Recurrent Erysipelas: Clinical Challenges and Strategies for Prevention—A Narrative Literature Review
Abstract
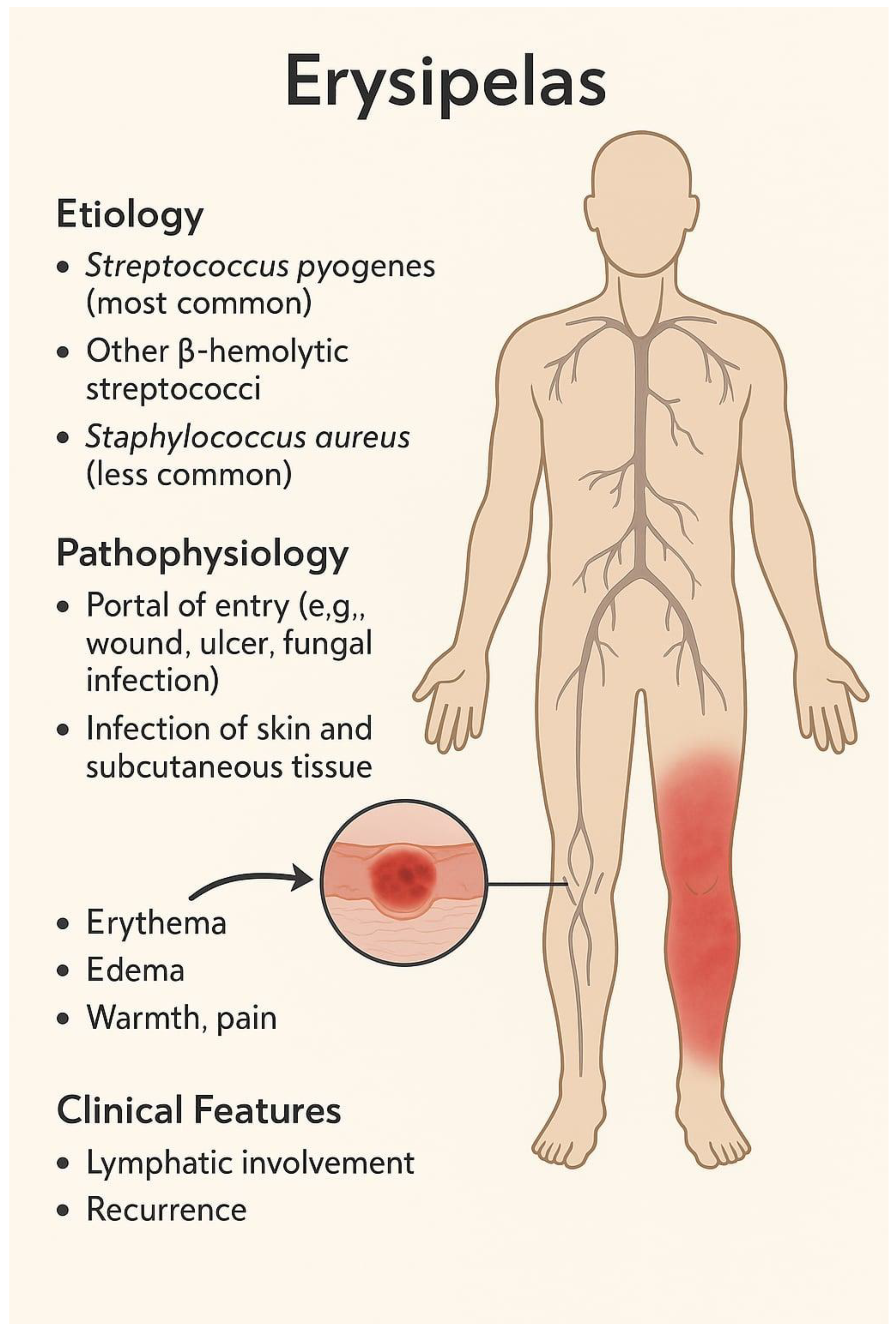
1. Introduction
2. Recurrent Erysipelas—Definition and Clinical Context
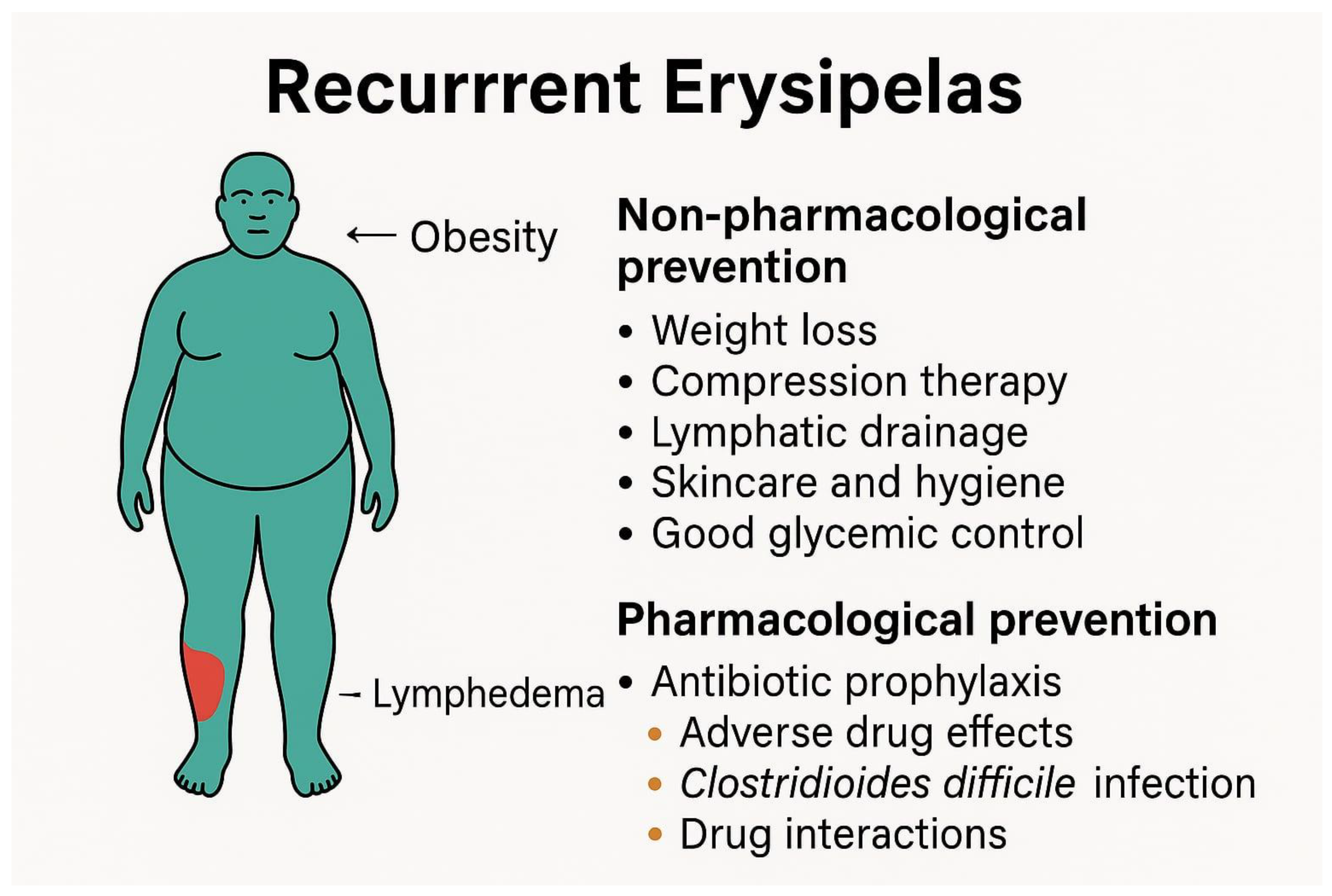
3. Risk Factors of Recurrent Erysipelas
4. Non-Pharmacological Strategies for the Prevention of Recurrent Erysipelas
5. Chemoprophylaxis of Recurrent Erysipelas—Available Treatment Modalities
6. Efficacy of Antibiotic Prophylaxis in Recurrent Erysipelas
7. Duration of Chemoprophylaxis for Recurrent Erysipelas
8. Chemoprophylaxis of Recurrent Erysipelas—Adverse Effects and Complications
9. Discussion
10. Conclusions
11. Broader Implications and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BID | lat. bis in die (eng. twice daily) |
| BLS | British Lymphology Society |
| BMI | Body Mass Index |
| BPG | Benzathine penicillin G |
| CDI | Clostridioides difficile infection |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-reactive protein |
| ECG | Electrocardiogram |
| GAS | Group A streptococcus |
| GCS | Graduated compression stockings |
| HR | Hazard ratio |
| IDSA | Infectious Diseases Society of America |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NICE | National Institute for Health and Care Excellence |
| NOAC | Novel Oral Anticoagulant |
| SSTI | Skin and soft tissue infection |
| TMP-SMX | Trimethoprim–sulfamethoxazole |
| RCT | Randomized Controlled Trial |
References
- Leszczyszyn-Pynka, M. Erysipelas. In Interna Szczeklika 2025, 19th ed.; Medycyna Praktyczna: Kraków, Poland, 2025; pp. 2490–2491. [Google Scholar]
- Sapuła, M.; Krankowska, D.; Wiercińska-Drapało, A. In Search of Risk Factors for Recurrent Erysipelas and Cellulitis of the Lower Limb: A Cross-Sectional Study of Epidemiological Characteristics of Patients Hospitalized due to Skin and Soft-Tissue Infections. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 1307232. [Google Scholar] [CrossRef] [PubMed]
