Distinctive Gene Expression Profiles and Biological Responses of Skin Fibroblasts to Nicotinamide Mononucleotide: Implications for Longevity Effects on Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Preparation
2.2. H2O2-Induced Aging Model and UV-Induced Photoaging Model
2.3. Measurement of Cellular NAD+ and ATP
2.4. Measurement of Cellular Viability and Wound Scratch Assay
2.5. Cellular Senescence and Oxidative Stress
2.6. Analysis of Genes, Transcriptome and Protein
2.7. Evaluation of Sirtuin and Autophagy Activation
2.8. Mitochondrial Membrane Potential Analysis
2.9. Statistical Analysis
3. Results
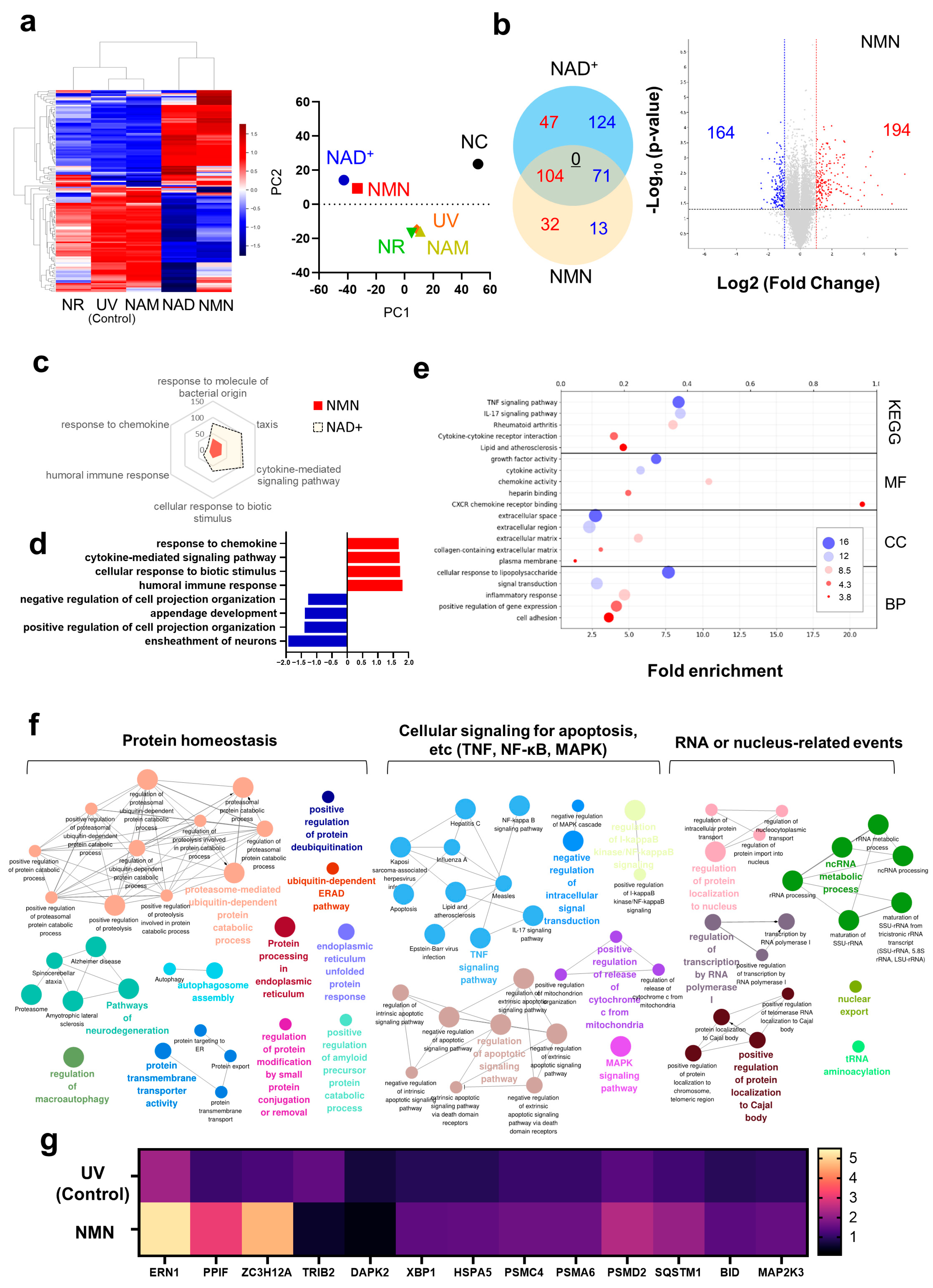
3.1. Transcriptome Analysis of NAD+ and Its Precursors
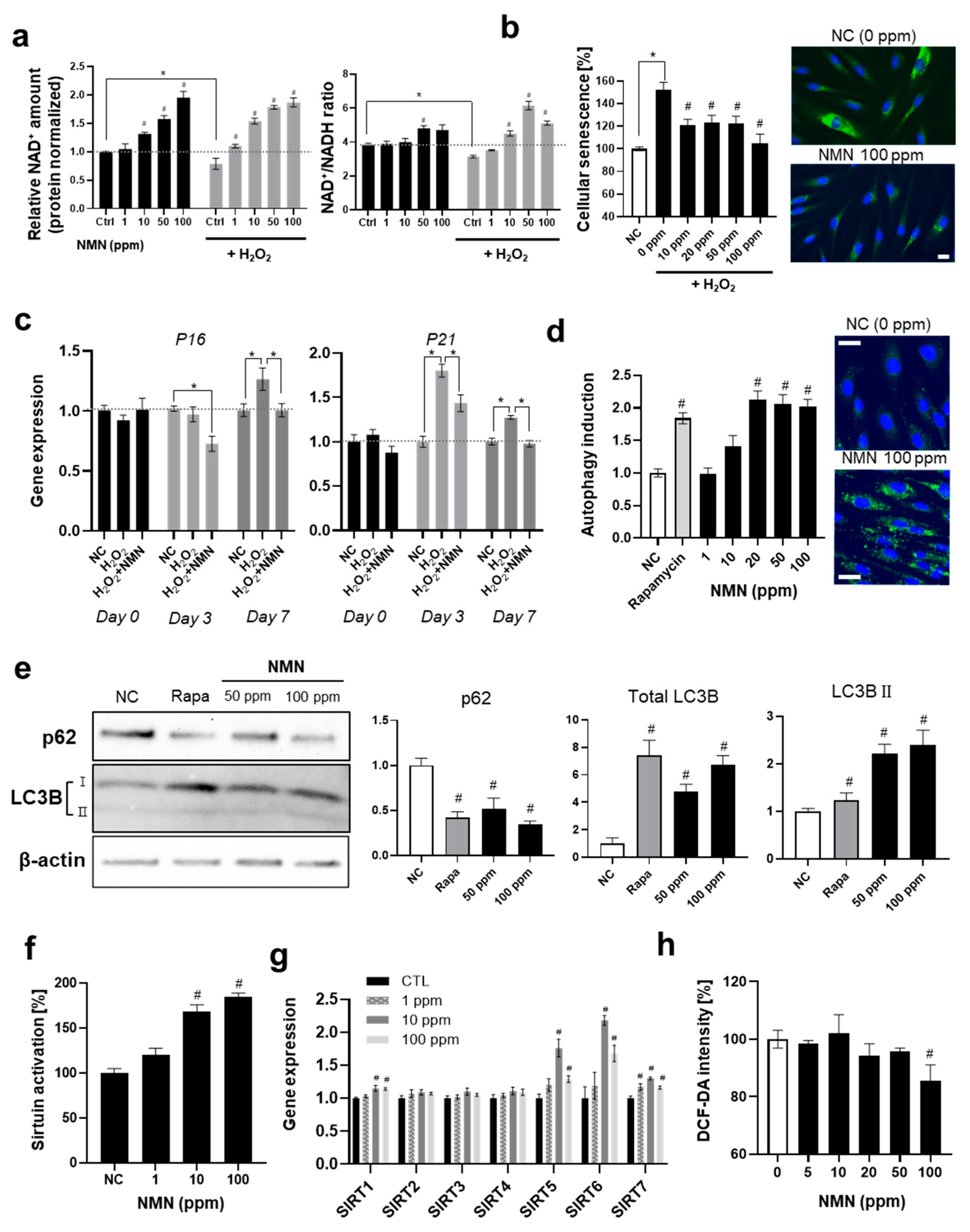
3.2. Biological Response of Human Skin Fibroblasts to NMN
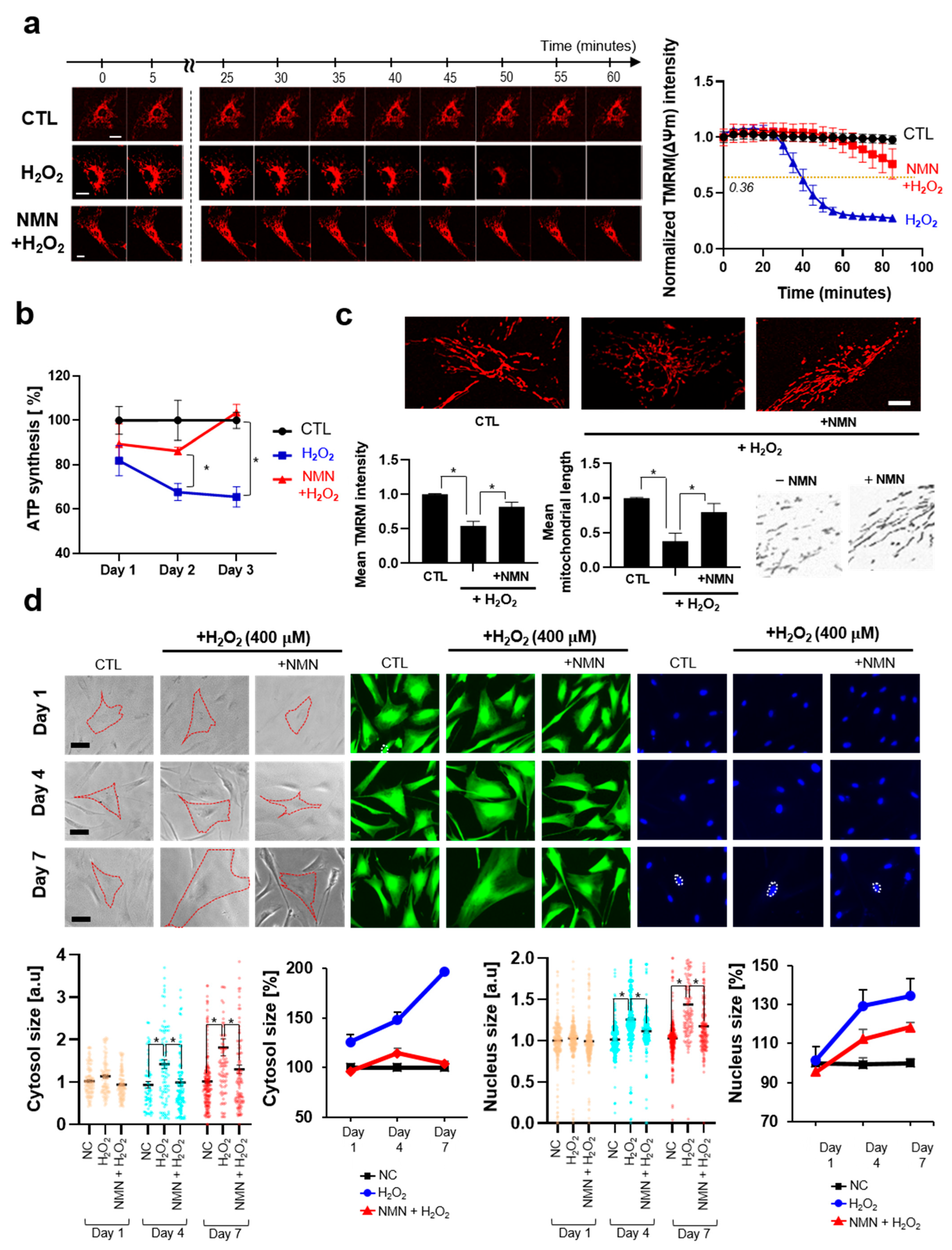
3.2.1. Suppression of Senescence and Activation of Longevity-Related Pathways by NMN
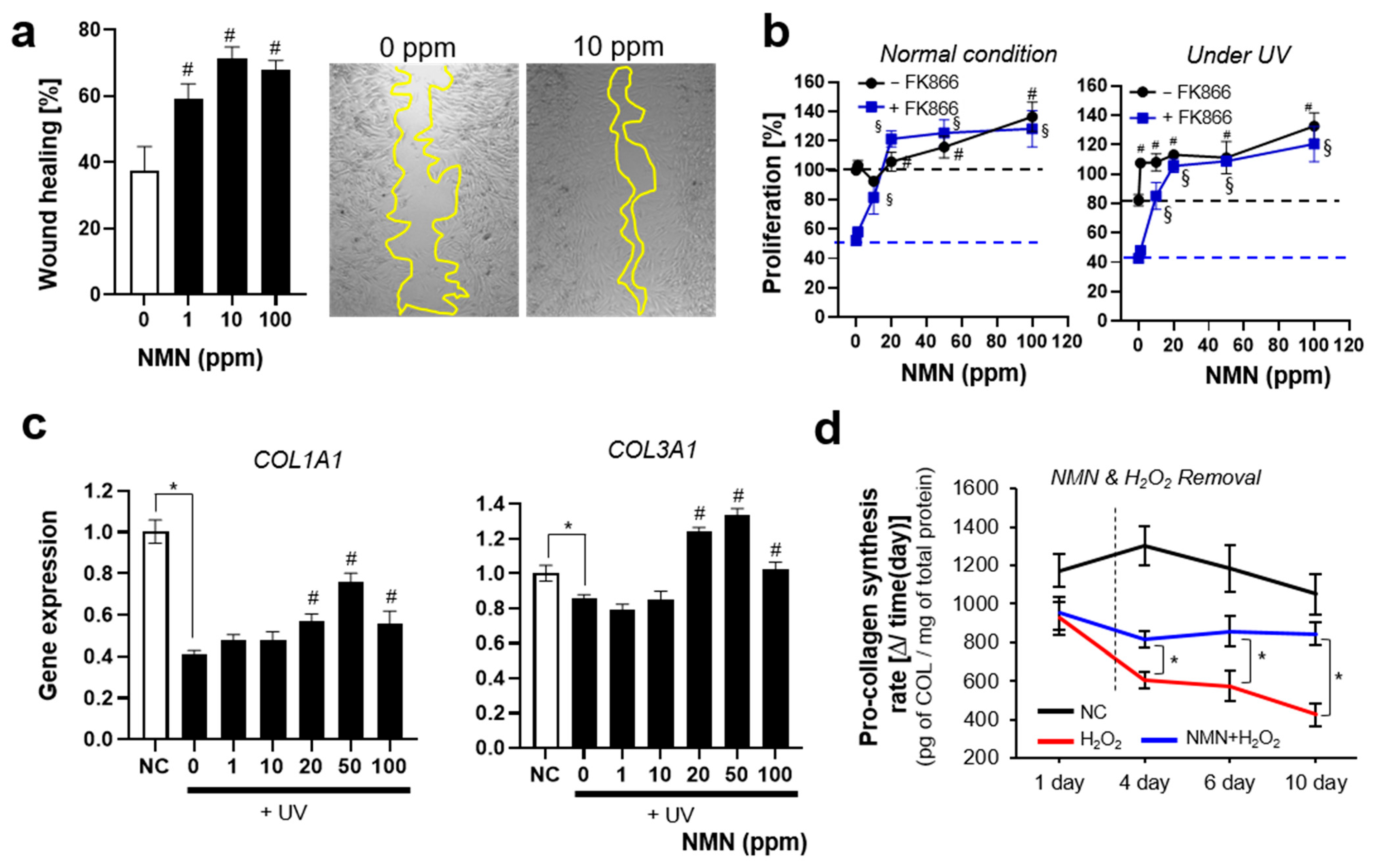
3.2.2. NMN Slows the Progression of Aging and Exerts Protective Effects
3.2.3. NMN Promotes Cell Proliferation and Restores UV-Induced Decrease in ECM Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaku, K.; Okabe, K.; Gulshan, M.; Takatsu, K.; Okamoto, H.; Nakagawa, T. Metabolism and biochemical properties of nicotinamide adenine dinucleotide (NAD) analogs, nicotinamide guanine dinucleotide (NGD) and nicotinamide hypoxanthine dinucleotide (NHD). Sci. Rep. 2019, 9, 13102. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; De Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of altered NAD metabolism in metabolic disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Odoh, C.K.; Guo, X.; Arnone, J.T.; Wang, X.; Zhao, Z.K. The role of NAD and NAD precursors on longevity and lifespan modulation in the budding yeast, Saccharomyces cerevisiae. Biogerontology 2022, 23, 169–199. