Cytokine Dynamics During Ustekinumab Induction as Predictors of Treatment Response in Crohn’s Disease: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Variables
2.3. Sample Collection and Measurement
2.4. Definition of Primary Response
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Baseline Characteristics
3.2. Biochemical Characteristics
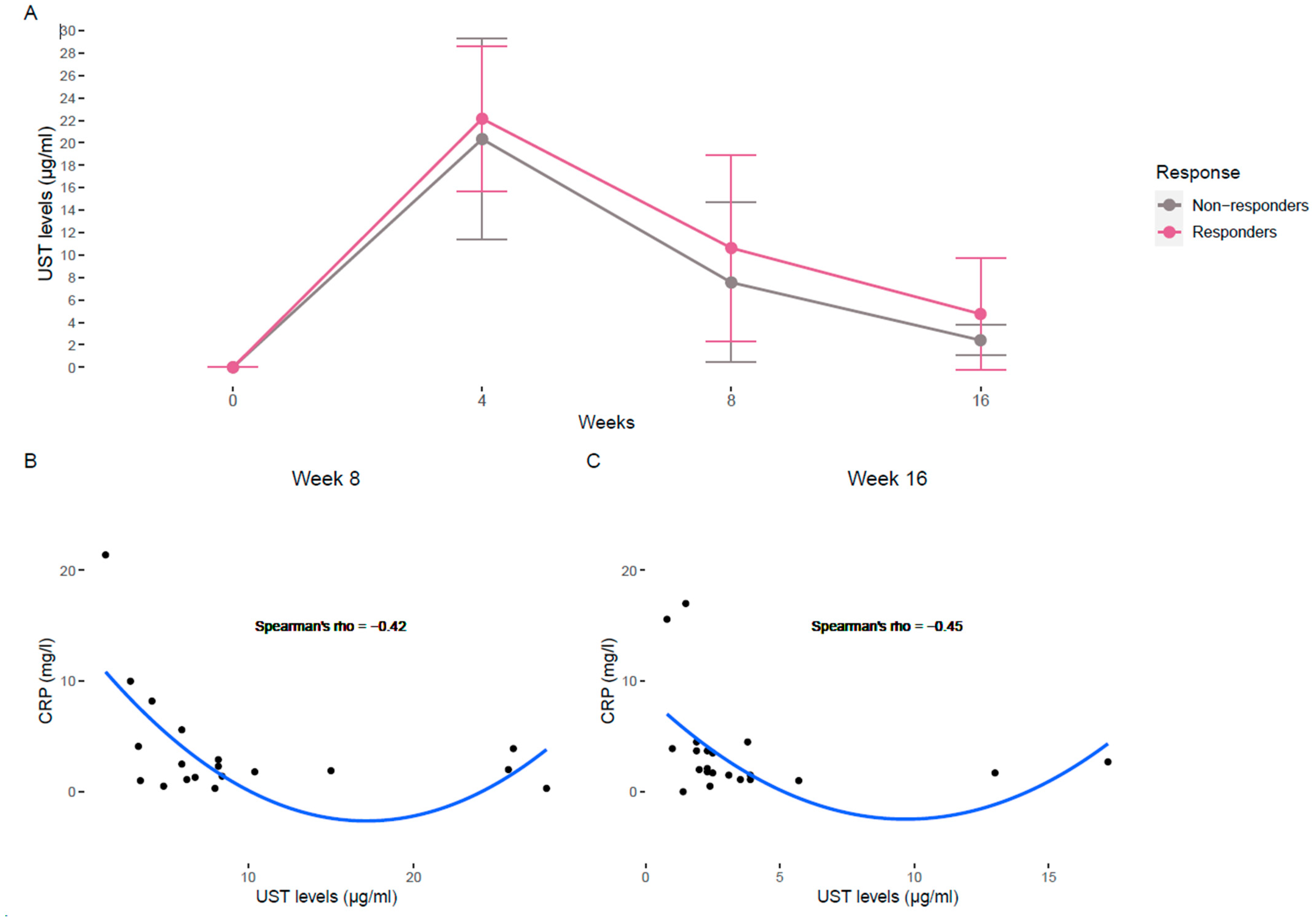
3.3. Evolution of Ustekinumab Levels and Correlation with Biochemical Profiles
3.4. Serum Cytokines and Biochemical Profiles
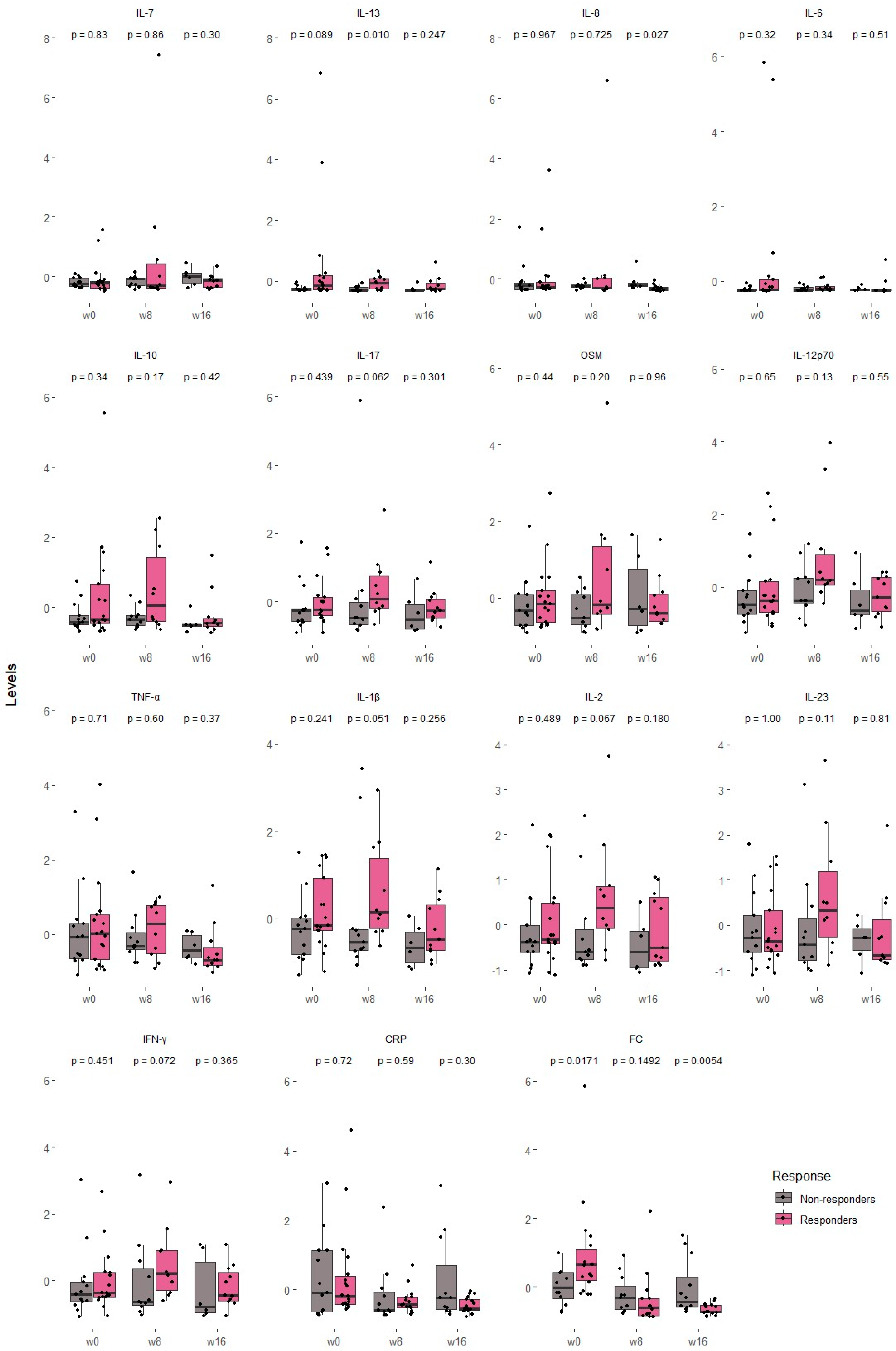
3.4.1. Overall Cytokine Behaviors
3.4.2. Clinical, Biochemical and Cytokine Differences Among Responders and Non-Responders
3.5. Ustekinumab Levels and Cytokine Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| FC | Fecal calprotectin |
| HBI | Harvey–Bradshaw Index |
| Hg | Hemoglobin |
| IBD | Inflammatory bowel disease |
| IL | Interleukin |
| INFγ | Interferon gamma |
| IQR | Interquartile range |
| OSM | Oncostatin M |
| TDM | Therapeutic drug monitoring |
| TNF-α | Tumor necrosis factor alpha |
| UST | Ustekinumab |
References
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim Care 2023, 50, 411–427. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokaria, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Salas, A.; Sands, B.E.; Abraham, C.; Leibovitzh, H.; Neurath, M.F.; Vande Casteele, N. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 433–446. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Gutiérrez, A.; Antolín, B.; Huguet, J.M.; De Francisco, R.; Merino, O.; Carpio, D.; García-López, S.; et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2019, 50, 278–288. [Google Scholar] [CrossRef]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Iglesias-Flores, E.; Navarro, P.; Rivero, M.; Gutiérrez, A.; Sierra-Ausin, M.; Mesonero, F.; Ferreiro-Iglesias, R.; et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2020, 52, 1017–1030. [Google Scholar] [CrossRef]
- Nigam, G.B.; Limdi, J.K. An update on the role of anti-IL-12/IL23 agents in the management of inflammatory bowel disease. Br. Med. Bull. 2021, 138, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, 675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2023, 69, 28–42. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.-L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef]
