Unveiling Major Depressive Disorder Through TMS-EEG: From Traditional to Emerging Approaches
Abstract
1. Introduction
2. Traditional TMS-EEG Measures in MDD
3. Emerging TMS-EEG Metric and Approaches in MDD
3.1. Oscillatory Dynamics
3.2. Microstate Analysis
3.3. Trial-by-Trial Variability
3.4. Source-Level Analysis
3.5. Multimodal/Multiscale TMS-EEG Approaches
3.6. Machine Learning Approaches to TMS-EEG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| DLPFC | dorsolateral prefrontal cortex |
| EEG | electroencephalography |
| GMFA | global mean field amplitude |
| GMFP | global mean field power |
| ICF | intracortical facilitation |
| IPL | Inferior Parietal Lobule |
| LMFA | local mean field amplitude |
| LMFP | local mean field power |
| MDD | Major Depressive Disorder |
| ML | Machine learning |
| NMDA | N-methyl-D-aspartate |
| rTMS | repetitive TMS |
| SCD | significant current density |
| SCS | significant current scattering |
| TEP | TMS-evoked potential |
| TMS-EEG | Transcranial magnetic stimulation—electroencephalography |
| TRD | Treatment-resistant Depression |
| TTV | Trial-by-trial variability |
References
- Wang, Y.; Qin, C.; Chen, H.; Liang, W.; Liu, M.; Liu, J. Global, Regional, and National Burden of Major Depressive Disorders in Adults Aged 60 Years and Older from 1990 to 2021, with Projections of Prevalence to 2050: Analyses from the Global Burden of Disease Study 2021. J. Affect. Disord. 2025, 374, 486–494. [Google Scholar] [CrossRef]
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 30 August 2025).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association Publishing: Washington, DC, USA, 2022; ISBN 0-89042-575-2. [Google Scholar]
- Greenberg, P.; Chitnis, A.; Louie, D.; Suthoff, E.; Chen, S.Y.; Maitland, J.; Gagnon-Sanschagrin, P.; Fournier, A.A.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2019). Adv. Ther. 2023, 40, 4460–4479. [Google Scholar] [CrossRef]
- Maletic, V.; Robinson, M.; Oakes, T.; Iyengar, S.; Ball, S.G.; Russell, J. Neurobiology of Depression: An Integrated View of Key Findings. Int. J. Clin. Pract. 2007, 61, 2030–2040. [Google Scholar] [CrossRef]
- Dev, A.; Roy, N.; Islam, M.K.; Biswas, C.; Ahmed, H.U.; Amin, M.A.; Sarker, F.; Vaidyanathan, R.; Mamun, K.A. Exploration of EEG-Based Depression Biomarkers Identification Techniques and Their Applications: A Systematic Review. IEEE Access 2022, 10, 16756–16781. [Google Scholar] [CrossRef]
- Hao, W.; Dai, X.; Wei, M.; Li, S.; Peng, M.; Xue, Q.; Lin, H.; Wang, H.; Song, P.; Wang, Y. Efficacy of Transcranial Photobiomodulation in the Treatment for Major Depressive Disorder: A TMS-EEG and Pilot Study. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12957. [Google Scholar] [CrossRef]
- Mcewen, B.S.; Gianaros, P.J. Central Role of the Brain in Stress and Adaptation: Links to Socioeconomic Status, Health, and Disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef] [PubMed]
