meta-Analysis of Controlled Studies. Med. Sci. 2025, 13, 115. [Google Scholar] [CrossRef]
- Nadeem, R.; Sockanathan, S.; Singh, M.; Hussain, T.; Kent, P.; AbuAlreesh, S. Impact of dobutamine in patients with septic shock: A meta-regression analysis. Am. J. Ther. 2017, 24, e333–e346. Available online: https://journals.lww.com/americantherapeutics/fulltext/2017/05000/impact_of_dobutamine_in_patients_with_septic.12.aspx (accessed on 8 August 2024). [CrossRef]
- Wilkman, E.; Kaukonen, K.M.; Pettilä, V.; Kuitunen, A.; Varpula, M. Association between inotrope treatment and 90-day mortality in patients with septic shock. Acta Anaesthesiol. Scand. 2013, 57, 431–442. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/aas.12056 (accessed on 8 August 2024). [CrossRef]
- Hernandez, G.; Bruhn, A.; Luengo, C.; Regueira, T.; Kattan, E.; Fuentealba, A.; Florez, J.; Castro, R.; Aquevedo, A.; Pairumani, R.; et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: A randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013, 39, 1435–1443. Available online: https://link.springer.com/article/10.1007/s00134-013-2982-0 (accessed on 8 August 2024). [CrossRef]
- Gattinoni, L.; Brazzi, L.; Pelosi, P.; Latini, R.; Tognoni, G.; Pesenti, A.; Fumagalli, R. A Trial of Goal-Oriented Hemodynamic Therapy in Critically Ill Patients. N. Engl. J. Med. 1995, 333, 1025–1032. Available online: https://www.nejm.org/doi/full/10.1056/NEJM199510193331601 (accessed on 8 August 2024). [CrossRef]
- Oba, Y.; Lone, N.A. Mortality benefit of vasopressor and inotropic agents in septic shock: A Bayesian network meta-analysis of randomized controlled trials. J. Crit. Care. 2014, 29, 706–710. [Google Scholar] [CrossRef]
- Belletti, A.; Benedetto, U.; Biondi-Zoccai, G.; Leggieri, C.; Silvani, P.; Angelini, G.D.; Zangrillo, A.; Landoni, G. The effect of vasoactive drugs on mortality in patients with severe sepsis and septic shock. A network meta-analysis of randomized trials. J. Crit. Care. 2017, 37, 91–98. [Google Scholar] [CrossRef]
- Morelli, A.; De Castro, S.; Teboul, J.-L.; Singer, M.; Rocco, M.; Conti, G.; De Luca, L.; Di Angelantonio, E.; Orecchioni, A.; Pandian, N.G.; et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005, 31, 638–644. Available online: https://link.springer.com/article/10.1007/s00134-005-2619-z (accessed on 4 July 2025). [CrossRef] [PubMed]
- Morelli, A.; Teboul, J.-L.; Maggiore, S.M.; Vieillard-Baron, A.; Rocco, M.; Conti, G.; De Gaetano, A.; Picchini, U.; Orecchioni, A.; Carbone, I.; et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: A pilot study. Crit. Care Med. 2006, 34, 2287–2293. Available online: https://journals.lww.com/ccmjournal/fulltext/2006/09000/effects_of_levosimendan_on_right_ventricular.3.aspx (accessed on 4 July 2025). [CrossRef] [PubMed]
- Gordon, A.C.; Perkins, G.D.; Singer, M.; McAuley, D.F.; Orme, R.M.; Santhakumaran, S.; Mason, A.J.; Cross, M.; Al-Beidh, F.; Best-Lane, J.; et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N. Engl. J. Med. 2016, 375, 1638–1648. Available online: https://www.nejm.org/doi/pdf/10.1056/NEJMoa1609409 (accessed on 4 July 2025). [CrossRef]
