Dissociation of Clinical Outcomes and CSF Proteinopathy Biomarkers in Parkinson’s Disease: Cognitive–Affective Dissociation with Specificity for Tau
Abstract
1. Introduction
2. Methods
2.1. Outcomes
2.2. Biomarkers
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biomarker Description and Validation
3.3. Cognition, Depression and Anxiety
3.4. Longitudinal Cognition and CSF Biomarkers
4. Discussion
4.1. Cognitive Impairment in PD
4.2. Depression and Anxiety in PD
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Xiang, C.; Zhang, S.; Zhang, T.; Wang, H.; Cong, S. Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci. Biobehav. Rev. 2022, 141, 104749. [Google Scholar] [CrossRef]
- Blundell, E.K.; Grover, L.E.; Stott, J.; Schrag, A. The experience of Anxiety for people with Parkinson’s disease. NPJ Park. Dis. 2023, 9, 75. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Ravina, B.; Camicioli, R.; Como, P.G.; Marsh, L.; Jankovic, J.; Weintraub, D.; Elm, J. The impact of depressive symptoms in early Parkinson disease. Neurology 2007, 69, 342–347. [Google Scholar] [CrossRef]
- Watson, G.S.; Leverenz, J.B. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 2010, 20, 640–645. [Google Scholar] [CrossRef]
- Global Parkinson’s Disease Survey (GPDS) Steering Committee. Factors impacting on quality of life in Parkinson’s disease: Results from an international survey. Mov. Disord. 2002, 17, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiao, L.; Li, M.; Wen, X.; Zhang, W.; Li, X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. Front Aging Neurosci. 2024, 16, 1498756. [Google Scholar] [CrossRef] [PubMed]
- Chikatimalla, R.; Dasaradhan, T.; Koneti, J.; Cherukuri, S.P.; Kalluru, R.; Gadde, S. Depression in Parkinson’s Disease: A Narrative Review. Cureus. 2022, 14, e27750. [Google Scholar] [CrossRef]
- Hatcher-Martin, J.M.; McKay, J.L.; Pybus, A.F.; Sommerfeld, B.; Howell, J.C.; Goldstein, F.C.; Wood, L.; Hu, W.T.; Factor, S.A. Cerebrospinal fluid biomarkers in Parkinson’s disease with freezing of gait: An exploratory analysis. NPJ Park. Dis. 2021, 7, 105. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Liampas, I.; Kyriakoulopoulou, P.; Siokas, V.; Tsiamaki, E.; Stamati, P.; Kefalopoulou, Z.; Chroni, E.; Dardiotis, E. Apolipoprotein E Gene in α-Synucleinopathies: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1795. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Bucci, G.; Emanuele, M.; Leo, D.; Sotnikova, T.D.; Mus, L.V.; Soubrane, C.H.; Dallas, M.L.; Thalhammer, A.; Cingolani, L.A.; et al. Exogenous α-synuclein decreases raft partitioning of Cav2.2 channels inducing dopamine release. J. Neurosci. 2014, 34, 10603–10615. [Google Scholar] [CrossRef] [PubMed]