- Toschi, A.; Giannella, M.; Viale, P. Recurrence of skin and soft tissue infections: Identifying risk factors and treatment strategies. Curr. Opin. Infect. Dis. 2025, 38, 71–77. [Google Scholar] [CrossRef]
- Inghammar, M.; Rasmussen, M.; Linder, A. Recurrent erysipelas-risk factors and clinical presentation. BMC Infect. Dis. 2014, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Michael, Y.; Shaukat, N.M. Erysipelas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532247/ (accessed on 30 August 2025).
- National Institute for Health and Care Excellence (NICE). Cellulitis and Erysipelas: Antimicrobial Prescribing. NICE Guideline NG141, UK, 27 September 2019. Available online: https://www.nice.org.uk/guidance/ng141 (accessed on 30 August 2025).
- Ong, B.S.; Dotel, R.; Ngian, V.J.J. Recurrent Cellulitis: Who is at Risk and How Effective is Antibiotic Prophylaxis? Int. J. Gen. Med. 2022, 15, 6561–6572. [Google Scholar] [CrossRef]
- Boucher, H. Erysipelas, Recurrent, Lymphedema. In Sanford Guide to Antimicrobial Therapy [Mobile App]; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2025. [Google Scholar]
- Li, A.; Wang, N.; Ge, L.; Xin, H.; Li, W. Risk factors of recurrent erysipelas in adult Chinese patients: A prospective cohort study. BMC Infect. Dis. 2021, 21, 26. [Google Scholar] [CrossRef]
- Matych, M.; Ciosek, A.; Miler, K.; Noweta, M.; Brzezińska, K.; Sarzała, M.; Narbutt, J.; Lesiak, A. Primary and Recurrent Erysipelas-Epidemiological Patterns in a Single-Centre Retrospective Analysis. J. Clin. Med. 2025, 14, 5299. [Google Scholar] [CrossRef]
- AMBOSS GmbH. Skin and Soft Tissue Infections. In AMBOSS; AMBOSS GmbH: Berlin, Germany, 2025. [Google Scholar]
- Ortiz-Lazo, E.; Arriagada-Egnen, C.; Poehls, C.; Concha-Rogazy, M. An Update on the Treatment and Management of Cellulitis. Actas Dermo-Sifiliogr. 2019, 110, 124–130. [Google Scholar] [CrossRef]
- Cross, E.L.A.; Quan, T.P.; Hayward, G.N.; Walker, A.S.; Llewelyn, M.J. Development and validation of the Baseline Recurrence Risk in Cellulitis (BRRISC) score. J. Infect. 2024, 88, 103–111. [Google Scholar] [CrossRef]