- Antcliffe, D.B.; Santhakumaran, S.; Orme, R.M.L.; Ward, J.K.; Al-Beidh, F.; O’dea, K.; Perkins, G.D.; Singer, M.; McAuley, D.F.; Mason, A.J.; et al. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: A subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med. 2019, 45, 1392–1400. Available online: https://link.springer.com/article/10.1007/s00134-019-05731-w (accessed on 4 July 2025). [CrossRef]
- Hasegawa, D.; Sato, R.; Prasitlumkum, N.; Nishida, K.; Takahashi, K.; Yatabe, T.; Nishida, O. Effect of Ultrashort-Acting β-Blockers on Mortality in Patients With Sepsis With Persistent Tachycardia Despite Initial Resuscitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest 2021, 159, 2289–2300. Available online: https://pubmed.ncbi.nlm.nih.gov/33434497/ (accessed on 8 August 2024). [CrossRef]
- Sato, R.; Messina, S.; Hasegawa, D.; Santonocito, C.; Scimonello, G.; Sanfilippo, G.; Morelli, A.; Dugar, S.; Sanfilippo, F. Mortality in Patients With Sepsis Treated With Esmolol or Landiolol: A Systematic Review and Meta-Analysis of Randomized Controlled Trials With Trial Sequential Analysis. Chest 2025, 167, 121–138. Available online: https://pubmed.ncbi.nlm.nih.gov/39197514/ (accessed on 6 July 2025). [CrossRef]
- Su, W.-L.; Chiu, S.-K.; Shen, C.-H.; Chen, Y.-T.; Lazar, A. Recent Data about the Use of Corticosteroids in Sepsis—Review of Recent Literature. Biomedical 2024, 12, 984. Available online: https://www.mdpi.com/2227-9059/12/5/984/htm (accessed on 22 July 2025).
- Bernard, G.R.; Francois, B.; Mira, J.-P.; Vincent, J.-L.; Dellinger, R.P.; Russell, J.A.; LaRosa, S.P.; Laterre, P.-F.; Levy, M.M.; Dankner, W.; et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α Fragment antibody AZD9773 in Adult patients with severe sepsis and/or septic shock: Randomized, double-blind, placebo-controlled phase IIb Study. Crit. Care Med. 2014, 42, 504–511. Available online: https://pubmed.ncbi.nlm.nih.gov/24335445/ (accessed on 20 August 2025). [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated with Reduced Mortality in Sepsis Patients with Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. Available online: https://pubmed.ncbi.nlm.nih.gov/26584195/ (accessed on 20 August 2025). [CrossRef] [PubMed]
- Leong, K.; Gaglani, B.; Khanna, A.K.; McCurdy, M.T. Novel Diagnostics and Therapeutics in Sepsis. Biomedical 2021, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; van der Poll, T.; Marshall, J.C. The End of “One Size Fits All” Sepsis Therapies: Toward an Individualized Approach. Biomedical 2022, 10, 2260. [Google Scholar] [CrossRef]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.-L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. Available online: https://link.springer.com/article/10.1007/s00134-019-05596-z (accessed on 4 July 2025). [CrossRef]
- Nguyen, L.S.; Squara, P. Non-Invasive Monitoring of Cardiac Output in Critical Care Medicine. Front. Med. 2017, 4, 200. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5715400/ (accessed on 20 August 2025). [CrossRef] [PubMed]
- Zaki, M.S.M.; Kassem, A.A.A.; Mohamed, R.H.A.; Guirguis, N.N.M. Non-invasive Hemodynamic Monitoring in Prediction of the Outcome and Prognosis in Sepsis. QJM An. Int. J. Med. 2020, 113 (Suppl. 1), hcaa039.078. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’Ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Devel Ther. 2015, 9, 3405. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4492638/ (accessed on 23 July 2025). [CrossRef]
