Gender- and Grade-Dependent Activation of Androgen Receptor Signaling in Adult-Type Diffuse Gliomas: Epigenetic Insights from a Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
- age ≥ 18 years old;
- (2)
- morphological and immunohistochemical profile consistent with the diagnosis of adult-type diffuse glioma (IDH-mutant glioma, grade 2 or 3 or 4 and GBM IDH-wildtype grade 4 according to the fifth edition of CNS WHO 2021 [2]);
- (3)
- histological slides/formalin-fixed paraffin-embedded tissue tumor (FFPE) blocks from the archive available to perform immunohistochemical assessment of androgen receptor, in situ hybridization, and molecular tests.
- (1)
- histological diagnosis different from adult-type diffuse gliomas;
- (2)
- unavailability of histological slides/formalin-fixed paraffin-embedded tissue tumor (FFPE) blocks;
- (3)
- molecular GBM IDH-wildtype and gliomas IDH-mutant with homozygous cyclin-dependent kinase inhibitor (CDKN2A/B) gene deletion were excluded from our study;
- (4)
- patients undergoing antiandrogen therapy for another malignancy.
2.2. Ethical Statement
2.3. AR Immunohistochemistry
2.4. Next-Generation Sequencing
2.5. FISH Analysis
3. Results
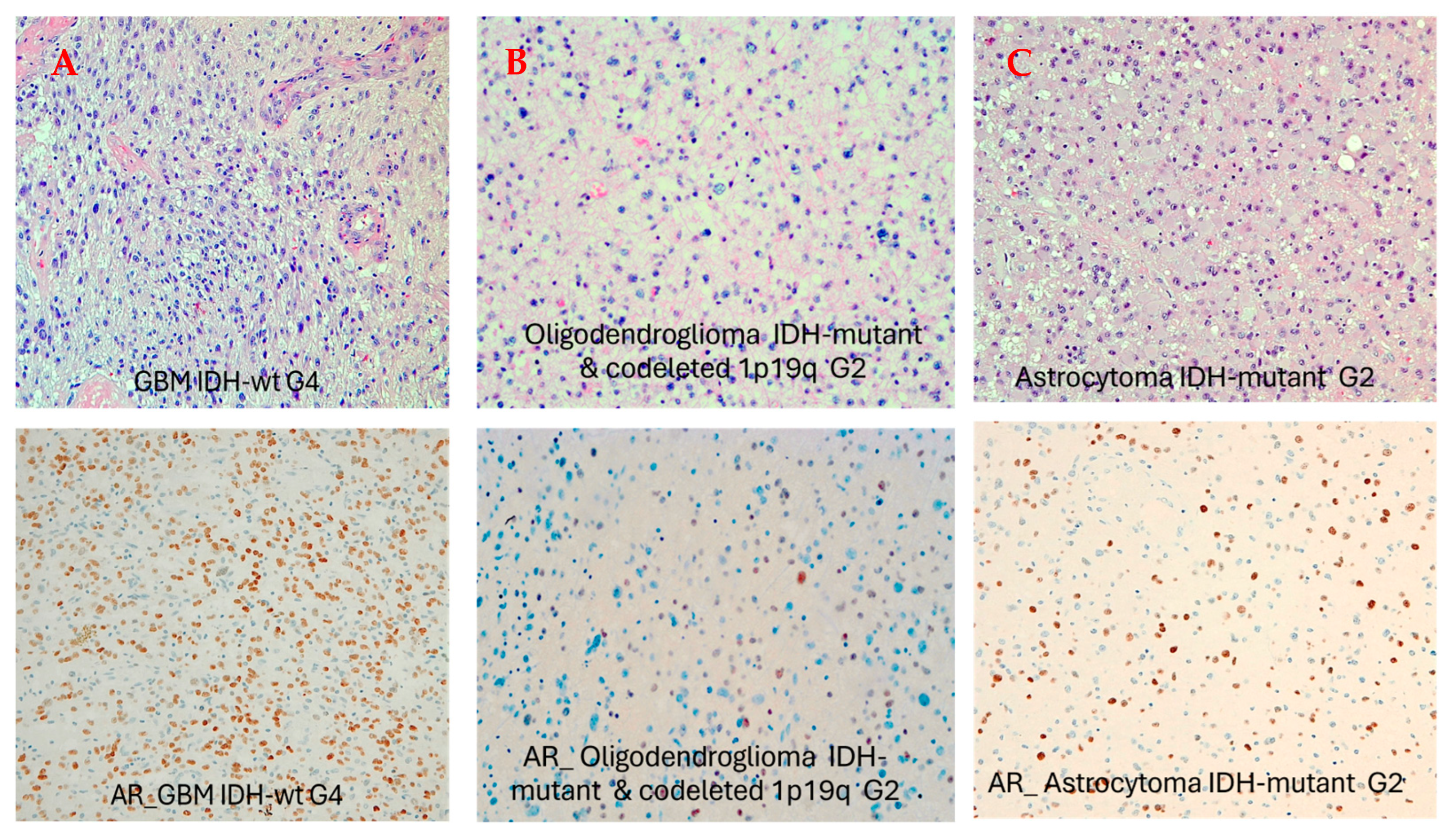
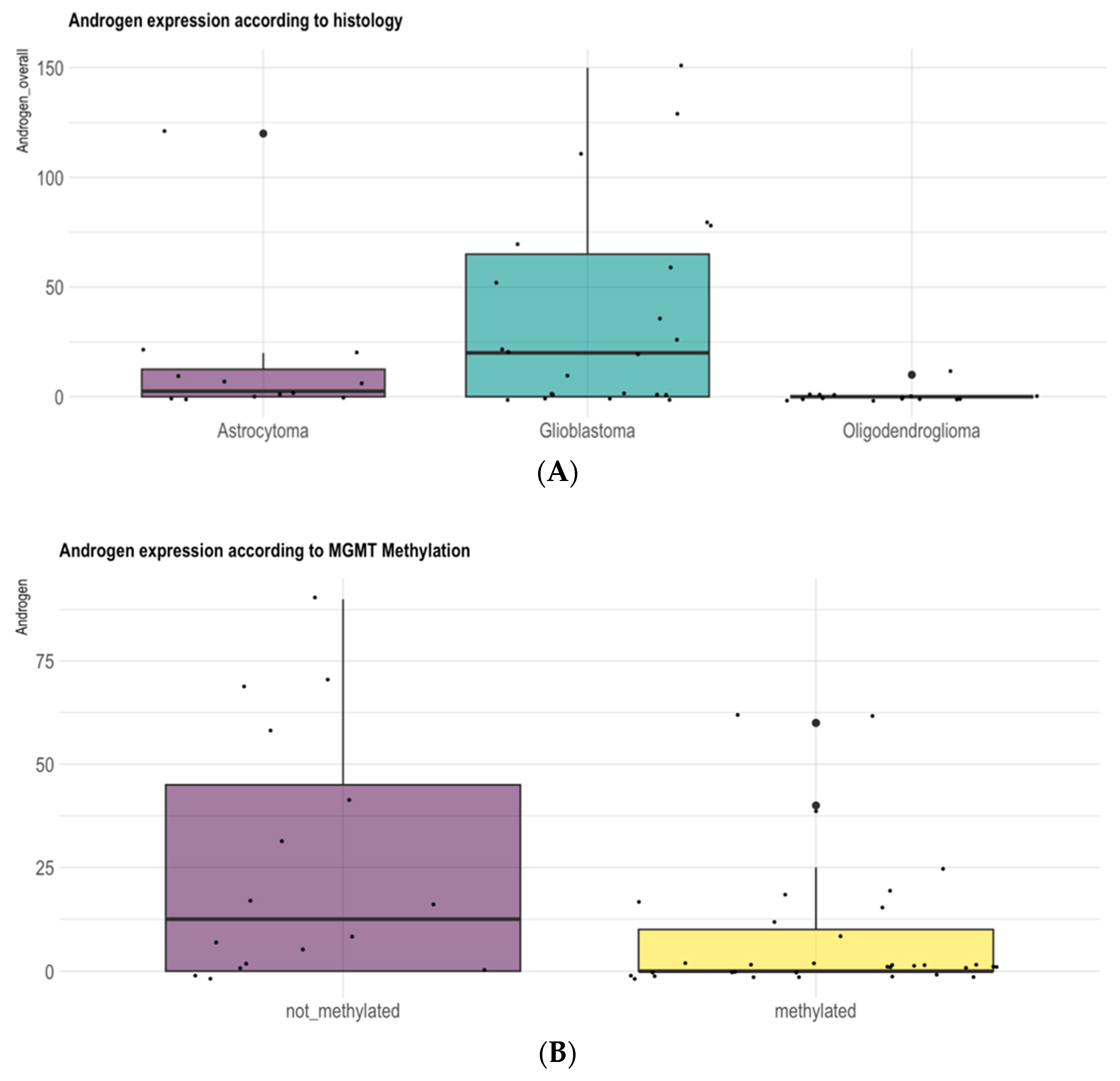
3.1. Gender and Pathologic Grade Are Associated with AR Expression Levels in Adult-Type Diffuse Gliomas
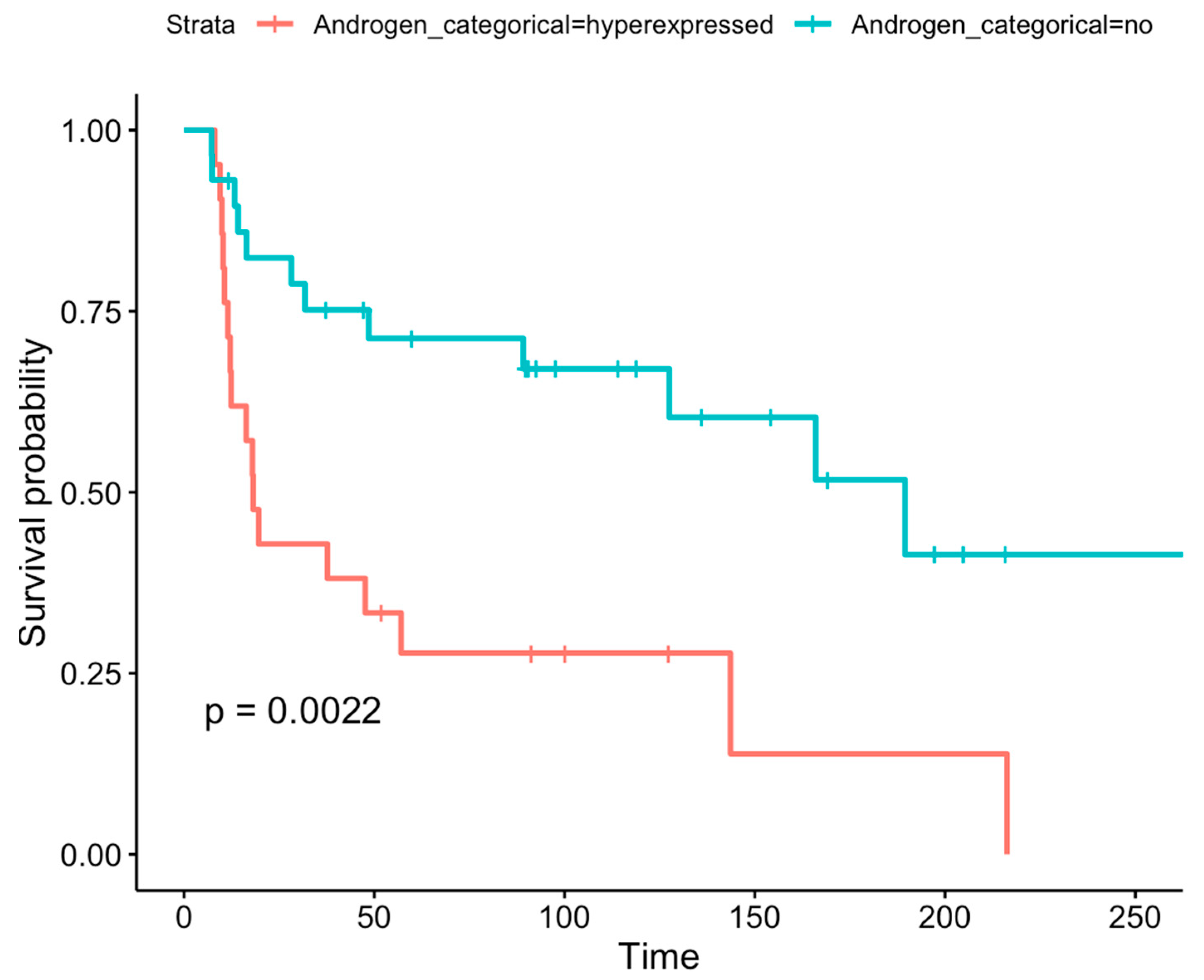
3.2. Prognostic Role of AR Expression Levels in Adult-Type Diffuse Gliomas
3.3. Differential DNA Methylation Analysis of AR Gene Promoter Region According to Immunohistochemical AR Expression
3.4. Differential Methylation Analysis of MAGEA1, MAGEA11, MAGEC1, MAGEC2, UXT, and FNLA Genes According to Immunohistochemical AR Expression
3.5. Differential Methylation Analysis of the MGMT Promoter According to Immunohistochemical Expression of AR
3.6. Differential Methylation Analysis of the AR Gene Promoter Region According to Pathologic Grade
3.7. Differential Methylation Analysis of MAGEA1, MAGEA11, MAGEC1, MAGEC2, UXT, and FNLA According to Pathologic Grade
