Abstract
Background/Objectives: Fabry disease (FD) is a rare X-linked disease caused by the deficient activity of the enzyme α-galactosidase A. Cardiac involvement is particularly critical, often determining the disease prognosis. Epidemiological data on FD in Portugal are limited and inconsistent, highlighting the need for targeted screening. The F-CHECK study aimed to determine the prevalence of FD through the systematic screening of a Portuguese cohort of patients with unexplained cardiomyopathies. Methods: This multicenter observational study (NCT05409846) assessed the prevalence and clinical characteristics of FD in a Portuguese cohort (n = 409) of patients from 10 central hospitals who presented with unexplained cardiomyopathies, including idiopathic hypertrophic cardiomyopathy (HCM), left ventricular hypertrophy, dilated-phase HCM, and dilated cardiomyopathy with late gadolinium enhancement in the inferolateral segment. Screening was performed using dried blood spot assays to measure α-galactosidase A activity and/or by GLA gene sequencing in whole-blood samples. Results: FD was diagnosed in 14 patients, corresponding to a prevalence of 3.4%. FD diagnosis was significantly associated with systemic manifestations such as acroparesthesias (p = 0.027) and angiokeratomas (p = 0.003), as well as an increased risk of prior arrhythmic events (p = 0.021) and cerebrovascular disease (p = 0.016). Most FD patients (57%) presented a non-founder mutation in the GLA gene; however, they were pathogenically relevant. Conclusions: The observed 3.4% prevalence highlights the importance of systematic FD screening among Portuguese patients with unexplained cardiomyopathy, extending beyond classic hypertrophic presentations to dilated forms. Specific clinical signs, electrocardiogram findings, and cardiac imaging features can serve as valuable indicators to guide targeted genetic testing for FD.
1. Introduction
Fabry disease (FD) is a rare X-linked lysosomal storage disease (LSD) caused by mutations in the GLA gene, which encodes the enzyme alpha-galactosidase A (α-Gal A). The deficiency or absence of α-Gal A leads to the intracellular accumulation of globotriaosylceramide and related glycosphingolipids within lysosomes across various cell types and organ systems, including the kidneys, heart, and nervous systems [1]. The accumulation triggers a complex cascade of pathophysiological processes, such as cellular hypertrophy, fibrosis, and inflammation, that ultimately cause progressive organ damage, life-threatening complications, and increased risk of premature death [2,3].
Clinically, FD is categorised into two major phenotypes: classic and later-onset. The classic phenotype is characterised by severely reduced (<3% of normal values) or absent α-Gal A activity and typically presents in early childhood with signs and symptoms such as cornea verticillata, acroparesthesias, and angiokeratomas [1,2]. Over time, patients often develop progressive multi-organ involvement, including chronic kidney disease with proteinuria, leading to end-stage renal failure, hypertrophic cardiomyopathy (HCM), sensorineural hearing loss, and cerebrovascular events. In contrast, the later-onset phenotype occurs in individuals with residual enzyme activity and usually lacks early symptoms. Clinical manifestations are often milder, delayed, or confined to a single organ, most commonly the heart, where significant pathology such as left ventricular hypertrophy (LVH) may emerge later in life [1].
Cardiac involvement is the primary determinant of morbidity and mortality in FD [3,4]. Multiple cardiac cell types may be affected, resulting in various cardiac phenotypes and clinical presentations. While concentric HCM is the most frequent cardiac manifestation, other variants such as asymmetric LVH, apical HCM, or even systolic left ventricular (LV) dysfunction may occur. Clinical consequences include heart failure (HF), arrhythmias, and ischemic events [1,5]. Given the progressive nature of FD and the availability of disease-specific therapies, early and accurate clinical diagnosis is critical for optimising treatment outcomes. Notably, studies have shown that enzyme replacement therapy (ERT) offers limited benefit when initiated after age 40 or in patients with advanced cardiac involvement, including significant LVH or established myocardial fibrosis [1].
Genetic testing is essential for diagnosing FD in males and females [1]. In males with the classic phenotype, a diagnosis can typically be confirmed by demonstrating severely reduced or absent α-Gal A activity [1,5]. DBS shows high sensitivity but lower specificity for FD in males, easily identifying true positives but also increasing the likelihood of false positives [6]. As such, is necessary to confirm positive cases with genetic analysis of the GLA gene. In females, diagnosis is more complex due to X-chromosome inactivation and variable expression. Enzyme activity may fall within the normal range, making genetic analysis of the GLA gene necessary for confirmation [5]. FD has been identified in approximately 0.5% to 1% of patients diagnosed with HCM, although distinguishing it from more common sarcomeric HCM forms remains challenging [3]. Due to FD’s heterogeneous and often nonspecific presentation, the systematic screening of patients with compatible phenotypes is considered the most effective approach to improve diagnostic accuracy.
In Portugal, data on FD prevalence remain limited but indicate a potentially significant disease burden, particularly in certain regions. A 2004 study by Pinto et al. estimated the national birth prevalence of LSDs in the country, identifying four FD cases diagnosed between 1982 and 2001, corresponding to a prevalence of 0.5% in the northern region of Portugal and 2.0% in other areas [7]. More recently, a Portuguese multicentre screening study conducted between 2008 and 2018, involving 780 patients with HCM, reported an FD prevalence of 4.7%, primarily attributed to the p.F113L founder variant [8]. In contrast, the Portuguese Registry of HCM (PRo-HCM), which included 1042 patients from 29 centre between 2013 and 2015, found no GLA pathogenic variants among the 528 patients who underwent genetic testing [9]. These conflicting findings underscore the importance of systematic and targeted screening to clarify FD epidemiology in Portugal and improve diagnostic precision. Table 1 summarises several FD screening studies in patients with cardiomyopathies.

Table 1.
Fabry disease cardiomyopathy: global screening summary.