- Pei XBin Liu, B. Research Progress on the Mechanism and Management of Septic Cardiomyopathy: A Comprehensive Review. Emerg. Med. Int. 2023, 2023, 8107336. Available online: https://pubmed.ncbi.nlm.nih.gov/38029224/ (accessed on 23 June 2025). [CrossRef]
- Kim, W.Y.; Jung, J.W.; Choi, J.C.; Shin, J.W.; Kim, J.Y. Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis. Nutrients 2019, 11, 2976. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, Q.; Zhong, J.; Xia, F.; Zheng, S.; Lu, J.; Deng, Y.; Hu, Y. Combination of melatonin and irisin ameliorates lipopolysaccharide-induced cardiac dysfunction through suppressing the Mst1–JNK pathways. J. Cell Physiol. 2020, 235, 6647–6659. Available online: https://pubmed.ncbi.nlm.nih.gov/31976559/ (accessed on 23 June 2025). [CrossRef]
- Zhen, G.; Liang, W.; Jia, H.; Zheng, X. Melatonin relieves sepsis-induced myocardial injury via regulating JAK2/STAT3 signaling pathway. Minerva Med. 2022, 113, 983–989. Available online: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2022N06A0983 (accessed on 23 June 2025). [CrossRef]
- Kim, E.H.; Wong, S.W.; Martinez, J. Programmed Necrosis and Disease:We interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 2019, 26, 25–40. Available online: https://pubmed.ncbi.nlm.nih.gov/30349078/ (accessed on 23 June 2025). [CrossRef]
- Sallinen, P.; Mänttäri, S.; Leskinen, H.; Ilves, M.; Vakkuri, O.; Ruskoaho, H.; Saarela, S. The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J. Pineal Res. 2007, 42, 254–260. Available online: https://pubmed.ncbi.nlm.nih.gov/17349023/ (accessed on 23 June 2025). [CrossRef]
- Taha, A.M.; Mahmoud, A.M.; Ghonaim, M.M.; Kamran, A.; AlSamhori, J.F.; AlBarakat, M.M.; Shrestha, A.B.; Jaiswal, V.; Reiter, R.J. Melatonin as a potential treatment for septic cardiomyopathy. Biomed. Pharmacother. 2023, 166, 115305. Available online: https://pubmed.ncbi.nlm.nih.gov/37619482/ (accessed on 23 June 2025). [CrossRef] [PubMed]
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res. Cardiol. 2015, 111, 8. Available online: https://link.springer.com/article/10.1007/s00395-015-0526-1 (accessed on 7 September 2025). [CrossRef] [PubMed]
- Wei, Z.; Chen, Z.; Zhao, Y.; Fan, F.; Xiong, W.; Song, S.; Yin, Y.; Hu, J.; Yang, K.; Yang, L.; et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials 2021, 275, 121000. Available online: https://pubmed.ncbi.nlm.nih.gov/34218049/ (accessed on 23 June 2025). [CrossRef]
- Gupta, D.; Wiklander, O.P.B.; Görgens, A.; Conceição, M.; Corso, G.; Liang, X.; Seow, Y.; Balusu, S.; Feldin, U.; Bostancioglu, B.; et al. Amelioration of systemic inflammation via the display of two different decoy protein receptors on extracellular vesicles. Nat. Biomed. Eng. 2021, 5, 1084–1098. Available online: https://pubmed.ncbi.nlm.nih.gov/34616047/ (accessed on 23 June 2025). [CrossRef]
- Gupta, D.; Wiklander, O.P.B.; Görgens, A.; Conceição, M.; Corso, G.; Liang, X.; Seow, Y.; Balusu, S.; Feldin, U.; Bostancioglu, B.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. Available online: https://pubmed.ncbi.nlm.nih.gov/30613290/ (accessed on 23 June 2025).