- Putzier, I.; Kullmann, P.H.; Horn, J.P.; Levitan, E.S. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J. Neurosci. 2009, 29, 15414–15419. [Google Scholar] [CrossRef]
- Wong, Y.C.; Luk, K.; Purtell, K.; Burke Nanni, S.; Stoessl, A.J.; Trudeau, L.E.; Yue, Z.; Krainc, D.; Oertel, W.; Obeso, J.A.; et al. Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals? Mov. Disord. 2019, 34, 1406–1422. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Fan, T.S.; Liu, S.C.; Wu, R.M. Alpha-Synuclein and Cognitive Decline in Parkinson Disease. Life 2021, 11, 1239. [Google Scholar] [CrossRef]
- Miquel-Rio, L.; Sarriés-Serrano, U.; Pavia-Collado, R.; Meana, J.J.; Bortolozzi, A. The Role of α-Synuclein in the Regulation of Serotonin System: Physiological and Pathological Features. Biomedicines 2023, 11, 541. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, H.H.; Liu, S.J.; Deng, Y.Q.; Zhang, Y.J.; Tian, Q.; Wang, X.C.; Chen, X.Q.; Yang, Y.; Zhang, J.Y.; et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Stokin, G.B.; Lillo, C.; Falzone, T.L.; Brusch, R.G.; Rockenstein, E.; Mount, S.L.; Raman, R.; Davies, P.; Masliah, E.; Williams, D.S.; et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 2005, 307, 1282–1288. [Google Scholar] [CrossRef]
- Iqbal, K.; Grundke-Iqbal, I.; Zaidi, T.; Merz, P.A.; Wen, G.Y.; Shaikh, S.S.; Wisniewski, H.M.; Alafuzoff, I.; Winblad, B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet 1986, 2, 421–426. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cholerton, B.; Shi, M.; Ginghina, C.; Cain, K.C.; Auinger, P.; Parkinson Study Group DATATOP Investigators; Zhang, J. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.H.; Tennagels, S.; Gold, R.; Gerwert, K.; Beyer, L.; Tönges, L. Update on CSF Biomarkers in Parkinson’s Disease. Biomolecules 2022, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Jennings, D.; Lasch, S.; Siderowf, A.; Tanner, C.; Simuni, T.; Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 2011, 95, 629–635. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Shin, C.; Park, M.H.; Lee, S.H.; Ko, Y.H.; Kim, Y.K.; Han, K.M.; Jeong, H.G.; Han, C. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J. Affect. Disord. 2019, 259, 370–375. [Google Scholar] [CrossRef]
- Oei, T.P.; Evans, L.; Crook, G.M. Utility and validity of the STAI with anxiety disorder patients. Br. J. Clin. Psychol. 1990, 29, 429–432. [Google Scholar] [CrossRef]
