sTREM-1, HMGB1, CRP, PCT, sCD14-ST, IL-6, IL-10, sHLA-G, and Vitamin D in Relation to Clinical Scores and Survival in SIRS/Sepsis
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects and Sample Collection
2.2. Laboratory Analyses
2.2.1. Humoral Pro- and Anti-Inflammatory Biomarkers sTREM-1, HMGB1, IL-6, IL-10, sHLA-G
2.2.2. Plasma Concentration of 25-hydroxyvitamin D (25(OH)D)
3. Statistics
4. Results
4.1. Comparison of Septic and Non-Infectious SIRS Patients
4.2. Logistic Regression Analysis of Most Predictive Biomarkers Distinguishing Septic (Sepsis + Septic Shock) and Non-Infectious SIRS Patients
4.3. Comparison of Inflammatory Markers Between Survivors and Non-Survivors on Day 7
4.4. Comparison of Inflammatory Markers Between Survivors and Non-Survivors on Day 28
4.5. Logistic Regression Analysis of 7-Day and 28-Day Survival
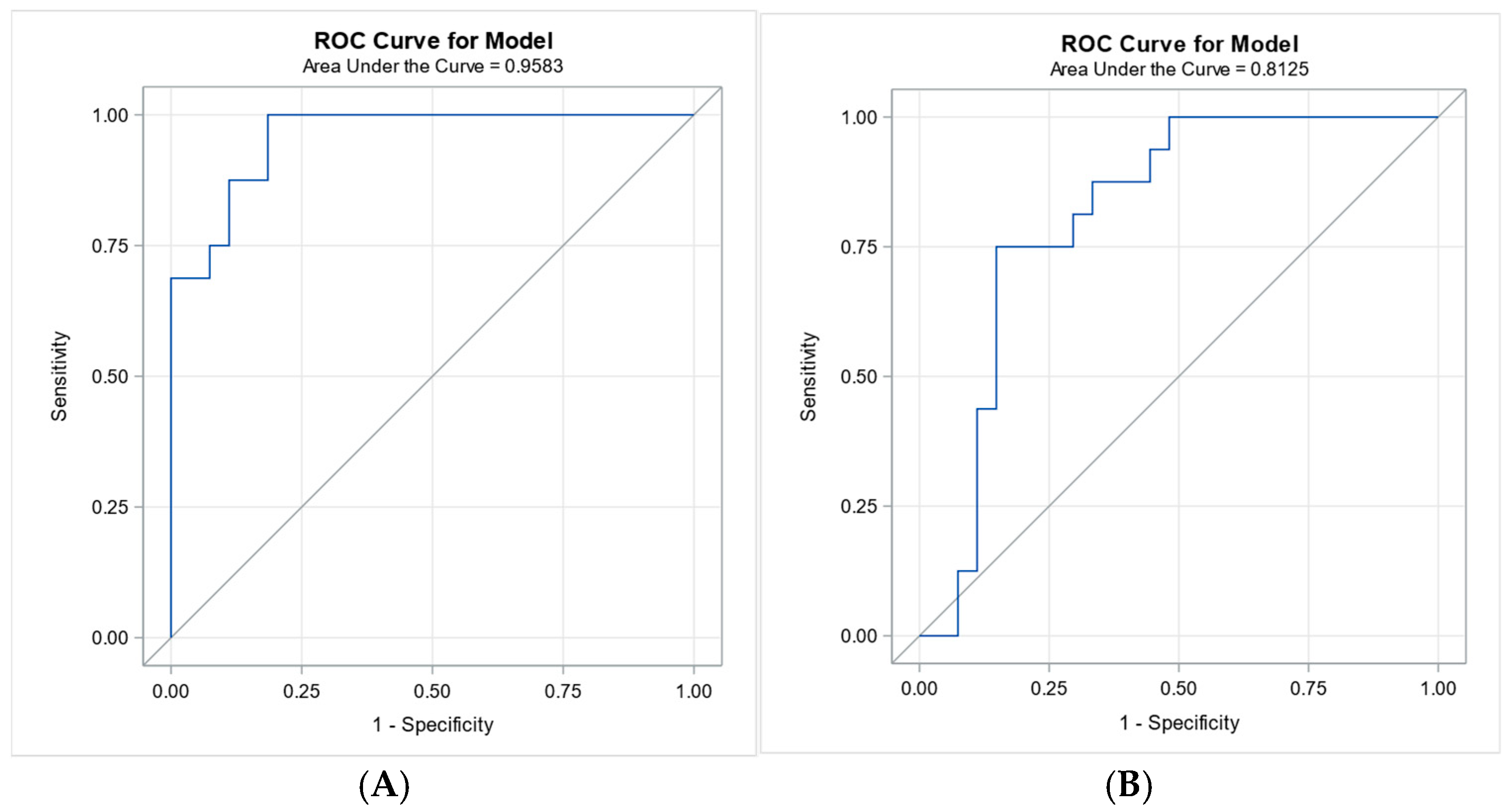
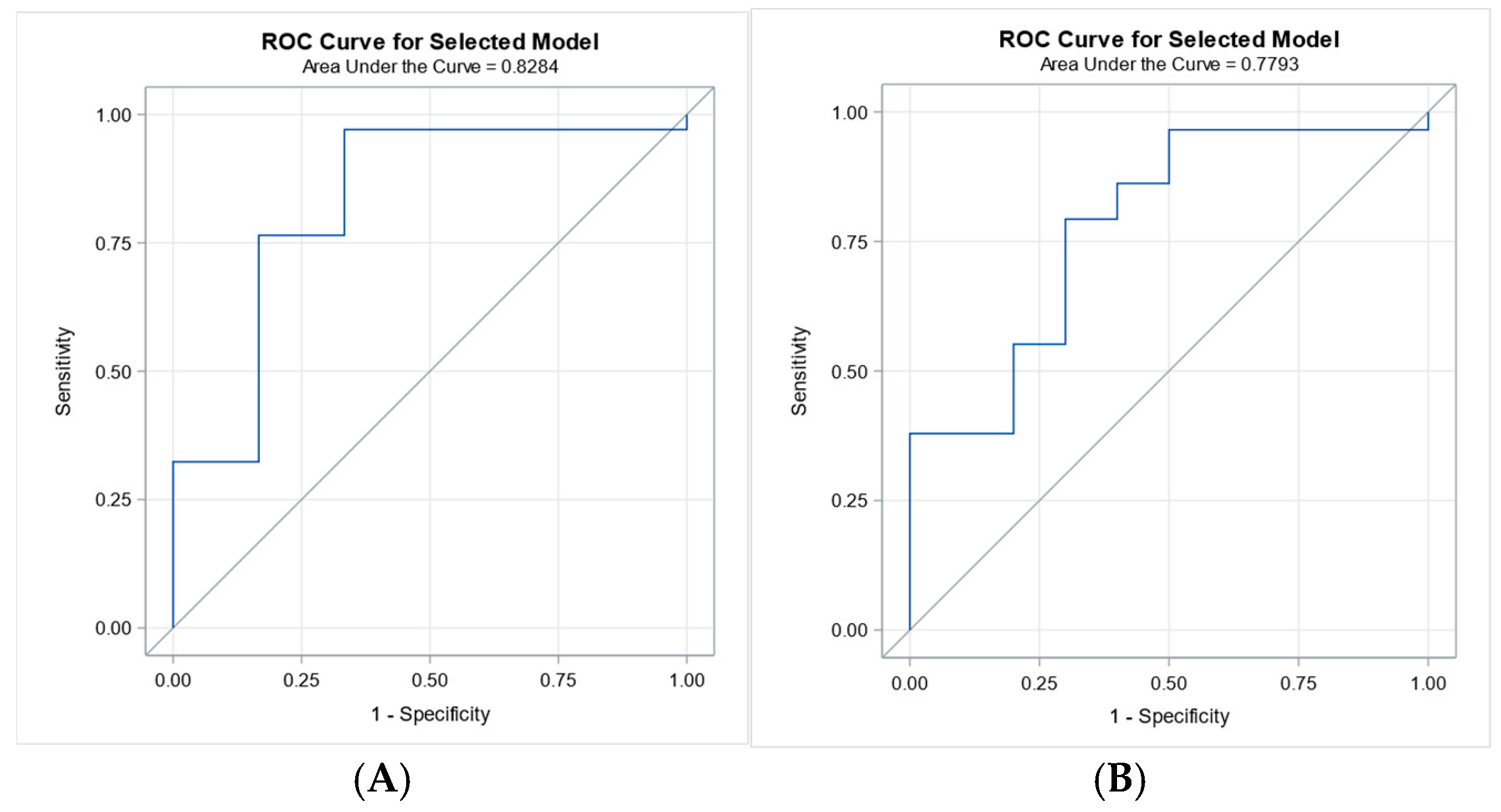
4.6. Receiver Operating Characteristic Analysis for 7-Day and 28-Day Survival
4.7. Relationship Between Biomarker Levels and 7-Day and 28-Day Survival
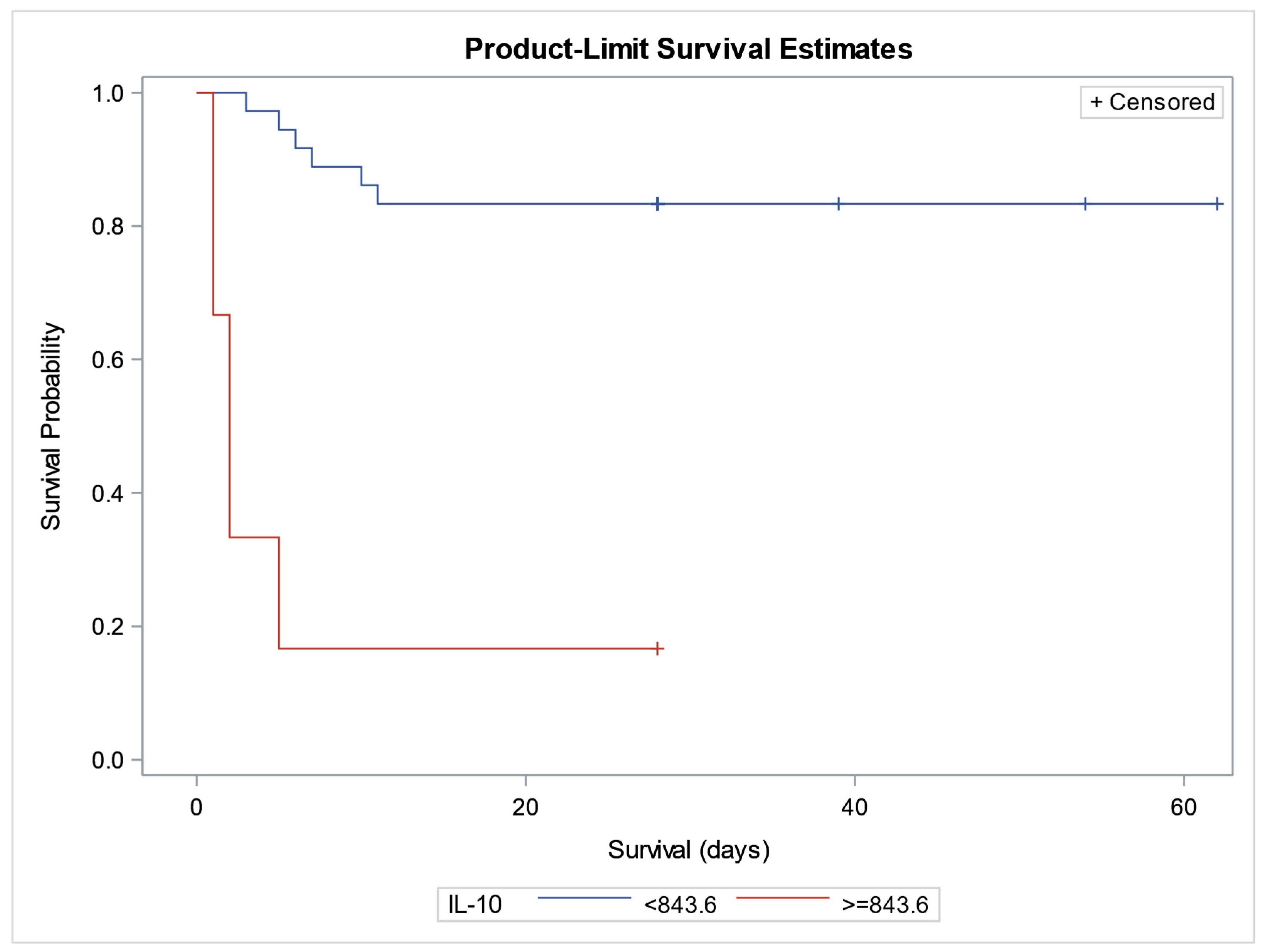
4.8. Kaplan–Meier Curves and Cox Proportional Hazards Regression Analysis for Prognostic Relevance of Biomarkers and Survival Analysis
4.9. Correlations of sTREM-1 with Other Estimated Parameters
4.10. Correlations of HMGB1 with Other Estimated Parameters
4.11. Correlations of Cytokines IL-6 and IL-10 with Other Estimated Parameters
4.12. Correlations of the Level of Anti-Inflammatory and Immunosuppressive Molecules sHLA-G and 25(OH) Vitamin D
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Vincent, J.L. Evolution of the Concept of Sepsis. Antibiotics 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis: Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ko, R.-E.; Lim, S.Y.; Park, S.; Suh, G.Y.; Lee, Y.J. Sepsis Alert Systems, Mortality, and Adherence in Emergency Departments: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2422823. [Google Scholar] [CrossRef]
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; Francois, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the Worldwide Burden of Critical Illness: The Intensive Care Over Nations (ICON) Audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Chadda, K.R.; Puthucheary, Z. Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS): A Review of Definitions, Potential Therapies, and Research Priorities. Br. J. Anaesth. 2024, 132, 507–518. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation and Immunosuppression: A Common Syndrome and New Horizon for Surgical Intensive Care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury Inflammation and Organ Dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef]