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. Available online: https://pubmed.ncbi.nlm.nih.gov/28158410/ (accessed on 23 June 2025). [CrossRef]
- Kwon, H.; Yang, S.; Lee, J.; Park, B.; Park, K.; Jung, J.; Bae, Y.; Park, G. Combination treatment with human adipose tissue stem cellderived exosomes and fractional co2 laser for acne scars: A 12-week prospective, double-blind, randomized, split-face study. Acta Derm. Venereol. 2020, 100, adv00310. Available online: https://pubmed.ncbi.nlm.nih.gov/33073298/ (accessed on 23 July 2025). [CrossRef]
- Cho, B.S.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Park, B.C.; et al. Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Cosmetics 2020, 7, 90. [Google Scholar] [CrossRef]
- Shapira, S.; Ben Shimon, M.; Hay-Levi, M.; Shenberg, G.; Choshen, G.; Bannon, L.; Tepper, M.; Kazanov, D.; Seni, J.; Lev-Ari, S.; et al. A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond. EMBO Mol. Med. 2022, 14, e15997. Available online: https://pubmed.ncbi.nlm.nih.gov/35776000/ (accessed on 23 July 2025). [CrossRef]
- Gao, S.; Li, H.; Xie, H.; Wu, S.; Yuan, Y.; Chu, L.; Sun, S.; Yang, H.; Wu, L.; Bai, Y.; et al. Therapeutic efficacy of Schistosoma japonicum cystatin on sepsis-induced cardiomyopathy in a mouse model. Parasites Vectors 2020, 13, 260. Available online: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-020-04104-3 (accessed on 23 June 2025). [CrossRef]
- Pantos, C.; Mourouzis, I.; Saranteas, T.; Clavé, G.; Ligeret, H.; Noack-Fraissignes, P.; Renard, P.Y.; Massonneau, M.; Perimenis, P.; Spanou, D.; et al. Thyroid hormone improves postischaemic recovery of function while limiting apoptosis: A new therapeutic approach to support hemodynamics in the setting of ischaemia-reperfusion? Basic Res. Cardiol. 2009, 104, 69–77. Available online: https://pubmed.ncbi.nlm.nih.gov/19101750/ (accessed on 24 July 2025). [CrossRef]
- Xie, Q.; Yi, Q.; Zhu, J.; Tan, B.; Xiang, H.; Wang, R.; Liu, H.; Chen, T.; Xu, H. Protective role of triiodothyronine in sepsis-induced cardiomyopathy through phospholamban downregulation. Int. J. Mol. Med. 2025, 55, 47. Available online: https://pubmed.ncbi.nlm.nih.gov/39821325/ (accessed on 19 July 2025). [CrossRef] [PubMed]
- Mourouzis, I.S.; Lourbopoulos, A.I.; Trikas, A.G.; Tseti, I.K.; Pantos, C.I. Triiodothyronine prevents tissue hypoxia in experimental sepsis: Potential therapeutic implications. Intensive Care Med. Exp. 2021, 9, 17. Available online: https://icm-experimental.springeropen.com/articles/10.1186/s40635-021-00382-y (accessed on 24 July 2025). [CrossRef] [PubMed]
- Kovacevic, M.; Adam, V.N.; Causevic, S. Triiodothyronine hormone supplementation therapy in septic shock patients with euthyroid sick syndrome: Two pilot, placebo-controlled, randomized trials. Anaesth. Crit. Care Pain. Med. 2024, 43, 101336. Available online: https://pubmed.ncbi.nlm.nih.gov/38061681/ (accessed on 28 July 2024). [CrossRef]