This study aims to screen for FD in patients presenting with a range of cardiac phenotypes, specifically those with cardiomyopathy of unknown or uncertain aetiology. The objectives are to facilitate the timely diagnosis of FD, enhance understanding of the disease’s national epidemiology, and raise awareness among clinicians managing patients whose clinical presentations may be attributable to FD.
2. Materials and Methods
The F-CHECK study was a multicentre, observational epidemiological study, conducted between January 2021 and January 2025, enrolling 409 patients referred from cardiomyopathy consultation across 10 Portuguese hospitals (NCT05409846). Patient recruitment commenced in April 2022, after obtaining ethical approval. Patients were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Inclusion criteria encompassed patients diagnosed with: idiopathic HCM, defined by LV wall thickness ≥ 15 mm (Group A); idiopathic LVH with wall thickness ≥ 13 mm (Group B); the dilated phase of HCM (Group C); and dilated cardiomyopathy of unknown aetiology, with late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) affecting the inferolateral basal segment (Group D). Patients were diagnosed according to the 2023 ESC guidelines for the management of cardiomyopathies [19].
Initially, DBS samples were collected for enzymatic analysis of α-Gal A activity, regardless of sex. Genetic testing of the GLA gene was performed in all female participants, in males with reduced α-Gal A activity, and in individuals with inconclusive enzymatic results or without prior DBS testing. The study protocol was originally designed to align with contemporary clinical practice in Portugal, in which DBS testing was routinely ordered prior to genetic analysis. Over the course of the study period, however, this practice evolved, and DBS testing was progressively discontinued in favour of direct genetic testing with HCM panels. To ensure adequate sample size, some participants who had undergone only genetic testing were therefore included. The flowchart in Figure 1 provides a detailed overview of the number of patients included through each pathway.
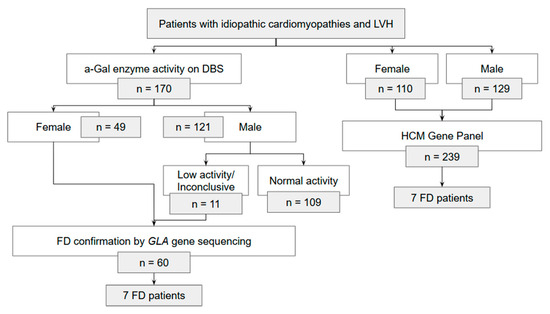
Figure 1.
Flowchart scheme of Fabry disease (FD) diagnosis in patients with idiopathic cardiomyopathies and left ventricular hypertrophy (LVH).
Genetic testing was performed by accredited diagnostic laboratories (ISO 15189) [20] using validated next-generation sequencing (NGS) cardiomyopathy panels, which included complete coverage of the GLA gene. Copy-number variation (CNV) analysis of GLA was performed. All variants classified as pathogenic or likely pathogenic were confirmed by Sanger sequencing before clinical reporting. Variant classification and description were performed by the certified laboratories, following international recommendations (PMID: 25741868, 23887774, 21681106), reducing subjective classification bias. Population frequencies were verified using gnomAD and DGV, and variant classification was conducted according to ACMG guidelines.
Sociodemographic and clinical data were collected from electronic clinical records, including cardiovascular history, current and past symptoms and signs, cardiovascular risk factors, and medication use. Additionally, the most recent findings from electrocardiogram (ECG), Holter monitoring, echocardiography, and CMR were analysed.
This project was approved by the Ethics Committee for Health of CHUSJ (CE/409/21) and other centres, according to the principles of the Helsinki Declaration, the Convention on Human Rights and Biomedicine, and the guidelines of the Council for International Organisations of Medical Sciences, and written informed consent was obtained from each patient.
No formal sample size calculation or power analysis was performed a priori. Statistical analyses were performed using R version 4.4.2 (R Foundation for Statistical Computing). Variables are presented as median and interquartile range (IQR). Due to the small number of FD cases, only non-parametric tests were used for group comparisons. The Mann–Whitney U test was applied to continuous variables, while categorical variables were analysed using the appropriate chi-square test or Fisher’s exact test (expected frequency < 5 in any cell). Statistical significance was defined as p < 0.05. Missing values were reported explicitly in tables using “-“ where data were not available. No imputation was performed. Analyses were conducted using available-case data for each variable.
Associations between FD and clinical or imaging features were assessed using logistic regression. For the overall cohort, unadjusted and multivariable-adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Adjusted models included age, sex, relevant comorbidities and medication use to account for potential confounding. Subgroup analyses were performed by LV phenotype (hypertrophic and dilated), using unadjusted logistic regression due to the limited number of FD patients in each subgroup.
The study included all consecutive patients meeting inclusion criteria during the recruitment period across participating centres to minimise sources of bias. Consequently, the sample size was determined by patient availability rather than statistical considerations. The relatively small number of FD cases, particularly in subgroup analyses, is acknowledged as a limitation and discussed in terms of precision and interpretability of results.
This study is reported in accordance with the STROBE guidelines for observational studies, and a completed STROBE checklist is provided in the Supplementary Materials (Supplemental Table S1).
3. Results
A total of 409 patients were enrolled in the study, including 250 males (61%), with a median age of 64 (range: 18–93) years. Group A comprised 72% of patients (n = 293), group B included 10% of patients (n = 78), group C accounted for 7.3% (n = 30), and group D for 2.0% (n = 8). FD was diagnosed in 14 patients, corresponding to an observed prevalence of genetically confirmed FD of 3.4% (95% CI [1.9, 5.7]) Among the 170 patients who underwent DBS testing, 71% were male (Figure 1). Of these, reduced α-Gal A activity was detected in 9% (n = 11). All female patients who underwent DBS and male patients with reduced enzyme activity were evaluated with GLA gene sequencing (n = 60). Seven GLA variants were identified in these patients (12%). Additionally, 239 patients underwent genetic testing alone, with pathogenic GLA variants identified in seven cases (2.9%). The full variant metadata is available in Appendix A Table A1.