- Shaw, L.M.; Waligorska, T.; Fields, L.; Korecka, M.; Figurski, M.; Trojanowski, J.Q.; Eichenlaub, U.; Wahl, S.; Quan, M.; Pontecorvo, M.J.; et al. Derivation of cutoffs for the Elecsys(®) amyloid β (1-42) assay in Alzheimer’s disease. Alzheimers Dement. 2018, 10, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.; Zetterberg, H.; Teunissen, C.E.; Ostlund, R.E., Jr.; Militello, M.; Andreasson, U.; Hubeek, I.; Gibson, D.; Chu, D.C.; Eichenlaub, U.; et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement. 2016, 12, 517–526. [Google Scholar] [CrossRef]
- Hansson, O.; Seibyl, J.; Stomrud, E.; Zetterberg, H.; Trojanowski, J.Q.; Bittner, T.; Lifke, V.; Corradini, V.; Eichenlaub, U.; Batrla, R.; et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018, 14, 1470–1481. [Google Scholar] [CrossRef]
- Lim, E.W.; Aarsland, D.; Ffytche, D.; Taddei, R.N.; van Wamelen, D.J.; Wan, Y.M.; Tan, E.K.; Ray Chaudhuri, K.; Kings Parcog groupMDSNonmotor Study Group. Amyloid-β and Parkinson’s disease. J. Neurol. 2019, 266, 2605–2619. [Google Scholar] [CrossRef]
- Goldman, J.G.; Williams-Gray, C.; Barker, R.A.; Duda, J.E.; Galvin, J.E. The spectrum of cognitive impairment in Lewy body diseases. Mov. Disord. 2014, 29, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C.; Growdon, W.; Gomez-Isla, T.; Newell, K.; George, J.M.; Clayton, D.F.; Hyman, B.T. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 1998, 57, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Sugeno, N.; Takeda, A.; Hasegawa, T.; Kobayashi, M.; Kikuchi, A.; Mori, F.; Wakabayashi, K.; Itoyama, Y. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J. Biol. Chem. 2008, 283, 23179–23188. [Google Scholar] [CrossRef]
- Power, J.H.; Barnes, O.L.; Chegini, F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2017, 27, 3–12. [Google Scholar] [CrossRef]
- Klucken, J.; Poehler, A.M.; Ebrahimi-Fakhari, D.; Schneider, J.; Nuber, S.; Rockenstein, E.; Schlötzer-Schrehardt, U.; Hyman, B.T.; McLean, P.J.; Masliah, E.; et al. Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy 2012, 8, 754–766. [Google Scholar] [CrossRef]
- Olanow, C.W.; Perl, D.P.; DeMartino, G.N.; McNaught, K.S. Lewy-body formation is an aggresome-related process: A hypothesis. Lancet Neurol. 2004, 3, 496–503. [Google Scholar] [CrossRef]
- Cooper, A.A.; Gitler, A.D.; Cashikar, A.; Haynes, C.M.; Hill, K.J.; Bhullar, B.; Liu, K.; Xu, K.; Strathearn, K.E.; Liu, F.; et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006, 313, 324–328. [Google Scholar] [CrossRef]