3.8. Differential Methylation Analysis of MGMT and TERT According to Pathologic Grade
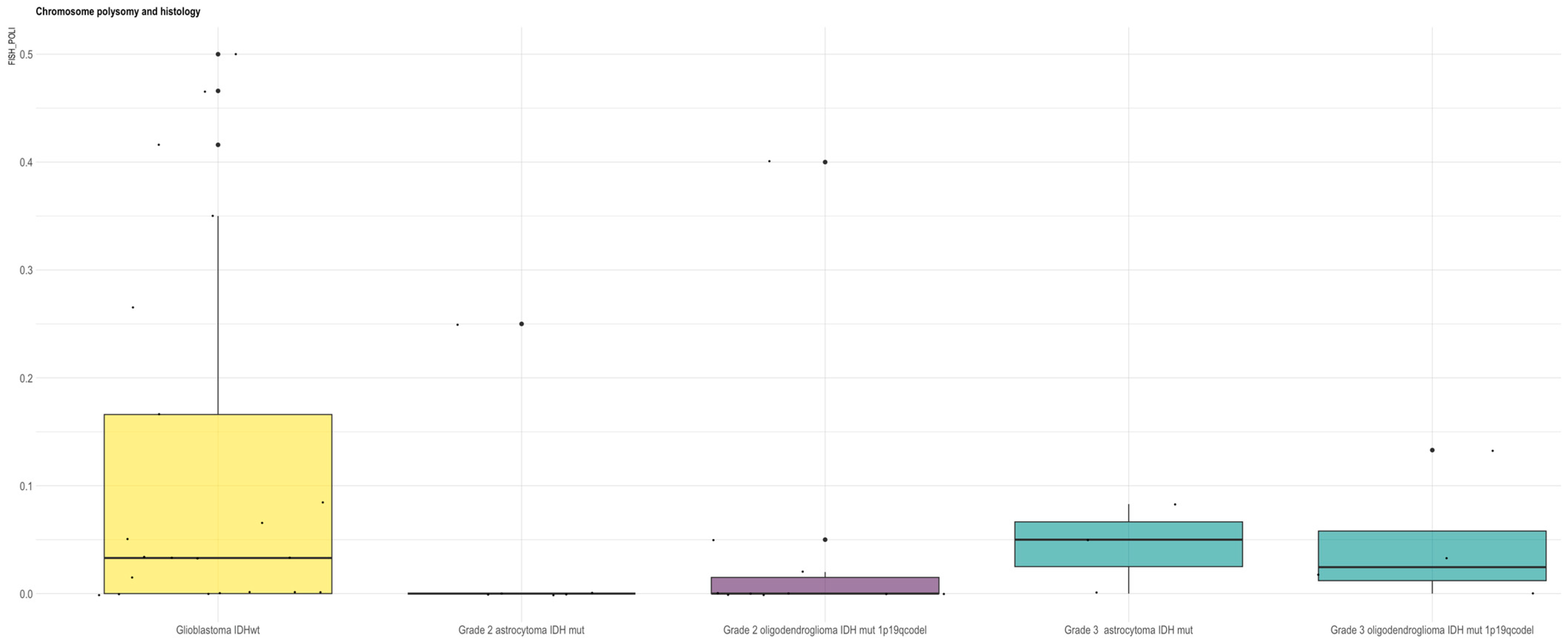
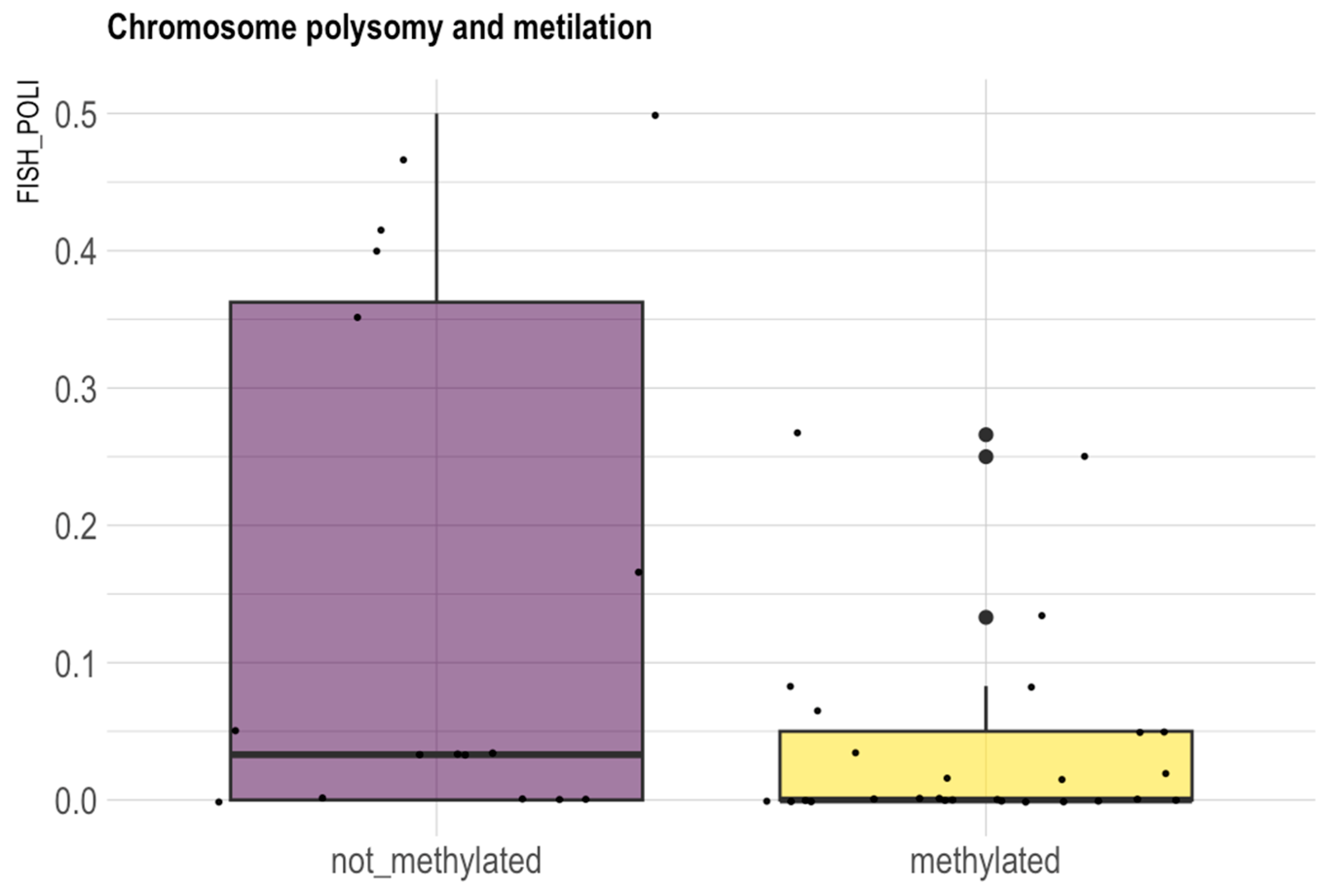
3.9. X-Chromosome Copy Number Analysis in Glioma
3.10. Differential Methylation Analysis of AR Promoter Regions According to X-Chromosome Copy Number
3.11. Differential Methylation Analysis of MAGEA1, MAGEA11, MAGEC1, MAGEC2, UXT, and FNLA Genes According to X-Chromosome Copy Number
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chakrabarti, I.; Mazumder, S. What Changed in CNS5? A Mini-Review on General Changes and Adult Diffuse Gliomas. Ann. Afr. Med. 2024, 23, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Yilmaz, A.; Taghizadehghalehjoughi, A.; Genc, S.; Yeni, Y.; Gecili, I.; Hacimuftuoglu, A. Ulipristal-temozolomide-hydroxyurea combination for glioblastoma: In-vitro studies. J. Neurosurg. Sci. 2024, 68, 468–481. [Google Scholar] [CrossRef]
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A., III; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Richards, L.M.; Whitley, O.K.N.; MacLeod, G.; Cavalli, F.M.G.; Coutinho, F.J.; Jaramillo, J.E.; Svergun, N.; Riverin, M.; Croucher, D.C.; Kushida, M.; et al. Gradient of Developmental and Injury Response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity. Nat. Cancer 2021, 2, 157–173. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Gan, X.; Liu, Y.; Wang, X. Targeting androgen receptor in glioblastoma. Crit. Rev. Oncol. Hematol. 2023, 191, 104142. [Google Scholar] [CrossRef]
- Bao, D.; Cheng, C.; Lan, X.; Xing, R.; Chen, Z.; Zhao, H.; Sun, J.; Wang, Y.; Niu, C.; Zhang, B.; et al. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget 2017, 8, 23142–23154. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Liu, Z.; Sun, Y.; Xu, C. Differential expression and function of AR isoforms in prostate cancer. Oncol. Rep. 2012, 27, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef]