3.1. Demographics and Clinical Characteristics of Patients
The demographic and clinical characteristics of FD and non-FD patients are summarised in Table 2. No significant differences were observed between FD and non-FD patients regarding age, sex, and body mass index (BMI). However, certain clinical features were significantly more common among FD patients, such as acroparesthesias (14 vs. 1.5%, p = 0.027) and angiokeratomas (14 vs. 0.3%, p = 0.003). FD patients had a significantly higher prevalence of prior arrhythmic events compared to non-FD patients (36 vs. 12%, p = 0.021). Cerebrovascular disease was more frequently observed in FD patients (29 vs. 6.9%, p = 0.016). Additionally, FD patients were less often prescribed β-blockers than non-FD patients (43% vs. 71%, p = 0.033), while anticoagulant use was significantly higher in the FD group (50% vs. 23%, p = 0.049).

Table 2.
Demographic and clinical characteristics of Fabry disease (FD) and non-FD patients.
3.2. Characteristics of Patients with FABRY DISEASE
FD patients had a median age of 63 (IQR 59, 65) years, and 57% were male (n = 8). The median BMI was 27 (25, 29) kg/m2. Cardiovascular symptoms/signs were present in 86% of patients (n = 12), with fatigue being the most frequently reported symptom, affecting 57% (n = 8). At least one comorbidity or cardiovascular risk factor was present in 86% (n = 12), with dyslipidaemia being the most common, observed in 57% of patients (n = 8), followed by hypertension, present in 50% (n = 7). Previous cardiovascular events were documented in 50% of FD patients (n = 7), with arrhythmias (primarily atrial fibrillation), being the most common, occurring in 36% (n = 4).
Among the 14 patients with genetically confirmed FD, seven distinct pathogenic mutations in the GLA gene were identified. The most frequent was the p.F113L variant, found in 43% (n = 6), followed by p.M290I in 21% (n = 3) and p.N215S in 14% (n = 2). The remaining four patients carried other distinct GLA gene variants. The characteristics of these 14 patients are summarised in Table A2. Some demographic and known FD red flags in patients with classical phenotype variants and patients with late-onset variants are summarised in Table A3.
3.3. Comparing FD Patients with Non-FD in Different Cardiac Phenotypic Spectra
When patients were stratified by LV phenotype, hypertrophic (groups A and B) versus dilated (groups C and D), FD was more frequent in patients with a dilated phenotype than those with a hypertrophic phenotype.
A comparison of ECG and Holter characteristics between FD and non-FD participants is presented in Table 3, dividing the patients according to LV phenotype. When comparing FD patients with non-FD patients, FD patients showed a significantly longer QRS duration in both the hypertrophic group (130 vs. 103 ms, p = 0.029) and the dilated group (169 vs. 112 ms, p = 0.013). However, the proportion of patients with a prolonged QRS duration (>110 ms) did not reach statistical significance between groups. FD patients in the hypertrophic group also had a higher prevalence of right bundle branch block (RBBB) compared to non-FD patients (56 vs. 9.1%, p < 0.001) and fascicular block (33% vs. 7.8%, p = 0.033). These ECG differences were also present in the dilated group but did not reach statistical significance.

Table 3.
Electrocardiogram (ECG) and Holter monitoring characteristics in Fabry disease (FD) and non-FD patients according to left ventricular phenotype.
In the 24 h Holter monitoring, FD patients in the hypertrophic group had significantly higher mean heart rates (HRs) than non-FD (89 vs. 66 bpm, p = 0.018). Maximum and minimum HR values were also higher in the hypertrophic FD patients; however, in the dilated group, FD patients had lower HRs than non-FD patients. These differences may reflect variations in medication use.
Table 4 compares imaging characteristics between FD and non-FD participants according to LV phenotype. Echocardiographic assessment revealed that FD patients had a smaller left atrial (LA) diameter than non-FD patients, with a more pronounced and statistically significant difference in the dilated group (39 vs. 48 mm, p = 0.013). Regarding LV function, FD patients in the hypertrophic group demonstrated a statistically significant trend toward lower LV ejection fraction (LVEF) than non-FD patients (57 vs. 62%, p = 0.042). In contrast, LVEF was higher in FD patients in the dilated group, although this difference was not statistically significant. While interventricular septum (IVS) thickness did not significantly differ between groups, the prevalence of IVS hypertrophy (IVS > 12 mm) was lower in FD patients in the hypertrophic group compared to non-FD patients (60 vs. 88%, p = 0.027).

Table 4.
Echocardiogram and cardiac magnetic resonance (CMR) characteristics in Fabry disease (FD) and non-FD patients according to left ventricular phenotype.
In the CMR assessment, the only statistically significant difference was the presence of LGE in the inferolateral basal segment, which was more frequently observed in FD patients than in non-FD patients in the hypertrophic group (78 vs. 19%, p < 0.001).
3.4. Clinical and Cardiac Outcomes According to Diagnosis and Ventricular Phenotype
Logistic regression analyses were performed to evaluate associations between FD and clinical as well as imaging features (Table 5). For the overall cohort, both unadjusted and multivariable-adjusted ORs with 95% CIs were calculated, adjusting for age, sex, comorbidities, and medication use. Subgroup analyses stratified by LV phenotype (hypertrophic vs. dilated) were performed using unadjusted ORs only, due to the limited number of FD patients in each subgroup, focusing on exam-derived variables (ECG, echocardiography, and CMR parameters).

Table 5.
Odds ratios (ORs) for clinical and cardiac features associated with Fabry disease.
