Acute Severe Ulcerative Colitis (ASUC): Clinical Features, Initial Management, and the Role of Advanced Therapies
Abstract
1. Introduction
2. Epidemiology
3. Diagnostic Workup
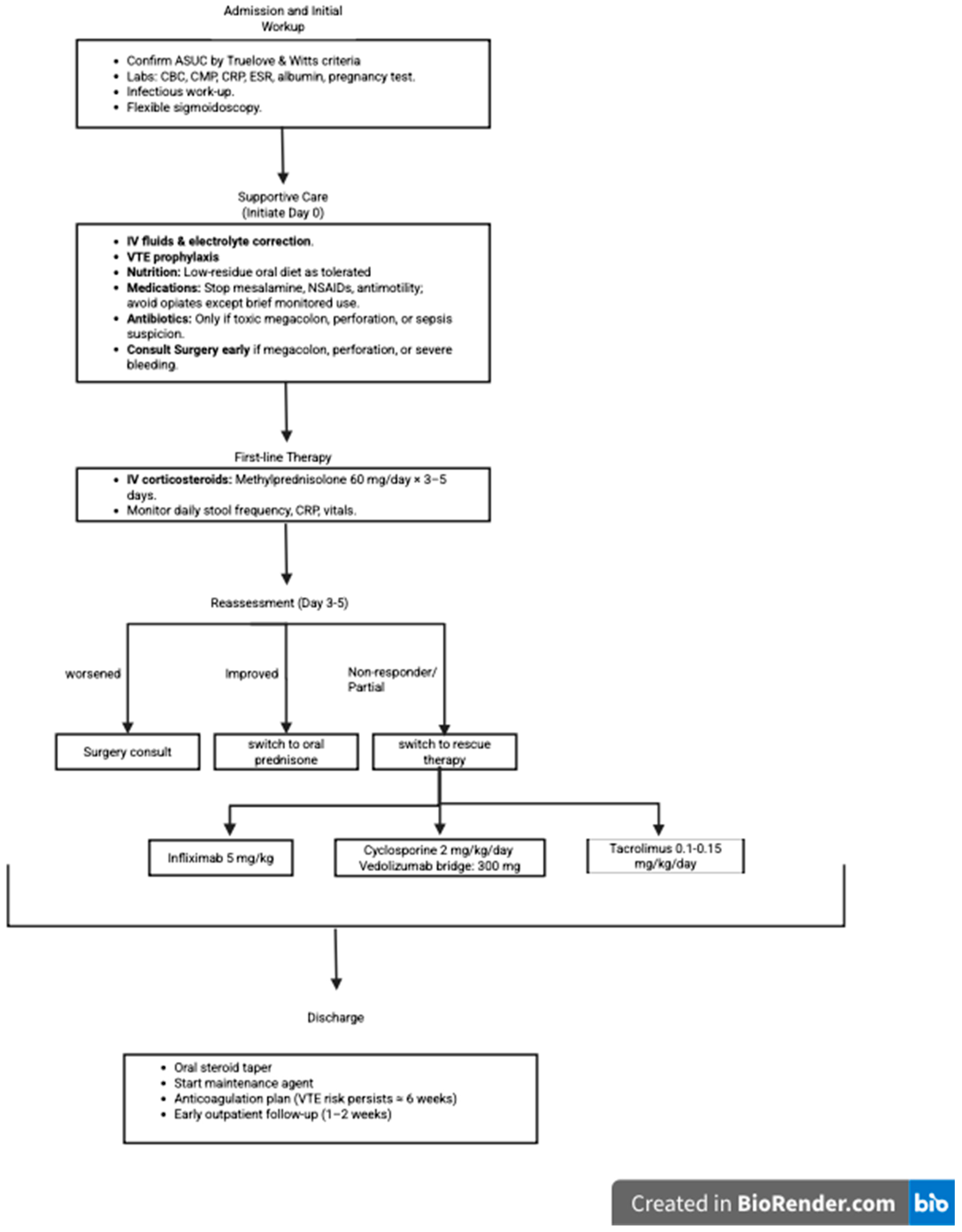
4. Initial Management
5. Response Assessment
6. Evidence for Use of Biologics and Small Molecules in ASUC
6.1. TNF Blockers (Infliximab)
6.1.1. Mechanism of Action
6.1.2. Clinical Data
6.1.3. Contraindications
6.1.4. Accelerated Dosing Rationale and Evidence
6.2. Calcineurin Inhibitors (Cyclosporine and Tacrolimus)
6.2.1. Mechanism of Action
6.2.2. Clinical Data
6.2.3. Contraindications
6.3. Infliximab vs. Cyclosporine
6.4. Infliximab/Cyclosporine Sequential Therapy
6.5. Vedolizumab with Cyclosporine
6.5.1. Mechanism of Action
6.5.2. Clinical Data
6.5.3. Contraindications
6.6. JAK Inhibitors (Tofacitinib and Upadacitinib)
6.6.1. Mechanism of Action
6.6.2. Clinical Data
6.6.3. Contraindications
6.7. IL-12/23 Inhibitor (Ustekinumab)
6.7.1. Mechanism of Action
6.7.2. Clinical Data
6.7.3. Contraindications
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5A–36A. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohsen, W.; Chapman, T.P.; Satsangi, J. Predicting Outcome in Acute Severe Colitis-Controversies in Clinical Practice in 2021. J. Crohns Colitis 2021, 15, 1211–1221. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; George, B.; Mortensen, N.J.; Travis, S.P. The pattern and outcome of acute severe colitis. J. Crohn’s Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef]
- Lynch, R.W.; Lowe, D.; Protheroe, A.; Driscoll, R.; Rhodes, J.M.; Arnott, I.D. Outcomes of rescue therapy in acute severe ulcerative colitis: Data from the United Kingdom inflammatory bowel disease audit. Aliment. Pharmacol. Ther. 2013, 38, 935–945. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Salameh, R.; Kirchgesner, J.; Allez, M.; Carbonnel, F.; Meyer, A.; Gornet, J.M.; Beaugerie, L.; Amiot, A. Long-term outcome of patients with acute severe ulcerative colitis responding to intravenous steroids. Aliment. Pharmacol. Ther. 2020, 51, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, S.; Fernandes, S.R.; Goncalves, A.R.; Valente, A.; Baldaia, C.; Santos, P.M.; Correia, L.A. Predicting the Course of Disease in Hospitalized Patients With Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 541–546. [Google Scholar] [CrossRef]
- Fudman, D.I.; Sattler, L.; Feuerstein, J.D. Inpatient Management of Acute Severe Ulcerative Colitis. J. Hosp. Med. 2019, 14, 766–773. [Google Scholar] [CrossRef]
- Jakobovits, S.L.; Travis, S.P. Management of acute severe colitis. Br. Med. Bull. 2005, 75–76, 131–144. [Google Scholar] [CrossRef]