- Farzan, F. Transcranial Magnetic Stimulation–Electroencephalography for Biomarker Discovery in Psychiatry. Biol. Psychiatry 2024, 95, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; George Niederehe, M.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and Other Tools (BEST) Resource in Neuro-Oncology. Neuro Oncol. 2018, 20, 1162–1172. [Google Scholar] [CrossRef]
- Bortoletto, M.; Veniero, D.; Thut, G.; Miniussi, C. The Contribution of TMS-EEG Coregistration in the Exploration of the Human Cortical Connectome. Neurosci. Biobehav. Rev. 2015, 49, 114–124. [Google Scholar] [CrossRef]
- Hernandez-Pavon, J.C.; Veniero, D.; Bergmann, T.O.; Belardinelli, P.; Bortoletto, M.; Casarotto, S.; Casula, E.P.; Farzan, F.; Fecchio, M.; Julkunen, P.; et al. TMS Combined with EEG: Recommendations and Open Issues for Data Collection and Analysis. Brain Stimul. 2023, 16, 567–593. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical Utility and Prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- George, M.S.; Ketter, T.A.; Post, R.M. Prefrontal Cortex Dysfunction I. Depression 1994, 72, 59–72. [Google Scholar] [CrossRef]
- Strafella, R.; Momi, D.; Zomorrodi, R.; Lissemore, J.; Noda, Y.; Chen, R.; Rajji, T.K.; Griffiths, J.D.; Vila-Rodriguez, F.; Downar, J.; et al. Identifying Neurophysiological Markers of Intermittent Theta Burst Stimulation in Treatment-Resistant Depression Using Transcranial Magnetic Stimulation–Electroencephalography. Biol. Psychiatry 2023, 94, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Atluri, S.; Lee, J.C.; Knyahnytska, Y.; Croarkin, P.E.; Blumberger, D.M.; Daskalakis, Z.J.; Farzan, F. Prefrontal Cortical Reactivity and Connectivity Markers Distinguish Youth Depression from Healthy Youth. Cereb. Cortex 2020, 30, 3884–3894. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Atluri, S.; Lee, J.; Knyahnytska, Y.; Croarkin, P.E.; Blumberger, D.M.; Daskalakis, Z.J.; Farzan, F. Neurophysiological Markers of Response to Theta Burst Stimulation in Youth Depression. Depress. Anxiety 2021, 38, 172–184. [Google Scholar] [CrossRef]
- Voineskos, D.; Blumberger, D.M.; Rogasch, N.C.; Zomorrodi, R.; Farzan, F.; Foussias, G.; Rajji, T.K.; Daskalakis, Z.J. Neurophysiological Effects of Repetitive Transcranial Magnetic Stimulation (RTMS) in Treatment Resistant Depression. Clin. Neurophysiol. 2021, 132, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Biermann, L.; Wunram, H.L.; Pokorny, L.; Breitinger, E.; Großheinrich, N.; Jarczok, T.A.; Bender, S. Changes in the TMS-Evoked Potential N100 in the Dorsolateral Prefrontal Cortex as a Function of Depression Severity in Adolescents. J. Neural Transm. 2022, 129, 1339–1352. [Google Scholar] [CrossRef]