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, M.; Nomura, T.; Sakamoto, K. Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology 2010, 11, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.-S.; Viswanathan, M.; Schoonjans, K. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; SenGupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 2019, 10, 5284. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.-i. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Auwerx, J. NAD+ metabolism: A therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Kadam, A.; Jubin, T.; Roychowdhury, R.; Begum, R. Role of PARP-1 in mitochondrial homeostasis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129669. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. Sirtuins at the service of healthy longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Kang, S.; Park, J.; Cheng, Z.; Ye, S.; Jun, S.-H.; Kang, N.-G. Novel Approach to Skin Anti-Aging: Boosting Pharmacological Effects of Exogenous Nicotinamide Adenine Dinucleotide (NAD+) by Synergistic Inhibition of CD38 Expression. Cells 2024, 13, 1799. [Google Scholar] [CrossRef]
- Poljšak, B.; Kovač, V.; Milisav, I. Current uncertainties and future challenges regarding NAD+ boosting strategies. Antioxidants 2022, 11, 1637. [Google Scholar] [CrossRef]
- Strømland, Ø.; Diab, J.; Ferrario, E.; Sverkeli, L.J.; Ziegler, M. The balance between NAD+ biosynthesis and consumption in ageing. Mech. Ageing Dev. 2021, 199, 111569. [Google Scholar] [CrossRef]
