What Is the Impact of Glyphosate on the Thyroid? An Updated Review
Abstract
1. Introduction
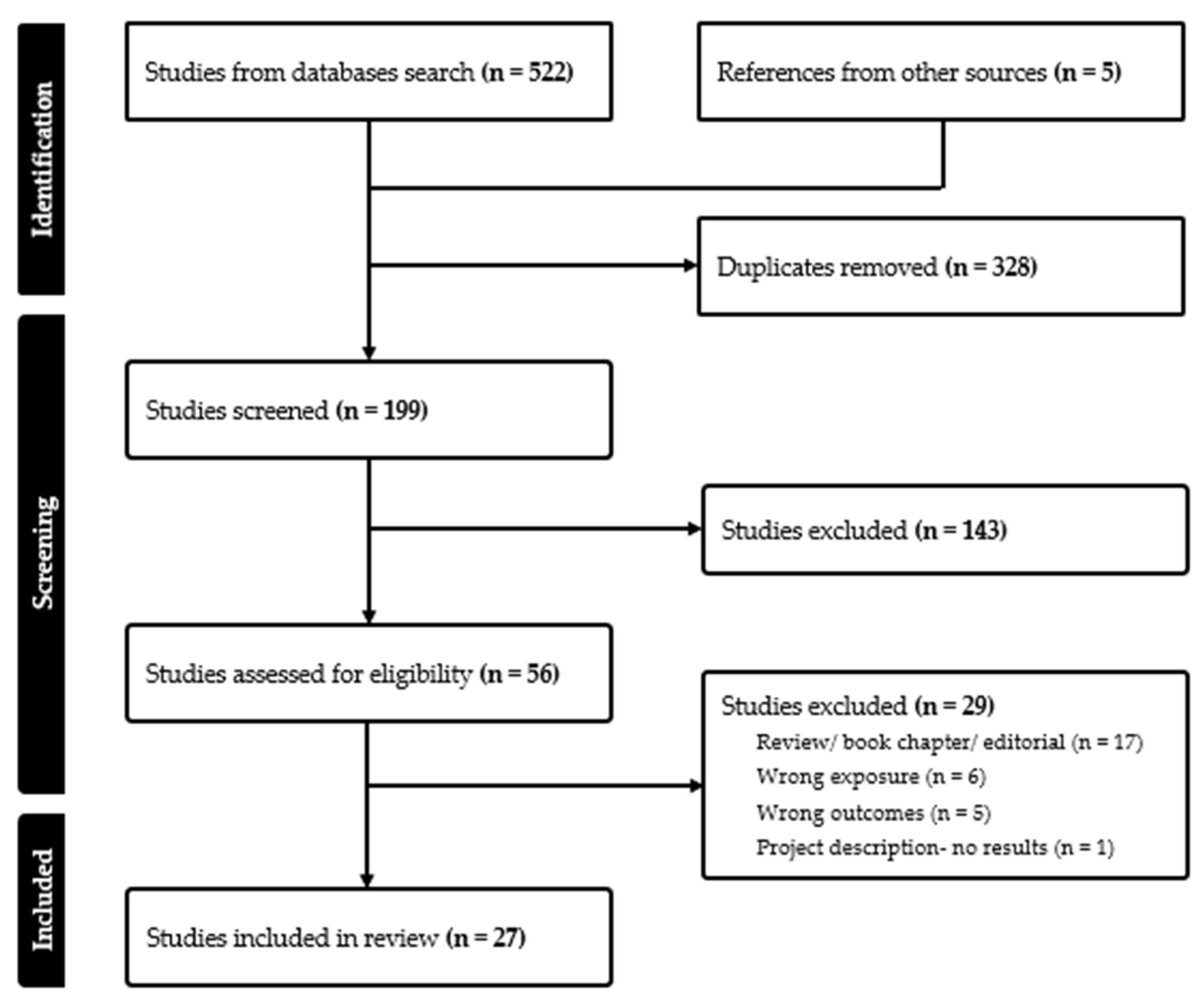
2. Methods
3. Results
3.1. Human Studies
3.1.1. Thyroid Hormone Disruption
3.1.2. Hypothyroidism
3.1.3. Hyperthyroidism
3.1.4. Thyroid Cancer
3.2. In Vitro Studies
3.3. Tadpole Studies
3.4. Rodent Studies
3.5. Other Animal Model Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHS | Agricultural Health Study |
| AMPA | Aminomethylphosphonic acid |
| Bw | Body weight |
| CI | Confidence Interval |
| EDC | Endocrine-disrupting chemical |
| FAE | Formulation acid equivalent |
| FRTL−5 | Fisher Rat Thyroid Cell Line−5 |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GD | Gestational day |
| GBH | Glyphosate-based herbicide |
| GS | Gosner stage |
| HR | Hazard stage |
| HPT | Hypothalamic–pituitary–thyroid (axis) |
| mRNA | Messenger Ribonucleic acid |
| NOAEL | No observed adverse effect level |
| OR | Odds ratio |
| PND | Postnatal day |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SE | Standard error |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TG | Thyroglobulin |
| Thrα1 | Thyroid hormone receptor alpha 1 |
| Thrβ1 | Thyroid hormone receptor beta 1 |
| Thrβ2 | Thyroid hormone receptor beta 2 |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| TSHR | Thyroid-stimulating hormone receptor |
| TTF−1 | Thyroid transcription factor 1 |
| VM | VisionMax® |
References
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, Q. Prevalence and Trends of Thyroid Disease Among Adults, 1999–2018. Endocr. Pract. 2023, 29, 875–880. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Rodrigues, V.G.; Henrique, G.; Sousa-Vidal, É.K.; de Souza, R.M.M.; Tavares, E.F.C.; Mezzalira, N.; Marques, T.D.O.; Alves, B.M.; Pinto, J.A.A.; Irikura, L.N.N.; et al. Thyroid under Attack: The Adverse Impact of Plasticizers, Pesticides, and PFASs on Thyroid Function. Endocrines 2024, 5, 430–453. [Google Scholar] [CrossRef]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid Disrupting Chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Glyphosate. Available online: https://www.epa.gov/ingredients-used-pesticide-products/glyphosate (accessed on 15 July 2025).