- Greene, A.K.; Grant, F.D.; Slavin, S.A.; Maclellan, R.A. Obesity-induced lymphedema: Clinical and lymphoscintigraphic features. Plast. Reconstr. Surg. 2015, 135, 1715–1719. [Google Scholar] [CrossRef]
- Bąk-Sosnowska, M.; Białkowska, M.; Bogdański, P.; Chomiuk, T.; Dobrowolski, P.; Gałązka-Sobotka, M.; Holecki, M.; Jankowska-Zduńczyk, A.; Jarosińska, A.; Jezierska, M.; et al. Zalecenia kliniczne dotyczące postępowania u chorych na otyłość 2024. Med. Prakt. Wyd. Specj. 2024; 1–116. Available online: https://ptlo.org.pl/resources/data/forms/aktualnosci/258/ws_ptlo_otylosc_2024_final.pdf (accessed on 30 August 2025).
- AMBOSS GmbH. Lymphedema. In AMBOSS; AMBOSS GmbH: Berlin, Germany, 2024. [Google Scholar]
- Webb, E.; Neeman, T.; Bowden, F.J.; Gaida, J.; Mumford, V.; Bissett, B. Compression Therapy to Prevent Recurrent Cellulitis of the Leg. N. Engl. J. Med. 2020, 383, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema:2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar] [CrossRef]
- Blackburn, J.; Kopecki, Z.; Ousey, K.J. Skin integrity, antimicrobial stewardship and infection control: A critical review of current best practice. Wound Pract. Res. 2024, 32, 34–43. [Google Scholar] [CrossRef]
- Krakauer, T.; Buckley, M. Doxycycline is anti-Inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003, 47, 3630–3633. [Google Scholar] [CrossRef]
- Taylor, E.; Nailor, M.D.; Feider, M.; Sullivan, S.; Goodlet, K.J. Doxycycline versus cephalexin treatment of presumed streptococcal skin and soft tissue infection among adults presenting to the emergency department. Antimicrob. Agents Chemother. 2024, 68, e01282-23. [Google Scholar] [CrossRef]
- Bowen, A.C.; Carapetis, J.R.; Currie, B.J.; Fowler, V., Jr.; Chambers, H.F.; Tong, S.Y.C. Sulfamethoxazole-Trimethoprim (Cotrimoxazole) for Skin and Soft Tissue Infections Including Impetigo, Cellulitis, and Abscess. Open Forum Infect. Dis. 2017, 4, ofx232. [Google Scholar] [CrossRef] [PubMed]