- Jinzhong Wang, M.S.; Jian Fu, M.S. STAT3/FoxO3a/Sirt1 pathway inhibition by ginsenoside Rc ameliorates cardiomyocyte damage in septic cardiomyopathy by altering macrophage polarization. J. Mol. Histol. 2025, 56, 148. Available online: https://link.springer.com/article/10.1007/s10735-025-10417-3 (accessed on 19 July 2025). [CrossRef] [PubMed]
- Wu, W.; Ma, Q.; Li, B.-T.; Shi, S.; Guan, G.-C.; Wang, J.-K.; Xue, B.-Y.; Liu, Z.-W. α-ketoglutarate protects against septic cardiomyopathy by improving mitochondrial mitophagy and fission. Mol. Med. Rep. 2025, 31, 146. Available online: https://pubmed.ncbi.nlm.nih.gov/40183404/ (accessed on 19 July 2025). [CrossRef] [PubMed]
- Mei, Y.; Chen, X.; Shi, S.; Lin, W.; Cheng, Z.; Fan, X.; Wu, W.; Han, J.; Huang, W.; Ye, B.; et al. GI-Y2, a novel gasdermin D inhibitor, attenuates sepsis-induced myocardial dysfunction by inhibiting gasdermin D-mediated pyroptosis in macrophages. Br. J. Pharmacol. 2025, 182, 3503–3521. Available online: https://pubmed.ncbi.nlm.nih.gov/40165368/ (accessed on 19 July 2025). [CrossRef]
- Lin, Y.; Zhang, W.; Jiang, X.; Wu, C.; Yang, J.; Tao, J.; Chen, Z.; He, J.; Zhu, R.; Zhong, H.; et al. Sodium octanoate mediates GPR84-dependent and independent protection against sepsis-induced myocardial dysfunction. Biomed. Pharmacother. 2024, 180, 117455. Available online: https://pubmed.ncbi.nlm.nih.gov/39341076/ (accessed on 19 July 2025). [CrossRef]
- Feng, S.; Cai, K.; Lin, S.; Chen, X.; Luo, Y.; Wang, J.; Lian, G.; Lin, Z.; Xie, L. Exploring potential therapeutic agents for lipopolysaccharide-induced septic cardiomyopathy based on transcriptomics using bioinformatics. Sci. Rep. 2023, 13, 20589. Available online: https://www.nature.com/articles/s41598-023-47699-0 (accessed on 23 July 2025). [CrossRef] [PubMed]
- Lee, M.T.; Jung, S.Y.; Baek, M.S.; Shin, J.; Kim, W.Y. Early vitamin c, hydrocortisone, and thiamine treatment for septic cardiomyopathy: A propensity score analysis. J. Pers. Med. 2021, 11, 610. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. Available online: https://pubmed.ncbi.nlm.nih.gov/22725668/ (accessed on 23 June 2025). [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal. Res. 2018, 65, e12525. Available online: https://pubmed.ncbi.nlm.nih.gov/30242884/ (accessed on 23 June 2025). [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J. Am. Heart Assoc. 2018, 7, e008737. Available online: https://pubmed.ncbi.nlm.nih.gov/30371236/ (accessed on 23 June 2025). [CrossRef]
- Valsamaki, A.; Vazgiourakis, V.; Mantzarlis, K.; Stamatiou, R.; Makris, D. MicroRNAs in Sepsis. Biomedicines 2024, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Fan, M.; Tu, F.; Yang, K.; Ha, T.; Liu, L.; Kalbfleisch, J.; Williams, D.; Li, C. Endothelial HSPA12B Exerts Protection Against Sepsis-Induced Severe Cardiomyopathy via Suppression of Adhesion Molecule Expression by miR-126. Front. Immunol. 2020, 11, 519735. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.00566/full (accessed on 22 July 2025). [CrossRef]