- Chin, K.S.; Yassi, N.; Churilov, L.; Masters, C.L.; Watson, R. Prevalence and clinical associations of tau in Lewy body dementias: A systematic review and meta-analysis. Park. Relat. Disord. 2020, 80, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gomperts, S.N. Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Continuum 2016, 22, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Førland, M.G.; Tysnes, O.B.; Aarsland, D.; Maple-Grødem, J.; Pedersen, K.F.; Alves, G.; Lange, J. The value of cerebrospinal fluid α-synuclein and the tau/α-synuclein ratio for diagnosis of neurodegenerative disorders with Lewy pathology. Eur. J. Neurol. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Fayyad, M.; Salim, S.; Majbour, N.; Erskine, D.; Stoops, E.; Mollenhauer, B.; El-Agnaf, O.M.A. Parkinson’s disease biomarkers based on α-synuclein. J. Neurochem. 2019, 150, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Abeta, tau, and alpha-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Palmio, J.; Suhonen, J.; Keränen, T.; Hulkkonen, J.; Peltola, J.; Pirttilä, T. Cerebrospinal fluid tau as a marker of neuronal damage after epileptic seizure. Seizure 2009, 18, 474–477. [Google Scholar] [CrossRef]
- Fischer, D.L.; Menard, M.; Abdelaziz, O.Z.; Kanaan, N.M.; Cobbs, V.G.; Kennedy, R.E.; Serrano, G.E.; Beach, T.G.; Volpicelli-Daley, L.A. Distinct subcellular localization of tau and alpha-synuclein in lewy body disease. Acta Neuropathol. Commun. 2025, 13, 14. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134 Pt 5, 1493–1505. [Google Scholar] [CrossRef]
- Irwin, D.J.; White, M.T.; Toledo, J.B.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.; Leverenz, J.B.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Stancu, I.C.; Buist, A.; Bird, M.; Wang, P.; Vanoosthuyse, A.; Van Kolen, K.; Verheyen, A.; Kienlen-Campard, P.; Octave, J.N.; et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 2016, 131, 549–569. [Google Scholar] [CrossRef]
- Williams, T.; Sorrentino, Z.; Weinrich, M.; Giasson, B.I.; Chakrabarty, P. Differential cross-seeding properties of tau and α-synuclein in mouse models of tauopathy and synucleinopathy. Brain Commun. 2020, 2, fcaa090. [Google Scholar] [CrossRef]
- Colloby, S.J.; McAleese, K.E.; Walker, L.; Erskine, D.; Toledo, J.B.; Donaghy, P.C.; McKeith, I.G.; Thomas, A.J.; Attems, J.; Taylor, J.P. Patterns of tau, amyloid and synuclein pathology in ageing, Alzheimer’s disease and synucleinopathies. Brain 2025, 148, 1562–1576. [Google Scholar] [CrossRef]