- Jiang, L.; Shan, J.; Shen, J.; Wang, Y.; Yan, P.; Liu, L.; Zhao, W.; Xu, Y.; Zhu, W.; Su, L.; et al. Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma. Oncotarget 2016, 7, 36814–36828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Kuang, X.Y.; Sun, W.L.; Xu, Y.; Zheng, Y.Z.; Liu, Y.R.; Lang, G.T.; Qiao, F.; Hu, X.; Shao, Z.M. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget 2016, 7, 41285–41293. [Google Scholar] [CrossRef]
- Zalcman, N.; Gutreiman, M.; Shahar, T.; Weller, M.; Lavon, I. Androgen Receptor Activation in Glioblastoma Can Be Achieved by Ligand-Independent Signaling through EGFR-A Potential Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 10954. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, N.; Khan, R.; Hung, M.Y.; Zhang, C.; Wang, S.; Wang, T.J.C.; Lin, C. The prognostic significance of androgen receptor expression in gliomas. Sci. Rep. 2024, 14, 22122. [Google Scholar] [CrossRef]
- Brown, C.J.; Goss, S.J.; Lubahn, D.B.; Joseph, D.R.; Wilson, E.M.; French, F.S.; Willard, H.F. Androgen receptor locus on the human X chromosome: Regional localization to Xq11-12 and description of a DNA polymorphism. Am. J. Hum. Genet. 1989, 44, 264–269. [Google Scholar] [PubMed]
- Foschini, M.P.; Morandi, L.; Sanchez, A.M.; Santoro, A.; Mulè, A.; Zannoni, G.F.; Varga, Z.; Moskovszky, L.; Cucchi, M.C.; Moelans, C.B.; et al. Methylation Profile of X-Chromosome-Related Genes in Male Breast Cancer. Front. Oncol. 2020, 10, 784. [Google Scholar] [CrossRef]
- Minges, J.T.; Su, S.; Grossman, G.; Blackwelder, A.J.; Pop, E.A.; Mohler, J.L.; Wilson, E.M. Melanoma antigen-A11 (MAGE-A11) enhances transcriptional activity by linking androgen receptor dimers. J. Biol. Chem. 2013, 288, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Meng, L.; Ding, P.; Sang, M. Epigenetic regulation of MAGE family in human cancer progression-DNA methylation, histone modification, and non-coding RNAs. Clin. Epigenetics 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Das, P.M.; Singal, R. DNA methylation and cancer. J. Clin. Oncol. 2004, 22, 4632–4642. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Stalpers, L.J.A.; Kaplan, E.L.; Edward, L. Kaplan and the Kaplan-Meier Survival Curve. BSHM Bull. J. Br. Soc. Hist. Math. 2018, 33, 109–135. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Zhang, K.; Bian, C.; Zhao, Y.; Zhang, J. Expression of estrogen receptors, androgen receptor and steroid receptor coactivator-3 is negatively correlated to the differentiation of astrocytic tumors. Cancer Epidemiol. 2014, 38, 291–297. [Google Scholar] [CrossRef]
- Gabusi, A.; Gissi, D.B.; Tarsitano, A.; Asioli, S.; Marchetti, C.; Montebugnoli, L.; Foschini, M.P.; Morandi, L. Intratumoral Heterogeneity in Recurrent Metastatic Squamous Cell Carcinoma of the Oral Cavity: New Perspectives Afforded by Multiregion DNA Sequencing and mtDNA Analysis. J. Oral Maxillofac. Surg. 2019, 77, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Gissi, D.B.; Tarsitano, A.; Gabusi, A.; Rossi, R.; Attardo, G.; Lenzi, J.; Marchetti, C.; Montebugnoli, L.; Foschini, M.P.; Morandi, L. 13-gene DNA Methylation Analysis from Oral Brushing: A Promising Non Invasive Tool in the Follow-up of Oral Cancer Patients. J. Clin. Med. 2019, 8, 2107. [Google Scholar] [CrossRef]
- Di Oto, E.; Monti, V.; Cucchi, M.C.; Masetti, R.; Varga, Z.; Foschini, M.P. X chromosome gain in male breast cancer. Hum. Pathol. 2015, 46, 1908–1912. [Google Scholar] [CrossRef]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef]
- Li, C.; Cheng, D.; Li, P. Androgen receptor dynamics in prostate cancer: From disease progression to treatment resistance. Front. Oncol. 2025, 15, 1542811. [Google Scholar] [CrossRef]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef]
- He, Y.; Xu, W.; Xiao, Y.T.; Huang, H.; Gu, D.; Ren, S. Targeting signaling pathways in prostate cancer: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.P.; Hakenberg, O.W.; Froehner, M. Antiandrogens in the treatment of prostate cancer. Eur. Urol. 2007, 51, 306–313, discussion 314. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sati, J.; Bhardwaj, V.; Kaur, T.; Dhawan, D.K.; Dhingra, N.; Chadha, V.D. Evaluation of Therapeutic Potential of a Selective 5α-reductase Inhibitor against Glioblastoma: Molecular Docking and In Vitro Insights. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, F.; Ahmed, S.; Liu, K.; Zhang, C.; Cathcart, S.J.; DiMaio, D.J.; Punsoni, M.; Guan, B.; Zhou, P.; et al. Androgen Receptor, Although Not a Specific Marker For, Is a Novel Target to Suppress Glioma Stem Cells as a Therapeutic Strategy for Glioblastoma. Front. Oncol. 2021, 11, 616625. [Google Scholar] [CrossRef]
- Alemán, O.R.; Quintero, J.C.; Camacho-Arroyo, I. The language of glioblastoma: A tale of cytokines and sex hormones communication. Neurooncol Adv. 2025, 7, vdaf017. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, M.; Demircan, T. Exploring the potentials of S4, a selective androgen receptor modulator, in glioblastoma multiforme therapy. Toxicol. Appl. Pharmacol. 2024, 490, 117029. [Google Scholar] [CrossRef]
- Rossi, J.; Zedde, M.; Napoli, M.; Pascarella, R.; Pisanello, A.; Biagini, G.; Valzania, F. Impact of Sex Hormones on Glioblastoma: Sex-Related Differences and Neuroradiological Insights. Life 2024, 14, 1523. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM-An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, D.C.; Piña-Medina, A.G.; Hansberg-Pastor, V.; Bello-Alvarez, C.; Camacho-Arroyo, I. Testosterone Promotes Glioblastoma Cell Proliferation, Migration, and Invasion Through Androgen Receptor Activation. Front. Endocrinol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, Y.; Wei, W.; Cong, P.; Ding, Y.; Xiang, L.; Wu, K. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol. 2015, 36, 967–972. [Google Scholar] [CrossRef]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef]
- Chung, Y.G.; Kim, H.K.; Lee, H.K.; Lee, K.C. Expression of androgen receptors in astrocytoma. J. Korean Med. Sci. 1996, 11, 517–521. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, J.T.; Chen, T.H.; Chen, R.M. Enzalutamide Induces Apoptotic Insults to Human Drug-Resistant and -Sensitive Glioblastoma Cells via an Intrinsic Bax-Mitochondrion-Cytochrome C Caspase Cascade Activation Pathway. Molecules 2022, 27, 6666. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018, 20, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Schiffgens, S.; Wilkens, L.; Brandes, A.A.; Meier, T.; Franceschi, E.; Ermani, M.; Hartmann, C.; Sandalcioglu, I.E.; Dumitru, C.A. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget 2016, 7, 55169–55180. [Google Scholar] [CrossRef]
- Franceschi, E.; Tosoni, A.; Minichillo, S.; Depenni, R.; Paccapelo, A.; Bartolini, S.; Michiara, M.; Pavesi, G.; Urbini, B.; Crisi, G.; et al. The Prognostic Roles of Gender and O6-Methylguanine-DNA Methyltransferase Methylation Status in Glioblastoma Patients: The Female Power. World Neurosurg. 2018, 112, e342–e347. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of sex in the prevalence and progression of glioblastomas: The role of gonadal steroid hormones. Biol. Sex. Differ. 2021, 12, 28. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Fariña-Jerónimo, H.; Martín-Ramírez, R.; González-Fernández, R.; Medina, L.; de Vera, A.; Martín-Vasallo, P.; Plata-Bello, J. Androgen deficiency is associated with a better prognosis in glioblastoma. Eur. J. Med. Res. 2024, 29, 57. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef]
- Karpf, A.R.; Matsui, S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res. 2005, 65, 8635–8639. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yokoo, H.; Yokoo, M.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Concurrent inactivation of RB1 and TP53 pathways in anaplastic oligodendrogliomas. J. Neuropathol. Exp. Neurol. 2001, 60, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Amatya, V.J.; Naumann, U.; Weller, M.; Ohgaki, H. TP53 promoter methylation in human gliomas. Acta Neuropathol. 2005, 110, 178–184. [Google Scholar] [CrossRef]