- Benbrook, C.M. Trends in Glyphosate Herbicide Use in the United States and Globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef]
- Mills, P.J.; Kania-Korwel, I.; Fagan, J.; McEvoy, L.K.; Laughlin, G.A.; Barrett-Connor, E. Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016. JAMA 2017, 318, 1610. [Google Scholar] [CrossRef]
- Ünlü Endirlik, B.; Bakır, E.; Ökçesiz, A.; Güler, A.; Hamurcu, Z.; Eken, A.; Dreij, K.; Gürbay, A. Investigation of the Toxicity of a Glyphosate-Based Herbicide in a Human Liver Cell Line: Assessing the Involvement of Nrf2 Pathway and Protective Effects of Vitamin E and α-Lipoic Acid. Environ. Toxicol. Pharmacol. 2022, 96, 103999. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.-C.; Séralini, G.-E. Glyphosate-Based Herbicides Are Toxic and Endocrine Disruptors in Human Cell Lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, Pathways to Modern Diseases II: Celiac Sprue and Gluten Intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Covidence Systematic Review Software. Veritas Health Innovation, Melbourne, Australia. Available online: https://www.covidence.org. (accessed on 29 September 2025).
- Kongtip, P.; Nankongnab, N.; Kallayanatham, N.; Pundee, R.; Choochouy, N.; Yimsabai, J.; Woskie, S. Thyroid Hormones in Conventional and Organic Farmers in Thailand. Int. J. Environ. Res. Public. Health 2019, 16, 2704. [Google Scholar] [CrossRef] [PubMed]
- Omidakhsh, N.; Heck, J.E.; Cockburn, M.; Ling, C.; Hershman, J.M.; Harari, A. Thyroid Cancer and Pesticide Use in a Central California Agricultural Area: A Case Control Study. J. Clin. Endocrinol. Metab. 2022, 107, e3574–e3582. [Google Scholar] [CrossRef]
- Goldner, W.S.; Sandler, D.P.; Yu, F.; Hoppin, J.A.; Kamel, F.; Levan, T.D. Pesticide Use and Thyroid Disease among Women in the Agricultural Health Study. Am. J. Epidemiol. 2010, 171, 455–464. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Pundee, R.; Kallayanatham, N.; Pengpumkiat, S.; Chungcharoen, J.; Phommalachai, C.; Konthonbut, P.; Choochouy, N.; Sowanthip, P.; et al. Acute Changes in Thyroid Hormone Levels among Thai Pesticide Sprayers. Toxics 2021, 9, 16. [Google Scholar] [CrossRef]
- Goldner, W.S.; Sandler, D.P.; Yu, F.; Shostrom, V.; Hoppin, J.A.; Kamel, F.; LeVan, T.D. Hypothyroidism and Pesticide Use among Male Private Pesticide Applicators in the Agricultural Health Study. J. Occup. Environ. Med. 2013, 55, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Parks, C.G.; Goldner, W.S.; Kamel, F.; Umbach, D.M.; Ward, M.H.; Lerro, C.C.; Koutros, S.; Hofmann, J.N.; Beane Freeman, L.E.; et al. Incident Thyroid Disease in Female Spouses of Private Pesticide Applicators. Environ. Int. 2018, 118, 282–292. [Google Scholar] [CrossRef]
- Lerro, C.C.; Beane Freeman, L.E.; DellaValle, C.T.; Kibriya, M.G.; Aschebrook-Kilfoy, B.; Jasmine, F.; Koutros, S.; Parks, C.G.; Sandler, D.P.; Alavanja, M.C.R.; et al. Occupational Pesticide Exposure and Subclinical Hypothyroidism among Male Pesticide Applicators. Occup. Environ. Med. 2018, 75, 79–89. [Google Scholar] [CrossRef]
- Shrestha, S.; Parks, C.G.; Goldner, W.S.; Kamel, F.; Umbach, D.M.; Ward, M.H.; Lerro, C.C.; Koutros, S.; Hofmann, J.N.; Beane Freeman, L.E.; et al. Pesticide Use and Incident Hypothyroidism in Pesticide Applicators in the Agricultural Health Study. Environ. Health Perspect. 2018, 126, 97008. [Google Scholar] [CrossRef]
- Santos, R.; Piccoli, C.; Cremonese, C.; Freire, C. Thyroid and Reproductive Hormones in Relation to Pesticide Use in an Agricultural Population in Southern Brazil. Environ. Res. 2019, 173, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Dal’ Bó, I.F.; Teixeira, E.S.; Rabi, L.T.; Peres, K.C.; Nascimento, M.; Chiamolera, M.I.; Máximo, V.; Bufalo, N.E.; Ward, L.S. Alternation between Toxic and Proliferative Effects of Roundup® on Human Thyroid Cells at Different Concentrations. Front. Endocrinol. 2022, 13, 904437. [Google Scholar] [CrossRef]
- Ward, L.S.; Rabi, L.T.; Teixeira, E.D.; de Oliveira, S.D.; da Silva, J.G.D.; Peres, K.C.; Nascimento, M.; Bufalo, N.E.; Dal’ Bó Cruz, I.F. ODP272 Roundup is an Important Disruptor That Causes Proliferative Effects on Normal and on Cancer Thyroid Cells. J. Endocr. Soc. 2022, 6, A433–A434. [Google Scholar] [CrossRef]
- Coperchini, F.; Greco, A.; Croce, L.; Denegri, M.; Magri, F.; Rotondi, M.; Chiovato, L. In Vitro Study of Glyphosate Effects on Thyroid Cells. Environ. Pollut. 2023, 317, 120801. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Peltzer, P.M.; Attademo, A.M.; Martinuzzi, C.S.; Simoniello, M.F.; Colussi, C.L.; Cuzziol Boccioni, A.P.; Sigrist, M. First Evaluation of Novel Potential Synergistic Effects of Glyphosate and Arsenic Mixture on Rhinella arenarum (Anura: Bufonidae) Tadpoles. Heliyon 2019, 5, e02601. [Google Scholar] [CrossRef]