- Bossa, F.; Fiorella, S.; Caruso, N.; Accadia, L.; Napolitano, G.; Valvano, M.R.; Andriulli, A.; Annese, V. Continuous infusion versus bolus administration of steroids in severe attacks of ulcerative colitis: A randomized, double-blind trial. Am. J. Gastroenterol. 2007, 102, 601–608. [Google Scholar] [CrossRef]
- Truelove, S.C.; Jewell, D.P. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1974, 1, 1067–1070. [Google Scholar] [CrossRef]
- Grainge, M.J.; West, J.; Card, T.R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Sam, J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 2008, 103, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnar, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Burr, N.E.; Smith, C.; West, R.; Hull, M.A.; Subramanian, V. Increasing Prescription of Opiates and Mortality in Patients With Inflammatory Bowel Diseases in England. Clin. Gastroenterol. Hepatol. 2018, 16, 534–541.e6. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.I.; Beck, P.L. A new look at toxic megacolon: An update and review of incidence, etiology, pathogenesis, and management. Am. J. Gastroenterol. 2003, 98, 2363–2371. [Google Scholar] [CrossRef]
- Takeuchi, K.; Smale, S.; Premchand, P.; Maiden, L.; Sherwood, R.; Thjodleifsson, B.; Bjornsson, E.; Bjarnason, I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Ransford, R.A.; Langman, M.J. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002, 51, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Lindgren, S.C.; Flood, L.M.; Kilander, A.F.; Lofberg, R.; Persson, T.B.; Sjodahl, R.I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 831–835. [Google Scholar] [CrossRef]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alsayegh, A.; Aldallal, U.; Ma, C.; Narula, N.; Jairath, V.; Singh, S.; Bessissow, T. Comparative Efficacy of Biologics and Small Molecule in Ulcerative Colitis: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2025, 23, 250–262. [Google Scholar] [CrossRef]
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630. [Google Scholar] [CrossRef]
- Sands, B.E.; Tremaine, W.J.; Sandborn, W.J.; Rutgeerts, P.J.; Hanauer, S.B.; Mayer, L.; Targan, S.R.; Podolsky, D.K. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: A pilot study. Inflamm. Bowel Dis. 2001, 7, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Jarnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlen, P.; Granno, C.; Vilien, M.; Strom, M.; Danielsson, A.; Verbaan, H.; et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology 2005, 128, 1805–1811. [Google Scholar] [CrossRef]