In the overall cohort, FD was associated with higher odds of arrhythmia (adjusted OR 4.6 [1.2, 17.6]), acroparesthesias (adjusted OR 12.6 [1.3, 97.9]), angiokeratomas (OR 65.5 [5.9, 1469]), and cerebrovascular disease (adjusted OR 5.8 [1.3, 21.6]). Other clinical outcomes, including heart failure, cardiac device use, and common symptoms showed non-significant trends.
Among the hypertrophic subgroup, FD patients demonstrated higher unadjusted odds of RBBB (OR 13.5 [3.1, 53.1]), fascicular block (OR 5.9 [1.2, 24.0]), and LGE in the inferolateral segment (OR 14.5 [3.4, 100]), while IVS hypertrophy was less frequent (OR 0.2 [0.1, 0.8]). In the dilated subgroup, OR estimates were generally less precise due to very small numbers, with wide confidence intervals.
These findings highlight that FD is associated with specific clinical and imaging features overall, with certain ECG and CMR abnormalities particularly pronounced in the hypertrophic phenotype. The subgroup analyses should be interpreted cautiously given the limited sample sizes.
4. Discussion
The importance of screening for FD in patients with cardiac involvement is well established, and several studies have examined its prevalence in different cohorts over the past years. Reported prevalence among patients with unexplained cardiomyopathy or LVH varies across international studies, reflecting differences in genetic backgrounds, inclusion criteria, and screening methodologies. A prospective study conducted in Edmonton and Hong Kong identified FD in 2.0% of patients (5/266) with undiagnosed LVH [21]. Similarly, two large Chinese cohorts reported prevalence rates of 0.9% (8/906) [22] and 1.8% (11/602) [23] among patients with LVH and HCM, respectively. In Europe, a multicentre study applying strict inclusion criteria (men aged ≥35 years and women aged ≥40 years with unexplained LVH) found a lower prevalence of 0.5% (7/1386) [12]. Of these, two patients had the p.N215S mutation, two had the p.A143T variant, and the remaining three had distinct mutations (p.R118C, p.D244N, and p.T410A). Additional studies in Spain [10] and the Czech Republic [17] reported prevalence rates of 1.0% (5/508 and 6/589, respectively) among HCM patients, with recurrent identification of later-onset variants such as p.N215S and p.A143T. In Portugal, the burden of FD appears to be higher, attributed mainly to the p.F113L founder mutation [8]. However, data from the PRo-HCM showed no GLA mutations among 528 genotyped patients [9]. These contrasting findings highlight the need for more targeted and systematic screening strategies to avoid underdiagnosis, particularly in regions with known founder effects. In our cohort, we identified a prevalence of 3.4% for FD, reinforcing the relevance of such strategies and contributing valuable epidemiological data to the national context. Notably, most studies to date have focused exclusively on patients with unexplained hypertrophic phenotypes, often neglecting those with a dilated LV. In our cohort, 29% of patients diagnosed with FD presented with a dilated cardiac phenotype, although the limited sample, it emphasises the need to broaden screening criteria beyond classical HCM presentations to capture the full clinical spectrum of FD-related cardiac disease.
The different methodologies used for FD screening should also be considered. DBS testing, though useful, has limitations due to its low specificity, particularly in females and in patients with late-onset variants, and often requires genetic confirmation. Nonetheless, it remains a valuable tool in settings with limited access to genetic testing. The growing availability of cardiomyopathy gene panels that include GLA as a standard gene has improved diagnostic efficiency and facilitated earlier identification of FD. However, identifying a GLA variant necessitates careful classification of pathogenicity, a process that is not always straightforward and may evolve as evidence accumulates. Monda et al. conducted a systematic review and meta-analysis to assess how the classification of GLA gene variants affects the estimated prevalence of FD in cardiac screening studies [24]. The results highlighted inconsistencies in variant interpretation across studies and the risk of overestimating FD prevalence if non-pathogenic variants are misclassified, as reclassification of variants, especially p.A143T, p.D313Y, and p.E66Q, significantly influenced prevalence estimates. Our cohort identified seven distinct mutations, each requiring consideration within a clinical and population-specific context. The p.F113L variant remains the most well-characterised in the Portuguese population, a known founder mutation. Additionally, the p.M290I variant has been described in the Madeira population [25], and the p.R118C mutation has been linked to increased stroke risk in Portuguese patients [26]. The identified mutations represent a broad spectrum of clinical phenotypes, ranging from classic and severe forms of FD to late-onset presentations, and even variants whose pathogenicity remains controversial or uncertain. This distribution highlights the considerable phenotypic heterogeneity inherent in cardiac FD.
Early recognition of FD is paramount for preventing irreversible organ damage and facilitating the timely initiation of specific therapies, such as ERT, which can significantly slow disease progression. The profound implications for patient management underscore the critical need for accurate GLA variant pathogenicity assignment. The identification of seven distinct GLA gene mutations in a cohort of 409 cardiomyopathy patients, yielding an approximate prevalence of 3.4%, suggests that a substantial number of FD cases within cardiac populations may remain undiagnosed through conventional clinical pathways or broader screening efforts. This finding reveals a notable genetic contribution of FD to this specific cardiac patient population, underscoring the diagnostic yield of focused genetic investigations in high-risk clinical settings.
Consequently, cardiologists must adopt a highly nuanced diagnostic approach, recognising that FD is a spectrum disorder. Relying solely on the classic presentation will inevitably lead to significant underdiagnosis, necessitating a broader suspicion for various cardiac and non-cardiac symptoms. Furthermore, for several specific mutations there is a geographical dimension to variant prevalence (e.g., p.F113L in Portugal/Southern Italy). Therefore, cardiologists and genetic counsellors in specific geographic regions should be particularly cognizant of the prevalence of specific GLA variants within their local or ancestral populations. This regional awareness can refine diagnostic suspicion and guide more targeted screening strategies, potentially improving diagnostic efficiency and patient identification in specific ethnic or geographical groups.