- Dutta, T.; Kapoor, N.; Mathew, M.; Chakraborty, S.; Ward, N.; Prieto-Farigua, N. Source of nicotinamide governs its metabolic fate in cultured cells, mice, and humans. Cell Rep. 2023, 42, 112218. [Google Scholar] [CrossRef]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Kropotov, A.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M. Degradation of extracellular NAD+ intermediates in cultures of human HEK293 cells. Metabolites 2019, 9, 293. [Google Scholar] [CrossRef]
- Naaz, T.; Kim, B.S. Use of Nicotinamide Mononucleotide as Non-Natural Cofactor. Catalysts 2025, 15, 37. [Google Scholar] [CrossRef]
- Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Park, J.W.; Kang, G.M.; Lee, C.H.; Dugu, H.; Gil, S.Y.; Kim, H.J.; Son, G.H.; Yu, R.; Kim, M.-S. Exogenous nicotinamide adenine dinucleotide regulates energy metabolism via hypothalamic connexin 43. Metabolism 2018, 88, 51–60. [Google Scholar] [CrossRef]
- Martínez-Morcillo, F.J.; Cantón-Sandoval, J.; Martínez-Navarro, F.J.; Cabas, I.; Martínez-Vicente, I.; Armistead, J.; Hatzold, J.; López-Muñoz, A.; Martínez-Menchón, T.; Corbalán-Vélez, R. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021, 19, e3001455. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, A.; Huang, L.; Zhu, X.; Hu, Q.; Zhang, Y.; Chen, X.; Li, F.; Wang, Q.; Wang, H. Illuminating NAD+ metabolism in live cells and in vivo using a genetically encoded fluorescent sensor. Dev. Cell 2020, 53, 240–252.e7. [Google Scholar] [CrossRef]
- Chang, T.-M.; Yang, T.-Y.; Huang, H.-C. Nicotinamide mononucleotide and coenzyme Q10 protects fibroblast senescence induced by particulate matter preconditioned mast cells. Int. J. Mol. Sci. 2022, 23, 7539. [Google Scholar] [CrossRef]
- Zhou, X.; Du, H.-H.; Long, X.; Pan, Y.; Hu, J.; Yu, J.; Zhao, X. β-Nicotinamide mononucleotide (NMN) administrated by intraperitoneal injection mediates protection against UVB-induced skin damage in mice. J. Inflamm. Res. 2021, 14, 5165–5182. [Google Scholar] [CrossRef]
