Targeting Cutaneous Leishmaniasis with Thiadiazine Thione Derivatives: An In Vivo Study of Its Anti-Inflammatory, Anti-Pyretic, Anti-Nociceptive, and Anti-Sedative Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
- 2-(5-propyl-6-thioxo-1,3,5-thiadiazinan-3-yl)acetic acid (C1),
- 2-(5-Butyl-6-thioxo-1,3,5-thiadiazinan-3-yl) acetic acid (C2),
- 2,2′,2″-(5,5′,5″-(nitrilotris(ethane-2,1-diyl))tris(6-thioxo-1,3,5 thiadiazinane-5,3-diyl)) tris(3-phenylpropanoic acid) (C3), 5,5′,5″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-ethylphenyl)-1,3,5 -thiadiazinane-2-thione) (C4), and 3,3′,3″-(nitrilotris(ethane-2,1-diyl))tris(5-mesityl-1,3,5-thiadiazinane-2-thione) (C5) (Figure 1).
2.2. Animals
Inclusion and Exclusion Criteria
2.3. Parasite Culture
2.4. Acute Toxicity Test
2.5. Therapeutic Dose Selection
2.6. In Vivo Antileishmanial Assay
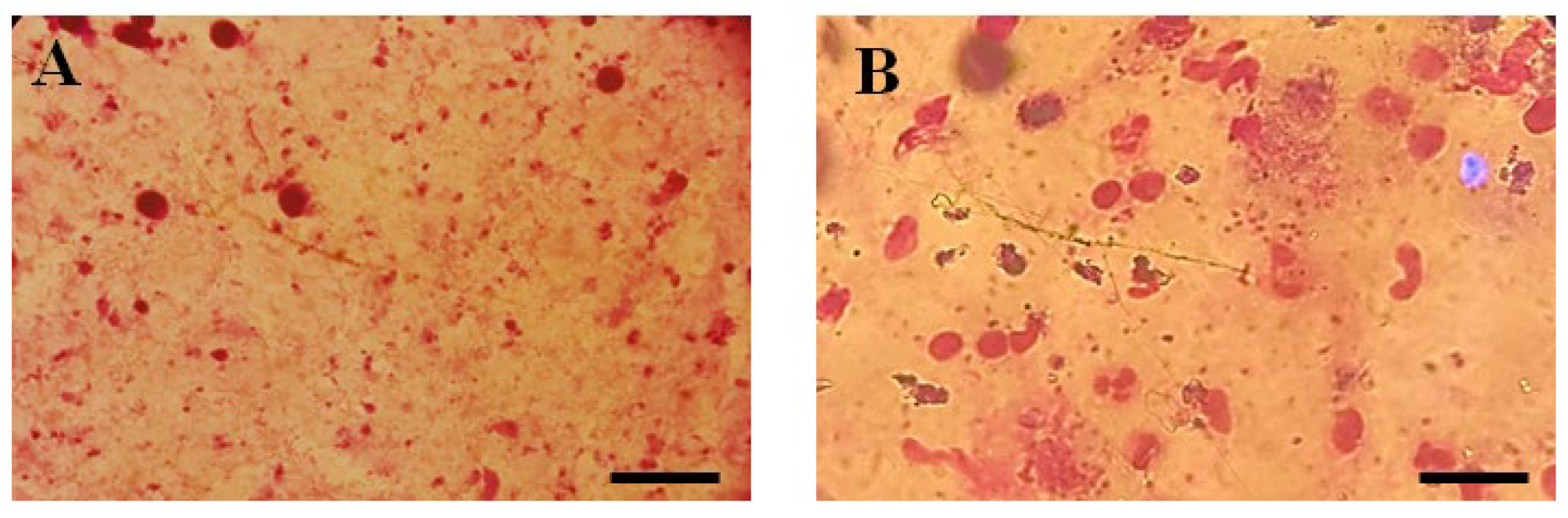
2.6.1. Parasite Burden by Microscopic Examination
2.6.2. Toxicological Study
2.7. Histopathology
Dissection Procedure of Animals and Slides Preparation