Well-established clinical red flags of FD were also observed in our cohort. Features such as acroparesthesias and angiokeratomas were significantly more prevalent among FD patients than non-FD individuals (p = 0.027 and p = 0.003, respectively). The clinical suspicion of FD frequently arises from the coexistence of these extracardiac manifestations along with certain features of cardiac disease. For example, disproportionate conduction abnormalities, early arrhythmic events, or stroke in a patient with LVH should prompt consideration of FD. Furthermore, FD patients exhibited increased prior arrhythmic events cerebrovascular disease (p = 0.016), highlighting the systemic nature of the disease and the importance of multidisciplinary recognition of these warning signs.
Although our study was limited by the relatively small number of patients with genetically confirmed FD, we conducted a subgroup exploratory analysis to compare FD and non-FD participants according to LV phenotype. FD patients demonstrated significantly longer QRS durations than non-FD patients in both the hypertrophic (p = 0.029) and dilated (p = 0.013) subgroups. In the hypertrophic group, FD patients presented more frequently with RBBB (p < 0.001) and fascicular block (p = 0.033). These findings are consistent with the literature, highlighting conduction system involvement as a hallmark of cardiac FD [2]. Interestingly, these differences persisted in the dilated phenotype, although they did not achieve statistical significance, likely due to the small number of patients in this subgroup. Echocardiographic analysis further revealed that FD patients had smaller LA diameters than non-FD patients, with the difference reaching statistical significance in the dilated group (p = 0.013). This aligns with prior studies comparing FD and HCM patients, in which FD was associated with smaller LA volumes [27]. However, existing literature focuses predominantly on the hypertrophic phenotype, and our findings offer new insight into LA remodelling in the context of the dilated presentation of FD, an area previously uncharacterised. Regarding LV systolic function, FD patients in the hypertrophic group exhibited a significantly lower LVEF than their non-FD counterparts (p = 0.042), suggesting early contractile impairment or less frequent supranormal ejection compared to other HCM aetiologies [28]. Conversely, in the dilated group, FD patients tended to have relatively higher values of LVEF. IVS thickness did not differ significantly between groups; however, FD patients in the hypertrophic subgroup were less likely to present with septal hypertrophy (p = 0.027). This finding may support the hypothesis that LVH in FD follows a different remodeling pattern, often with less prominent septal involvement than in sarcomeric HCM. On cardiac MRI, LGE in the inferolateral basal segment was more frequent in FD patients within the hypertrophic group than non-FD patients (p < 0.001). This specific LGE distribution is well recognized in FD and supports its diagnostic value. However, the role of this feature in the diagnosis of FD in patients with dilated phenotype is still unclear.
In addition to the comparative analyses, logistic regression was performed to further explore the association between FD and specific clinical, ECG, and imaging features. In the overall cohort, both unadjusted and adjusted models were applied, with adjustment for age, sex, hypertension, dyslipidemia, B-blocker, and anticoagulant use. In the subgroup analyses, unadjusted models were employed due to the limited number of FD cases within each LV phenotype. The regression results demonstrated that several features, such as arrhythmia, cerebrovascular disease, acroparesthesias, and angiokeratomas, were significantly associated with FD in the overall analysis. Within the hypertrophic and dilated subgroups, conduction abnormalities, particularly right bundle branch block and fascicular block, were strongly associated with FD, corroborating the descriptive findings. Conversely, LV hypertrophy and LGE inferolateral distribution exhibited distinct trends depending on the LV phenotype, suggesting differing structural remodeling mechanisms across disease stages. Nevertheless, the wide CIs observed across several ORs reflect the small number of FD patients, introducing statistical uncertainty despite consistent effect directions. In some models, ORs could not be reliably estimated due to zero events or sparse data, underscoring the challenges of regression analysis in rare diseases. These findings highlight the need for larger, multicentric studies to validate the identified associations and refine the phenotypic predictors of FD across the hypertrophic and dilated spectrum.
5. Conclusions
In conclusion, our findings underscore the clinical relevance of systematic screening for FD in patients with unexplained cardiac phenotypes, including both hypertrophic and dilated presentations. The observed prevalence of 3.4% in our cohort, along with distinct ECG and imaging features (by echocardiography and CMR), reinforces the need to integrate FD into the differential diagnosis of cardiomyopathies. The inclusion of the GLA gene in cardiomyopathy genetic panels, combined with heightened clinical suspicion based on cardiac and extracardiac red flags, may enhance early diagnosis and appropriate management. However, the interpretation of genetic variants remains complex and context-dependent, requiring continued efforts in variant classification and population-specific characterisation. Our results contribute novel insights into the phenotypic variability of cardiac FD, particularly in the underexplored dilated phenotype, and highlight the importance of comprehensive, multidisciplinary approaches to improve detection and care for affected individuals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13102530/s1. Table S1. STROBE Statement—checklist of items that should be included in reports of observational studies.
Author Contributions
Conceptualization, E.M. and J.Q.-S.; methodology, E.M. and R.M.; validation, E.M. and J.Q.-S.; formal analysis, R.M. and I.F.; investigation, R.M.; resources, S.S., C.C., J.C., A.F.A., P.R., D.B., M.V., N.A., V.L., C.G., A.S.C., C.Q., A.T., A.S., R.F.-C., A.L., I.S. and E.M.; data curation, R.M. and I.F.; writing—original draft preparation, R.M., E.M. and J.Q.-S.; writing—review and editing, R.M., E.M., J.Q.-S. and D.B.; visualisation, R.M.; supervision, E.M. and J.Q.-S.; project administration, E.M.; funding acquisition, E.M. and J.Q.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SANOFI—Pharmaceutical Products, Lda.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of São João Hospital/FMUP (approval code: CE/409/21 and approval date: 23 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this article are not readily available due to privacy and ethical reason. Requests for access to these data should be made to the corresponding author, Elisabete Martins, who can be reached at ebernardes@med.up.pt.