- Macy, E. Penicillin allergy: Optimizing diagnostic protocols, public health implications, and future research needs. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Macy, E. Addressing the epidemic of antibiotic “allergy” over-diagnosis. Ann. Allergy Asthma Immunol. 2020, 124, 550–557. [Google Scholar] [CrossRef]
- Dalal, A.; Eskin-Schwartz, M.; Mimouni, D.; Ray, S.; Days, W.; Hodak, E.; Leibovici, L.; Paul, M. Interventions for the prevention of recurrent erysipelas and cellulitis. Cochrane Database Syst. Rev. 2017, 6, CD009758. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Zuckerman, R.; Avraham, Z.; Raz, R. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J. Infect. 1991, 22, 37–40. [Google Scholar] [CrossRef]
- Nguyen, L.; Rowland, K. Low-dose penicillin for recurrent cellulitis? J. Fam. Pract. 2014, 63, E10–E12. [Google Scholar]
- Olszewski, W.L.; Zaleska, M.T. Long-Term Benzathine Penicillin Prophylaxis Lasting for Years Effectively Prevents Recurrence of Dermato-Lymphangio-Adenitis (Cellulitis) in Limb Lymphedema. Lymphat. Res. Biol. 2021, 19, 545–552. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- UK Dermatology Clinical Trials Network’s PATCH Trial Team; Thomas, K.; Crook, A.; Foster, K.; Mason, J.; Chalmers, J.; Bourke, J.; Ferguson, A.; Level, N.; Nunn, A.; et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: Results of the UK Dermatology Clinical Trials Network’s PATCH II trial. Br. J. Dermatol. 2012, 166, 169–178. [Google Scholar] [CrossRef]
- Thomas, K.S.; Crook, A.M.; Nunn, A.J.; Foster, K.A.; Mason, J.M.; Chalmers, J.R.; Nasr, I.S.; Brindle, R.J.; English, J.; Meredith, S.K.; et al. Penicillin to Prevent Recurrent Leg Cellulitis. N. Engl. J. Med. 2013, 368, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, A.; Panasiuk, A.; Stępień, P.M. Clinical practice guidelines for Clostridioides (Clostridium) difficile infection and fecal microbiota transplant protocol—Recommendations of the Polish Society of Epidemiology and Infectious Diseases. Przegl. Epidemiol. 2020, 74, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Goldman, J.L. Erythromycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532910/ (accessed on 30 August 2025).