- Mínguez, A.; Coello, E.; Garrido, A.; Ripoll, P.; Gomez, M.; Aguas, M.; Iborra, M.; Cerrillo, E.; Tortosa, L.; Bayarri, V.; et al. Optimizing outcomes with maintenance IV UST in highly bio-exposed patients with IBD. Efficacy and adjusted regimen in real world. Gastroenterol. Hepatol. 2024, 48, 502253. [Google Scholar] [CrossRef]
- Digby-Bell, J.L.; Atreya, R.; Monteleone, G.; Powell, N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 9–20. [Google Scholar] [CrossRef]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol. Res. Acad. Press 2020, 158, 104835. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Eberl, A.; Hallinen, T.; af Björkesten, C.G.; Heikkinen, M.; Hirsi, E.; Kellokumpu, M.; Koskinen, I.; Moilanen, V.; Nielsen, C.; Nuutinen, H.; et al. Ustekinumab for Crohn’s disease: A nationwide real-life cohort study from Finland (FINUSTE). Scand. J. Gastroenterol. 2019, 54, 718–725. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients with Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Parigi, T.L.; Iacucci, M.; Ghosh, S. Blockade of IL-23: What is in the Pipeline? J. Crohn’s Colitis 2022, 16, II64–II72. [Google Scholar] [CrossRef]
- Bertin, L.; Barberio, B.; Gubbiotti, A.; Bertani, L.; Costa, F.; Ceccarelli, L.; Visaggi, P.; Bodini, G.; Pasta, A.; Sablich, R.; et al. Association between Ustekinumab Trough Levels, Serum IL-22, and Oncostatin M Levels and Clinical and Biochemical Outcomes in Patients with Crohn’s Disease. J. Clin. Med. 2024, 13, 1539. [Google Scholar] [CrossRef]
- Mateos, B.; Sáez-González, E.; Moret, I.; Hervás, D.; Iborra, M.; Cerrillo, E.; Tortosa, L.; Nos, P.; Beltrán, B. Plasma Oncostatin M, TNF-α, IL-7, and IL-13 Network Predicts Crohn’s Disease Response to Infliximab, as Assessed by Calprotectin Log Drop. Dig. Dis. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Niu, R.; Xiong, S.; He, J.; Wang, Y.; Zhang, P.; Su, F.; Liu, Z.; Zhou, L.; et al. Multi-Omics Biomarkers for Predicting Efficacy of Biologic and Small-Molecule Therapies in Adults with Inflammatory Bowel Disease: A Systematic Review. United Eur. Gastroenterol. J. 2024, 13, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Krisam, J.; Wehling, C.; Kloeters-Plachky, P.; Leopold, Y.; Belling, N.; Gauss, A. Ustekinumab: “Real-world” outcomes and potential predictors of nonresponse in treatment-refractory Crohn’s disease. World J. Gastroenterol. 