Acknowledgments
The authors would like to thank all clinical and research staff from the collaborating hospitals for their invaluable contribution to the F-CHECK study.
Conflicts of Interest
The authors declare no conflicts of interest. Sanofi had no role in the study design, data collection, analysis, interpretation, and manuscript preparation.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| CMR | Cardiac magnetic resonance |
| DBS | Dried blood spot |
| ECG | Electrocardiogram |
| ERT | Enzyme replacement therapy |
| FD | Fabry disease |
| HCM | Hypertrophic cardiomyopathy |
| HF | Heart failure |
| HR | Heart rates |
| IQR | Interquartile range |
| IVS | Interventricular septum |
| LA | Left atrium |
| LGE | Late gadolinium enhancement |
| LSD | Lysosomal storage disease |
| LV | Left ventricle |
| LVD | Left ventricle diastolic |
| LVEDV | Left ventricle end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVESV | Left ventricle end-systolic volume |
| LVH | Left ventricular hypertrophy |
| LVOTO | Left ventricular outflow tract obstruction |
| LVS | Left ventricle systolic |
| OR | Odds Ratio |
| PW | Posterior wall |
| RBBB | Right bundle branch block |
| RVEDV | Right ventricle end-diastolic volume |
| RVEF | Right ventricular ejection fraction |
| RVESV | Right ventricle end-systolic volume |
| SD | Standard deviation |
| α-GAL A | α-galactosidase A |
Appendix A

Table A1.
Summary of GLA gene variants detected in patients with Fabry disease, including HGVS nomenclature, ClinVar accession numbers, and pathogenicity classification according to ACMG/ClinVar criteria.
Table A1.
Summary of GLA gene variants detected in patients with Fabry disease, including HGVS nomenclature, ClinVar accession numbers, and pathogenicity classification according to ACMG/ClinVar criteria.
| Variant (HGVS) | ClinVar Accession (VCV) | Population Frequency (gnomAD) | ACMG Classification |
|---|---|---|---|
| NM_000169.3(GLA):c.337T>C (p.Phe113Leu) | VCV000222218.26 | Not available | Pathogenic |
| NM_000169.3(GLA):c.916C>T (p.Gln306Ter) | VCV000198052.6 | Not available | Pathogenic |
| NM_000169.3(GLA):c.644A>G (p.Asn215Ser) | VCV000010730.49 | 0.00003 | Pathogenic |
| NM_000169.3(GLA):c.870G>A (p.Met.290Ile) | VCV000222435.20 | 0.000003 | Pathogenic |
| NM_000169.3(GLA):c.937G>T (p.Asp313Tyr) | VCV000010738.87 | Not available | VUS |
| NM_000169.3(GLA):c.352C>T (p.Arg118Cys) | VCV000042454.74 | 0.0006 | Likely pathogenic |

Table A2.
Demographic and clinical characteristics of the Fabry disease patients.
Table A2.
Demographic and clinical characteristics of the Fabry disease patients.
| Mutation, Phenotype Association n (%) | Group | Gender, Age | Birth City/Country | Family History | α-GAL Activity (pmol/h/spot) | Symptoms | Events | Risk Factors | RBBB | IVS (mm) | LGE Inferolateral |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NM_000169.3(GLA):c.337T>C (p.Phe113Leu) Late-onset 6 (43%) | H | F, 60 | VN de Famalicão | No | 1.6 | Dyspnea Chest pain | None | None | Yes | 12 | Yes |
| H | M, 62 | Porto | No | - | Fatigue | None | DLP | - | 18 | No | |
| H | M, 58 | Angola | Yes | 2.8 | Fatigue Murmur | HF ICD | DLP CVD | Yes | 22 | Yes | |
| H | M, 66 | Lisboa | No | - | Fatigue Dyspnea | HF | HTA | Yes | 21 | No | |
| H | M, 54 | Guimarães | No | - | Fatigue Acroparesthesias; Angiokeratomes | None | DLP | No | 16 | Yes | |
| D | M, 74 | Porto | No | - | Angiokeratomas | None | HTA | Yes | 16 | No | |
| NM_000169.3(GLA):c.916C>T (p.Gln306Ter) Classic 1 (7.1%) | H | F, 67 | VN de Famalicão | Yes | - | Dyspnea Murmur | None | DLP CVD | No | 15 | Yes |
| NM_000169.3(GLA):c.644A>G (p.Asn215Ser) Late on-set 1 (7.1%) | H | M, 43 | Brazil | Yes | 0.8 | Palpitations Acroparesthesias | AF | Smoking | Yes | 12 | Yes |
| p. N215S + p.M290I Late on-set/classic 1 (7.1%) | D | F, 65 | Vila Real | Yes | - | None | cAVB ICD | HTA DLP | Yes | 13 | Yes |
| NM_000169.3(GLA):c.870G>A (p.Met290Ile) Classic 3 (21%) | H | M, 56 | VN de Gaia | Yes | 3.0 | Fatigue | Cardioembolic | HTA DLP | Yes | 12 | Yes |
| D | M, 64 | Vila Real | No | - | None | cAVB PM | HTA DLP CVD | No | 10 | - | |
| NM_000169.3(GLA):c.937G>T (p.Asp313Tyr) Classic 1 (7.1%) | H | F, 63 | Gondomar | Yes | - | Fatigue | Atrial Flutter | DM HTA CVD | No | 12 | No |
| NM_000169.3(GLA):c.352C>T (p.Arg118Cys) Late on-set 1 (7.1%) | D | F, 64 | Coimbra | Yes | - | Fatigue Murmur | ICD | None | - | 22 | No |
| New (TBA) Classic 1 (7.1%) | H | F, 61 | Penafiel | No | - | Fatigue Chest pain Edema | None | DM HTA DLP Cornea verticillata | No | 16 | Yes |
AF, Atrial fibrillation; cAVB, complete Atrioventricular block; CVD, Cerebrovascular disease; D, dilated; DLP, dyslipidaemia; DM, diabetes mellitus; F, female; H, hypertrophic; HF, Heart failure; HTA, hypertension; ICD, Implantable cardioverter defibrillator; LGE, Late gadolinium enhancement; IVS, Interventricular septum; M, male; PM, pacemaker; RBBB, Right bundle branch block. “-” indicates data not available or not applicable.