- European Medicines Agency. Azithromycin—Summary of Product Characteristics. Available online: https://www.geneesmiddeleninformatiebank.nl/smpc/h110860_smpc_en.pdf (accessed on 30 August 2025).
- Omran, A.; Wood, M. Clarithromycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549780/ (accessed on 30 August 2025).
- Ashley, E.A.; Martens, R.J.H. Doxycycline. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556117/ (accessed on 30 August 2025).
- Berbel, D.; González-Díaz, A.; López de Egea, G.; Càmara, J.; Ardanuy, C. An Overview of Macrolide Resistance in Streptococci: Prevalence, Mobile Elements and Dynamics. Microorganisms 2022, 10, 2316. [Google Scholar] [CrossRef]
- Villalón, P.; Bárcena, M.; Medina-Pascual, M.J.; Garrido, N.; Pino-Rosa, S.; Carrasco, G.; Valdezate, S. National Surveillance of Tetracycline, Erythromycin, and Clindamycin Resistance in Invasive Streptococcus pyogenes: A Retrospective Study of the Situation in Spain, 2007–2020. Antibiotics 2023, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Ünübol, N.; Caglayan, N.; Cebeci, S.; Beşli, Y.; Sancak, B.; Uyar, N.Y.; Ahrabi, S.S.; Alebouyeh, M.; Kocagöz, T. Antimicrobial resistance and epidemiological patterns of Streptococcus pyogenes in Türkiye. J. Infect. Public Health 2025, 18, 102633. [Google Scholar] [CrossRef]
- Alexandrova, A.S.; Muhtarova, A.A.; Boyanov, V.S.; Gergova, R.T. Burden of Streptococcus pyogenes and emm12 Type in Severe Otitis Media Among Children. Microbiol. Res. 2025, 16, 181. [Google Scholar] [CrossRef]
- Fox, E.; Misko, J.; Rawlins, M.; Manning, L. The risk of intramuscular haematoma is low following injection of benzathine penicillin G in patients receiving concomitant anticoagulant therapy. J. Thromb. Thrombolysis 2020, 50, 237–238. [Google Scholar] [CrossRef]
- British Lymphology Society (BLS). Consensus Document on the Management of Cellulitis in Lymphoedema; BLS: Lichfield, UK, 2016; Available online: https://www.thebls.com/public/uploads/documents/document-75091530863967.pdf (accessed on 30 September 2025).