- Cuzziol Boccioni, A.P.; Lajmanovich, R.C.; Peltzer, P.M.; Attademo, A.M.; Martinuzzi, C.S. Toxicity Assessment at Different Experimental Scenarios with Glyphosate, Chlorpyrifos and Antibiotics in Rhinella arenarum (Anura: Bufonidae) Tadpoles. Chemosphere 2021, 273, 128475. [Google Scholar] [CrossRef]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of Glyphosate-Based Pesticides to Four North American Frog Species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef]
- Elkattan, A.N.; El-Saadany, S.; Azzazy, M.; Okda, T.M.; Mamdouh, M.; Ahmed, O.; El-Far, A.H.; ElKhayat, M.; Albadrani, G.M.; Al-Ghadi, M.Q.; et al. Ameliorative Effect of Licorice Extract against the Detrimental Effect of Glyphosate-Based Pesticide: Toxicity and Health. Heliyon 2024, 10, e31623. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Lanctôt, C.; Jackman, P.; Park, B.J.; Doe, K.; Pauli, B.D.; Trudeau, V.L. Effects of Glyphosate-Based Herbicides on Survival, Development, Growth and Sex Ratios of Wood Frogs (Lithobates sylvaticus) Tadpoles. I: Chronic Laboratory Exposures to VisionMax®. Aquat. Toxicol. 2014, 154, 278–290. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.S.; Kizys, M.M.L.; da Conceição, R.R.; Glebocki, G.; Romano, R.M.; Ortiga-Carvalho, T.M.; Giannocco, G.; da Silva, I.D.C.G.; Dias da Silva, M.R.; Romano, M.A.; et al. Perinatal Exposure to Glyphosate-Based Herbicide Alters the Thyrotrophic Axis and Causes Thyroid Hormone Homeostasis Imbalance in Male Rats. Toxicology 2017, 377, 25–37. [Google Scholar] [CrossRef]
- Manservisi, F.; Lesseur, C.; Panzacchi, S.; Mandrioli, D.; Falcioni, L.; Bufea, L.; Manservigi, M.; Spinaci, M.; Galeati, G.; Mantovani, A.; et al. The Ramazzini Institute 13-Week Pilot Study Glyphosate-Based Herbicides Administered at Human-Equivalent Dose to Sprague Dawley Rats: Effects on Development and Endocrine System. Environ. Health 2019, 18, 15. [Google Scholar] [CrossRef]
- Hamdaoui, L.; Oudadesse, H.; Lefeuvre, B.; Mahmoud, A.; Naifer, M.; Badraoui, R.; Ayadi, F.; Rebai, T. Sub-Chronic Exposure to Kalach 360 SL, Glyphosate-Based Herbicide, Induced Bone Rarefaction in Female Wistar Rats. Toxicology 2020, 436, 152412. [Google Scholar] [CrossRef] [PubMed]
- Costa Reis, L.T.; Sena de Souza, J.; Hirochi Herai, R.; Cunha, E.B.; Ribeiro Pereira Soares, J.; Santos El-Bachá, R.; Diogenes Amaral da Silva, V.; Aurelio Romano, M.; Marino Romano, R.; Izabel Chiamolera, M.; et al. Intergenerational Thyroid Hormone Homeostasis Imbalance in Cerebellum of Rats Perinatally Exposed to Glyphosate-Based Herbicide. Environ. Toxicol. 2021, 36, 1031–1042. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Shi, F.; Zhang, S.; Wang, S.; Feng, X. Melatonin Alleviates the Deterioration of Oocytes and Hormonal Disorders from Mice Subjected to Glyphosate. Mol. Cell Endocrinol. 2021, 520, 111073. [Google Scholar] [CrossRef]
- Docea, A.O.; Cirstea, A.E.; Cercelaru, L.; Drocas, A.I.; Dinca, V.; Mesnage, R.; Marginean, C.; Radu, A.; Popa, D.G.; Rogoveanu, O.; et al. Effect of Perinatal Exposure to Glyphosate and Its Mixture with 2,4-D and Dicamba on Rat Dam Kidney and Thyroid Function and Offspring’s Health. Environ. Res. 2023, 237, 116908. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Zenzeluk, J.; Bargi-Souza, P.; Szawka, R.E.; Romano, M.A.; Romano, R.M. The Effects of Glyphosate-Based Herbicide on the Hypothalamic-Pituitary Thyroid Axis Are Tissue-Specific and Dependent on Age Exposure. Environ. Pollut. 2023, 334, 122216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shangguan, Y.; Zhu, P.; Sultan, Y.; Feng, Y.; Li, X.; Ma, J. Developmental Toxicity of Glyphosate on Embryo-Larval Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 236, 113493. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, P.; Veskoukis, A.S.; Rossiou, D.; Gournikis, C.; Kapetanopoulou, T.; Karzi, V.; Docea, A.O.; Tsatsakis, A.; Kouretas, D. A Mixture of Endocrine Disruptors and the Pesticide Roundup® Induce Oxidative Stress in Rabbit Liver When Administered under the Long-Term Low-Dose Regimen: Reinforcing the Notion of Real-Life Risk Simulation. Toxics 2022, 10, 190. [Google Scholar] [CrossRef]
- Killian, D.; Faheem, M.; Reh, B.; Wang, X.; Bhandari, R.K. Effects of Chronic Roundup Exposure on Medaka Larvae. J. Xenobiot. 2023, 13, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Aranda, A. Thyroid Hormone Receptors, Cell Growth and Differentiation. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3908–3916. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Bleak, T.C.; Calaf, G.M. Glyphosate and the Key Characteristics of an Endocrine Disruptor: A Review. Chemosphere 2021, 270, 128619. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; de Oliveira, J.M.; de Oliveira, V.M.; de Oliveira, I.M.; Torres, Y.R.; Bargi-Souza, P.; Martino Andrade, A.J.; Romano, M.A. Could Glyphosate and Glyphosate-Based Herbicides Be Associated With Increased Thyroid Diseases Worldwide? Front. Endocrinol. 2021, 12, 627167. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Study Design | Study Population (n) | Country | Number of Cases and Controls | Association with Thyroid Disease |