Table A3.
Comparison of demographics and clinical Fabry disease (FD) red flags between patients with the classical phenotype variants and patients with late-onset GLA mutations.
Table A3.
Comparison of demographics and clinical Fabry disease (FD) red flags between patients with the classical phenotype variants and patients with late-onset GLA mutations.
| Classical Phenotype n = 6 | Late on-Set Variants n = 8 | |
|---|---|---|
| Age, median [range] | 63.5 [56, 67] | 62 [43, 74] |
| Male sex | 2 (33%) | 6 (75%) |
| LV Phenotype | ||
| Dilated | 2 (33%) | 2 (25%) |
| Hypertrophic | 4 (67%) | 6 (75%) |
| Family history | 4 (67%) | 3 (38%) |
| At least one FD red flag | 4 (67%) | 5 (71%) |
| Arrhythmic events | 3 (50%) | 2 (25%) |
| Acroparesthesias | 0 (0%) | 2 (25%) |
| Angiokeratomas | 0 (0%) | 2 (25%) |
| Cornea verticillata | 1 (17%) | 0 (0%) |
| Cerebrovascular disease | 3 (50%) | 1 (13%) |
| At least one FD exam red flag | 5 (83%) | 7 (88%) |
| QRS, median [range] | 164 [110, 169] | 149.5 [105, 209] |
| QRS > 110 | 2 (67%) | 3 (75%) |
| Right Bundle Branch Block | 2 (33%) | 4 (80%) |
| Fascicular block | 2 (33%) | 1 (20%) |
| Papillary hypertrophy | 0 (0%) | 1 (17%) |
| LGE Inferolateral | 4 (80%) | 4 (50%) |
FD, Fabry disease; LGE, Late gadolinium enhancement; LV, Left ventricle.
References
- Germain, D.P.; Altarescu, G.; Barriales-Villa, R.; Mignani, R.; Pawlaczyk, K.; Pieruzzi, F.; Terryn, W.; Vujkovac, B.; Ortiz, A. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol. Genet. Metab. 2022, 137, 49–61. [Google Scholar] [CrossRef]
- Azevedo, O.; Cordeiro, F.; Gago, M.F.; Miltenberger-Miltenyi, G.; Ferreira, C.; Sousa, N.; Cunha, D. Fabry Disease and the Heart: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4434. [Google Scholar] [CrossRef]
- Pieroni, M.; Namdar, M.; Olivotto, I.; Desnick, R.J. Anderson-Fabry disease management: Role of the cardiologist. Eur. Heart J. 2024, 45, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.; Brand, E.; Hughes, D.; Kantola, I.; Krämer, J.; Nowak, A.; Tøndel, C.; Wanner, C.; Spada, M. An expert consensus on the recommendations for the use of biomarkers in Fabry disease. Mol. Genet. Metab. 2023, 139, 107585. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, I.; Tuttolomondo, A.; Daidone, M.; Pinto, A. Biomarkers in Anderson–Fabry Disease. Int. J. Mol. Sci. 2020, 21, 8080. [Google Scholar] [CrossRef] [PubMed]
- Stiles, A.R.; Zhang, H.; Dai, J.; McCaw, P.; Beasley, J.; Rehder, C.; Koeberl, D.D.; McDonald, M.; Bali, D.S.; Young, S.P. A comprehensive testing algorithm for the diagnosis of Fabry disease in males and females. Mol. Genet. Metab. 2020, 130, 209–214. [Google Scholar] [CrossRef]
- Pinto, R.; Caseiro, C.; Lemos, M.; Lopes, L.; Fontes, A.; Ribeiro, H.; Pinto, E.; Silva, E.; Rocha, S.; Marcão, A.; et al. Prevalence of lysosomal storage diseases in Portugal. Eur. J. Hum. Genet. 2004, 12, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, O.; Marques, N.; Reis, L.; Cruz, I.; Craveiro, N.; Antunes, H.; Lourenço, C.; Gomes, R.; Guerreiro, R.A.; Faria, R.; et al. Predictors of Fabry disease in patients with hypertrophic cardiomyopathy: How to guide the diagnostic strategy? Am. Heart J. 2020, 226, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cardim, N.; Brito, D.; Rocha Lopes, L.; Freitas, A.; Araújo, C.; Belo, A.; Gonçalves, L.; Mimoso, J.; Olivotto, I.; Elliott, P.; et al. The Portuguese Registry of Hypertrophic Cardiomyopathy: Overall results. Rev. Port. Cardiol. 2018, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, L.; Gimeno-Blanes, J.R.; Marín, F.; Hermida-Prieto, M.; García-Honrubia, A.; Pérez, I.; Fernández, X.; de Nicolas, R.; de la Morena, G.; Payá, E.; et al. Prevalence of fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2007, 50, 2399–2403. [Google Scholar] [CrossRef]
- Havndrup, O.; Christiansen, M.; Stoevring, B.; Jensen, M.; Hoffman-Bang, J.; Andersen, P.S.; Hasholt, L.; Nørremølle, A.; Feldt-Rasmussen, U.; Køber, L.; et al. Fabry disease mimicking hypertrophic cardiomyopathy: Genetic screening needed for establishing the diagnosis in women. Eur. J. Heart Fail. 2010, 12, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Baker, R.; Pasquale, F.; Quarta, G.; Ebrahim, H.; Mehta, A.B.; Hughes, D.A.; ACES Study Group. Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: The European Anderson-Fabry Disease survey. Heart 2011, 97, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Hagège, A.A.; Caudron, E.; Damy, T.; Roudaut, R.; Millaire, A.; Etchecopar-Chevreuil, C.; Tran, T.-C.; Jabbour, F.; Boucly, C.; Prognon, P.; et al. Screening patients with hypertrophic cardiomyopathy for Fabry disease using a filter-paper test: The FOCUS study. Heart 2011, 97, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Terryn, W.; Deschoenmakere, G.; De Keyser, J.; Meersseman, W.; Van Biesen, W.; Wuyts, B.; Hemelsoet, D.; Pascale, H.; De Backer, J.; De Paepe, A.; et al. Prevalence of Fabry disease in a predominantly hypertensive population with left ventricular hypertrophy. Int. J. Cardiol. 2013, 167, 2555–2560. [Google Scholar] [CrossRef]