| Source/Guideline | Definition of Recurrence | Notes |
|---|---|---|
| NICE (2019) [6] | ≥2 distinct, documented episodes within 12 months | Applies to cellulitis and erysipelas; triggers consideration of prophylaxis [6] |
| Australian Lymphology Association (2015) [7] | ≥2 episodes per year despite skin care and treatment of risk factors | Focuses on chronic edema/lymphedema patients [7] |
| BLS (2016) [7] | ≥2 episodes per year | Similar to Australian guideline [7] |
| IDSA (2014) [7] | 3–4 episodes per year despite control of risk factors | Stricter threshold [7] |
| South Korean SSTI guideline (2017) [7] | 3–4 episodes per year | Similar to IDSA [7] |
| Sanford Guide (2025) [8] | ≥2 episodes within 3 years | Less strict; “frequent episodes” definition [8] |
| Inghammar et al. (2014) [4] | >1 episode, irrespective of time interval | No fixed temporal criterion [4] |
| Li et al. (2021, China) [9] | Recurrence defined only if in the same anatomical location | Excludes new episodes at different sites [9] |
| Matych et al. (2025, Poland) [10] | Recurrent erysipelas accounted for 75.7% of cases | No time-based threshold provided [10] |
| Type of Risk Factor | Risk Factor | Pathomechanism |
|---|---|---|
| non-modifiable | age | Age-related skin changes. |
| sex | Physique, hormonal and differences. | |
| history of previous erysipelas episodes | Recovery from erysipelas does not confer immunity, and the risk of complications can predispose individuals to recurrent episodes. | |
| modifiable | lymphedema | Impairs lymphatic function and tissue viability, increasing the risk of infection. |
| obesity | Adverse effects on lymphatic function and lymph node architecture, increasing susceptibility to skin infections. | |
| diabetes | Suboptimal glycemic control increases the risk of infection, diabetic complications lead to ulcer formation. | |
| venous insufficiency | Sustained venous hypertension leads to chronic inflammatory changes and edema. | |
| cancer | Edema resulting from tumor invasion, lymph node resection, or radiation therapy increases the risk of infection. | |
| immunodeficiency | Weakened immune defenses. Increases the risk of infection. | |
| skin trauma | Provides a portal of entry for bacteria. | |
| dermatological conditions | Harboring pathogenic bacteria, including group A streptococcus. |
| Intervention | Potential Benefits | Limitations/Challenges |
|---|---|---|
| Weight reduction(diet, physical activity, bariatric or pharmacological treatment) | Lowers recurrence risk, improves metabolic profile, enhances success of antibiotic prophylaxis | Requires long-term adherence; weight loss is difficult to sustain; variable access to obesity treatments |
| Glycemic control in diabetes | Improves wound healing, reduces infection portals, lowers recurrence risk | Demands strict glucose monitoring; suboptimal control remains common |
| Smoking cessation | Improves microcirculation, reduces chronic inflammation, lowers risk of COPD and delayshealing | High relapse rates; requires behavioral and/or pharmacological support |
| Lymphedema management(compression, manual drainage, physiotherapy, skin care) | Reduces recurrence (HR ~0.23 in RCT with compression therapy); improves quality of life | Compliance often poor (<50%); discomfort, skin irritation, cosmetic concerns |
| Treatment of fungal infections(tinea pedis, onychomycosis) | Eliminates common entry portals; reduces recurrence | Risk of reinfection; requires long-term topical or systemic therapy |
| Venous insufficiency treatment(conservative or stenting) | May reduce edema and recurrence in selected cases | Limited availability; requires specialized expertise |
| General skin care&hygiene(moisturization, footwear, wound protection) | Reduces microfissures and entry points for pathogens | Requiresongoingpatient education and daily effort |
| Route | Antibiotic | Standard Dose | Alternative/High-Risk Dosing | Notes | Sources |