- Etcheverry, A.; Aubry, M.; de Tayrac, M.; Vauleon, E.; Boniface, R.; Guenot, F.; Saikali, S.; Hamlat, A.; Riffaud, L.; Menei, P.; et al. DNA methylation in glioblastoma: Impact on gene expression and clinical outcome. BMC Genom. 2010, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gorlia, T.; van den Bent, M.J.; Hegi, M.E.; Mirimanoff, R.O.; Weller, M.; Cairncross, J.G.; Eisenhauer, E.; Belanger, K.; Brandes, A.A.; Allgeier, A.; et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008, 9, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Saini, S.; Chadha, V.D.; Saini, A.; Dhawan, D.K. A Computational and In Vitro Appraisal of Ostarine to Target Androgen Receptor in Glioma C6 Cells. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Orozco, M.; Valdez, R.A.; Ramos, L.; Cabeza, M.; Segovia, J.; Romano, M.C. Dutasteride combined with androgen receptor antagonists inhibit glioblastoma U87 cell metabolism, proliferation, and invasion capacity: Androgen regulation. Steroids 2020, 164, 108733. [Google Scholar] [CrossRef]
- Díaz Méndez, A.B.; Di Giuliani, M.; Sacconi, A.; Tremante, E.; Lulli, V.; Di Martile, M.; Vari, G.; De Bacco, F.; Boccaccio, C.; Regazzo, G.; et al. Androgen receptor inhibition sensitizes glioblastoma stem cells to temozolomide by the miR-1/miR-26a-1/miR-487b signature mediated WT1 and FOXA1 silencing. Cell Death Discov. 2025, 11, 248. [Google Scholar] [CrossRef]
| AR-Negative (n = 29) | AR-Positive (n = 21) | p-Value (Chi-Squared Test with Yates Continuity Correction) | |
|---|---|---|---|
| Gender | |||
| Male | 11 (37.9%) | 15 (71.4%) | 0.04 |
| Female | 18 (62.1%) | 6 (28.6%) | |
| MGMT | |||
| Unmethylated | 5 (17.2%) | 11 (52.4%) | 0.02 |
| Methylated | 24 (82.8%) | 10 (47.6%) | |
| IDH1 | |||
| WT | 10 (34.4%) | 15 (71.4%) | 0.018 |
| Mutated | 19 (65.5%) | 6 (28.6%) | |
| Age | |||
| Mean (SD) | 48.0 | 54.0 | 0.126 |
| Median (Min, Max) | 46.3 (26.3, 69.1) | 53.7 (29.3, 76.7) | |
| Histology | |||
| Grade 3 astrocytoma IDH-mut | 1 (3.4%) | 2 (9.5%) | 0.018 |
| Grade 3 oligodendroglioma IDH-mut | 4 (13.8%) | 0 (0%) | |
| Grade 2 astrocytoma IDH-mut | 5 (17.2%) | 4 (19.0%) | |
| Grade 2 oligodendroglioma IDH-mut | 10 (34.5%) | 1 (4.8%) | |
| Glioblastoma IDHwt | 9 (31.0%) | 14 (66.7%) | 0.04 |