- Mawatari, K.; Yasukawa, H.; Oba, T.; Nagata, T.; Togawa, T.; Tsukimura, T.; Kyogoku, S.; Ohshima, H.; Minami, T.; Sugi, Y.; et al. Screening for Fabry disease in patients with left ventricular hypertrophy. Int. J. Cardiol. 2013, 167, 1059–1061. [Google Scholar] [CrossRef]
- Citro, R.; Prota, C.; Ferraioli, D.; Iuliano, G.; Bellino, M.; Radano, I.; Silverio, A.; Migliarino, S.; Polito, M.V.; Ruggiero, A.; et al. Importance of Echocardiography and Clinical “Red Flags” in Guiding Genetic Screening for Fabry Disease. Front. Cardiovasc. Med. 2022, 9, 838200. [Google Scholar] [CrossRef] [PubMed]
- Zemánek, D.; Januška, J.; Honěk, T.; Čurila, K.; Kubánek, M.; Šindelářová, Š.; Zahálková, L.; Klofáč, P.; Laštůvková, E.; Lichnerová, E.; et al. Nationwide screening of Fabry disease in patients with hypertrophic cardiomyopathy in Czech Republic. ESC Heart Fail. 2022, 9, 4160–4166. [Google Scholar] [CrossRef]
- Leung, S.P.Y.; Dougherty, S.; Zhang, X.Y.; Kam, K.K.H.; Chi, W.K.; Chan, J.Y.S.; Fung, E.; Wong, J.K.T.; Choi, P.C.L.; Chan, D.K.H.; et al. The Asian Fabry Cardiomyopathy High-Risk Screening Study 2 (ASIAN-FAME-2): Prevalence of Fabry Disease in Patients with Left Ventricular Hypertrophy. J. Clin. Med. 2024, 13, 3896. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; De Winter, T.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- ISO 15189:2022; Medical Laboratories Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2022.
- Sadasivan, C.; Chow, J.T.Y.; Sheng, B.; Chan, D.K.H.; Fan, Y.; Choi, P.C.L.; Wong, J.K.T.; Tong, M.M.B.; Chan, T.-N.; Fung, E.; et al. Screening for Fabry Disease in patients with unexplained left ventricular hypertrophy. PLoS ONE 2020, 15, e0239675. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Liu, Y.; Miao, D.; Zhang, H.; Zhang, T.; Zhang, F.; Li, P.; Dai, H.; Jiang, G.; et al. Screening for Fabry disease in patients with left ventricular hypertrophy in China: A multicentre and prospective study. ESC Heart Fail. 2024, 11, 4381–4389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, L.; Xu, Z.; Wan, K.; Xu, Y.; Wang, J.; Han, Y.; Chen, Y. Screening for Fabry disease in patients with hypertrophic cardiomyopathy using cardiac magnetic resonance imaging. Eur. Radiol. 2024, 35, 2888–2898. [Google Scholar] [CrossRef]
- Monda, E.; Diana, G.; Graziani, F.; Rubino, M.; Bakalakos, A.; Linhart, A.; Germain, D.P.; Scarpa, M.; Biagini, E.; Pieroni, M.; et al. Impact of GLA Variant Classification on the Estimated Prevalence of Fabry Disease: A Systematic Review and Meta-Analysis of Screening Studies. Circ. Genom. Precis. Med. 2023, 16, e004252. [Google Scholar] [CrossRef]
- Silva, F.; Pestana, N.; Durães, J.; Rosa, N.G.; Silva, G. Fabry Disease p.M290I Mutation is Related to Organ Involvement: A Case Report. Cureus 2021, 13, e14100. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.V.; Ferreira, S.; Pinho-e-Melo, T.; Carvalho, M.; Cruz, V.T.; Carmona, C.; Silva, F.A.; Tuna, A.; Rodrigues, M.; Ferreia, C.; et al. Mutations of the GLA Gene in Young Patients with Stroke. Stroke 2010, 41, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.; Tondi, L.; Milani, V.; Pieroni, M.; Pieruzzi, F.; Bevilacqua, F.; Pasqualin, G.; Chow, K.; Pica, S.; Lombardi, M.; et al. Left atrial remodeling in hypertrophic cardiomyopathy and Fabry disease: A CMR-based head-to-head comparison and outcome analysis. Int. J. Cardiol. 2023, 393, 131357. [Google Scholar] [CrossRef] [PubMed]
- Landucci, L.; Faxén, U.L.; Benson, L.; Rosano, G.M.C.; Dahlström, U.; Lund, L.H.; Savarese, G. Characterizing Heart Failure Across the Spectrum of the Preserved Ejection Fraction: Does Heart Failure with Supranormal Ejection Fraction Exist? Data from the Swedish Heart Failure Registry. J. Am. Heart Assoc. 2025, 14, e037502. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).