- Esteras, N.; Adjobo-Hermans, M.J.; Abramov, A.Y.; Koopman, W.J. Visualization of mitochondrial membrane potential in mammalian cells. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 155, pp. 221–245. [Google Scholar]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Romani, M.; Sorrentino, V.; Oh, C.-M.; Li, H.; de Lima, T.I.; Zhang, H.; Shong, M.; Auwerx, J. NAD+ boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Rep. 2021, 34, 108660. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 2011, 91, 1219–1243. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, E.; Ung, T.P.L.; Alarcón del Carmen, A.; del Toro-Ríos, X.; Fajardo-Orduña, G.R.; Noriega, L.G.; Cortés-Morales, V.A.; Tovar, A.R.; Montesinos, J.J.; Orozco-Solís, R. Coordinated metabolic transitions and gene expression by NAD+ during adipogenesis. J. Cell Biol. 2022, 221, e202111137. [Google Scholar] [CrossRef]
- Hill, S.E.; Esquivel, A.R.; Ospina, S.R.; Rahal, L.M.; Dickey, C.A.; Blair, L.J. Chaperoning activity of the cyclophilin family prevents tau aggregation. Protein Sci. 2022, 31, e4448. [Google Scholar] [CrossRef]
- Cifuentes, R.A.; Cruz-Tapias, P.; Rojas-Villarraga, A.; Anaya, J.-M. ZC3H12A (MCPIP1): Molecular characteristics and clinical implications. Clin. Chim. Acta 2010, 411, 1862–1868. [Google Scholar] [CrossRef]
- Matsushita, K.; Takeuchi, O.; Standley, D.M.; Kumagai, Y.; Kawagoe, T.; Miyake, T.; Satoh, T.; Kato, H.; Tsujimura, T.; Nakamura, H. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 2009, 458, 1185–1190. [Google Scholar] [CrossRef]
- Uehata, T.; Takeuchi, O. Regnase-1 is an endoribonuclease essential for the maintenance of immune homeostasis. J. Interferon Cytokine Res. 2017, 37, 220–229. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, B.B.; Tan, C.Y.R.; Ho, C.Y.; Soon, A.L.; Lam, T.T.; Yang, X.; Nguyen, C.; Guo, W.; Chew, Y.C.; DeAngelis, Y.M. Early onset of senescence and imbalanced epidermal homeostasis across the decades in photoexposed human skin: Fingerprints of inflammaging. Exp. Dermatol. 2022, 31, 1748–1760. [Google Scholar] [CrossRef]