- Gustavsson, A.; Jarnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlen, P.; Granno, C.; Vilien, M.; Strom, M.; Verbaan, H.; et al. Clinical trial: Colectomy after rescue therapy in ulcerative colitis-3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment. Pharmacol. Ther. 2010, 32, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, M.; Magnuson, A.; Bjork, J.; Benoni, C.; Almer, S.; Friis-Liby, I.; Hertervig, E.; Olsson, M.; Karlen, P.; Eriksson, A.; et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: A long-term follow-up of 211 Swedish patients. Aliment. Pharmacol. Ther. 2013, 38, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Janssen Biotech Inc. REMICADE® (Infliximab) [Prescribing Information]; Janssen Biotech Inc.: Horsham, PA, USA, 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/103772s5412lbl.pdf (accessed on 1 September 2025).
- Gibson, D.J.; Heetun, Z.S.; Redmond, C.E.; Nanda, K.S.; Keegan, D.; Byrne, K.; Mulcahy, H.E.; Cullen, G.; Doherty, G.A. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 330–335.e1. [Google Scholar] [CrossRef] [PubMed]
- Hindryckx, P.; Novak, G.; Vande Casteele, N.; Laukens, D.; Parker, C.; Shackelton, L.M.; Narula, N.; Khanna, R.; Dulai, P.; Levesque, B.G.; et al. Review article: Dose optimisation of infliximab for acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.C.; Li Wai Suen, C.F.D.; Con, D.; Boyd, K.; Pena, R.; Burrell, K.; Rosella, O.; Proud, D.; Brouwer, R.; Gorelik, A.; et al. Intensified versus standard dose infliximab induction therapy for steroid-refractory acute severe ulcerative colitis (PREDICT-UC): An open-label, multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 981–996. [Google Scholar] [CrossRef]
- Lee, H.; Myoung, H.; Kim, S.M. Review of two immunosuppressants: Tacrolimus and cyclosporine. J. Korean Assoc. Oral. Maxillofac. Surg. 2023, 49, 311–323. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Van Assche, G.; D’Haens, G.; Noman, M.; Vermeire, S.; Hiele, M.; Asnong, K.; Arts, J.; D’Hoore, A.; Penninckx, F.; Rutgeerts, P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003, 125, 1025–1031. [Google Scholar] [CrossRef]
- Ogata, H.; Matsui, T.; Nakamura, M.; Iida, M.; Takazoe, M.; Suzuki, Y.; Hibi, T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006, 55, 1255–1262. [Google Scholar] [CrossRef]
- Ogata, H.; Kato, J.; Hirai, F.; Hida, N.; Matsui, T.; Matsumoto, T.; Koyanagi, K.; Hibi, T. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 803–808. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals Corporation. Sandimmune® (Cyclosporine) [Prescribing Information]; Novartis Pharma K G: Basel, Switzerland, 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/050573s044%2C050574s057%2C050625s061lbl.pdf (accessed on 1 September 2025).