|---|---|---|---|---|---|
| Goldner et al. (2010) [15] | Prospective cohort study | Female spouses of licensed pesticide applicators (n = 16,529) | US | 5071 exposed cases and 11458 control cases | Hyperthyroidism: OR 0.98 (95% CI: 0.78–1.2) Hypothyroidism: OR 1.00 (95% CI: 0.91–1.2) Other thyroid disease: OR 0.97 (95% CI: 0.81–1.2) |
| Goldner et al. (2013) [17] | Retrospective cohort study | Private male pesticide applicators (n = 22,246) | US (AHS); Iowa and North Carolina | 919 exposed cases and 21,327 controls) | Hypothyroidism: 1.18 (95% CI 0.94–1.49) |
| Lerro et al. (2018) [19] | Retrospective cohort study | Male pesticide applicators (n = 679) | US (AHS); Iowa and North Carolina | 552 total exposed, 127 total controls | Glyphosate exposure intensity-weighted days (0 days: reference): 20–315 days: Subclinical hypothyroidism: OR 1.28 (95% CI: 0.71–2.32). Natural log of hormones: TSH: beta: 1.02 (95% CI: 0.88–1.17), T4: beta: 1.01, (95% CI: 0.95–1.07), and T3: beta: 0.98, (95%CI: 0.92–1.04). >315–917 days: Subclinical hypothyroidism: OR 1.28 (95% CI: 0.6–1.93). Natural log of hormones: TSH: beta: 1.00 (95% CI: 0.87–1.15), T4: beta: 1.01 (95% CI: 0.95–1.07), and T3: beta: 1.01 (95% CI: 0.95 to 1.07). >907–2622 days: Subclinical hypothyroidism: OR: 0.95 (95% CI: 0.51–1.77). Natural log of hormones: TSH: beta: 1.00 (95% CI: 0.87–1.15), T4: beta: 1.01 (95% CI: 0.95–1.07), and T3: beta: 1.01 (95% CI: 0.95–1.07). >2622–113,400 days: Subclinical hypothyroidism: OR: 1.21 (95% CI: 0.66–2.24). Natural log of hormones: TSH: beta: 1.14 (95% CI: 0.99–1.33), T4: beta: 0.99 (95% CI: 0.94–1.05), and T3: beta: 0.97 (95% CI: 0.91–1.01). p-trend for subclinical hypothyroidism: 0.70 p-trend for TSH: 0.05. p-trend for T4: 0.68. p-trend for T3: 0.41 |
| Shrestha et al. (2018) [20] | Prospective cohort study | Licensed pesticide applicators (n = 821) | US | 663 exposed cases with hypothyroidism, 158 controls | Ever use of glyphosate and hypothyroidism risk: 1.28 (95% CI 1.07–1.52) Intensity-weighted lifetime days of use of glyphosate and hypothyroidism risk: >0–≤686 intensity-exposed days: HR: 1.27 (95% CI: 1.03–1.56) >686–≤2604 intensity-exposed days: HR: 1.38 (95% CI: 1.12–1.69) >2604 intensity-exposed days: HR: 1.17 (95% CI: 0.94–1.45) |
| Shresta et al. (2018) [18] | Prospective cohort study | Female spouses of licensed pesticide applicators (n = 2087) | US | 755 exposed cases (170 with hyperthyroidism and 585 with hypothyroidism), 1332 controls (345 with hyperthyroidism and 987 with hypothyroidism) | Ever use of pesticides and risk of incident hypothyroidism: HR: 1.05 (95% CI 0.94–1.16) when adjusted for education, state, and smoking, and HR: 1.07 (95% CI 0.95–1.20) when additionally adjusted for correlated pesticides, wherever applicable. Ever use of pesticides and risk of incident hyperthyroidism: HR: 0.93 (95% CI 0.77–1.12) when adjusted for education, state, and smoking, and HR: 0.90 (95% CI 0.73–1.11) when additionally adjusted for correlated pesticides. |
| Kongtip et al. (2019) [13] | Cross-sectional study | Farmers (n = 417) | Thailand | 222 non-exposed participants (114 male, 108 female), 195 exposed participants (144 male, 51 female) | TSH: beta: 0.992 (95% CI 0.957–1.027) FT3: beta: 1.002 (95% CI 0.998–1.007) FT4: beta: 0.999 (95% CI 0.993–1.005) T3: beta: 1.006 (95% CI 0.999–1.012) T4: beta: 1.007 (95% CI 1.001–1.014) |
| Santos et al. (2019) [21] | Retrospective cohort study | Farm residents (n = 122) | Brazil | 97 cases and 25 controls | Percent change in serum hormone levels with recent glyphosate exposure when comparing exposed to unexposed in the past 7 days: TSH: −22% (95% CI: −56 to 37); Free T4: −1% (95% CI: −10 to 8); Total T4: −7%(95% CI: −20 to 8); Free T3: −5% (95% CI: −13 to 5); Total T3: 0% (95% CI: −13 to 15) |
| Kongtip et al. (2021) [16] | Prospective cohort study | Pesticide sprayers (n = 48) | Thailand | 48 exposed | Change in thyroid hormone levels as a function of changes in urinary glyphosate levels after herbicide spraying (both logged morning after spraying—logged morning before spraying (ΔLN)) ΔLNTSH (nIU/mL). Beta = 68.1. SE = 51.1. p-value = 0.183. ΔLNFT3 (pg/dL). Beta = 1.7. SE = 12.1. p-value = 0.888. ΔLNFT4 (pg/dL). Beta = 11.1. SE = 10.9. p-value = 0.311. ΔLNT3 (ng/dL). Beta = 11.7. SE = 25.1. p-value = 0.642. ΔLNT4 (ng/dL). Beta = 25.5. SE = 12.1. p-value = 0.045. |