- Dhami, P.; Moreno, S.; Croarkin, P.E.; Blumberger, D.M.; Daskalakis, Z.J.; Farzan, F. Baseline Markers of Cortical Excitation and Inhibition Predict Response to Theta Burst Stimulation Treatment for Youth Depression. Sci. Rep. 2023, 13, 19115. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Liu, Q.; Zheng, C.; Yu, C.; Hou, G.; Chen, Z.; Chen, Y.; Chen, Y.; Zhu, G.; et al. TMS-Evoked Potential in the Dorsolateral Prefrontal Cortex to Assess the Severity of Depression Disease: A TMS-EEG Study. Front. Pharmacol. 2023, 14, 1207020. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Li, J.; Liu, J.; Luo, R.; Li, Y.; Wang, D.; Zhou, D.; Zhang, X.Y. Neurophysiological Markers of Disease Severity and Cognitive Dysfunction in Major Depressive Disorder: A TMS-EEG Study. Int. J. Clin. Health Psychol. 2024, 24, 100495. [Google Scholar] [CrossRef]
- Sheen, J.Z.; Mazza, F.; Momi, D.; Miron, J.P.; Mansouri, F.; Russell, T.; Zhou, R.; Hyde, M.; Fox, L.; Voetterl, H.; et al. N100 as a Response Prediction Biomarker for Accelerated 1 Hz Right DLPFC-RTMS in Major Depression. J. Affect. Disord. 2024, 363, 174–181. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, J.; Wei, S.; Zhou, D.; Wang, D.; Zhang, X. Relationships between Clinical Symptoms, Cognitive Functioning, and TMS-Evoked Potential Features in Patients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 136, 111184. [Google Scholar] [CrossRef]
- Hadas, I.; Zomorrodi, R.; Hill, A.T.; Sun, Y.; Fitzgerald, P.B.; Blumberger, D.M.; Daskalakis, Z.J. Subgenual Cingulate Connectivity and Hippocampal Activation Are Related to MST Therapeutic and Adverse Effects. Transl. Psychiatry 2020, 10, 392. [Google Scholar] [CrossRef]
- Hill, A.T.; Hadas, I.; Zomorrodi, R.; Voineskos, D.; Fitzgerald, P.B.; Blumberger, D.M.; Daskalakis, Z.J. Characterizing Cortical Oscillatory Responses in Major Depressive Disorder Before and After Convulsive Therapy: A TMS-EEG Study. J. Affect. Disord. 2021, 287, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nakajima, S.; Honda, S.; Takano, M.; Taniguchi, K.; Tsugawa, S.; Mimura, Y.; Hattori, N.; Koike, S.; Zomorrodi, R.; et al. Reduced Signal Propagation Elicited by Frontal Transcranial Magnetic Stimulation Is Associated with Oligodendrocyte Abnormalities in Treatment-Resistant Depression. J. Psychiatry Neurosci. 2022, 47, E325–E335. [Google Scholar] [CrossRef]
- Niu, Z.; Jia, L.; Li, Y.; Yang, L.; Liu, Y.; Lian, S.; Wang, D.; Wang, W.; Yang, L.; Pan, W.; et al. Trial-by-Trial Variability of TMS-EEG in Healthy Controls and Patients With Depressive Disorder. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nakajima, S.; Honda, S.; Takano, M.; Taniguchi, K.; Homma, S.; Ueda, R.; Tobari, Y.; Mimura, Y.; Fujii, S.; et al. Decreased Prefrontal Glutamatergic Function Is Associated with a Reduced Astrocyte-Related Gene Expression in Treatment-Resistant Depression. Transl. Psychiatry 2024, 14, 478. [Google Scholar] [CrossRef]