- Xiong, D.; Geng, H.; Lv, X.; Wang, S.; Jia, L. Inflammatory Response and Anti-Inflammatory Treatment in Persistent Inflammation-Immunosuppression-Catabolism Syndrome (PICS). J. Inflamm. Res. 2025, 18, 2267–2281. [Google Scholar] [CrossRef]
- François, B.; Lambden, S.; Fivez, T.; Gibot, S.; Derive, M.; Grouin, J.M.; Salcedo-Magguilli, M.; Lemarié, J.; De Schryver, N.; Jalkanen, V.; et al. Prospective Evaluation of the Efficacy, Safety, and Optimal Biomarker Enrichment Strategy for Nangibotide, a TREM-1 Inhibitor, in Patients with Septic Shock (ASTONISH): A Double-Blind, Randomised, Controlled, Phase 2b Trial. Lancet Respir. Med. 2023, 11, 894–904. [Google Scholar] [CrossRef]
- Read, C.B.; Kuijper, J.L.; Hjorth, S.A.; Heipel, M.D.; Tang, X.; Fleetwood, A.J.; Dantzler, J.L.; Grell, S.N.; Kastrup, J.; Wang, C.; et al. Cutting Edge: Identification of Neutrophil PGLYRP1 as a Ligand for TREM-1. J. Immunol. 2015, 194, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Baykara, R.A.; Kiran, T.R.; Otlu, Ö.; Erdem, M.; Pihtili Taş, N. Could TREM-1 Be a Novel Marker in the Diagnosis of Fibromyalgia? A Cross-Sectional Study. Medicine 2024, 103, e38806. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Li, C.; Cai, C.; Xu, D.; Chen, X.; Song, J. TREM1: Activation, Signaling, Cancer and Therapy. Pharmacol. Res. 2024, 204, 107212. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Zhang, B.; Mao, W.; Liu, T.; Sun, M.; Wu, Y. TREM1: A Positive Regulator for Inflammatory Response via NF-κB Pathway in A549 Cells Infected with Mycoplasma pneumoniae. Biomed. Pharmacother. 2018, 107, 1466–1472. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Samant, S.; Bakhos, J.J.; Liu, M.; Khan, B.; Makadia, J.; Muhammad, F.; Kievit, F.M.; Agrawal, D.K.; Chatzizisis, Y.S. Triggering Receptor Expressed on Myeloid Cells-1 (TREM-1) Inhibition in Atherosclerosis. Pharmacol. Ther. 2022, 238, 108182. [Google Scholar] [CrossRef]
- Bucova, M.; Suchankova, M.; Dzurilla, M.; Vrlik, M.; Novosadova, H.; Tedlova, E.; Urban, S.; Hornakova, E.; Seligova, M.; Durmanova, V.; et al. Inflammatory Marker sTREM-1 Reflects the Clinical Stage and Respiratory Tract Obstruction in Allergic Asthma Bronchiale Patients and Correlates with Number of Neutrophils. Mediat. Inflamm. 2012, 2012, 628754. [Google Scholar] [CrossRef]
- Sun, Y.; Aliyari, S.R.; Parvatiyar, K.; Wang, L.; Zhen, A.; Sun, W.; Han, X.; Zhang, A.; Kato, E.; Shi, H.; et al. STING Directly Interacts with PAR to Promote Apoptosis upon Acute Ionizing Radiation-Mediated DNA Damage. Cell Death Differ. 2025, 32, 1167–1179. [Google Scholar] [CrossRef]