- Green, O.; Shenberg, G.; Baruch, R.; Argaman, L.; Levin, T.; Michelson, I.; Hadary, R.; Isakovich, B.; Golos, M.; Schwartz, R.; et al. Inhaled Exosomes Genetically Manipulated to Overexpress CD24 (EXO-CD24) as a Compassionate Use in Severe ARDS Patients. Biomedical 2023, 11, 2523. [Google Scholar] [CrossRef] [PubMed]
- Boonen, E.; Van Den Berghe, G. Endocrine Responses to Critical Illness: Novel Insights and Therapeutic Implications. J. Clin. Endocrinol. Metab. 2014, 99, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Chatzitomaris, A.; Hoermann, R.; Midgley, J.E.; Hering, S.; Urban, A.; Dietrich, B.; Abood, A.; Klein, H.H.; Dietrich, J.W. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front. Endocrinol. 2017, 8, 273787. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2017.00163/full (accessed on 9 August 2024). [CrossRef] [PubMed]
- Pantos, C.I.; Trikas, A.G.; Pissimisis, E.G.; Grigoriou, K.P.; Stougiannos, P.N.; Dimopoulos, A.K.; Linardakis, S.I.; Alexopoulos, N.A.; Evdoridis, C.G.; Gavrielatos, G.D.; et al. Effects of Acute Triiodothyronine Treatment in Patients with Anterior Myocardial Infarction Undergoing Primary Angioplasty: Evidence from a Pilot Randomized Clinical Trial (ThyRepair Study). Thyroid 2022, 32, 714–724. Available online: https://pubmed.ncbi.nlm.nih.gov/35297659/ (accessed on 28 July 2024). [CrossRef]
- Lei, I.; Tian, S.; Gao, W.; Liu, L.; Guo, Y.; Tang, P.; Chen, E.; Wang, Z. Acetyl-CoA production by specific metabolites promotes cardiac repair after myocardial infarction via histone acetylation. eLife 2021, 10, e60311. Available online: https://pubmed.ncbi.nlm.nih.gov/34939931/ (accessed on 19 July 2025). [CrossRef]


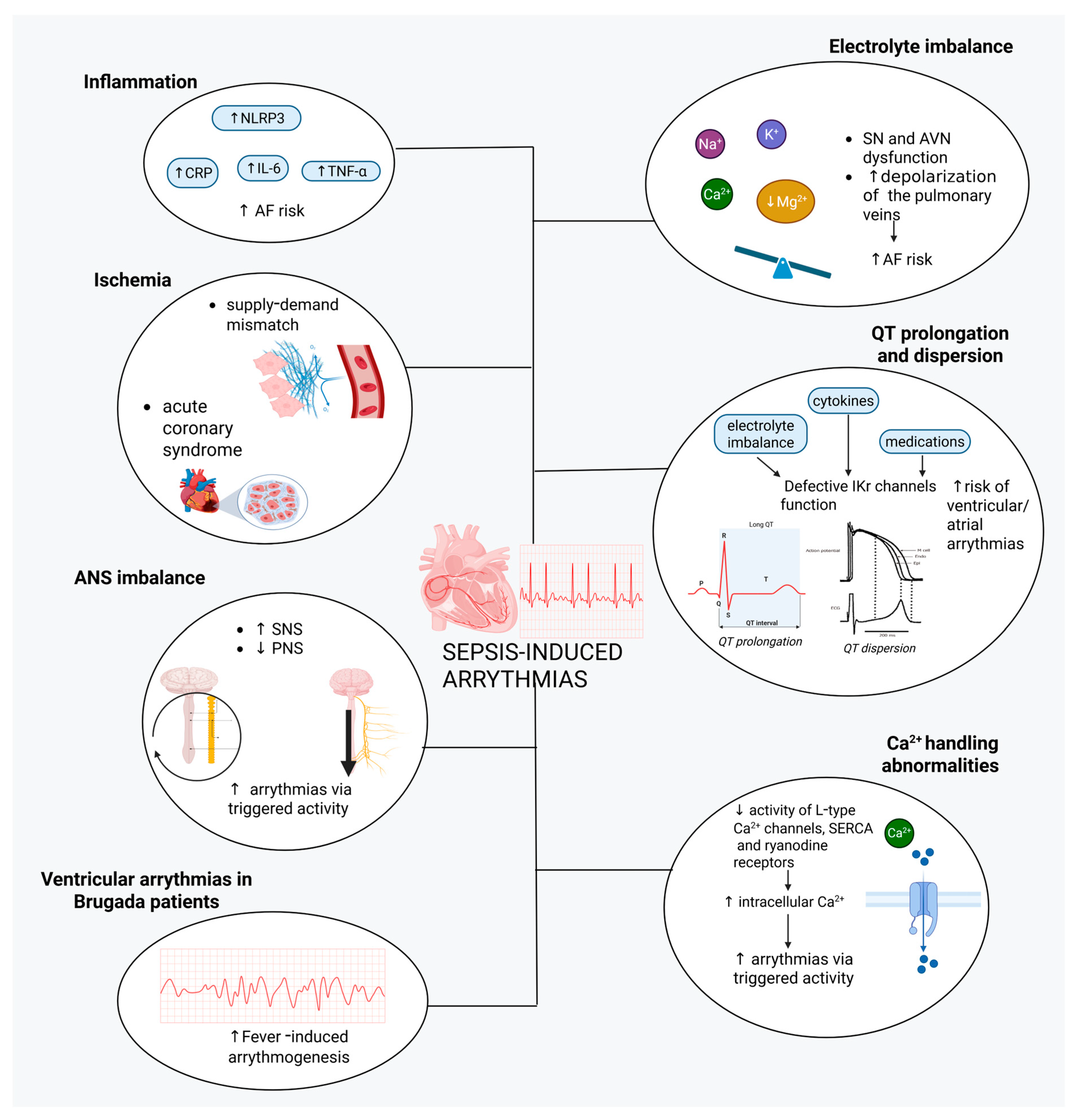
| First Author, Year | Estimation-Diagnostic Criteria | Prevalence |
|---|---|---|
| Endo T, 2013 [9] | LVEF < 50% (TTE) | 23/93 (25%) after 24 h |
| Orde SR, 2014 [10] | RV GLS > −21% LV GLS > −17% (TTE) | 60 patients analyzed: 72% RV dysfunction 69% LV dysfunction 50% LV and RV dysfunction |
| Lanspa MJ, 2015 [11] | LV GLS > −17% (TTE) | 41/68 (60%) after 6 h |
| De Geer L, 2015 [12] | LV GLS > −15% +/− LVEF < 50% E/é > 15 and/or é < 0.08 m/s (TTE) | 31/44 (70%) after 24 h |
| Dalla K, 2015 [13] | LV GLS > −15% RV GLS > −19% | 17/34 (50%) after 48 h |
| Sato R, 2016 [14] | LVEF < 50% and a ≥10% decrease compared to the baseline LVEF (TTE) | 29/210 (14%) after 24 h |
| Jayaprakash N, 2018 [15] | LVEF < 50% (TTE), cTnT > 0.01 ng/mL or NT-pro-BNP > 500 pg/mL in first 24 h ICU for diagnosis of myocardial dysfunction or LVEF < 50% for myocardial depression | 169/578 (29%) myocardial dysfunction and 23/578 (4%) myocardial depression in 24 h. |