- Noda, Y.; Sakaue, K.; Wada, M.; Takano, M.; Nakajima, S. Development of Artificial Intelligence for Determining Major Depressive Disorder Based on Resting-State EEG and Single-Pulse Transcranial Magnetic Stimulation-Evoked EEG Indices. J. Pers. Med. 2024, 14, 101. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, X.; Zhang, J.; Fan, J.; Zhong, D.; Li, J.; Zheng, Z.; Jin, R. Brain Microstate Features of Patients with Depression: A Transcranial Magnetic Stimulation and Electroencephalographic (TMS-EEG) Study. Psychiatry Res. 2025, 351, 116616. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Zhuang, W.; Xu, Y.; Pei, Z.; Liu, J.; Zhang, Y.; Yu, C.; Wang, Y.; Liu, X.; et al. Heightened Effective Connectivity of DLPFC-MPFC and DLPFC-ACC Circuits in Major Depressive Disorder with Suicidal Ideation: Evidence from a TMS-EEG Study. Transl. Psychiatry 2025, 15, 332. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Kičić, D. Methodology for Combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Voineskos, D.; Blumberger, D.M.; Zomorrodi, R.; Rogasch, N.C.; Farzan, F.; Foussias, G.; Rajji, T.K.; Daskalakis, Z.J. Altered Transcranial Magnetic Stimulation–Electroencephalographic Markers of Inhibition and Excitation in the Dorsolateral Prefrontal Cortex in Major Depressive Disorder. Biol. Psychiatry 2019, 85, 477–486. [Google Scholar] [CrossRef]
- Cao, K.X.; Ma, M.L.; Wang, C.Z.; Iqbal, J.; Si, J.J.; Xue, Y.X.; Yang, J.L. TMS-EEG: An Emerging Tool to Study the Neurophysiologic Biomarkers of Psychiatric Disorders. Neuropharmacology 2021, 197, 108574. [Google Scholar] [CrossRef]
- Strafella, R.; Chen, R.; Rajji, T.K. Resting and TMS-EEG Markers of Treatment Response in Major Depressive Disorder: A Systematic Review. Front. Hum. Neurosci. 2022, 16, 940759. [Google Scholar] [CrossRef]
- Premoli, I.; Rivolta, D.; Espenhahn, S.; Castellanos, N.; Belardinelli, P.; Ziemann, U.; Müller-Dahlhaus, F. Characterization of GABAB-Receptor Mediated Neurotransmission in the Human Cortex by Paired-Pulse TMS-EEG. Neuroimage 2014, 103, 152–162. [Google Scholar] [CrossRef]
- Belardinelli, P.; König, F.; Liang, C.; Premoli, I.; Desideri, D.; Müller-Dahlhaus, F.; Gordon, P.C.; Zipser, C.; Zrenner, C.; Ziemann, U. TMS-EEG Signatures of Glutamatergic Neurotransmission in Human Cortex. Sci. Rep. 2021, 11, 8159. [Google Scholar] [CrossRef] [PubMed]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural Frequencies of Human Corticothalamic Circuits. J. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, J.; Veniero, D.; Hanslmayr, S.; Palva, S. Top-down and Bottom-up Interactions Rely on Nested Brain Oscillations to Shape Rhythmic Visual Attention Sampling. PLoS Biol. 2025, 23, e3002688. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-Analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- David, O.; Kilner, J.M.; Friston, K.J. Mechanisms of Evoked and Induced Responses in MEG/EEG. Neuroimage 2006, 31, 1580–1591. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in Resting-State EEG: Current Status and Future Directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Strik, W.K.; Henggeler, B.; Koenig, T.; Koukkou, M. Brain Electric Microstates and Momentary Conscious Mind States as Building Blocks of Spontaneous Thinking: I. Visual Imagery and Abstract Thoughts. Int. J. Psychophysiol. 1998, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic ERP Analyses: A Step-by-Step Tutorial Review. Brain Topogr. 2008, 20, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, D.; Guidali, G.; Sulcova, D.; Zazio, A.; Bonfiglio, N.S.; Stango, A.; Barchiesi, G.; Bortoletto, M. Stimulation Parameters Recruit Distinct Cortico-Cortical Pathways: Insights from Microstate Analysis on TMS-Evoked Potentials. Brain Topogr. 2025, 38, 39. [Google Scholar] [CrossRef]
- Sulcova, D.; Salatino, A.; Ivanoiu, A.; Mouraux, A. Investigating the Origin of TMS-Evoked Brain Potentials Using Topographic Analysis. Brain Topogr. 2022, 35, 583–598. [Google Scholar] [CrossRef]
- da Cruz, J.R.; Favrod, O.; Roinishvili, M.; Chkonia, E.; Brand, A.; Mohr, C.; Figueiredo, P.; Herzog, M.H. EEG Microstates Are a Candidate Endophenotype for Schizophrenia. Nat. Commun. 2020, 11, 3089. [Google Scholar] [CrossRef]
- Murphy, M.; Whitton, A.E.; Deccy, S.; Ironside, M.L.; Rutherford, A.; Beltzer, M.; Sacchet, M.; Pizzagalli, D.A. Abnormalities in Electroencephalographic Microstates Are State and Trait Markers of Major Depressive Disorder. Neuropsychopharmacology 2020, 45, 2030–2037. [Google Scholar] [CrossRef]
- Zanesco, A.P. Normative Temporal Dynamics of Resting EEG Microstates. Brain Topogr. 2024, 37, 243–264. [Google Scholar] [CrossRef]
- Goris, R.L.T.; Movshon, J.A.; Simoncelli, E.P. Partitioning Neuronal Variability. Nat. Neurosci. 2014, 17, 858–865. [Google Scholar] [CrossRef]
- Miniussi, C.; Bortoletto, M. Harnessing Neural Variability: Implications for Brain Research and Non-Invasive Brain Stimulation. Neurosci. Biobehav. Rev. 2025, 176, 106312. [Google Scholar] [CrossRef] [PubMed]
- Arazi, A.; Gonen-Yaacovi, G.; Dinstein, I. The Magnitude of Trial-by-Trial Neural Variability Is Reproducible over Time and across Tasks in Humans. eNeuro 2017, 4, ENEURO.0292-17.2017. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Tadayon, E.; Boucher, P.; Momi, D.; Karakhanyan, K.A.; Fox, M.D.; Halko, M.A.; Pascual-Leone, A.; Shafi, M.M.; Santarnecchi, E. Individualized Perturbation of the Human Connectome Reveals Reproducible Biomarkers of Network Dynamics Relevant to Cognition. Proc. Natl. Acad. Sci. USA 2020, 117, 8115–8125. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Nakajima, S.; Takano, M.; Wada, M.; Taniguchi, K.; Honda, S.; Uchida, H.; Mimura, M.; Noda, Y. Long-Interval Intracortical Inhibition in Individuals with Autism Spectrum Disorders: A TMS-EEG Study with Source Estimation Analyses. Clin. Neurophysiol. 2025, 178, 2110936. [Google Scholar] [CrossRef] [PubMed]