- Tian, W.; Alsaadi, R.; Guo, Z.; Kalinina, A.; Carrier, M.; Tremblay, M.-E.; Lacoste, B.; Lagace, D.; Russell, R.C. An antibody for analysis of autophagy induction. Nat. Methods. 2020, 17, 232–239. [Google Scholar] [CrossRef]
- Orhon, I.; Reggiori, F. Assays to monitor autophagy progression in cell cultures. Cells 2017, 6, 20. [Google Scholar] [CrossRef]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Lin, S.; Xing, H.; Zang, T.; Ruan, X.; Wo, L.; He, M. Sirtuins in mitochondrial stress: Indispensable helpers behind the scenes. Ageing Res. Rev. 2018, 44, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Hua, F.; Fang, P.; Li, C.; Deng, F.; Chen, S.; Ying, J.; Wang, X. Regulation of mitophagy by sirtuin family proteins: A vital role in aging and age-related diseases. Front. Aging Neurosci. 2022, 14, 845330. [Google Scholar] [CrossRef] [PubMed]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging 2013, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.; da Costa Martins, P.A.; Bitsch, N.; Pintelon, I.; De Meyer, G.R.; Martinet, W.; Schrijvers, D.M. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 2015, 11, 2014–2032. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Koh, E.-S.; Kim, H.W.; Kim, S.; Woo, J.J.; Kim, Y.K. The impairment of endothelial autophagy accelerates renal senescence by ferroptosis and NLRP3 inflammasome signaling pathways with the disruption of endothelial barrier. Antioxidants 2024, 13, 886. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Gilbert, M.M.; Mathes, S.C.; Mahajan, A.S.; Rohan, C.A.; Travers, J.B.; Thyagarajan, A. The role of sirtuins in dermal fibroblast function. Front. Med. 2023, 10, 1021908. [Google Scholar] [CrossRef] [PubMed]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015, 15, 19. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The mitochondrial basis of aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.; Allard, J.; Ingram, D.K.; Navas, P. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.-G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Rovini, A.; Heslop, K.; Hunt, E.G.; Morris, M.E.; Fang, D.; Gooz, M.; Gerencser, A.A.; Maldonado, E.N. Quantitative analysis of mitochondrial membrane potential heterogeneity in unsynchronized and synchronized cancer cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21148. [Google Scholar] [CrossRef]
- Begum, H.M.; Ta, H.P.; Zhou, H.; Ando, Y.; Kang, D.; Nemes, K.; Mariano, C.F.; Hao, J.; Yu, M.; Shen, K. Spatial regulation of mitochondrial heterogeneity by stromal confinement in micropatterned tumor models. Sci. Rep. 2019, 9, 11187. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Bakowska, J.C. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. JoVE (J. Vis. Exp.) 2011, 51, 2704. [Google Scholar]
- Al-Khaldi, A.; Sultan, S. The expression of sirtuins, superoxide dismutase, and lipid peroxidation status in peripheral blood from patients with diabetes and hypothyroidism. BMC Endocr. Disord. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, S.; Li, Z.; Hao, D.; Zhao, X.; Zhang, Z.; Liu, D. Sirt6 mediates antioxidative functions by increasing Nrf2 abundance. Exp. Cell Res. 2023, 422, 113409. [Google Scholar] [CrossRef]
- Guedouari, H.; Daigle, T.; Scorrano, L.; Hebert-Chatelain, E. Sirtuin 5 protects mitochondria from fragmentation and degradation during starvation. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 169–176. [Google Scholar] [CrossRef]