| Jeong HS, 2018 [16] | LVEF < 50% and/or ≥10% decrease from baseline LVEF (TTE) | 25/325 (8%) after 24 h |
| Narváez I, 2018 [17] | LV systolic dysfunction (LVEF < 50%) attributable to sepsis, excluding patients with previous heart disease, associated or not to RV systolic dysfunction or LV diastolic dysfunction | 13/57 (23%) in 24 h |
| Cheng MM, 2019 [18] | Sepsis + LVEF ≤ 50% or LVEDD > 50 mm-TTE) or ≥2 of: cTnI > 3x ULN, NT-ProBNP > 3x ULN, low cardiac output manifestations, requirement for inotropes | 36/88 (41%) in 24 h |
| Lu NF, 2019 [19] | Sepsis + one of: LV-Sm < 8 cm/s or LVEF < 50%, RV-Sm < 12 cm/s, E/e′ > 15 or e′ < 8 cm/s, with no history of heart disease | 48/93 (52%) over the course of 7 days since admission |
| Chen FC, 2020 [20] | LVEF < 50% (TTE) or need for inotropes (milrinone/dobutamine) or vasopressors + biomarkers (h-FABP, MPO, cTnI) | 70/147 (48%) in 24 h |
| Wang L, 2021 [21] | LVEF < 50% (TTE), hs-TnI > 0.78 ng/mL or NT-proBNP > 500 pg/mL in first 24 h ICU (Mayo Clinic criteria) | 35/75 (47%) in 24 h |
| Tucker RV, 2022 [22] | LVEF ≤ 55% or decrease from baseline resulting in recategorization of patients from a higher LVEF category to a lower using thresholds: normal: >55%; mildly reduced: 41–55%; moderately reduced: 30–40%; severely reduced: <30% (TTE) | 9/110 (8%) in 24 h |
| Cutuli SL, 2023 [23] | New-onset cardiac dysfunction unrelated to ischemia + at least 1 of: LVSD (LVEF <45%), LVDD (lateral e′ < 8 cm/s), RVD (TAPSE < 16 mm with systolic pulmonary arterial pressure < 35 mm Hg)-using TTE | 60/148 (41%) |
| Zhang J, 2023 [24] | Septic patients with LVEF < 50% (TTE) | 22/79 (28%) in 24 h |
| Hendrickson KW, 2024 [25] | Septic shock patients with LVEF ≤ 55% or decrease in LVEF ≥ 10% from baseline (TTE) | 207/1229 (17%) in 72 h |
| Chang X, 2024 [26] | Sepsis + no pre-existing heart conditions + LVEF < 50% (TTE) | 56/270 (21%) in 24 h |
| Yang X, 2025 [27] | Acute reversible cardiac function changes within 5 days ICU + global or unilateral ventricular dysfunction [LVEF < 50% OR (TRV > 2.8 m/s, LAVi > 34 mL/m2, septal e′ wave < 7 cm/s or lateral e′ < 10 cm/s, and E/e′ ratio >13-lateral or >15-septal) OR (RV TAPSE < 16 mm/s or sTDI < 10 cm/s)] + exclusion of myocardial ischemia (TTE) | 110/181 (61%) after 5 days since admission |
| Zhou YT, 2025 [28] | Infection + organ dysfunction + elevated troponin I + ≥ 1 of: myoglobin, CK-MB, α-HBDH (SAMI diagnostic criteria) | 316/517 (61%) in 24 h |
| Therapeutic Agent | Mechanism | Evidence |
|---|---|---|
| Vitamin C |
| Small trials with relatively few patients [232]; potential benefit of early administration (<2 h) in patients with marked inflammatory response (observational evidence) [233] |
| Melatonin | Preclinical SICM models in mice with promising efficacy and safety; lack of evidence in humans (including route and dose of administration), though with relatively high anticipated safety | |