- Hadas, I.; Sun, Y.; Lioumis, P.; Zomorrodi, R.; Jones, B.; Voineskos, D.; Downar, J.; Fitzgerald, P.B.; Blumberger, D.M.; Daskalakis, Z.J. Association of Repetitive Transcranial Magnetic Stimulation Treatment With Subgenual Cingulate Hyperactivity in Patients With Major Depressive Disorder: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e195578. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Xu, T. Working Memory Function in Patients with Major Depression Disorder: A Narrative Review. Clin. Psychol. Psychother. 2023, 30, 281–293. [Google Scholar] [CrossRef]
- Casali, A.G.; Casarotto, S.; Rosanova, M.; Mariotti, M.; Massimini, M. General Indices to Characterize the Electrical Response of the Cerebral Cortex to TMS. Neuroimage 2010, 49, 1459–1468. [Google Scholar] [CrossRef]
- Goldman, R.I.; Stern, J.M.; Engel, J.; Cohen, M.S. Acquiring Simultaneous EEG and Functional MRI. Clin. Neurophysiol. 2000, 111, 1974–1980. [Google Scholar] [CrossRef]
- Neuling, T.; Ruhnau, P.; Fuscà, M.; Demarchi, G.; Herrmann, C.S.; Weisz, N. Friends, Not Foes: Magnetoencephalography as a Tool to Uncover Brain Dynamics during Transcranial Alternating Current Stimulation. Neuroimage 2015, 118, 406–413. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal Responses to Magnetic Stimulation Reveal Cortical Reactivity and Connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef]
- Huang, S.S.; Huang, H.L.; Lin, T.P.; Kuo, P.H.; Hou, P.H.; Peng, S.J. Predicting the Longitudinal Efficacy of Medication for Depression Using Electroencephalography and Machine Learning. J. Psychiatr. Res. 2025, 190, 372–379. [Google Scholar] [CrossRef]
- Zhao, Z.; Ran, X.; Niu, Y.; Qiu, M.; Lv, S.; Zhu, M.; Wang, J.; Li, M.; Gao, Z.; Wang, C.; et al. Predicting Treatment Response of Repetitive Transcranial Magnetic Stimulation in Major Depressive Disorder Using an Explainable Machine Learning Model Based on Electroencephalography and Clinical Features. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2025, 10, 656–665. [Google Scholar] [CrossRef]
- Watts, D.; Pulice, R.F.; Reilly, J.; Brunoni, A.R.; Kapczinski, F.; Passos, I.C. Predicting Treatment Response Using EEG in Major Depressive Disorder: A Machine-Learning Meta-Analysis. Transl. Psychiatry 2022, 12, 332. [Google Scholar] [CrossRef]
- Noda, Y.; Takano, M.; Wada, M.; Mimura, Y. Neuroscience Validation of the Number of Pulses Required for TMS-EEG in the Prefrontal Cortex Considering Test Feasibility. Neuroscience 2024, 554, 63–71. [Google Scholar] [CrossRef]
- Mulc, D.; Vukojevic, J.; Kalafatic, E.; Cifrek, M.; Vidovic, D.; Jovic, A. Opportunities and Challenges for Clinical Practice in Detecting Depression Using EEG and Machine Learning. Sensors 2025, 25, 409. [Google Scholar] [CrossRef]