- Sahoo, T.A.; Chand, J.; Kandy, A.T.; Antony, S.; Subramanian, G. Unravelling the Proteinopathic Engagement of α-Synuclein, Tau, and Amyloid Beta in Parkinson’s Disease: Mitochondrial Collapse as a Pivotal Driver of Neurodegeneration. Neurochem. Res. 2025, 50, 145. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Agarwal, P. Depression and Anxiety in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Moberg, P.J.; Duda, J.E.; Katz, I.R.; Stern, M.B. Recognition and treatment of depression in Parkinson’s disease. J. Geriatr. Psychiatry Neurol. 2003, 16, 178–183. [Google Scholar] [CrossRef]
- Patriquin, M.A.; Mathew, S.J. The Neurobiological Mechanisms of Generalized Anxiety Disorder and Chronic Stress. Chronic Stress 2017, 1, 2470547017703993. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001, 26, S11–S16. [Google Scholar] [CrossRef]
- van Dooren, F.E.; Schram, M.T.; Schalkwijk, C.G.; Stehouwer, C.D.; Henry, R.M.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.; Koster, A.; Sep, S.J.; et al. Associations of low grade inflammation and endothelial dysfunction with depression—The Maastricht Study. Brain Behav. Immun. 2016, 56, 390–396. [Google Scholar] [CrossRef]
- Watt, D.F.; Panksepp, J. Depression: An evolutionarily conserved mechanism to terminate separation distress? A review of aminergic, peptidergic, and neural network perspectives. Neuropsychoanalysis 2009, 11, 7–51. [Google Scholar] [CrossRef]
- Caudal, D.; Alvarsson, A.; Björklund, A.; Svenningsson, P. Depressive-like phenotype induced by AAV-mediated overexpression of human α-synuclein in midbrain dopaminergic neurons. Exp. Neurol. 2015, 273, 243–252. [Google Scholar] [CrossRef]
- Kennis, M.; Gerritsen, L.; van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef]
- Fregni, F.; Santos, C.M.; Myczkowski, M.L.; Rigolino, R.; Gallucci-Neto, J.; Barbosa, E.R.; Valente, K.D.; Pascual-Leone, A.; Marcolin, M.A. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Twait, E.L.; Wu, J.H.; Kamarioti, M.; Basten, M.; van der Flier, W.M.; Gerritsen, L.; Geerlings, M.I. Association of amyloid-beta with depression or depressive symptoms in older adults without dementia: A systematic review and meta-analysis. Transl. Psychiatry 2024, 14, 25. [Google Scholar] [CrossRef]
- Rotter, A.; Lenz, B.; Pitsch, R.; Richter-Schmidinger, T.; Kornhuber, J.; Rhein, C. Alpha-Synuclein RNA Expression is Increased in Major Depression. Int. J. Mol. Sci. 2019, 20, 2029. [Google Scholar] [CrossRef]
- Du, T.; Li, G.; Luo, H.; Pan, Y.; Xu, Q.; Ma, K. Hippocampal alpha-synuclein mediates depressive-like behaviors. Brain Behav. Immun. 2021, 95, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Singleton, A.; Shaw, L.M.; Trojanowski, J.Q.; Frasier, M.; Simuni, T.; Iranzo, A.; et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019, 34, 1354–1364. [Google Scholar] [CrossRef] [PubMed]

| MoCA Test | |||