| Omidakhsh et al. (2022) [14] | Retrospective case–control study | Residents of central California agricultural area (n = 3070) | US, central California | 2067 cases and 1003 controls | Primary thyroid cancer of all subtypes: 1.33 (95% CI: 1.12–1.58) Distant/regional thyroid cancer: OR 1.37 (95% CI: 95% 1.08, 1.73) Localized thyroid cancer: OR 1.26 (95% CI: 1.04, 1.52). (Disease associations did not remain after adjustment for paraquat) |
| Author (Year) | Chemical Exposure | Experimental Model | Exposure Duration | Association with Thyroid Disease |
|---|---|---|---|---|
| Dal’ Bó et al. (2019) [22] | Roundup Original Dl | Nthy-or 3–1 (from thyroid normal follicular cells) TPC−1 (from papillary carcinoma) | 24 and 48 h | Percent mortality of Nthy-ori 3–1 and TPC−1 cells measured by trypan blue exclusion test. 24 h: 6.5 µg/L Roundup: 49.5 +/− 1.5% for Nthy-ori 3–1. 54 +/− 9.5% for TPC−1. 65 µg/L Roundup: 38 +/− 3.7% for Nthy-ori 3–1. 63 +/− 8.9% for TPC−1. 160 µg/L Roundup: 43 +/− 9.2% for Nthy-ori 3–1. 50 +/− 7.5% for TPC−1. 830 µg/L Roundup: 35 +/− 8.4% for Nthy-ori 3–1. 58 +/− 3.7% for TPC−1. 6500 µg/L Roundup: 53 +/− 3% for Nthy-ori 3–1. 57 +/− 9.6% for TPC−1. 48 h: 6.5 µg/L Roundup: 26 +/− 12.6% for Nthy-ori 3–1. 22 +/− 6% for TPC−1. 65 µg/L Roundup: 34 +/− 5.7% for Nthy-ori 3–1. 31 +/− 4.8% for TPC−1. 160 µg/L Roundup: 33 +/− 15.1% for Nthy-ori 3–1. 33 +/− 4.9% for TPC−1. 830 µg/L Roundup: 18 +/− 3.8% for Nthy-ori 3–1. 37 +/− 14.7% for TPC−1. 6500 µg/L Roundup: 23 +/− 3.8% for Nthy-ori 3–1. 15 +/− 1.7% for TPC−1. Percent of viable cells after Roundup exposure measured by CCK−8 assay. 24 h. 6.5 µg/L Roundup: 113 +/− 13.44% for Nthy-ori 3–1. 105 +/− 15.3% for TPC−1. 65 µg/L Roundup: 104 +/− 7.55% for Nthy-ori 3–1. 94 +/− 6.6% for TPC−1. 160 µg/L Roundup: 87 +/− 3.6% for Nthy-ori 3–1. 88 +/− 6.6% for TPC−1. 830 µg/L Roundup: 87 + /− 5.36% for Nthy-ori 3–1. 97 +/− 11.1% for TPC−1. 6500 µg/L Roundup: 86 +/− 6.23% for Nthy-ori 3–1. 91 +/− 6.9% for TPC−1. 48 h. 6.5 µg/L Roundup: 101 +/− 1.2% for Nthy-ori 3–1. 79 +/− 24.8% for TPC−1. 65 µg/L Roundup: 102 +/− 1.73% for Nthy-ori 3–1. 79 +/− 16.2% for TPC−1. 160 µg/L Roundup: 84 +/− 4.18% for Nthy-ori 3–1. 100 +/− 7.83% for TPC−1. 830 µg/L Roundup: 91 +/− 4.48% for Nthy-ori 3–1. 82 +/− 12.3% for TPC−1. 6500 µg/L Roundup: 92 +/− 1.76% for Nthy-ori 3–1. 106 +/− 4.93% for TPC−1. |
| Ward et al. (2022) [23] | Roundup Original Dl | Thyroid-derived cell lines Nthy-or 3–1 (from thyroid normal follicular cells) and TPC−1 (from papillary carcinoma) | 24 and 48 h | At 24 h, 160 µg/L resulted in 52% and 58% cell death in Nthy-ori 3–1 and TPC−1 cells, respectively. 830 µg/L resulted in 43% and 58% cell death in Nthy-ori 3–1 and TPC−1 cells, respectively. At 48 h, 160 µg/L resulted in 19% and 29% cell death in Nthy-ori 3–1 and TPC−1 cells, respectively. 830 µg/L resulted in 15% and 23% cell death in Nthy-ori 3–1 and TPC−1 cells, respectively. After 24 h of 6.5 µg/L exposure, cell viability increased to 113% in Nthy-ori 3–1 cells and 118% in TPC−1 cells. This proliferative effect persisted at 48 h as measured by the CCK−8 assay |
| Coperchini et al. (2023) [24] | Glyphosate | Adherent−2D and spheroid−3D models derived from the Fisher rat thyroid cell line−5 (FRTL−5) cell strain | 24 h | Changes in the mRNA levels of thyroid-related genes after Adherent and spheroid FRTL−5 cells were exposed to 0.5 mM glyphosate: FRTL−5 Adherent−2D Model: NIS (sodium/iodide symporter): 1.85-fold change (95% CI: 1.13–3.17) Thyroid transcription factor gene Pax8: 2.34-fold change (95% CI: 1.77–6.53) TG(thyroglobulin): 2.49-fold change (95% CI: 1.58–3.36) TPO (thyroid peroxidase): 1.85-fold change (95% CI: 0.94- 2.94) TSHR(thyroid-stimulating hormone receptor): 2.75-fold change (95% CI: 1.51–8.34) TTF−1 (thyroid transcription factor 1): 3.55-fold change (95% CI: 1.92–4.11) FRTL−5 Spheroid−3D Model: TG: 3.82-fold change (95% CI: 2.31–5.60) TPO: 4.27-fold change (95% CI: 3.02–7.29) TSHR: 14.76-fold change (95% CI: 1.69–25.78) |
| Author (Year) | Chemical Exposure and Dose | Experimental Model and Exposure Route | Exposure Duration and Age at Experiment End | Association with Thyroid Disease |
|---|---|---|---|---|
| Howe et al. (2004) [27] | Glyphosate technical and Roundup Original; 0.6 and 1.8 mg FAE/L (formulation acid equivalents) | North American amphibian species (Rana clamitans, R. pipiens, R. sylvatica, and Bufo americanus); Immersion in aquaria filled with filtered river water and the test compounds added once a week in a static renewal system | Acute exposure had 24 and 96 h exposures; chronic exposure groups were exposed until 80% or more of the surviving tadpoles in each control group reached metamorphic climax (Gosner stage 42). | Relative expression levels (copy number/5 ng total RNA) of thyroid hormone receptor ß (TRß) mRNA in the tails of Gosner stage 25: Control: 901.8 +/− 34.9 0.6 mg FAE/L glyphosate: 913.5 +/− 87.2 1.8 mg FAE/L glyphosate: 1189.8 +/− 75.7 Gosner stage 42: Control: 10349 +/− 1327 0.6 mg FAE/L glyphosate: 0 1.8 mg FAE/L glyphosate: 8874.7 +/− 884.5 |