- Zhou, R.; Xie, X.; Li, X.; Qin, Z.; Wei, C.; Liu, J.; Luo, Y. The Triggers of the cGAS–STING Pathway and the Connection with Inflammatory and Autoimmune Diseases. Infect. Genet. Evol. 2020, 77, 104094. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a Late Mediator of Endotoxin Lethality in Mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.J.; Wang, C.Y.; Shen, M.Z.; Jiang, S.; Li, T.; Di, W.C.; Chen, Y.; et al. Targeting HMGB1 for the Treatment of Sepsis and Sepsis-Induced Organ Injury. Acta Pharmacol. Sin. 2022, 43, 520–528. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Yin, X.Y.; Tang, X.H.; Wang, S.X.; Zhao, Y.C.; Jia, M.; Yang, J.J.; Ji, M.H.; Shen, J.C. HMGB1 Mediates Synaptic Loss and Cognitive Impairment in an Animal Model of Sepsis-Associated Encephalopathy. J. Neuroinflamm. 2023, 20, 69. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Pitts, S.L.; Maish, G.O.; Schroeppel, T.J.; Magnotti, L.J.; Croce, M.A.; Minard, G.; Brown, R.O. Vitamin D Deficiency in Critically Ill Patients with Traumatic Injuries. Burn. Trauma 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Olejarova, M.; Dobisova, A.; Suchankova, M.; Tibenska, E.; Szaboova, K.; Koutun, J.; Vlnieskova, K.; Bucova, M. Vitamin D Deficiency—A Potential Risk Factor for Sepsis Development, Correlation with Inflammatory Markers, SOFA Score and Higher Early Mortality Risk in Sepsis. Bratisl. Lek. Listy 2019, 120, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, S. The Cytokine Storm and Factors Determining the Sequence and Severity of Organ Dysfunction in Multiple Organ Dysfunction Syndrome. Am. J. Emerg. Med. 2008, 26, 711–715. [Google Scholar] [CrossRef]
- Essa, E.S.; Elzorkany, K.M. sTREM-1 in Patients with Chronic Kidney Disease on Hemodialysis. APMIS 2015, 123, 969–974. [Google Scholar] [CrossRef]
- Rohde, G.; Radsak, M.P.; Borg, I.; Buhl, R.; Schultze-Werninghaus, G.; Taube, C. Levels of Soluble Triggering Receptor Expressed on Myeloid Cells 1 in Infectious Exacerbations of Chronic Obstructive Pulmonary Disease. Respiration 2012, 83, 133–139. [Google Scholar] [CrossRef]
- Aksaray, S.; Alagoz, P.; Inan, A.; Cevan, S.; Ozgultekin, A. Diagnostic Value of sTREM-1 and Procalcitonin Levels in the Early Diagnosis of Sepsis. North. Clin. Istanb. 2017, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Kalogirou, M.S.; Patoulias, I. Triggering Receptor Expressed on Myeloid Cells-1 (TREM-1) and Its Soluble Plasma Form (sTREM-1) as a Diagnostic Biomarker in Neonatal Sepsis. Folia Med. Cracov. 2018, 58, 15–19. [Google Scholar]
- Zahorec, R. Ratio of Neutrophil to Lymphocyte Counts—Rapid and Simple Parameter of Systemic Inflammation and Stress in Critically Ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Girardot, T.; Rimmelé, T.; Venet, F.; Monneret, G. Apoptosis-Induced Lymphopenia in Sepsis and Other Severe Injuries. Apoptosis 2017, 22, 295–305. [Google Scholar] [CrossRef]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J. Immunol. 2018, 200, 1543–1553. [Google Scholar] [CrossRef]