2019, 25, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Colombel, J.F.; Ungaro, R. Review article: Predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 47, 896–905. [Google Scholar] [CrossRef]
- Schulte, L.; Beck, A.; Hofelich, J.; Reuther, H.S.; Klaus, J. Real-Life Ustekinumab Response and Blood Cytokine Profiles in Patients with Crohn’s Disease. Am. J. Gastroenterol. Hepatol. 2020, 1, 1006. [Google Scholar]
- Van den Berghe, N.; Alsoud, D.; Verstockt, B.; Vermeire, S.; Declerck, P.; Thomas, D. Evaluation of serum cytokines and acute phase proteins as possible pharmacodynamic biomarkers to monitor endoscopic remission during ustekinumab therapy in patients with Crohn’s disease. Therap. Adv. Gastroenterol. 2023, 16, 17562848231189110. [Google Scholar] [CrossRef]
- Murate, K.; Maeda, K.; Nakamura, M.; Sugiyama, D.; Wada, H.; Yamamura, T.; Sawada, T.; Mizutani, Y.; Ishikawa, T.; Furukawa, K.; et al. Endoscopic activity and serum TNF-α level at baseline are associated with clinical response to ustekinumab in crohn’s disease patients. Inflamm. Bowel Dis. 2020, 26, 1669–1681. [Google Scholar] [CrossRef]
- McDonald, C.; Kerr, H.; Gibbons, E.; Lukose, T.; Cheriyan, D.; Harewood, G.; Patchett, S.; O’toole, A.; Kelly, O.; Boland, K. Higher Ustekinumab Levels in Maintenance Therapy are Associated with Greater Mucosal Healing and Mucosal Response in Crohn’s Disease: An Experience of 2 IBD Centers. Inflamm. Bowel Dis. 2024, 30, 423–428. [Google Scholar] [CrossRef]
- Thomann, A.K.; Schulte, L.A.; Globig, A.M.; Hoffmann, P.; Klag, T.; Itzel, T.; Teufel, A.; Schreiner, R.; Scheffe, N.; Ebert, M.P.; et al. Ustekinumab serum concentrations are associated with clinical outcomes in Crohn’s disease—A regional multi-center pilot study. Z. Gastroenterol. 2020, 58, 439–444. [Google Scholar] [CrossRef]
- Rodríguez-Moranta, F.; Argüelles-Arias, F.; Hinojosa del Val, J.; Iborra Colomino, M.; Martín-Arranz, M.D.; Menchén Viso, L.; Núñez, F.M.; Gómez, E.R.; Sánchez-Hernández, J.G.; Valdés-Delgado, T.; et al. Therapeutic drug monitoring in inflammatory bowel diseases. Position statement of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis. Gastroenterol. Hepatol. 2024, 47, 522–552. [Google Scholar] [CrossRef]