| Navarro-Martína et al. (2014) [29] | VisionMax (glyphosate-based herbicide): 0.021 mg acid equivalents (a.e.)/L and 2.9 mg a.e./L. | Wood frog tadpoles (Lithobates sylvaticus); Water | Exposure started at GS 25; GS 42 at the end of the experiment. | Fold change of trß, dio2, dio3 mRNA levels in the brain from GS30 to GS42: trß: Increased trß expression across developmental stages (F(2,69) = 3.475, p = 0.037) dio2: Significant interactions between treatment and Gosner developmental stage were detected for dio2 (F(4,69) = 16.163, p < 0.001). dio3: Not significant by treatment (F(2) = 2.249, p = 0.118). Fold change of trß, dio2, dio3 mRNA levels in the tails of tadpoles at GS30 to GS42 chronically exposed to VisionMax® (VM®), determined by real-time RT-PCR. trß: Significant treatment effect: (F(2,69) = 27.569, p < 0.001) dio2: Significant treatment and stage interaction: (F(4,69) = 11.157, p < 0.001) dio3: Not significant by treatment (F(2) = 2.653, p = 0.079) |
| Lajmanovich et al. (2019) [25] | Glyphosate-based herbicide (GBH): Acute toxicity: 1.25 mg/L of GBH. Chronic toxicity: 1.25 mg/L of GBH | Tadpoles (Rhinella arenarum); Immersion in test solutions containing the GBH in glass flasks with dechlorinated tap water | Acute toxicity was exposed for 48 h, and chronic toxicity was exposed for 22 days; All tadpoles were at GS 26–30 at the start of the experiment | T3 levels in the 48 h toxicity group: Control: 1.714 +/− 0.187 ng/g GBH: 1.592 +/− 0.248 ng/g T3 levels in the 22 days toxicity group: Control: 1.149 +/− 0.232 ng/g GBH: 1.199 +/− 0.215 ng/g T4 levels in the 48 h toxicity group: Control: 6.441 +/− 1.299 ng/g GBH: 6.723 +/− 1.695 ng/g T4 levels in the 22 days toxicity group: Control: 3.842 +/− 1.808 ng/g GBH: 5.819 +/− 2.034 ng/g |
| Cuzziol Boccioni et al. (2021) [26] | Glyphosate: 1.25 mg/L (GHB1) and 2.5 mg/ L (GHB2) | Tadpoles (Rhinella arenarum); Water | 2 weeks GS (Gosner stage) 26 at the end of the experiment. | Control: mean T4: 2.4 +/− 0.75 ng/g GHB1: 2.66 +/− 0.36 ng/g GHB2: mean T4: 1.22 +/− 0.1 ng/g (p > 0.005) |
| Author (Year) | Chemical Exposure and Dose | Experimental Model and Exposure Route | Age at the Beginning of the Exposure and Exposure Duration | Association with Thyroid Disease |
|---|---|---|---|---|
| de Souza et al. (2017) [30] | Roundup Transorb; 5 mg/kg/day and 50 mg/kg/day | Female pregnant Wistar rats were exposed, male offspring were analyzed; By Gavage | GD (gestational day) 18 at the beginning of the exposure; Exposure lasted until PND (postnatal day) 5 | TSH (ng/dL): Control: 962.5 +/− 152.1 5 mg/kg/day: 526.3 +/− 96.32 (p < 0.05) 50 mg/kg/day: 507.7 +/− 91.49 (p < 0.05) T3 (ng/dL): control: 58.63 +/− 4.590 5 mg/kg/day: 48.13 +/− 2.730 50 mg/kg/day: 52.36 +/− 2.123 T4 (ng/dL): control: 4.674 +/− 0.2476; 5 mg/kg/day: 4.335 +/− 0.2404; 50 mg/kg/day:4.801 +/− 0.1748 |
| Manservisi et al. (2019) [31] | Glyphosate or Roundup Bioflow; Dose of 1.75 mg/kg bw/day | Sprague Dawley rats; Drinking water | GD 6 (in utero) up to PND 120; Exposed for 6 weeks and 13 weeks. | TSH (males; mean ± SEM): 6-week cohort: control 4.23 ± 0.76, glyphosate 8.17 ± 1.58 (p < 0.05), Roundup 5.57 ± 0.31 13-week cohort: control 1.89 ± 0.20, glyphosate 2.53 ± 0.25, Roundup 3.69 ± 0.42 (p < 0.01) TSH (females; mean ± SEM): 6-week cohort: control 2.70 ± 1.13, glyphosate 3.02 ± 2.00, Roundup 3.04 ± 1.53 13-week cohort: control 1.29 ± 0.69, glyphosate 1.93 ± 0.89, Roundup 3.03 ± 2.22 |
| Hamdaoui et al. (2020) [32] | Kalach 360 SL herbicide (KL); 126 mg/Kg and 315 mg/Kg dissolved in water | Female Wistar rats; Gavage, dissolved in water. | Rats were exposed for 60 days | Plasma-Free T3 Levels (pmol/L). control: 4.82 +/− 0.76 126 mg/Kg: 2.89 +/− 1.27 (p < 0.01) 315 mg/Kg: 2.35 +/− 0.53 (p < 0.001) Plasma-Free T4 levels (pmol/L): control: 25.83 +/− 2.2 126 mg/Kg: 16.85 +/− 2.61 (p < 0.05) 315 mg/Kg: 13.26 +/− 4.23 (p < 0.01) Plasma TSH levels (pmol/L) control 0.457: +/− 0.123 126 mg/Kg: 0.733 +/− 0.6 (p < 0.01) 315 mg/Kg: 0.917 +/− 0.083 (p < 0.001) |