- Scumpia, P.O.; Moldawer, L.L. Biology of Interleukin-10 and Its Regulatory Roles in Sepsis Syndromes. Crit. Care Med. 2005, 33 (Suppl. S12), S468–S471. [Google Scholar] [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus Anti-Inflammatory Cytokine Profile in Patients with Severe Sepsis: A Marker for Prognosis and Future Therapeutic Options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Heper, Y.; Akalin, E.H.; Mistik, R.; Akgöz, S.; Töre, O.; Göral, G.; Oral, B.; Budak, F.; Helvaci, S. Evaluation of Serum C-Reactive Protein, Procalcitonin, Tumor Necrosis Factor Alpha, and Interleukin-10 Levels as Diagnostic and Prognostic Parameters in Patients with Community-Acquired Sepsis, Severe Sepsis, and Septic Shock. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 481–491. [Google Scholar] [CrossRef]
- Chuang, T.Y.; Chang, H.T.; Chung, K.P.; Cheng, H.S.; Liu, C.Y.; Liu, Y.C.; Huang, H.H.; Chou, T.C.; Chang, B.L.; Lee, M.R.; et al. High Levels of Serum Macrophage Migration Inhibitory Factor and Interleukin 10 Are Associated with a Rapidly Fatal Outcome in Patients with Severe Sepsis. Int. J. Infect. Dis. 2014, 20, 13–17. [Google Scholar] [CrossRef]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine Profiles as Markers of Disease Severity in Sepsis: A Multiplex Analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Mera, S.; Tatulescu, D.; Cismaru, C.; Bondor, C.; Slavcovici, A.; Zanc, V.; Carstina, D.; Oltean, M. Multiplex Cytokine Profiling in Patients with Sepsis. APMIS 2011, 119, 155–163. [Google Scholar] [CrossRef]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low Serum 25-Hydroxyvitamin D at Critical Care Initiation Is Associated with Increased Mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Trongtrakul, K.; Feemuchang, C. Prevalence and Association of Vitamin D Deficiency and Mortality in Patients with Severe Sepsis. Int. J. Gen. Med. 2017, 10, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhang, N.; Xu, N.; Pan, Y.; Xiao, M.; Wu, J.; Zhou, H.; Yang, S.; Chen, Y. Corrigendum: 1,25-Dihydroxyvitamin D Inhibits LPS-Induced High-Mobility Group Box 1 (HMGB1) Secretion via Targeting the NF-E2-Related Factor 2-Heme Oxygenase-1-HMGB1 Pathway in Macrophages. Front. Immunol. 2018, 9, 357. [Google Scholar] [CrossRef]
- Amodio, G.; Sales de Albuquerque, R.; Gregori, S. New Insights into HLA-G Mediated Tolerance. Tissue Antigens 2014, 84, 255–263. [Google Scholar] [CrossRef]
- Loustau, M.; Anna, F.; Dréan, R.; Lecomte, M.; Langlade-Demoyen, P.; Caumartin, J. HLA-G Neo-Expression on Tumors. Front. Immunol. 2020, 11, 1685. [Google Scholar] [CrossRef]
- Amiot, L.; Vu, N.; Samson, M. Immunomodulatory Properties of HLA-G in Infectious Diseases. J. Immunol. Res. 2014, 2014, 298569. [Google Scholar] [CrossRef]