- Alsoud, D.; Vermeire, S.; Verstockt, B. Biomarker discovery for personalized therapy selection in inflammatory bowel diseases: Challenges and promises. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100089. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Antonioli, L.; Fornili, M.; D’Antongiovanni, V.; Ceccarelli, L.; Carmisciano, L.; Benvenuti, L.; Mumolo, M.G.; Bottari, A.; Pardi, V.; et al. Baseline Assessment of Serum Cytokines Predicts Clinical and Endoscopic Response to Ustekinumab in Patients with Crohn’s Disease: A Prospective Pilot Study. Inflamm. Bowel Dis. 2024, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Pauwels, R.W.M.; Van Der Woude, C.J.; Doukas, M.; Oudijk, L.; Peppelenbosch, M.P.; Grohmann, U.; Crombag, M.-R.B.S.; de Vries, A.C.; Fuhler, G.M. Ustekinumab Tissue and Serum Levels in Patients with Crohn’s Disease Are Closely Correlated though Not Consistently Associated with Objective Response after Induction. Inflamm. Bowel Dis. 2023, 29, 1038–1046. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Sollelis, E.; Quinard, R.M.; Bouguen, G.; Goutte, M.; Goutorbe, F.; Bouvier, D.; Pereira, B.; Bommelaer, G.; Buisson, A. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J. Gastroenterol. 2019, 25, 2354–2364. [Google Scholar] [CrossRef]
- Zubin, G.; Peter, L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: A comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm. Bowel Dis. 2015, 21, 1386–1391. [Google Scholar] [PubMed]
| Variable | Total (n = 31) | Responders (n = 18) | Non-Responders (n = 13) | p-Value |
|---|---|---|---|---|
| Sex, n (%) | 1 | |||
| Male | 19 (61.3%) | 11 (61.1%) | 8 (61.5%) | |
| Female | 12 (38.7%) | 7 (38.7%) | 5 (38.5%) | |
| Height (cm) | 170 (165–177) | 169 (165–179) | 170 (165–175) | |
| Body weight (kg) | 70 (62–82) | 70 (62–81) | 71 (66–80) | |
| Age at diagnosis, n (%) | 0.213 | |||
| A1 | 4 (12.9%) | 3 (16.7%) | 1 (7.7%) | |
| A2 | 16 (51.6%) | 11 (61.1%) | 5 (38.5%) | |
| A3 | 11 (35.5%) | 4 (22.2%) | 7 (53.8%) | |
| Location, n (%) | 0.051 | |||
| L1 | 10 (32.3%) | 7 (38.9%) | 3 (23.1%) | |
| L2 | 8 (25.8%) | 7 (38.9%) | 1 (7.7%) | |