| Costa Reis et al. (2021) [33] | Roundup Transorb; 5 mg/kg/day and 50 mg/kg/day | Female pregnant Wistar rats were exposed, male offspring were analyzed; By Gavage | GD 18 to PND 5 | Thyroid hormone receptor alpha 1 (Thrα1) mRNA levels: control 0.961 ± 0.272, 5 mg/kg 0.906 ± 0.238, 50 mg/kg 1.513 ± 0.223 (p < 0.05) Receptor Thrβ1 gene expression: control 1.147 ± 0.647, 5 mg/kg 0.591 ± 0.343 (p < 0.05), 50 mg/kg 0.957 ± 0.308 Receptor Thrβ2 gene expression: control 1.116 ± 0.562, 5 mg/kg 0.698 ± 0.280, and 50 mg/kg 0.835 ± 0.209 Thyroid hormone transporter Slco1c (Oatp1c1) expression: control 1.302 ± 0.988, 5 mg/kg 1.386 ± 0.855, and 50 mg/kg 1.277 ± 0.950 Thyroid hormone transporter, Mct8, expression: control 1.114 ± 0.662, 5 mg/kg 2.353 ± 0.482 (p < 0.05), 50 mg/kg 1.909 ± 0.988 Deiodinase 1 gene expression: control 1.335 ± 0.896, 5 mg/kg 1.257 ± 1.513, and 50 mg/kg 1.184 ± 0.850 Deiodinases 2 (Dio2) gene expression: controls 1.027 ± 0.236, 5 mg/kg 0.968 ± 0.692, and 50 mg/kg 1.008 ± 0.606 Dio3 gene expression: controls 1.554 ± 0.866, 5 mg/kg 0.534 ± 0.173 (p < 0.05), 50 mg/kg 0.891 ± 0.568 |
| Zhang et al. (2021) [34] | Glyphosate; Low dose: 250 mg/kg High dose: 500 mg/kg | Female Kunming mice; Intragastric | 4 weeks old at the beginning of exposure; 7 days of exposure | TRH (µ IU/mL): control: 7.8 +/− 1.92, Low dose: 14.93 +/− 1.08 (p < 0.0001), High dose: 14.93 +/− 1.12 (p < 0.0001) TSH (mU/L): control: 20.37 +/− 0.63, Low dose: 17.02 +/− 0.64 (p < 0.01), High dose: 13.61 +/− 1.5 (p < 0.0001) T4 (ng/mL): control: 109.75 +/− 2.25, Low dose: 96 +/− 2.75 (p < 0.01), High dose: 83.25 +/− 3.5 (p < 0.0001) T3 (pmol/L): control: 21.30 +/− 0.51, Low dose: 19.64 +/− 0.85 (p < 0.01), High dose: 15.77 +/− 0.21 (p < 0.0001) Relative expression of genes in the HPT axis. Relative Dio2 mRNA expression in the hypothalamus: control: 1.008 +/− 0.113, Low dose: 0.121 +/− 0.008 (p < 0.0001), High dose: 0.0726 +/− 0.008 (p < 0.0001) Relative Mct8 mRNA expression in hypothalamus: control: 1.003 +/− 0.054, Low dose: 0.1554 +/− 0.0156 (p < 0.0001), High dose: 0.1865 +/− 0.0389 (p < 0.0001) Relative Dio2 mRNA expression in pituitary: control: 1.027 +/− 0.165, Low dose: 1.438 +/− 0.494, High dose: 5.219 +/− 2.055 (p < 0.001) [there was also a significant difference between Low dose and H-gly groups, p < 0.001] Relative Mct8 mRNA expression in pituitary: control: 0.993 +/− 0.124, Low dose: 1.821 +/− 0.165 (p < 0.001), High dose: 2.793 +/− 0.207 (p < 0.0001) Relative Thrh mRNA expression in pituitary: control: 1.005 +/− 0.078, Low dose: 1.881 +/− 0.387 (p < 0.01), High dose: 3.557 +/− 0.309 (p < 0.0001) Relative Nis mRNA expression in thyroid: control: 1.031 +/− 0.177, Low dose: 0.5307 +/− 0.1 (p < 0.001), High dose: 0.1923 +/− 0.085 (p < 0.0001) Relative Tpo mRNA expression in thyroid: control: 1.000 +/− 0.0825, Low dose: 0.629 +/− 0.08234, High dose: 0.443 +/− 0.227 (p < 0.01) Relative Tg mRNA expression in thyroid: control: 0.943 +/− 0.140, Low dose: 0.534 +/− 0.085 (p < 0.001), High dose: 0.062 +/− 0.008 (p < 0.0001) Relative Tshr mRNA expression in thyroid: control: 1.023 +/− 0.269, Low dose: 0.185 +/− 0.054 (p < 0.0001), High dose: 0.054 +/− 0.008 (p < 0.0001) |
| Docea et al. (2023) [35] | Glyphosate PRESTANA; ADI: 0.5 mg/kg body weight (bw)/day glyphosate. NOAEL: 50 mg/kg bw/day glyphosate | Female Wistar rats; Drinking water | Female rats were initially 3 months, acclimated to the laboratory environment for 2 weeks and were then mated; Exposure from GD 6 until PND 28. | Total T3 (ng/ mL). control: 39.94 ± 1.11 ADI: 8.95 ± 0.59 (p < 0.001) NOAEL: 46.15 ± 2.83 (p < 0.01) Total T4 (nmol/L). control: 13.30 ± 0.56 ADI 19.97 ± 1.73 (p < 0.01) NOAEL 31.99 ± 1.17 (p < 0.001) TSH (uUI/mL). control: 0.41 ± 0.01 ADI: 0.42 ± 0.01 (p < 0.05) NOAEL: 0.40 ± 0.01 |
| Oliveira et al. (2023) [36] | Roundup Transorb; | Male Wistar rats; By Gavage | PND 23 at the beginning of the exposure; Exposure lasted until PND 60 (37 days of exposure) or PND 90 (67 days of exposure) | Serum TSH concentrations (ng/mL). PND 60: 0 mg GBH/kg/day: 1.00 +/− 0.14 ng/mL 0.5 mg GBH/kg/day: 1.27 +/− 0.32 ng/mL 5 mg GBH/kg/day: 1.85 +/− 0.3 ng/mL. PND 90: 0 mg GBH/kg/day: 1.45 +/− 0.43 ng/mL 0.5 mg GBH/kg/day: 1.24 +/− 0.35 ng/mL 5 mg GBH/kg/day: 0.91 +/− 0.18 ng/mL. Serum T4 concentrations (µg/dL). PND 60: 0 mg GBH/kg/day: 5.25 +/− 0.34 µg/dL 0.5 mg GBH/kg/day: 4.78 +/− 0.18 µg/dL 5 mg GBH/kg/day: 5.05 +/− 0.28 µg/dL PND 90: 0 mg GBH/kg/day: 5.05 +/− 0.28 µg/dL 0.5 mg GBH/kg/day: 5.31 +/− 0.3 µg/dL 5 mg GBH/kg/day: 5.93 +/− 0.32 µg/dL (p < 0.05) Serum T3 concentrations (ng/mL) PND 60: 0 mg GBH/kg/day: 0.44 +/− 0.017 ng/mL 0.5 mg GBH/kg/day: 0.45 +/− 0.02 ng/mL 5 mg GBH/kg/day: 0.53 +/− 0.05 ng/mL PND 90: 0 mg GBH/kg/day: 0.46 +/− 0.03 ng/mL 0.5 mg GBH/kg/day: 0.46 +/− 0.04 ng/mL 5 mg GBH/kg/day: 0.49 +/− 0.05 ng/mL There were no significant differences in relative mRNA expression of Trh in the hypothalamus and Trhr, and Tshb in the pituitary glands. This was consistent across the different PND groups and at various concentrations of GBH. |