- Bertazzoli, G.; Esposito, R.; Mutanen, T.P.; Ferrari, C.; Ilmoniemi, R.J.; Miniussi, C.; Bortoletto, M. The Impact of Artifact Removal Approaches on TMS–EEG Signal. Neuroimage 2021, 239, 118272. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, A.; Tabarelli, D.; Zazio, A.; Bertazzoli, G.; Metsomaa, J.; Ziemann, U.; Bortoletto, M.; Belardinelli, P. Towards the Definition of a Standard in TMS-EEG Data Preprocessing. Neuroimage 2024, 301, 120874. [Google Scholar] [CrossRef]
- Bortoletto, M.; Veniero, D.; Julkunen, P.; Hernandez-Pavon, J.C.; Mutanen, T.P.; Zazio, A.; Bagattini, C. T4TE: Team for TMS−EEG to Improve Reproducibility through an Open Collaborative Initiative. Brain Stimul. 2023, 16, 20–22. [Google Scholar] [CrossRef]
- Beck, M.M.; Christiansen, L.; Madsen, M.A.J.; Jadidi, A.F.; Vinding, M.C.; Thielscher, A.; Bergmann, T.O.; Siebner, H.R.; Tomasevic, L. Transcranial Magnetic Stimulation of Primary Motor Cortex Elicits an Immediate Transcranial Evoked Potential. Brain Stimul. 2024, 17, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Stango, A.; Zazio, A.; Barchiesi, G.; Bonfiglio, N.S.; Dognini, E.; Marcantoni, E.; Bortoletto, M. Immediate TMS-EEG Responses Reveal Motor Cortex Excitability 2024. bioRxiv 2024. [Google Scholar] [CrossRef]
- Peters, J.C.; Reithler, J.; de Graaf, T.A.; Schuhmann, T.; Goebel, R.; Sack, A.T. Concurrent Human TMS-EEG-FMRI Enables Monitoring of Oscillatory Brain State-Dependent Gating of Cortico-Subcortical Network Activity. Commun. Biol. 2020, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Wischnewski, M.; Shirinpour, S.; Alekseichuk, I.; Lapid, M.I.; Nahas, Z.; Lim, K.O.; Croarkin, P.E.; Opitz, A. Real-Time TMS-EEG for Brain State-Controlled Research and Precision Treatment: A Narrative Review and Guide. J. Neural Eng. 2024, 21, 061001. [Google Scholar] [CrossRef] [PubMed]
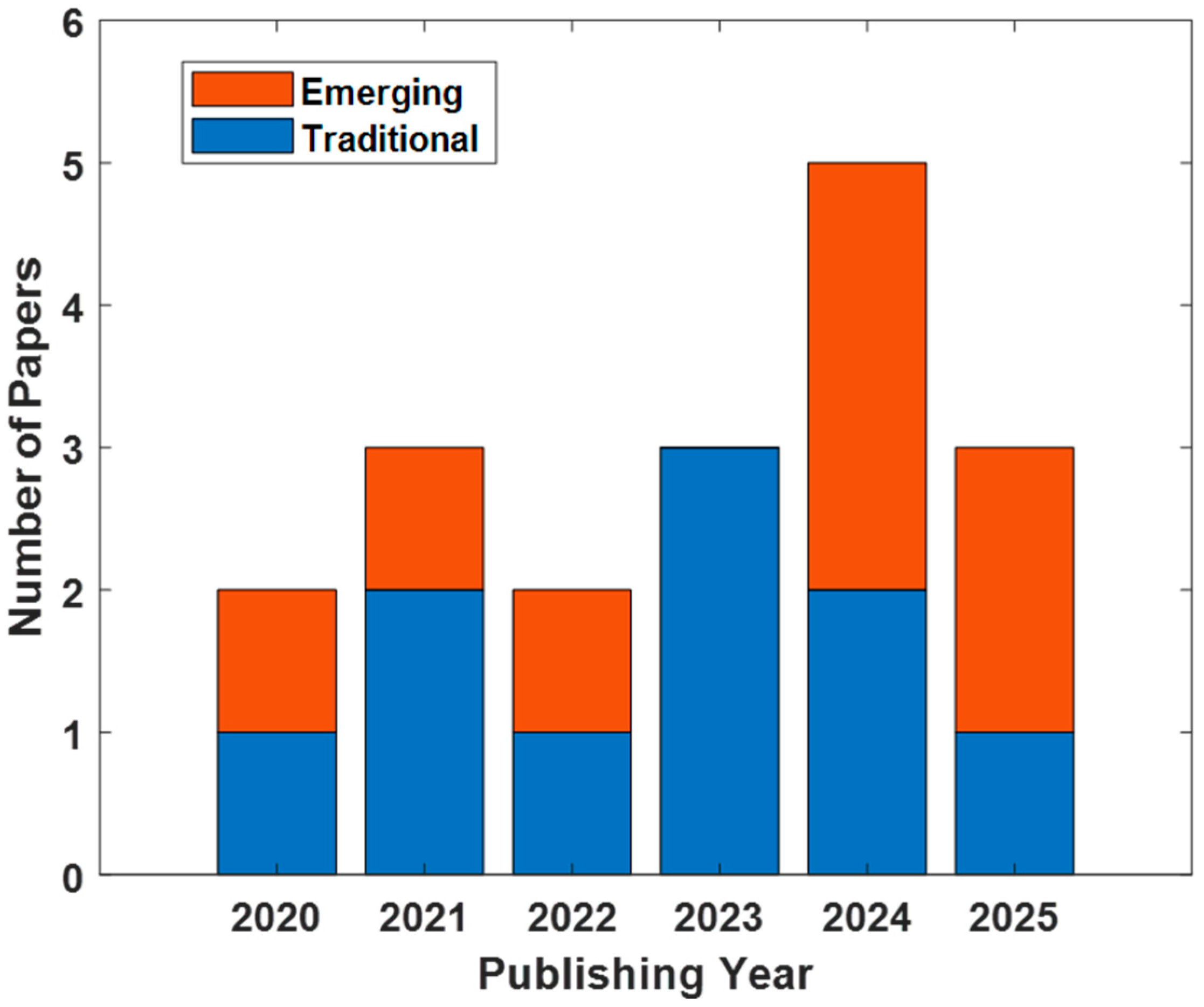
| Reference Year | Authors | TEP Components | Sample | Stimulation Sites | Medications | Coil Type/Intensity (% rMT) |
|---|---|---|---|---|---|---|
| [17] 2020 | Dhami et al. | N45, N100 | 45 MDD, 20 HC | Bilateral DLPFC, Motor Cortex, Inferior parietal lobule (IPL) | Stable treatment (medication or psychotherapy) | 70 mm Figure-of-eight/120% |
| [18] 2021 | Dhami et al. | N45 | 16 MDD, 16 HC | Bilateral DLPFC, Motor Cortex, IPL | Stable treatment (medication or psychotherapy) | 70 mm Figure-of-eight/120% |
| [19] 2021 | Voineskos et al. | N45, N100 | 30 Treatment-resistant depression (TRD) | Left DLPFC | yes | 70 mm Figure-of-eight/120% |
| [20] 2022 | Biermann et al. | N100 | 38 MDD | Bilateral DLPFC | yes | 75 mm Figure-of-eight/120% |
| [16] 2023 | Strafella et al. | N45, N100 | 185 MDD | Left DLPFC | yes | 70 mm Figure-of-eight/120% |