- Monneret, G.; Voirin, N.; Krawice-Radanne, I.; Bohé, J.; Lepape, A.; Rouas-Freiss, N.; Carosella, E.D. Soluble Human Leukocyte Antigen-G5 in Septic Shock: Marked and Persisting Elevation as a Predictor of Survival. Crit. Care Med. 2007, 35, 1942–1947. [Google Scholar] [CrossRef]
- Wang, H.; Ward, M.F.; Sama, A.E. Novel HMGB1-Inhibiting Therapeutic Agents for Experimental Sepsis. Shock 2009, 32, 348–357. [Google Scholar] [CrossRef]
- Grégoire, M.; Tadié, J.M.; Uhel, F.; Gacouin, A.; Piau, C.; Bone, N.; Le Tulzo, Y.; Abraham, E.; Tarte, K.; Zmijewski, J.W. Frontline Science: HMGB1 Induces Neutrophil Dysfunction in Experimental Sepsis and in Patients Who Survive Septic Shock. J. Leukoc. Biol. 2017, 101, 1281–1287. [Google Scholar] [CrossRef]

| All Patients | SIRS | Sepsis | Septic Shock | |
|---|---|---|---|---|
| Number | 43 | 16 | 11 | 16 |
| Mean age (years) | 58.977 ± 12.229 | 56.875 ± 16.342 | 60.545 ± 9.554 | 60.0 ± 9.121 |
| Sex (male/female) | 28/15 | 10/6 | 8/3 | 10/6 |
| Comorbidities: | ||||
| NYHA III-IV | 15 | 7 | 5 | 3 |
| AH | 27 | 9 | 8 | 10 |
| COPD | 2 | 0 | 1 | 1 |
| Diabetes mellitus | 12 | 1 | 4 | 7 |
| CHRI | 5 | 2 | 2 | 1 |
| Hepatopathy | 7 | 1 | 3 | 3 |
| Malignancy | 4 | 0 | 2 | 2 |
| APACHE II | 30.395 ± 9.396 | 24.563 ± 9.899 | 32.000 ± 8.173 | 35.125 ± 6.531 |
| SOFA | 12.674 ± 2.146 | 11.938 ± 2.175 | 12.273 ± 1.902 | 13.688 ± 1.991 |
| Marker | Group | N | Median/Mean | IQR/SD | p (Mann-Whitney/t-Test with Welch’s Correction *) | Adjusted p-Value (FDR Correction) |
|---|---|---|---|---|---|---|
| sTREM-1 (ng/L) | SIRS | 16 | 96.512 | 75.083 | 0.0055 | 0.0138 |
| sepsis | 27 | 201.48 | 154.71 | |||
| HMGB1 (ng/L) | SIRS | 14 | 788.12 | 243.18 | 0.0951 * | 0.1359 |
| sepsis | 26 | 919.55 | 194.33 | |||
| CRP (mg/L) | SIRS | 16 | 62.120 | 35.654 | <0.0001 * | <0.0010 |
| sepsis | 27 | 265.17 | 155.63 | |||
| PCT (ng/L) | SIRS | 16 | 10.094 | 14.291 | 0.0007 | 0.0023 |
| sepsis | 27 | 53.593 | 40.992 | |||
| sCD14-ST (ng/L) | SIRS | 16 | 579.75 | 637.37 | 0.0001 | 0.0005 |
| sepsis | 27 | 2399.0 | 5232.5 | |||
| IL-6 (ng/L) | SIRS | 14 | 187.80 | 149.45 | 0.7430 | 0.7430 |
| sepsis | 20 | 242.64 | 220.93 | |||
| IL-10 (ng/L) | SIRS | 16 | 296.29 | 262.45 | 0.7156 | 0.7951 |
| sepsis | 27 | 443.95 | 500.11 | |||
| VD (μg/L) | SIRS | 16 | 15.719 | 7.382 | 0.0148 * | 0.0247 |
| sepsis | 27 | 10.246 | 4.856 | |||
| sHLA-G (U/mL) | SIRS | 16 | 48.159 | 55.134 | 0.4435 | 0.5544 |
| sepsis | 27 | 58.872 | 50.553 | |||
| Neu/Ly | SIRS | 16 | 8.125 | 5.303 | 0.0057 | 0.0114 |
| sepsis | 25 | 17.243 | 14.218 |
| Number of Variables | Variables Included in Model | Score Chi-Square |
|---|---|---|
| 1 | CRP | 15.97 |
| 1 | PCT | 13.68 |