| L3 | 10 (32.8%) | 3 (16.7%) | 7 (53.8%) | |
| L1 + L4 | 3 (9.7%) | 1 (5.6%) | 2 (15.4%) | |
| Behaviour, n (%) | 0.753 | |||
| B1 | 21 (67.7%) | 12 (66.7%) | 9 (69.2%) | |
| B2 | 4 (12.9%) | 3 (16.7%) | 1 (7.7%) | |
| B3 | 6 (19.4%) | 3 (16.7%) | 3 (23.1%) | |
| Perianal disease, n (%) | 6 (19.4%) | 3 (16.7%) | 3 (23.1%) | |
| n of prior anti-TNF-α exposure, n (%) | 0.393 | |||
| 0 | 4 (12.9%) | 2 (11.1%) | 2 (15.4%) | |
| 1 | 18 (58.1%) | 9 (50.0%) | 9 (69.2%) | |
| 2 | 9 (29.0%) | 7 (38.9%) | 2 (15.4%) | |
| Other prior biologic exposure, n (%) | 0.245 | |||
| 0 | 28 (90.3%) | 15 (83.3%) | 13 (100%) | |
| 1 | 3 (9.6%) | 3 (16.7%) | ||
| Concomitant drugs at the start of ust, n (%) | 0.027 | |||
| Corticosteroids | 5 (16.1%) | 5 (27.8%) | ||
| Immunosuppressants | 7 (22.6%) | 2 (11.1%) | 5 (38.5%) | |
| Smoking, n (%) | 1 | |||
| Yes | 10 (32.3%) | 6 (33.3%) | 4 (30.8%) | |
| No | 12 (38.7%) | 7 (38.9%) | 5 (38.5%) | |
| Ex | 9 (29.0%) | 5 (27.8%) | 4 (30.8%) | |
| Surgery, n (%) | 15 (48.4%) | 8 (44.4%) | 7 (53.8%) | |
| Need ust intensification/ reinduction, n (%) | 13 (41.9%) | 6 (33.3%) | 7 (53.8%) |
| Variable | Responders | Non-Responders | ||||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 8 | Week 16 | p-Value | Week 0 | Week 8 | Week 16 | p-Value | |
| HBI | 5.12 5 (4–7) | 2.44 2 (0.75–4) | 1.75 1 (0–3.25) | 0.002 | 4.15 5 (2–6) | 2.27 2 (0–4) | 1.31 1 (0–2) | 0.032 |
| CRP (mg/L) | 7.83 4.15 (2.48–7.93) | 3.21 2.5 (1.85–3.85) | 2.36 1.7 (1.4–3.5) | 0.012 | 7.7 4.8 (1–12.9) | 4.28 1.4 (1–4.8) | 6.95 3.7 (1.45–10.05) | 0.55 |
| FC (µg/g) | 1906.4 1623 (1144–2098) | 564.3 296.6 (53.3–586.5) | 246 177.5 (142.5–369.7) | 2.40 × 10−6 | 944.4 928.1 (575.2–1337.8) | 709.9 620 (249.4–956.5) | 911.6 493 (304.8–1240) | 0.47 |
| Hemoglobin (g/dL) | 14.33 14.3 (12.9–15.8) | 14.68 14.7 (13.5–16) | 14.25 14.5 (13.5–15.6) | 0.76 | 13.68 13.4 (13.1–14.2) | 13.81 13.8 (13.4–14.8) | 13.66 13.8 (13.4–14.7) | 0.9 |
| Albumin (g/dL) | 4.29 4.5 (3.9–4.7) | 4.407 4.45 (4.2–4.5) | 4.45 4.5 (4.4–4.6) | 0.52 | 4.24 4.3 (4.1–4.5) | 4.31 4.3 (4.2–4.5) | 4.45 4.5 (4.35–4.55) | 0.32 |
| UST levels (µg/mL) | 10.627 8.2 (5.6–12.7) | 4.75 2.8 (2.37–3.9) | 7.55 6 (3.85–7.7) | 2.41 2.1 (1.9–2.3) | ||||
| Cytokine | Cohen’s d | Lower CI | Upper CI |
|---|---|---|---|