- Astellas Pharma Inc. Prograf® (Tacrolimus) [Prescribing Information]; Astellas Pharma Inc.: Deerfield, IL, USA, 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/050708s053%2C050709s045%2C210115s005lbl.pdf (accessed on 1 September 2025).
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Nachury, M.; Moreau, J.; et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet 2012, 380, 1909–1915. [Google Scholar] [CrossRef]
- Croft, A.; Walsh, A.; Doecke, J.; Cooley, R.; Howlett, M.; Radford-Smith, G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: Ciclosporin vs. infliximab. Aliment. Pharmacol. Ther. 2013, 38, 294–302. [Google Scholar] [CrossRef]
- Williams, J.G.; Alam, M.F.; Alrubaiy, L.; Arnott, I.; Clement, C.; Cohen, D.; Gordon, J.N.; Hawthorne, A.B.; Hilton, M.; Hutchings, H.A.; et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): A mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol. Hepatol. 2016, 1, 15–24. [Google Scholar] [CrossRef]
- Narula, N.; Marshall, J.K.; Colombel, J.F.; Leontiadis, G.I.; Williams, J.G.; Muqtadir, Z.; Reinisch, W. Systematic Review and Meta-Analysis: Infliximab or Cyclosporine as Rescue Therapy in Patients With Severe Ulcerative Colitis Refractory to Steroids. Am. J. Gastroenterol. 2016, 111, 477–491. [Google Scholar] [CrossRef]
- Ordas, I.; Domenech, E.; Manosa, M.; Garcia-Sanchez, V.; Iglesias-Flores, E.; Penalva, M.; Canas-Ventura, A.; Merino, O.; Fernandez-Banares, F.; Gomollon, F.; et al. Long-Term Efficacy and Safety of Cyclosporine in a Cohort of Steroid-Refractory Acute Severe Ulcerative Colitis Patients from the ENEIDA Registry (1989–2013): A Nationwide Multicenter Study. Am. J. Gastroenterol. 2017, 112, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg, M.; Duijvis, N.W.; Ponsioen, C.; van den Brink, G.R.; Fockens, P.; D’Haens, G.R. Length of hospital stay and associated hospital costs with infliximab versus cyclosporine in severe ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1240–1246. [Google Scholar] [CrossRef]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohns Colitis 2022, 16, 179–189. [Google Scholar] [CrossRef]
- Chaparro, M.; Burgueno, P.; Iglesias, E.; Panes, J.; Munoz, F.; Bastida, G.; Castro, L.; Jimenez, C.; Mendoza, J.L.; Barreiro-de Acosta, M.; et al. Infliximab salvage therapy after failure of ciclosporin in corticosteroid-refractory ulcerative colitis: A multicentre study. Aliment. Pharmacol. Ther. 2012, 35, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S.; Allez, M.; Seksik, P.; Flourie, B.; Peeters, H.; Dupas, J.L.; Bouguen, G.; Peyrin-Biroulet, L.; Duclos, B.; Bourreille, A.; et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am. J. Gastroenterol. 2011, 106, 771–777. [Google Scholar] [CrossRef]
- Luzentales-Simpson, M.; Pang, Y.C.F.; Zhang, A.; Sousa, J.A.; Sly, L.M. Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases. Front. Cell Dev. Biol. 2021, 9, 612830. [Google Scholar] [CrossRef]
- Pellet, G.; Stefanescu, C.; Carbonnel, F.; Peyrin-Biroulet, L.; Roblin, X.; Allimant, C.; Nachury, M.; Nancey, S.; Filippi, J.; Altwegg, R.; et al. Efficacy and Safety of Induction Therapy With Calcineurin Inhibitors in Combination With Vedolizumab in Patients With Refractory Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Ollech, J.E.; Dwadasi, S.; Rai, V.; Peleg, N.; Normatov, I.; Israel, A.; Sossenheimer, P.H.; Christensen, B.; Pekow, J.; Dalal, S.R.; et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 637–643. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rubin, D.T.; Danese, S.; Vermeire, S.; Abhyankar, B.; Sankoh, S.; James, A.; Smyth, M. Efficacy of Vedolizumab Induction and Maintenance Therapy in Patients with Ulcerative Colitis, Regardless of Prior Exposure to Tumor Necrosis Factor Antagonists. Clin. Gastroenterol. Hepatol. 2017, 15, 229–239.e5. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Colman, R.J.; Micic, D.; Gibson, P.R.; Goeppinger, S.R.; Yarur, A.; Weber, C.R.; Cohen, R.D.; Rubin, D.T. Vedolizumab as Induction and Maintenance for Inflammatory Bowel Disease: 12-month Effectiveness and Safety. Inflamm. Bowel Dis. 2018, 24, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- D’Amico, F.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Tofacitinib in the treatment of ulcerative colitis: Efficacy and safety from clinical trials to real-world experience. Therap. Adv. Gastroenterol. 2019, 12, 1756284819848631. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Bhatnagar, S.; Parmentier, J.M.; Nakasato, P.; Wung, P. Upadacitinib: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2024, 17, e13688. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Goyal, M.K.; Midha, V.; Mahajan, R.; Kaur, K.; Gupta, Y.K.; Singh, D.; Bansal, N.; Kaur, R.; Kalra, S.; et al. Tofacitinib in Acute Severe Ulcerative Colitis (TACOS): A Randomized Controlled Trial. Am. J. Gastroenterol. 2024, 119, 1365–1372. [Google Scholar] [CrossRef]
- Narula, N.; Pray, C.; Hamam, H.; Peerani, F.; Hansen, T.; Bessissow, T.; Bressler, B.; Arun, A.; Schmit, M.; Castelli, J.; et al. Tofacitinib for Hospitalized Acute Severe Ulcerative Colitis Management (The TRIUMPH Study). Crohn’s Colitis 360 2025, 7, otaf013. [Google Scholar] [CrossRef]
- Berinstein, J.A.; Steiner, C.A.; Regal, R.E.; Allen, J.I.; Kinnucan, J.A.R.; Stidham, R.W.; Waljee, A.K.; Bishu, S.; Aldrich, L.B.; Higgins, P.D.R. Efficacy of Induction Therapy With High-Intensity Tofacitinib in 4 Patients With Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 988–990.e1. [Google Scholar] [CrossRef]
- Hanauer, S.; Panaccione, R.; Danese, S.; Cheifetz, A.; Reinisch, W.; Higgins, P.D.R.; Woodworth, D.A.; Zhang, H.; Friedman, G.S.; Lawendy, N.; et al. Tofacitinib Induction Therapy Reduces Symptoms Within 3 Days for Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 139–147. [Google Scholar] [CrossRef]
- Berinstein, J.A.; Karl, T.; Patel, A.; Dolinger, M.; Barrett, T.A.; Ahmed, W.; Click, B.; Steiner, C.A.; Dulaney, D.; Levine, J.; et al. Effectiveness of Upadacitinib for Patients with Acute Severe Ulcerative Colitis: A Multicenter Experience. Am. J. Gastroenterol. 2024, 119, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Damianos, J.A.; Osikoya, O.; Brennan, G. Upadacitinib for Acute Severe Ulcerative Colitis: A Systematic Review. Inflamm. Bowel Dis. 2025, 31, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. XELJANZ® (Tofacitinib) [Prescribing Information]. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028%2C208246s013%2C213082s003lbl.pdf (accessed on 1 September 2025).
- AbbVie Inc. Rinvoq® (Upadacitinib) [Prescribing Information]; AbbVie Inc.: North Chicago, IL, USA, 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/211675s015lbl.pdf (accessed on 1 September 2025).
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs 2011, 3, 535–545. [Google Scholar] [CrossRef]
- Ochsenkuhn, T.; Tillack, C.; Szokodi, D.; Janelidze, S.; Schnitzler, F. Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United Eur. Gastroenterol. J. 2020, 8, 91–98. [Google Scholar] [CrossRef]
- Vitali, F.; Rath, T.; Klenske, E.; Vogele, A.L.; Ganzleben, I.; Zundler, S.; Strobel, D.; Geppert, C.; Hartmann, A.; Neurath, M.F.; et al. Long-term outcomes of cyclosporin induction and ustekinumab maintenance combination therapy in patients with steroid-refractory acute severe ulcerative colitis. Therap. Adv. Gastroenterol. 2024, 17, 17562848231218555. [Google Scholar] [CrossRef]
- Janssen Biotech Inc. STELARA® (Ustekinumab) [Prescribing Information]; Janssen Biotech Inc.: Horsham, PA, USA, 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761379s001lbl.pdf (accessed on 1 September 2025).