| 2 | CRP, PCT | 19.99 |
| 3 | CRP, PCT, Ne/Ly | 20.57 |
| 4 | CRP, PCT, sCD14-ST, VD (μg/L) | 20.19 |
| 5 | sTREM1, CRP, PCT, sCD14-ST, Ne/Ly | 20.89 |
| Biomarker | Estimate (β) | Standard Error | p-Value | Interpretation |
|---|---|---|---|---|
| Vitamin D | –0.3004 | 0.1343 | 0.025 | Significant, protective effect |
| sCD14 | 0.00188 | 0.00094 | 0.045 | Significant positive predictor |
| PCT | 0.0414 | 0.0205 | 0.044 | Significant positive predictor |
| CRP | 0.2579 | 0.1778 | 0.147 | Not significant |
| sTREM-1 | 0.00799 | 0.0059 | 0.176 | Not significant |
| Marker | 7th Day Survival | N | Median/Mean | IQR/SD | p (Mann-Whitney/t-Test with Welch’s Correction *) | Adjusted p-Value (FDR Correction) |
|---|---|---|---|---|---|---|
| sTREM-1 (ng/L) | + | 37 | 147.42 | 111.45 | 0.3096 | 0.3483 |
| − | 6 | 254.92 | 249.28 | |||
| HMGB1 (ng/L) | + | 34 | 858.77 | 227.99 | 0.1951 * | 0.2508 |
| − | 6 | 957.30 | 145.02 | |||
| CRP (mg/L) | + | 37 | 178.90 | 163.17 | 0.1298 | 0.1947 |
| − | 6 | 255.74 | 121.68 | |||
| PCT (ng/L) | + | 37 | 37.195 | 40.364 | 0.4833 | 0.4833 |
| − | 6 | 38.712 | 37.674 | |||
| sCD14-ST (ng/L) | + | 37 | 1734.2 | 4559.3 | 0.0704 | 0.1267 |
| − | 6 | 1647.7 | 841.92 | |||
| IL-10 (ng/L) | + | 37 | 291.98 | 315.36 | 0.0057 | 0.0257 |
| − | 6 | 987.36 | 579.38 | |||
| VD (μg/L) | + | 37 | 13.205 | 6.331 | 0.0035 * | 0.0315 |
| − | 6 | 6.597 | 3.550 | |||
| sHLA-G (U/mL) | + | 37 | 59.471 | 54.438 | 0.0546 | 0.1638 |
| − | 6 | 26.613 | 13.677 | |||
| Eo (109/L) | + | 36 | 0.04944 | 0.09568 | 0.0583 | 0.1312 |
| − | 4 | 0.2200 | 0.2855 |
| Marker | 28th Day Survival | N | Median/Mean | IQR/SD | p (Mann-Whitney/ t-Test with Welch’s Correction *) | Adjusted p-Value (FDR Correction) |
|---|---|---|---|---|---|---|
| sTREM-1 (ng/L) | + | 31 | 156.91 | 118.36 | 0.7530 | 0.8367 |
| − | 11 | 186.10 | 195.48 | |||
| HMGB1 (ng/L) | + | 29 | 859.93 | 228.02 | 0.5973 * | 0.8533 |
| − | 10 | 902.02 | 207.80 | |||
| CRP (mg/L) | + | 31 | 197.21 | 167.17 | 0.9544 | 0.9544 |
| − | 11 | 184.37 | 138.21 | |||
| PCT (ng/L) | + | 31 | 43.256 | 41.284 | 0.3525 | 0.8813 |
| − | 11 | 24.189 | 32.183 | |||
| sCD14-ST (ng/L) | + | 31 | 1907.8 | 4956.9 | 0.4570 | 0.9140 |
| − | 11 | 1327.8 | 999.92 | |||
| IL-6 (ng/L) | + | 27 | 208.96 | 177.81 | 0.6326 | 0.7908 |
| − | 6 | 305.89 | 255.50 | |||
| IL-10 (ng/L) | + | 31 | 278.27 | 319.51 | 0.0176 | 0.1760 |
| − | 11 | 711.95 | 561.34 | |||
| VD (μg/L) | + | 31 | 13.248 | 6.534 | 0.0500 * | 0.2500 |
| − | 11 | 9.105 | 5.342 | |||
| sHLA-G (U/mL) | + | 24 | 61.024 | 42.160 | 0.0614 | 0.2047 |
| − | 11 | 47.573 | 56.588 | |||
| Eo (109/L) | + | 30 | 0.05667 | 0.1032 | 0.5591 | 0.9318 |
| − | 9 | 0.1067 | 0.2059 |
| Survival | N | Markers | Score Chi-Square |