| Variable | HR (95%CI) | p-Value |
|---|---|---|
| GBM MGMT-methylated vs. unmethylated | 0.34 (0.12–0.95) | 0.04 |
| GBM AR-positive vs. negative | 1.68 (0.67–4.26) | 0.27 |
| Variable | HR (95%CI) | p-Value |
|---|---|---|
| GBM MGMT-methylated vs. unmethylated | 0.33 (0.11–0.93) | 0.04 |
| GBM AR-positive vs. negative | 2.25 (0.79–6.39) | 0.12 |
| Gender female vs. male | 1.9 (0.71–5.17) | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, L.; Asioli, S.; Morandi, L.; Di Oto, E.; Di Nunno, V.; Tosoni, A.; Aprile, M.; Bartolini, S.; Griva, L.; Melotti, S.; et al. Gender- and Grade-Dependent Activation of Androgen Receptor Signaling in Adult-Type Diffuse Gliomas: Epigenetic Insights from a Retrospective Cohort Study. Biomedicines 2025, 13, 2379. https://doi.org/10.3390/biomedicines13102379
Gatto L, Asioli S, Morandi L, Di Oto E, Di Nunno V, Tosoni A, Aprile M, Bartolini S, Griva L, Melotti S, et al. Gender- and Grade-Dependent Activation of Androgen Receptor Signaling in Adult-Type Diffuse Gliomas: Epigenetic Insights from a Retrospective Cohort Study. Biomedicines. 2025; 13(10):2379. https://doi.org/10.3390/biomedicines13102379
Chicago/Turabian StyleGatto, Lidia, Sofia Asioli, Luca Morandi, Enrico Di Oto, Vincenzo Di Nunno, Alicia Tosoni, Marta Aprile, Stefania Bartolini, Lucia Griva, Sofia Melotti, and et al. 2025. "Gender- and Grade-Dependent Activation of Androgen Receptor Signaling in Adult-Type Diffuse Gliomas: Epigenetic Insights from a Retrospective Cohort Study" Biomedicines 13, no. 10: 2379. https://doi.org/10.3390/biomedicines13102379
APA StyleGatto, L., Asioli, S., Morandi, L., Di Oto, E., Di Nunno, V., Tosoni, A., Aprile, M., Bartolini, S., Griva, L., Melotti, S., Gentilini, F., Pinto, G., Casadei, F., Foschini, M. P., Tonon, C., Lodi, R., & Franceschi, E. (2025). Gender- and Grade-Dependent Activation of Androgen Receptor Signaling in Adult-Type Diffuse Gliomas: Epigenetic Insights from a Retrospective Cohort Study. Biomedicines, 13(10), 2379. https://doi.org/10.3390/biomedicines13102379