| [21] 2023 | Dhami et al. | P30, N45, P60, N100, P200 | 20 MDD-30 MDD | Bilateral DLPFC, bilateral IPL | Stable treatment (medication or psychotherapy | 70 mm Figure-of-eight/120% |
| [22] 2023 | Li et al. | P60 | 41 MDD, 42 HC | Left DLPFC | No info | 70 mm Figure-of-eight/100% |
| [23] 2024 | Li et al. | P180, P30 | 133 MDD, 76 HC | Left DLPFC | Stable medication | 70 mm Figure-of-eight/100% |
| [24] 2024 | Sheen et al. | N100 | 23 MDD | Right DLPFC | Stable medication | 70 mm Figure-of-eight/100% |
| [25] 2025 | Li et al. | P30, N45, P60, N100, P180, N280 | 59 MDD, 58 HC | Left DLFPC | Stable medication | 70 mm Figure-of-eight/100% |
| Reference Year | Authors | Metrics/Approaches | Sample | Stimulation Sites | Medication | Coil Type/Intensity (% rMT) |
|---|---|---|---|---|---|---|
| [26] 2020 | Hadas et al. | Source-level analysis | 31 TRD | Left DLPFC | yes | 70 mm Figure-of-eight/100% |
| [27] 2021 | Hill et al. | Oscillatory dynamics | 38 MDD, 22 HC | Left DLPFC, Left primary motor cortex | yes | 70 mm Figure-of-eight/120% |
| [28] 2022 | Wada et al. | Multimodal/multiscale TMS-EEG approaches | 60 TRD, 30 HC | Left DLPFC | yes | 70 mm Figure-of-eight/120% |
| [29] 2024 | Niu et al. | Trial-by-trial variability | 34 MDD, 36 HC | Left DLPFC | yes | 70 mm Figure-of-eight/110% |
| [30] 2024 | Wada et al. | Multimodal/multiscale TMS-EEG | 60 TRD, 30 HC | Left DLPFC | yes | 70 mm Figure-of-eight/80, 120% |
| [31] 2024 | Noda et al. | Machine learning approaches to TMS-EEG | 60 MDD, 60 HC | Left DLPFC | yes | 70 mm Figure-of-eight/- |
| [32] 2025 | Zhang et al. | Microstate analysis | 60 MDD, 60 HC | Left primary motor cortex | No (antidepressant) | 70 mm Figure-of-eight/90% |
| [33] 2025 | Chen et al. | Source-level analysis | 166 MDD, 61 HC | Left DLPFC | yes | 70 mm Figure-of-eight/100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stango, A.; Fracassi, C.; Cesareni, A.; Borroni, B.; Zazio, A. Unveiling Major Depressive Disorder Through TMS-EEG: From Traditional to Emerging Approaches. Biomedicines 2025, 13, 2474. https://doi.org/10.3390/biomedicines13102474
Stango A, Fracassi C, Cesareni A, Borroni B, Zazio A. Unveiling Major Depressive Disorder Through TMS-EEG: From Traditional to Emerging Approaches. Biomedicines. 2025; 13(10):2474. https://doi.org/10.3390/biomedicines13102474
Chicago/Turabian StyleStango, Antonietta, Claudia Fracassi, Andrea Cesareni, Barbara Borroni, and Agnese Zazio. 2025. "Unveiling Major Depressive Disorder Through TMS-EEG: From Traditional to Emerging Approaches" Biomedicines 13, no. 10: 2474. https://doi.org/10.3390/biomedicines13102474
APA StyleStango, A., Fracassi, C., Cesareni, A., Borroni, B., & Zazio, A. (2025). Unveiling Major Depressive Disorder Through TMS-EEG: From Traditional to Emerging Approaches. Biomedicines, 13(10), 2474. https://doi.org/10.3390/biomedicines13102474