|---|---|---|---|
| 7-day | 1 | IL-10 | 12.56 |
| 7-day | 1 | VD | 5.67 |
| 7-day | 2 | HMGB1, IL-10 | 14.38 |
| 7-day | 3 | HMGB1, IL-10, VD (μg/L) | 15.02 |
| 7-day | 4 | HMGB1, IL-10, sHLA-G, VD (μg/L) | 15.27 |
| 28-day | 1 | IL-10 | 9 |
| 28-day | 1 | VD | 5.04 |
| 28-day | 2 | IL-10, VD (μg/L) | 10.37 |
| 28-day | 3 | PCT, IL-10, VD (μg/L) | 12.21 |
| 28-day | 4 | HMGB1, PCT, IL-10, VD (μg/L) | 13.29 |
| Biomarker | Survival Time | Estimate (β) | Standard Error | p-Value | Odds Ratio (OR) | 95% CI for OR | Interpretation |
|---|---|---|---|---|---|---|---|
| IL-10 | 7-day | –0.00492 | 0.00235 | 0.037 | 0.995 | 0.991–1.000 | Significant predictor |
| IL-10 | 28-day | –0.00262 | 0.0012 | 0.029 | 0.997 | 0.995–1.000 | Significant predictor |
| Vitamin D | 28-day | 0.1645 | 0.0912 | 0.071 | 1.179 | 0.986–1.409 | Borderline significance |
| Variable | Estimate (β) | Standard Error | Hazard Ratio (HR) | p-Value | Interpretation |
|---|---|---|---|---|---|
| IL-10 | 0.00241 | 0.00093 | 1.002 | 0.0096 | Significant predictor of increased mortality risk |
| Vitamin D | –0.14138 | 0.07619 | 0.868 | 0.0635 | Borderline protective effect for survival |
| Age | –0.01617 | 0.04853 | 0.984 | 0.7389 | Not significant |
| Sex (male vs. female) | –0.32945 | 1.11768 | 0.719 | 0.7682 | Not significant |
| BMI | –0.03247 | 0.11118 | 0.968 | 0.7702 | Not significant |
| NYHA III-IV | 0.27215 | 0.96465 | 1.313 | 0.7778 | Not significant |
| AH | 1.3456 | 1.10075 | 3.841 | 0.2215 | Not significant |
| COPD | –16.1199 | 4330 | ~0 | 0.997 | Unstable estimate due to low event count |
| Diabetes | –0.31623 | 0.97971 | 0.729 | 0.7469 | Not significant |
| CHRI | 0.45045 | 1.14936 | 1.569 | 0.6951 | Not significant |
| Hepatic Cirrhosis | 1.14233 | 0.90567 | 3.134 | 0.2072 | Not significant |
| Malignancy | –16.18316 | 2825 | ~0 | 0.9954 | Unstable estimate due to low event count |
| Marker | N | SR | 95% CI | p (Spearman Test) | Adjusted p-Value (FDR Correction) | |
|---|---|---|---|---|---|---|
| sTREM-1 | CRP | 43 | 0.5747 | 0.3233–0.7503 | <0.0001 | 0.004 |
| PCT | 43 | 0.4564 | 0.1719–0.6706 | 0.0021 | 0.021 | |
| sCD14-ST | 43 | 0.3908 | 0.09328–0.6242 | 0.0096 | 0.0384 | |
| HMGB1 | 40 | 0.2950 | −0.02774–0.5621 | 0.0646 | 0.078303 | |
| VD | 43 | −0.4211 | −0.6458–0.1291 | 0.0049 | 0.028 | |
| Comorbid | 43 | 0.4388 | 0.1504–0.6583 | 0.0032 | 0.0256 | |
| APACHE II | 43 | 0.4722 | 0.1913–0.6815 | 0.0014 | 0.018667 | |
| SOFA | 43 | 0.3820 | 0.08308–0.6179 | 0.0115 | 0.041818 | |
| HMGB1 | CRP | 40 | 0.3822 | 0.07066–0.6258 | 0.0149 | 0.049667 |
| PCT | 40 | 0.3676 | 0.05374–0.6153 | 0.0196 | 0.041263 | |
| sTREM-1 | 40 | 0.2950 | −0.02774–0.5621 | 0.0646 | 0.076 | |
| sHLA-G | 40 | −0.3694 | −0.6166–0.05587 | 0.0190 | 0.0475 | |