- Shefa, U.; Jeong, N.Y.; Song, I.O.; Chung, H.-J.; Kim, D.; Jung, J.; Huh, Y. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen. Res. 2019, 14, 749–756. [Google Scholar] [CrossRef]
- Tak, H.; Cha, S.; Hong, Y.; Jung, M.; Ryu, S.; Han, S.; Jeong, S.M.; Kim, W.; Lee, E.K. The miR-30-5p/TIA-1 axis directs cellular senescence by regulating mitochondrial dynamics. Cell Death Dis. 2024, 15, 404. [Google Scholar] [CrossRef]
- Iqbal, S.; Hood, D.A. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am. J. Physiol.-Cell Physiol. 2014, 306, C1176–C1183. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Gu, X.; Patel, R.J.; Chuphal, P.; Viana, M.P.; Brown, A.I.; Zid, B.M.; Tsuboi, T. Mitochondrial protein heterogeneity stems from the stochastic nature of co-translational protein targeting in cell senescence. Nat. Commun. 2024, 15, 8274. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive oxygen species and mitochondrial dynamics: The yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Klinkenberg, M.; Auburger, G.; Bereiter-Hahn, J.; Jendrach, M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 2010, 123, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Mellem, D.; Sattler, M.; Pagel-Wolff, S.; Jaspers, S.; Wenck, H.; Rübhausen, M.A.; Fischer, F. Fragmentation of the mitochondrial network in skin in vivo. PLoS ONE 2017, 12, e0174469. [Google Scholar] [CrossRef]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Belhadj, J.; Surina, S.; Hengstschläger, M.; Lomakin, A.J. Form follows function: Nuclear morphology as a quantifiable predictor of cellular senescence. Aging Cell 2023, 22, e14012. [Google Scholar] [CrossRef]