|---|---|---|---|
| ≥26 (n = 304) | <26 (n = 134) | p-Value | |
| Age | 59.09 (±9.01) | 64.28 (±8.96) | <0.001 |
| Sex (Male) | 179 (58.9%) | 79 (59.0%) | 1 |
| White | 301 (99.0%) | 124 (92.5%) | <0.001 |
| Hispanic/Latino | 19 (6.2%) | 8 (6.0%) | 1 |
| Asian | 2 (0.7%) | 4 (3.0%) | 0.14 |
| Black | 0 | 3 (2.2%) | 0.05 |
| Education Years | 16.21 (±3.34) | 14.81 (±4.02) | <0.001 |
| MDS UPDRS Part III | 19.64 (±9.54) | 19.58 (±9.83) | 0.96 |
| Total STAI | 47.12 (±5.02) | 46.66 (±6.32) | 0.45 |
| Total GDS | 5.39 (±1.55) | 5.85 (±1.68) | 0.008 |
| MoCA Test | 28.01 (±1.37) | 22.66 (±3.09) | <0.001 |
| LRRK2 | 6 (5.6%) | 8 (13.8%) | 0.13 |
| Depression Screening | |||
|---|---|---|---|
| Positive (GDS ≥ 5) (n = 349) | Negative (GDS < 5) (n = 96) | p-Value | |
| Age | 60.4 (±9.5) | 62.0 (±8.2) | 0.09 |
| Sex (Male) | 200 (57.3%) | 63 (65.6%) | 0.18 |
| Ethnicity | |||
| White | 336 (96.3%) | 95 (99.0%) | 0.32 |
| Hispanic/Latino | 24 (6.9%) | 4 (4.2%) | 0.47 |
| Asian | 5 (1.4%) | 1 (1.0%) | 1 |
| Black | 4 (1.1%) | 0 | 0.66 |
| Other | 8 (2.3%) | 0 | 0.53 |
| MDS UPDRS Part III | 19.5 (±9.9) | 20.1 (±8.9) | 0.59 |
| STAI | 47.5 (±5.2) | 45.1 (±5.9) | <0.001 |
| MoCA Test | 26.2 (±3.4) | 26.9 (±2.2) | 0.01 |
| LRRK2 | 12 (9.0%) | 2 (6.1%) | 0.84 |
| Education (Years) | 15.6 (±3.7) | 16.7 (±3.1) | 0.003 |
| Anxiety Screening | |||
|---|---|---|---|
| Positive (STAI > 40) (n = 395) | Negative (STAI ≤ 40) (n = 50) | p-Value | |
| Age | 60.6 (±9.3) | 61.8 (±8.6) | 0.35 |
| Sex (Male) | 231 (58.5%) | 32 (64.0%) | 0.55 |
| Ethnicity | |||
| White | 382 (96.7%) | 49 (98.0%) | 0.95 |
| Hispanic/Latino | 25 (6.3%) | 3 (6.0%) | 1 |
| Asian | 5 (1.3%) | 1 (2.0%) | 1 |
| Black | 4 (1.0%) | 0 | 1 |
| Other | 8 (2.1%) | 0 | 0.93 |
| Education Years | 15.9 (±3.7) | 15.2 (±2.7) | 0.09 |
| MDS UPDRS Part III | 19.4 (±9.7) | 21.9 (±9.3) | 0.08 |
| MoCA Test | 26.5 (±3.1) | 25.1 (±3.8) | 0.02 |
| LRRK2 | 12 (8.5%) | 2 (8.3%) | 1 |
| Healthy Subjects (n = 108) | Parkinson’s Disease (n = 300) | p-Value | |
|---|---|---|---|
| Alpha-synuclein | 119.7 (±51.6) | 100.62 (±47.0) | <0.01 |
| pTau | 17.9 (±9.3) | 14.1 (±5.0) | <0.001 |
| tTau | 195.0 (±83.7) | 163.0 (±55.5) | <0.001 |
| Aβ-42 | 1031.3 (±527.8) | 923.0 (±398.3) | 0.05 |
| Aβ-42 < 1100 | 71 (65.7%) | 217 (72.3%) | 0.244 |
| pTau/Aβ-42 > 0.022 | 14 (14.3%) | 19 (6.9%) | 0.05 |
| tTau/Aβ-42 > 0.26 | 16 (15.2%) | 21 (7.2%) | 0.03 |
| Overall (n = 300) | MoCA ≥ 26 (n = 213) | MoCA < 26 (n = 87) | p-Value | |
|---|---|---|---|---|
| Alpha-synuclein | 100.6 (±47.0) | 98.7 (±42.3) | 106.2 (±58.8) | 0.46 |
| Alpha-synuclein > p75 | 27 (17.2%) | 20 (17.1%) | 7 (17.5%) | 1 |