| Engineered exosomes | Vehicles facilitating targeted molecular transportation | Preclinical mouse models in SICM; small-scale studies have examined exosomes in humans in various clinical conditions with promising results [244,245,246], though large-scale studies are lacking |
| Schistosoma japonicum-produced cystatin (Sj-Cys) | LPS-MyD88 pathway inhibition → anti-inflammatory action:
| CLP model of sepsis in mice [247] |
| Triiodothyronine (T3) | Preclinical models in mice with SICM [250]; promising results in an RCT of patients with septic shock (not specifically examined in SICM) [251] | |
| Ginsenoside Rc (substance isolated from ginseng) | STAT3/FoxO3a/Sirt1 pathway modulation → anti-inflammatory action via inhibition of macrophage activation | Mouse model of SICM [252] |
| a-Ketoglutarate |
| Mouse model of SICM [253] |
| Gasmerdin-D inhibitor Y2 (GI-Y2) | GI-Y2 binding and blocking gasmerdin-D
| Mouse model of CLP- or LPS-induced SICM [254] |
| Sodium octanoate (hydrophilic product of saturated fatty acid) |
| Mouse model of LPS-induced SICM [255] |
| Gene-targeted therapies |
| Gene identification in a mouse model of LPS-induced SICM [256] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamporis, K.; Karakasis, P.; Pantelidaki, A.; Goutis, P.A.; Grigoriou, K.; Theofilis, P.; Katsaouni, A.; Botis, M.; Karanikola, A.-E.; Milaras, N.; et al. Sepsis-Induced Cardiomyopathy and Cardiac Arrhythmias: Pathophysiology and Implications for Novel Therapeutic Approaches. Biomedicines 2025, 13, 2643. https://doi.org/10.3390/biomedicines13112643
Pamporis K, Karakasis P, Pantelidaki A, Goutis PA, Grigoriou K, Theofilis P, Katsaouni A, Botis M, Karanikola A-E, Milaras N, et al. Sepsis-Induced Cardiomyopathy and Cardiac Arrhythmias: Pathophysiology and Implications for Novel Therapeutic Approaches. Biomedicines. 2025; 13(11):2643. https://doi.org/10.3390/biomedicines13112643
Chicago/Turabian StylePamporis, Konstantinos, Paschalis Karakasis, Antonia Pantelidaki, Panagiotis Antonios Goutis, Konstantinos Grigoriou, Panagiotis Theofilis, Athanasia Katsaouni, Michail Botis, Aikaterini-Eleftheria Karanikola, Nikias Milaras, and et al. 2025. "Sepsis-Induced Cardiomyopathy and Cardiac Arrhythmias: Pathophysiology and Implications for Novel Therapeutic Approaches" Biomedicines 13, no. 11: 2643. https://doi.org/10.3390/biomedicines13112643
APA StylePamporis, K., Karakasis, P., Pantelidaki, A., Goutis, P. A., Grigoriou, K., Theofilis, P., Katsaouni, A., Botis, M., Karanikola, A.-E., Milaras, N., Vlachos, K., Tsiachris, D., Pantos, C., & Mourouzis, I. (2025). Sepsis-Induced Cardiomyopathy and Cardiac Arrhythmias: Pathophysiology and Implications for Novel Therapeutic Approaches. Biomedicines, 13(11), 2643. https://doi.org/10.3390/biomedicines13112643