| Elkattan et al. (2024) [28] | Glyphosate; 1 mL glyphosate solution 24% | Adult male albino rats; Daily, orally | Adult rats; Exposed for 3 weeks | Free serum T3 levels (ng/dL). Week 1: control: 4.853 +/− 0.147, Gly: 3.637 +/− 0.364 Week 2: control 5.059 +/− 0.235, Gly 3.02 +/− 0.215 (p < 0.05) Week 3: control 5.183 +/− 0.201, Gly 3.118 +/− 0.245 Free serum T4 levels (pg/mL). Week 1: control 2.686 +/− 0.41, Gly 1.776 +/− 0.188 (p < 0.05) Week 2: control 3.018 +/− 0.189, Gly 1.46 +/− 0.329 (p < 0.05) Week 3: control 3.109 +/− 0.137, Gly 1.542 +/− 0.436 (p < 0.05) Total T3 (ng/dL). Week 1: control 126.9 +/− 7.2, Gly 93.9 +/− 13.1 (p < 0.05) Week 2: control 125.3 +/− 8.9, Gly 80.56 +/− 3.88 (p < 0.05) Week 3: control 129.7 +/− 8.3, Gly 86.4 +/− 0.82(p < 0.05) Total T4 (µg/dL). Week 1: control 7.789 +/− 1.024, Gly 3.935 +/− 0.667 (p < 0.05) Week 2: control 7.268 +/− 0.862, Gly 3.789 +/− 0.683 (p < 0.05) Week 3: control 7.333 +/− 0.927, Gly 4.325 +/− 0.504 (p < 0.05) TSH (μU/mL). Week 1: control 0.0144 +/− 0.0098, Gly 0.00862 +/− 0.00123. Week 2: control 0.0128 +/− 0.0065, Gly 0.00903 +/− 0.0013. Week 3: control 0.0226 +/− 0.0065, Gly 0.199 +/− 0.0004 (p < 0.05) |
| Author (Year) | Chemical Exposure and Dose | Experimental Model and Exposure Route | Age at the Beginning of the Exposure and Exposure Duration | Association with Thyroid Disease |
|---|---|---|---|---|
| Liu et al. (2022) [37] | Glyphosate; 0.7, 7, and 35 mL/L of glyphosate | Embryo-larval zebrafish (Danio rerio); Water | 1–1.5 h post-fertilization; Exposure from 3 to 120 h post-fertilization | Ratio of T3 to T4 Control 0.0209 +/− 0.0013 0.7 mg/L gly 0.0167 +/− 0.0029 7 mg/L gly 0.0196 +/− 0.0026 35 mg/L gly 0.0148 +/− 0.0009 (p < 0.05) T3: Control: 9.31 +/− 0.65 pmol/L 0.7 mg/L gly: 8.45 +/− 1.29 pmol/L 7 mg/L: 8.52 +/− 1.08 pmol/L, 35 mg/L: 7.61 +/− 0.37 pmol/L (p < 0.05) T4: Control: 441.7 +/− 7.9 pmol/L 0.7 mg/L gly: 504.4 +/− 9.6 pmol/L (p < 0.01) 7 mg/L: 435.5 +/− 9.2 pmol/L 35 mg/L: 513.6 +/− 25.4 pmol/L (p < 0.01) |
| Vardakas et al. (2022) [38] | Glyphosate and Roundup; 5 mg/kg/day | New Zealand rabbits; Dissolved in a 5% ethanol/water solution and administered once daily | 2–3 months old; Exposed for 12 months | Thyroid gland GSH (reduced form of glutathione) concentrations (µmol/mg protein). Control 0.0075 +/− 0.0018 Glyphosate 0.0096 +/− 0.0018 Roundup 0.0132 +/− 0.0044 Thyroid gland catalase activity (U/mg protein). Control 67.84 +/− 7.36 Glyphosate 62.29 +/− 6.43 Roundup 48.78 +/− 6.61 Thyroid gland total antioxidant capacity (mmol DPPH/mg protein). Control 0.0681 +/− 0.0096 Glyphosate 0.0764 +/− 0.01 Roundup 0.0747 +/− 0.0066 Thyroid gland thiobarbituric acid reactive substances (nmol/mg protein). Control 1.37 +/− 0.06 Glyphosate 1.15 +/− 0.11 Roundup 0.99 +/− 0.05 Thyroid gland protein carbonyls concentration (nmol/mg protein) Control 1.51 +/− 0.44 Glyphosate 0.952 +/− 0.198 Roundup 1.28 +/− 0.4 |
| Killian et al. (2023) [39] | Roundup; 0.05, 0.5, 5, 10, and 20 mg/L of glyphosate | Japanese medaka larvae (Oryzias latipes); Well plate containing exposure solution | 8 h post fertilization; Exposure from 8 h post-fertilization to 14 days post-fertilization | Relative expression of thyroid hormone receptor alpha. Control (0 mg/L) 1.523 +/− 0.746 0.05 mg/L gly 0.274 +/− 0.182 (p < 0.05) 0.5 mg/L gly 1.53 +/− 0.909 5 mg/L gly 0.544 +/− 0.233 0 mg/L gly 0.989 +/− 0.419 20 mg/L gly 0.306 +/− 0.17 (p < 0.05) Relative expression of thyroid hormone receptor beta. Control (0 mg/L) 1.22 +/− 0.437 0.05 mg/L gly 0.426 +/− 0.181 (p < 0.05) 0.5 mg/L gly 2.20 +/− 1.045 5 mg/L gly 0.757 +/− 0.379 10 mg/L gly 1.45 +/− 0.59 20 mg/L gly 0.264 +/− 0.132 (p < 0.05) Relative expression of thyroid-stimulating hormone beta. Control (0 mg/L) 1.44 +/− 0.55 0.05 mg/L gly 1.59 +/− 0.77 (p < 0.05) 0.5 mg/L gly 6.30 +/− 3.89 5 mg/L gly 1.09 +/− 0.82 10 mg/L gly 2.55 +/− 1.01 20 mg/L gly 0.290 +/− 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, L.; Monaghan, M.; Schweppe, R.; Franco, A.T.; Goldner, W.; van Gerwen, M. What Is the Impact of Glyphosate on the Thyroid? An Updated Review. Biomedicines 2025, 13, 2402. https://doi.org/10.3390/biomedicines13102402
Choudhary L, Monaghan M, Schweppe R, Franco AT, Goldner W, van Gerwen M. What Is the Impact of Glyphosate on the Thyroid? An Updated Review. Biomedicines. 2025; 13(10):2402. https://doi.org/10.3390/biomedicines13102402
Chicago/Turabian StyleChoudhary, Lomesh, Mathilda Monaghan, Rebecca Schweppe, Aime T. Franco, Whitney Goldner, and Maaike van Gerwen. 2025. "What Is the Impact of Glyphosate on the Thyroid? An Updated Review" Biomedicines 13, no. 10: 2402. https://doi.org/10.3390/biomedicines13102402
APA StyleChoudhary, L., Monaghan, M., Schweppe, R., Franco, A. T., Goldner, W., & van Gerwen, M. (2025). What Is the Impact of Glyphosate on the Thyroid? An Updated Review. Biomedicines, 13(10), 2402. https://doi.org/10.3390/biomedicines13102402