| pTau | 14.09 (±4.97) | 13.64 (±4.49) | 15.1 (±5.8) | 0.02 |
| tTau | 163.0 (±55.4) | 158.1 (±51.9) | 174.2 (±61.6) | 0.01 |
| Aβ-42 | 923.0 (±398.3) | 928.6 (±388.4) | 909.1 (±423.6) | 0.71 |
| Aβ-42 < 1100 | 217 (72.3%) | 152 (71.4%) | 65 (74.7%) | 0.65 |
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 7 (3.6%) | 12 (14.6%) | 0.003 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 8 (3.9%) | 13 (15.1%) | 0.002 |
| Univariable | OR | 95% Confidence Interval | p-Value | |
| Alpha-synuclein | 1.003 | 0.996 | 1.011 | 0.38 |
| pTau | 1.06 | 1.01 | 1.11 | 0.01 |
| tTau | 1.005 | 1.001 | 1.009 | 0.01 |
| Aβ-42 | 0.999 | 0.999 | 1.000 | 0.70 |
| Alpha-synuclein > p75 | 1.03 | 0.37 | 2.56 | 0.95 |
| Aβ-42 < 1100 | 1.19 | 0.68 | 2.12 | 0.56 |
| pTau/Aβ-42 > 0.022 | 4.53 | 1.75 | 12.6 | <0.01 |
| tTau/Aβ-42 > 0.26 | 4.40 | 1.78 | 11.5 | <0.01 |
| Multivariable * | OR | 95% Confidence Interval | p-Value | |
| Alpha-synuclein | 1.00 | 0.99 | 1.01 | 0.69 |
| pTau | 1.02 | 0.98 | 1.07 | 0.31 |
| tTau | 1.00 | 0.99 | 1.01 | 0.36 |
| Aβ-42 | 0.999 | 0.998 | 1.00 | 0.35 |
| Alpha-synuclein > p75 | 0.79 | 0.26 | 2.11 | 0.65 |
| Aβ-42 < 1100 | 1.35 | 0.75 | 2.51 | 0.32 |
| pTau/Aβ-42 > 0.022 | 4.64 | 1.67 | 13.8 | <0.01 |
| tTau/Aβ-42 > 0.26 | 4.18 | 1.60 | 11.5 | <0.01 |
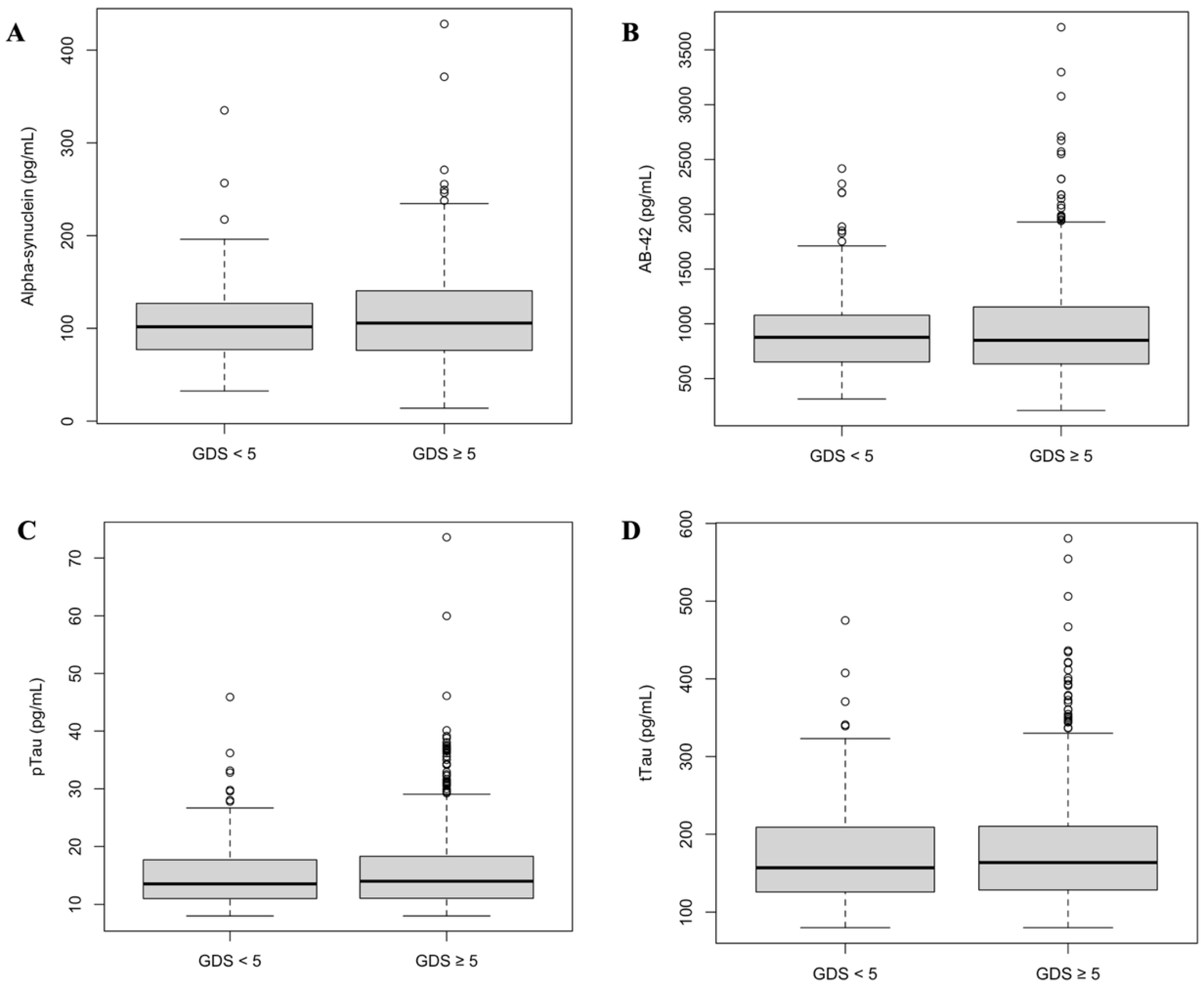
| Overall | GDS ≥ 5 | GDS < 5 | p-Value | |
| Alpha-synuclein | 100.62 (±47.0) | 102.9 (±49.6) | 94.5 (±39.2) | 0.27 |
| pTau | 14.09 (±5.0) | 14.0 (±4.8) | 14.3 (±5.5) | 0.62 |
| tTau | 163.0 (±55.5) | 161.1 (±53.8) | 169.5 (±60.5) | 0.25 |
| Aβ-42 | 924.2 (±397.8) | 917.7 (±413.1) | 946.1 (±343.7) | 0.57 |
| Alpha-synuclein > p75 | 27 (17.2%) | 22 (19.3%) | 5 (11.6%) | 0.37 |
| Aβ-42 < 1100 | 217 (72.3%) | 167 (72.3%) | 50 (72.5%) | 0.99 |
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 16 (7.7%) | 3 (4.5%) | 0.53 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 17 (7.6%) | 4 (5.9%) | 0.83 |
| Overall | GDS ≥ 9 | GDS < 9 | p-Value | |
| Alpha-synuclein | 100.62 (±47.0) | 100.60 (±47.3) | 101.39 (±33.4) | 0.97 |
| pTau | 14.09 (±5.0) | 14.14 (±5.0) | 12.57 (±4.1) | 0.20 |
| tTau | 163.0 (±55.5) | 163.7 (±55.3) | 146.85 (±58.3) | 0.29 |
| Aβ-42 | 924.2 (±397.8) | 930.0 (±400.7) | 770.4 (±286.0) | 0.1 |
| Alpha-synuclein > p75 | 27 (17.2%) | 27 (17.5%) | 0 | 0.98 |
| Aβ-42 < 1100 | 217 (72.3%) | 208 (72.0%) | 9 (81.8%) | 0.71 |
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 19 (7.2%) | 0 | 0.87 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 21 (7.5%) | 0 | 0.73 |
| Univariable | OR | 95% Confidence Interval | p-Value | |