- Naharro-Rodriguez, J.; Bacci, S.; Hernandez-Bule, M.L.; Perez-Gonzalez, A.; Fernandez-Guarino, M. Decoding Skin Aging: A Review of Mechanisms, Markers, and Modern Therapies. Cosmetics 2025, 12, 144. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the senescence-associated phenotype in human skin fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bonifant, H.; Holloway, S. A review of the effects of ageing on skin integrity and wound healing. Br. J. Community Nurs. 2019, 24, S28–S33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Park, J.; Cho, E.; Kim, D.; Ye, S.; Jeong, E.T.; Jun, S.-H.; Kang, N.-G. Distinctive Gene Expression Profiles and Biological Responses of Skin Fibroblasts to Nicotinamide Mononucleotide: Implications for Longevity Effects on Skin. Biomedicines 2025, 13, 2395. https://doi.org/10.3390/biomedicines13102395
Kang S, Park J, Cho E, Kim D, Ye S, Jeong ET, Jun S-H, Kang N-G. Distinctive Gene Expression Profiles and Biological Responses of Skin Fibroblasts to Nicotinamide Mononucleotide: Implications for Longevity Effects on Skin. Biomedicines. 2025; 13(10):2395. https://doi.org/10.3390/biomedicines13102395
Chicago/Turabian StyleKang, Seongsu, Jiwon Park, Eunbyul Cho, Dohyun Kim, Sanghyun Ye, Eui Taek Jeong, Seung-Hyun Jun, and Nae-Gyu Kang. 2025. "Distinctive Gene Expression Profiles and Biological Responses of Skin Fibroblasts to Nicotinamide Mononucleotide: Implications for Longevity Effects on Skin" Biomedicines 13, no. 10: 2395. https://doi.org/10.3390/biomedicines13102395
APA StyleKang, S., Park, J., Cho, E., Kim, D., Ye, S., Jeong, E. T., Jun, S.-H., & Kang, N.-G. (2025). Distinctive Gene Expression Profiles and Biological Responses of Skin Fibroblasts to Nicotinamide Mononucleotide: Implications for Longevity Effects on Skin. Biomedicines, 13(10), 2395. https://doi.org/10.3390/biomedicines13102395