| TNF-α | −0.58 | −1.20 | 0.04 |
| IL-1β | −0.47 | −1.09 | 0.15 |
| IL-10 | −0.38 | −1.00 | 0.24 |
| IL-8 | −0.37 | −0.99 | 0.24 |
| IL-6 | −0.33 | −0.95 | 0.28 |
| IL-13 | −0.33 | −0.94 | 0.29 |
| IL-17 | −0.24 | −0.85 | 0.38 |
| IL-2 | −0.20 | −0.82 | 0.41 |
| IL-12p70 | −0.20 | −0.81 | 0.41 |
| IL-23 | −0.19 | −0.80 | 0.43 |
| INF-Γ | −0.18 | −0.79 | 0.43 |
| OSM | 0 | −0.61 | 0.61 |
| IL-7 | 0.04 | −0.57 | 0.65 |
| Spearman Coefficient | ||
|---|---|---|
| Cytokines | w8 | w16 |
| IL-6 | −0.45 | −0.22 |
| IL-8 | −0.40 | −0.49 |
| IL-13 | −0.39 | −0.08 |
| IL-23 | −0.38 | −0.38 |
| IL-2 | −0.30 | −0.26 |
| IL-1β | −0.25 | −0.34 |
| IL-12p70 | −0.23 | −0.31 |
| INF-γ | −0.23 | −0.29 |
| OSM | −0.18 | −0.40 |
| IL-17 | −0.09 | −0.12 |
| IL-10 | −0.08 | −0.21 |
| IL-7 | −0.05 | −0.23 |
| TNF-α | 0.19 | −0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mínguez, A.; Mateos, B.; Iborra, M.; Aguas, M.; Bastida, G.; Garrido, A.; Cerrillo, E.; García, S.; Tortosa, L.; Moret, I.; et al. Cytokine Dynamics During Ustekinumab Induction as Predictors of Treatment Response in Crohn’s Disease: An Observational Study. Biomedicines 2025, 13, 2608. https://doi.org/10.3390/biomedicines13112608
Mínguez A, Mateos B, Iborra M, Aguas M, Bastida G, Garrido A, Cerrillo E, García S, Tortosa L, Moret I, et al. Cytokine Dynamics During Ustekinumab Induction as Predictors of Treatment Response in Crohn’s Disease: An Observational Study. Biomedicines. 2025; 13(11):2608. https://doi.org/10.3390/biomedicines13112608
Chicago/Turabian StyleMínguez, Alejandro, Beatriz Mateos, Marisa Iborra, Mariam Aguas, Guillermo Bastida, Alejandro Garrido, Elena Cerrillo, Sonia García, Lluís Tortosa, Inés Moret, and et al. 2025. "Cytokine Dynamics During Ustekinumab Induction as Predictors of Treatment Response in Crohn’s Disease: An Observational Study" Biomedicines 13, no. 11: 2608. https://doi.org/10.3390/biomedicines13112608
APA StyleMínguez, A., Mateos, B., Iborra, M., Aguas, M., Bastida, G., Garrido, A., Cerrillo, E., García, S., Tortosa, L., Moret, I., & Nos, P. (2025). Cytokine Dynamics During Ustekinumab Induction as Predictors of Treatment Response in Crohn’s Disease: An Observational Study. Biomedicines, 13(11), 2608. https://doi.org/10.3390/biomedicines13112608