| Alpha-synuclein | 1.004 | 0.996 | 1.013 | 0.32 |
| pTau | 0.987 | 0.942 | 1.037 | 0.59 |
| tTau | 0.997 | 0.993 | 1.002 | 0.22 |
| Aβ-42 | 0.999 | 0.999 | 1.001 | 0.60 |
| Alpha-synuclein > p75 | 1.82 | 0.68 | 5.74 | 0.26 |
| Aβ-42 < 1100 | 0.99 | 0.53 | 1.79 | 0.98 |
| pTau/Aβ-42 > 0.022 | 1.79 | 0.57 | 7.86 | 0.37 |
| tTau/Aβ-42 > 0.26 | 1.31 | 0.47 | 4.69 | 0.63 |
| Multivariable * | OR | 95% Confidence Interval | p-Value | |
| Alpha-synuclein | 1.005 | 0.997 | 1.014 | 0.25 |
| pTau | 0.990 | 0.943 | 1.042 | 0.71 |
| tTau | 0.997 | 0.993 | 1.002 | 0.30 |
| Aβ-42 | 0.999 | 0.999 | 1.001 | 0.60 |
| Alpha-synuclein > p75 | 2.03 | 0.75 | 6.54 | 0.19 |
| Aβ-42 < 1100 | 0.98 | 0.53 | 1.78 | 0.95 |
| pTau/Aβ-42 > 0.022 | 2.03 | 0.64 | 9.08 | 0.28 |
| tTau/Aβ-42 > 0.26 | 1.48 | 0.51 | 5.36 | 0.50 |
| Anxiety Screening | |||
|---|---|---|---|
| Positive (STAI > 40) (n = 265) | Negative (STAI ≤ 40) (n = 35) | p-Value | |
| Alpha-synuclein | 100.7 (±47.7) | 100.3 (±41.6) | 0.97 |
| Alpha-synuclein > p75 | 23 (16.4%) | 4 (23.5%) | 0.69 |
| pTau | 14.1 (±5.03) | 14.2 (±4.6) | 0.87 |
| tTau | 163.1 (±56.6) | 162.6 (±47.3) | 0.95 |
| Aβ-42 | 922.0 (±404.8) | 930.4 (±350.5) | 0.90 |
| Aβ-42 < 1100 | 192 (72.5%) | 25 (71.4%) | 1 |
| pTau/Aβ-42 > 0.022 | 18 (7.5%) | 1 (3.0%) | 0.57 |
| tTau/Aβ-42 > 0.26 | 20 (7.8%) | 1 (2.9%) | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota Telles, J.P.; Camargo, L.; Gianlorenço, A.C.; Fregni, F. Dissociation of Clinical Outcomes and CSF Proteinopathy Biomarkers in Parkinson’s Disease: Cognitive–Affective Dissociation with Specificity for Tau. Biomedicines 2025, 13, 2478. https://doi.org/10.3390/biomedicines13102478
Mota Telles JP, Camargo L, Gianlorenço AC, Fregni F. Dissociation of Clinical Outcomes and CSF Proteinopathy Biomarkers in Parkinson’s Disease: Cognitive–Affective Dissociation with Specificity for Tau. Biomedicines. 2025; 13(10):2478. https://doi.org/10.3390/biomedicines13102478
Chicago/Turabian StyleMota Telles, João Paulo, Lucas Camargo, Anna Carolyna Gianlorenço, and Felipe Fregni. 2025. "Dissociation of Clinical Outcomes and CSF Proteinopathy Biomarkers in Parkinson’s Disease: Cognitive–Affective Dissociation with Specificity for Tau" Biomedicines 13, no. 10: 2478. https://doi.org/10.3390/biomedicines13102478
APA StyleMota Telles, J. P., Camargo, L., Gianlorenço, A. C., & Fregni, F. (2025). Dissociation of Clinical Outcomes and CSF Proteinopathy Biomarkers in Parkinson’s Disease: Cognitive–Affective Dissociation with Specificity for Tau. Biomedicines, 13(10), 2478. https://doi.org/10.3390/biomedicines13102478