|---|---|---|---|---|---|
| Intramuscular | Benzathinepenicillin G (debecillin) | 1.2–2.4 million units every 4 weeks (±1 week) | Interval shortened to every 2–3 weeks in patients with breakthrough recurrences | First-line; independent of GI absorption; convenient monthly dosing | [6,7,8] |
| Oral | Penicillin V (phenoxymethylpenicillin) | 250 mg twice daily | 500 mg twice daily if BMI ≥ 33; case reports of up to 2 g twice daily; double dose if >100 kg | First-line oral prophylaxis | |
| Erythromycin | 250–500 mg once daily | — | Preferred in penicillin-allergic patients | ||
| Azithromycin | 250 mg once daily | — | Option in penicillin allergy | ||
| Clarithromycin | 500 mg once daily | — | Option in penicillin allergy | ||
| Cephalexin | 125–250 mg twice daily | 125 mg once daily (alternative regimen) | For non-severe penicillin allergy | ||
| Cefadroxil | Not standardized | — | For non-severe penicillin allergy | ||
| Clindamycin | 150 mg once daily | — | Considered if first-line fails | [7] | |
| Doxycycline | 50 mg once daily | — | Active vs S. aureus & GAS; option if intolerance to other agents | [7,20,21] | |
| TMP-SMX | Low prophylactic doses (not standardized) | — | Efficacy in GAS infections; theoretical prophylaxis role but limited evidence | [7,22] |
| Study/Source | Population | Intervention | Duration | Main Results | Notes |
|---|---|---|---|---|---|
| PATCH II (Thomas et al., 2012, Br J Dermatol) n = 123 [30] | Patients with ≥1 previous episode of leg cellulitis | Penicillin V 250 mg BID (vs. placebo) | 6 months | Recurrence: 20% vs. 33% (HR 0.53, 95% CI 0.26–1.07, p = 0.08) | Trend toward benefit; effect only during prophylaxis; underpowered |
| PATCH I (Thomas et al., 2013, NEJM) n = 274 [31] | Patients with ≥2 episodes in the previous 3 years | Penicillin V 250 mg BID (vs. placebo) | 12 months | Recurrence: 22% vs. 37% (HR 0.55, 95% CI 0.35–0.86, p = 0.01) | 45% risk reduction; no significant benefit in chronic edema, BMI ≥ 33 kg/m2, or ≥3 prior episodes |
| Kremer et al., 1991, J Infect n = 32 [26] | Patients with ≥2 episodes in the previous year | Erythromycin 250 mg BID (vs. no prophylaxis) | 18 months | 0/16 relapses vs. 8/16 (50%) in controls (p < 0.001) | Open-label RCT; 3 pts (20%) developed GI side effects → discontinued treatment |
| Olszewski et al., 2021, Lymphat Res Biol [28] | Patients with limb lymphedema and recurrent dermato-lymphangio-adenitis | Benzathinepenicillin 1.2–2.4 million units IM every 14–21 days | Several years | Recurrence risk reduced by ~95%; excellent tolerability; no resistance observed | Observational, single-center; long-term prophylaxis in lymphedema |
| Cochrane Review (Dalal et al., 2017, CD009758) [25] | 5 RCTs (n ≈ 513) of recurrent cellulitis | Oral or IM penicillin, erythromycin | 6–18 months | Pooled RR 0.46 (95% CI 0.26–0.79): prophylaxis effective during active treatment | Benefit lost after discontinuation; uncertainty about long-term efficacy, cost-effectiveness, and resistance |
| Limitation | Details/Examples | Clinical Implications |
|---|---|---|
| Adherence issues (oral regimens) | Daily dosing with penicillin V, macrolides, or cephalosporins may be inconvenient; reduced compliance in patients with polypharmacy or malabsorption | Decreased effectiveness, risk of recurrence during/after treatment |
| Injection-related complications | Intramuscular benzathinepenicillin convenient (every 2–4 weeks), but carries risk of hematoma, especially in patients on anticoagulants (e.g., atrial fibrillation, obesity-related thromboembolism) | May limit use in elderly and high-risk patients |
| Gastrointestinal side effects | Nausea, diarrhea, abdominal pain, especially with macrolides or higher penicillin doses in obese patients | Reduced tolerance and adherence |
| CDI | Long-term antibiotic exposure increases risk of CDI | Potentially severe complication; requires careful risk–benefit assessment |