- Li, M.; Yeung, K. Managing high-priced biologic agents: Challenges and potential solutions. J. Manag. Care Spec. Pharm. 2021, 27, 411–414. [Google Scholar] [CrossRef] [PubMed]
| Score | When to Use | Components | Interpretation |
|---|---|---|---|
| Mayo Clinic Score | General disease activity assessment (outpatient or inpatient). | Stool frequency, rectal bleeding, endoscopy, physician global (0–3 each; total 0–12). | 0–2 remission; 3–5 mild; 6–10 moderate; 11–12 severe. |
| Partial/Clinical Mayo Score | Outpatient follow-up, when endoscopy is not available. | Stool frequency, rectal bleeding, physician global (0–3 each; total 0–9). | ≤2 remission; 3–5 mild; 6–7 moderate; 8–9 severe. |
| Truelove and Witts Criteria | Initial assessment of suspected ASUC (hospital/ER). | ≥6 bloody stools/day plus ≥ 1: fever > 37.8 °C, HR > 90, Hb < 10.5 g/dL, ESR > 30 mm/h (or CRP > 30 mg/L). | Meets criteria = ASUC; if >10 stools + systemic toxicity = fulminant colitis. |
| Oxford Day-3 Index | Inpatients on IV steroids; predicts steroid failure. | Stool count (day 3), CRP. | ≥8 stools/day, or 3–8 stools/day + CRP ≥ 45 → approx. 85% risk colectomy → plan rescue therapy. |
| ACG 2019 Severity Classification | Framework for UC severity (broader than ASUC). | Combines symptoms, CRP/ESR, Hb, endoscopy. | Mild, moderate–severe, or severe–fulminant → guides outpatient vs. inpatient and escalation to biologics/small molecules. |
| Category | Do | Do Not/Caution | Notes/Evidence Gaps |
|---|---|---|---|
| Anticoagulation | Initiate LMWH (e.g., enoxaparin 40 mg SC daily) or UFH unless contraindicated | Withhold only if bleeding is life-threatening | Optimal dose and duration post-discharge remain undefined |
| Stool testing | Rule out C. difficile, CMV, and other infections before escalation | Delay testing once biologic started | Diagnostic yield decreases after immunosuppression |
| Endoscopic assessment | Perform limited flexible sigmoidoscopy to confirm severity and exclude CMV | Avoid full colonoscopy due to perforation risk | Early endoscopic confirmation supports timely escalation |
| Nutritional support | Maintain low-fiber oral diet if tolerated | Avoid TPN unless toxic megacolon or perforation | No data favor specific diet or formula |
| Medications to stop | Discontinue mesalamine, NSAIDs, anti-motility agents | Use opiates only for short-term, monitored pain | Based on ECCO and ACG guidance |
| Discharge planning | Resume prophylactic anticoagulation if risk persists; ensure steroid taper and follow-up | - | VTE risk persists up to 6 weeks post-hospitalization |
| Agent | Mechanism | Key Evidence | Onset of Effect | Guideline Stance in ASUC |
|---|---|---|---|---|
| Infliximab | Neutralizes soluble and membrane TNF; Fc-mediated macrophage effects | RCT-based | Days–1–2 weeks | Preferred rescue option (ECCO/ACG) |
| Cyclosporine | Calcineurin/NFAT blockade → ↓IL-2 T-cell activation | RCT-based | Days | Preferred rescue option (ECCO/ACG) |
| Tacrolimus | Calcineurin inhibition via FKBP | RCT-based | Days | Considered alternative (expert use) |
| Vedolizumab | Blocks lymphocyte gut homing (MAdCAM-1) | Cohort-based | Weeks | Not standard rescue; reasonable as maintenance after CNI |