| Leu | 40 | −0.3683 | −0.6158–0.05457 | 0.0194 | 0.043111 | |
| Ly | 38 | −0.2898 | −0.5646–0.04282 | 0.0776 | 0.081684 | |
| Neu | 38 | −0.3840 | −0.6327–−0.06344 | 0.0173 | 0.046133 | |
| Mo | 38 | −0.3032 | −0.5745–0.02811 | 0.0642 | 0.08025 | |
| IL-6 | CRP | 34 | 0.3213 | −0.02940–0.6016 | 0.0639 | 0.088138 |
| PCT | 34 | 0.3695 | 0.02538–0.6354 | 0.0315 | 0.057273 | |
| VD | 34 | −0.3675 | −0.6340–−0.02302 | 0.0325 | 0.056522 | |
| IL-10 | Age | 43 | 0.2683 | −0.04407–0.5329 | 0.0819 | 0.0819 |
| APACHE II | 43 | 0.2780 | −0.03366–0.5403 | 0.0711 | 0.079 | |
| SOFA | 43 | 0.4172 | 0.1245–0.6431 | 0.0054 | 0.024 | |
| sHLA-G | HMGB1 | 40 | −0.3694 | −0.6166–0.05587 | 0.0190 | 0.044706 |
| Eo | 40 | −0.3819 | −0.6256–0.07033 | 0.0150 | 0.046154 | |
| SOFA | 43 | −0.3364 | −0.5845–0.03091 | 0.0274 | 0.0548 | |
| VD | CRP | 43 | −0.4963 | −0.6981–0.2215 | 0.0007 | 0.014 |
| PCT | 43 | −0.2694 | −0.5337–0.04293 | 0.0807 | 0.082769 | |
| sCD14-ST | 43 | −0.4294 | −0.6517–0.1391 | 0.0041 | 0.027333 | |
| sTREM-1 | 43 | −0.4211 | −0.6458–0.1291 | 0.0049 | 0.0245 | |
| IL-6 | 34 | −0.3675 | −0.6340–−0.02302 | 0.0325 | 0.054167 | |
| Leu | 43 | 0.2744 | −0.03746–0.5376 | 0.0749 | 0.080973 | |
| Neu | 41 | 0.3726 | 0.06393–0.6162 | 0.0164 | 0.046857 | |
| Mo | 41 | 0.3133 | −0.003224–0.5728 | 0.0461 | 0.065857 | |
| APACHE II | 43 | −0.2793 | −0.5414–0.03217 | 0.0697 | 0.079657 | |
| SOFA | 43 | −0.3100 | −0.5647–0.001403 | 0.0431 | 0.063852 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopcova, M.; Dobisova, A.; Suchankova, M.; Tibenska, E.; Szaboova, K.; Koutun, J.; Bucova, M. sTREM-1, HMGB1, CRP, PCT, sCD14-ST, IL-6, IL-10, sHLA-G, and Vitamin D in Relation to Clinical Scores and Survival in SIRS/Sepsis. Biomedicines 2025, 13, 2481. https://doi.org/10.3390/biomedicines13102481
Kopcova M, Dobisova A, Suchankova M, Tibenska E, Szaboova K, Koutun J, Bucova M. sTREM-1, HMGB1, CRP, PCT, sCD14-ST, IL-6, IL-10, sHLA-G, and Vitamin D in Relation to Clinical Scores and Survival in SIRS/Sepsis. Biomedicines. 2025; 13(10):2481. https://doi.org/10.3390/biomedicines13102481
Chicago/Turabian StyleKopcova, Michaela, Anna Dobisova, Magda Suchankova, Elena Tibenska, Kinga Szaboova, Juraj Koutun, and Maria Bucova. 2025. "sTREM-1, HMGB1, CRP, PCT, sCD14-ST, IL-6, IL-10, sHLA-G, and Vitamin D in Relation to Clinical Scores and Survival in SIRS/Sepsis" Biomedicines 13, no. 10: 2481. https://doi.org/10.3390/biomedicines13102481
APA StyleKopcova, M., Dobisova, A., Suchankova, M., Tibenska, E., Szaboova, K., Koutun, J., & Bucova, M. (2025). sTREM-1, HMGB1, CRP, PCT, sCD14-ST, IL-6, IL-10, sHLA-G, and Vitamin D in Relation to Clinical Scores and Survival in SIRS/Sepsis. Biomedicines, 13(10), 2481. https://doi.org/10.3390/biomedicines13102481