| Microbiota disruption & resistance | Selective pressure → colonization with MRSA, resistant Enterobacteriaceae | Reduced future treatment options, public health concern |
| Rising macrolide resistance | Resistance to erythromycin/azithromycin exceeds 20–30% in parts of Europe; higher rates in pediatric isolates | Limits usefulness of macrolides as alternatives to penicillin |
| Drug–drug interactions | Macrolides interact with statins, anticoagulants, QT-prolonging drugs | Requires regular medication review |
| Obesity-related reduced efficacy | Higher BMI often necessitates increased penicillin doses (e.g., ≥500 mg bid if BMI ≥ 33), yet prophylaxis may still fail | Obesity management is crucial adjunct to prophylaxis |
| Limited evidence for optimal duration | Studies suggest 6–18 months; relapse common after discontinuation; some guidelines recommend lifelong prophylaxis in high-risk patients | Individualized treatment duration and regular reassessment needed |
| Over reported penicillin allergy | Up to 90% of self-reported allergies not confirmed after testing; many patients can safely tolerateβ-lactams | Mislabeling leads to unnecessary use of macrolides, fueling resistance and reducing efficacy |
| Category | Issue | Examples/Details |
|---|---|---|
| Gastrointestinal intolerance | More frequent at higher doses, particularly in obese patients requiring double dosing | Nausea, diarrhea, abdominal pain; adherence problems [7] |
| CDI | Disruption of intestinal microbiota | Highest risk with clindamycin, moderate with macrolides/penicillins, low with tetracyclines [32] |
| Drug-specific toxicities | Cardiac, hepatic, hematologic, dermatologic adverse effects | Erythromycin: QT prolongation, arrhythmia, agranulocytosis [33]; Azithromycin: rash, cholestasis [34]; Clarithromycin: candidiasis, leukopenia, QT prolongation [35]; Doxycycline: photosensitivity, contraindicated in pregnancy [36] |
| Antimicrobial resistance | Rising macrolide resistance in S. pyogenes | <5% in some northern European countries vs. 20–40% in southern Europe [37,38,39,40] |
| Ecological impact | Selection pressure → multidrug-resistant organisms | Colonization with MRSA, resistant Enterobacteriaceae [7] |
| Penicillin allergy (often over reported) | Up to 90% of patients with reported allergy can tolerateβ-lactams | Allergy testing and carefulhistory recommended before switching to alternatives [23,24] |
| Drug–drug interactions | Increased toxicity when combined with anticoagulants or other drugs | Erythromycin ↔ apixaban/rivaroxaban (↑ bleeding risk) [33] |
| Route of administration | IM injections vs. oral dosing | Benzathinepenicillin IM: convenient monthly schedule but risk of hematoma in anticoagulated patients [41]; Oral: daily dosing, adherence issues, absorption problems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskóła-Polkowska, D.M.; Blok, K.; Skibińska, A.; Chciałowski, A. Recurrent Erysipelas: Clinical Challenges and Strategies for Prevention—A Narrative Literature Review. Biomedicines 2025, 13, 2448. https://doi.org/10.3390/biomedicines13102448
Jaskóła-Polkowska DM, Blok K, Skibińska A, Chciałowski A. Recurrent Erysipelas: Clinical Challenges and Strategies for Prevention—A Narrative Literature Review. Biomedicines. 2025; 13(10):2448. https://doi.org/10.3390/biomedicines13102448
Chicago/Turabian StyleJaskóła-Polkowska, Dominika Maria, Krystian Blok, Anna Skibińska, and Andrzej Chciałowski. 2025. "Recurrent Erysipelas: Clinical Challenges and Strategies for Prevention—A Narrative Literature Review" Biomedicines 13, no. 10: 2448. https://doi.org/10.3390/biomedicines13102448
APA StyleJaskóła-Polkowska, D. M., Blok, K., Skibińska, A., & Chciałowski, A. (2025). Recurrent Erysipelas: Clinical Challenges and Strategies for Prevention—A Narrative Literature Review. Biomedicines, 13(10), 2448. https://doi.org/10.3390/biomedicines13102448