| Tofacitinib | JAK-STAT blockade → broad cytokine down-regulation | RCT and prospective study based | Days (≤3) | Investigational; center-selected cases |
| Upadacitinib | JAK1 inhibition | Cohort-based | Days | Investigational |
| Ustekinumab | Targets p40 subunit → ↓Th1/Th17 signaling | Cohort-based | Weeks | Investigational |
| Agent | Trial (First Author, Year) | Population/n | Design | Comparison Arm | Induction/Dosing | Primary Endpoint | Key Result | Short-Term Colectomy | Long-Term Colectomy/Follow-Up | Serious AEs | 95% CI/p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infliximab | Sands et al., 2001 [28] | Steroid-refractory UC; n = 11 | RCT, double-blind, placebo-controlled pilot | Placebo | Single infusion ≈ 5 mg/kg IV | Clinical response at 2 weeks | 50% vs. 0% placebo | — | — | Not reported | — |
| Järnerot et al., 2005 [29,30] | Steroid-refractory ASUC; n = 45 | RCT, double-blind, placebo-controlled | Placebo | Single infusion 5 mg/kg IV | Colectomy or death at 3 months | 29% vs. 66%, placebo | 3 mo: 29% vs. 66% | 3 yrs: 50% vs. 76% | Comparable to placebo | p = 0.017 (3 mo); p = 0.012 (3 yrs) | |
| Cyclosporine | Lichtiger et al., 1994 [37] | Steroid-refractory ASUC; n = 20 | RCT, double-blind, placebo-controlled | Placebo | IV 4 mg/kg/day × 7 days | Clinical response on day 7 | 82% vs. 0% placebo | 7% vs. 44% | — | Seizures, paresthesia, hypertension (manageable) | p < 0.001 |
| Tacrolimus | Ogata et al., 2006 [39] | Steroid-refractory UC; n = 60 | RCT, double-blind, placebo-controlled | Placebo | Oral tacrolimus titrated to high (10–15 ng/mL) or low (5–10 ng/mL) trough | Clinical response | 68.4% vs. 10% placebo | — | — | Serious gastroenteritis (n=1) | p < 0.001 |
| Tofacitinib | TACOS Trial, 2024 [60] | Hospitalized ASUC on IV steroids; n = 104 | RCT, double-blind, placebo-controlled | Placebo + IV steroids | Oral 10 mg TID × 7 days | Clinical response on day 7 | 83% vs. 58.8% placebo | — | Between 7 days and 9 days: 2% vs. 6% | Dural venous sinus thrombosis (n = 1) | p = 0.007 |
| TRIUMPH Study, 2024 [61] | Steroid-refractory ASUC; n = 24 | Prospective open-label | None | Oral 10 mg TID | Clinical response on day 7 | 58.3% | 16.7% (day 7) | 25% (6 mo) | None major | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, F.; Ivanov, M.; Elmasry, S.; Gonzalez, A.J.; Malik, T.A. Acute Severe Ulcerative Colitis (ASUC): Clinical Features, Initial Management, and the Role of Advanced Therapies. Biomedicines 2025, 13, 2544. https://doi.org/10.3390/biomedicines13102544
Jamal F, Ivanov M, Elmasry S, Gonzalez AJ, Malik TA. Acute Severe Ulcerative Colitis (ASUC): Clinical Features, Initial Management, and the Role of Advanced Therapies. Biomedicines. 2025; 13(10):2544. https://doi.org/10.3390/biomedicines13102544
Chicago/Turabian StyleJamal, Fares, Marina Ivanov, Sandra Elmasry, Alejandro J. Gonzalez, and Talha A. Malik. 2025. "Acute Severe Ulcerative Colitis (ASUC): Clinical Features, Initial Management, and the Role of Advanced Therapies" Biomedicines 13, no. 10: 2544. https://doi.org/10.3390/biomedicines13102544
APA StyleJamal, F., Ivanov, M., Elmasry, S., Gonzalez, A. J., & Malik, T. A. (2025). Acute Severe Ulcerative Colitis (ASUC): Clinical Features, Initial Management, and the Role of Advanced Therapies. Biomedicines, 13(10), 2544. https://doi.org/10.3390/biomedicines13102544