2.8. In Vivo Pharmacological Efficacy Assessment
2.8.1. Carrageenan-Induced Inflammation
2.8.2. Antinociceptive Study
2.8.3. Antipyretic Activity by Brewer’s Yeast Method
2.8.4. Antisedative Effect
Statistical Analysis
3. Results
3.1. Assessment of Safety Profile
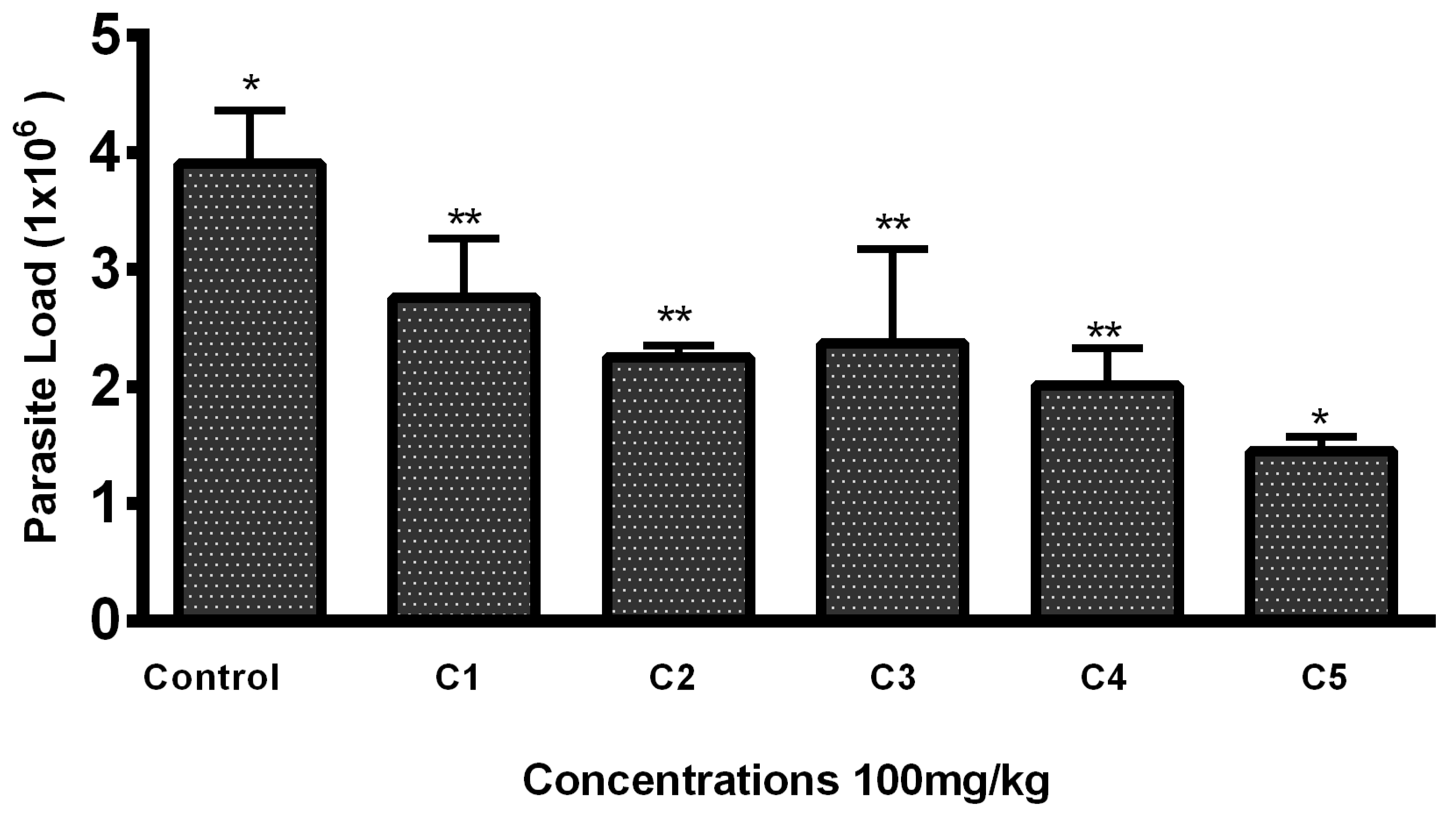
3.2. In Vivo Antileishmanial Activity
Lesion Size and Parasite Load
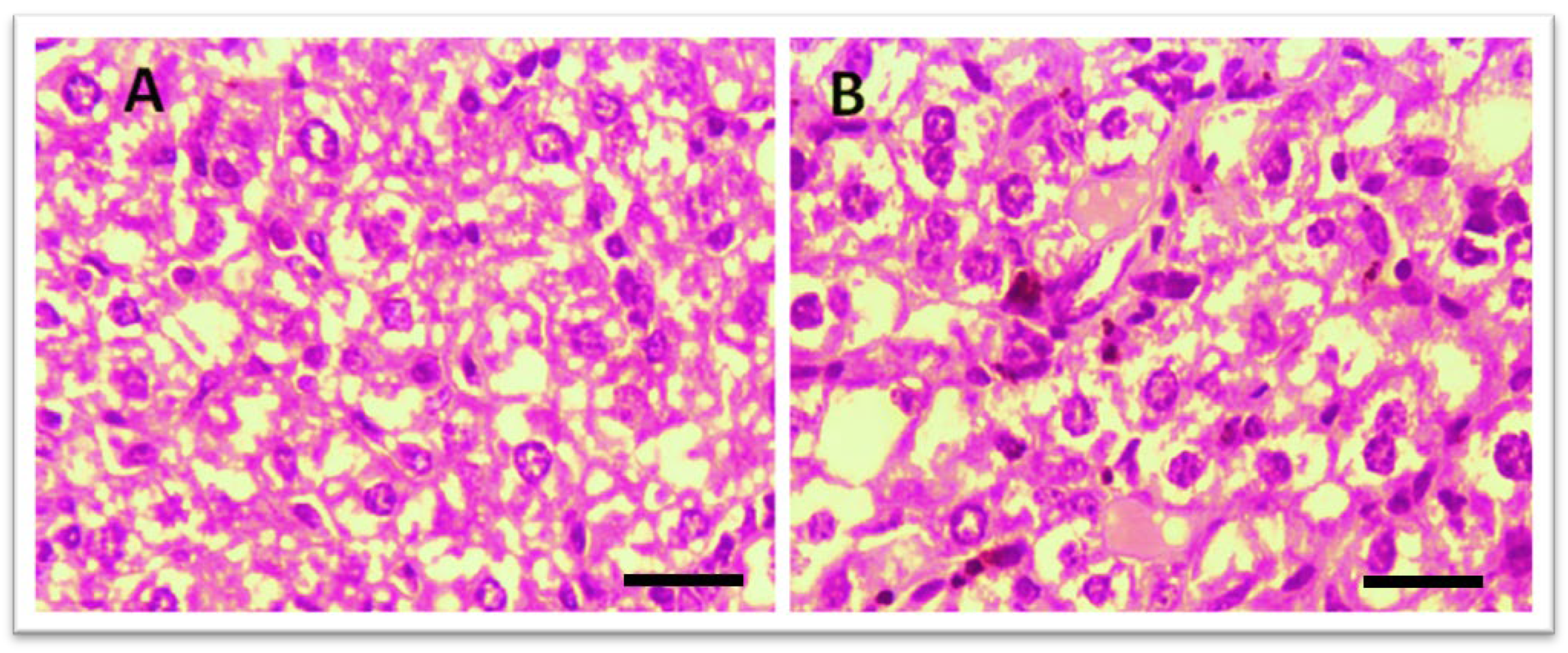
3.3. Histopathology
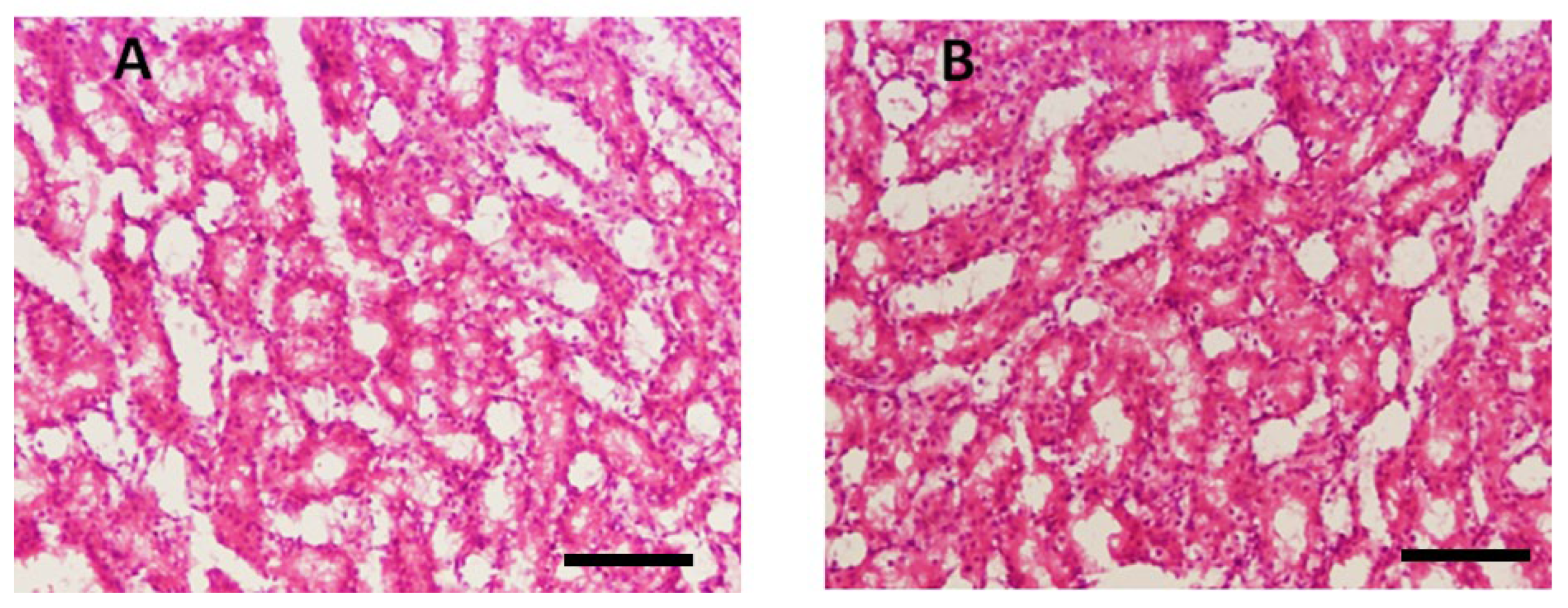
3.3.1. Effect of Test Compounds (THTT Derivatives) on Histology of the Liver
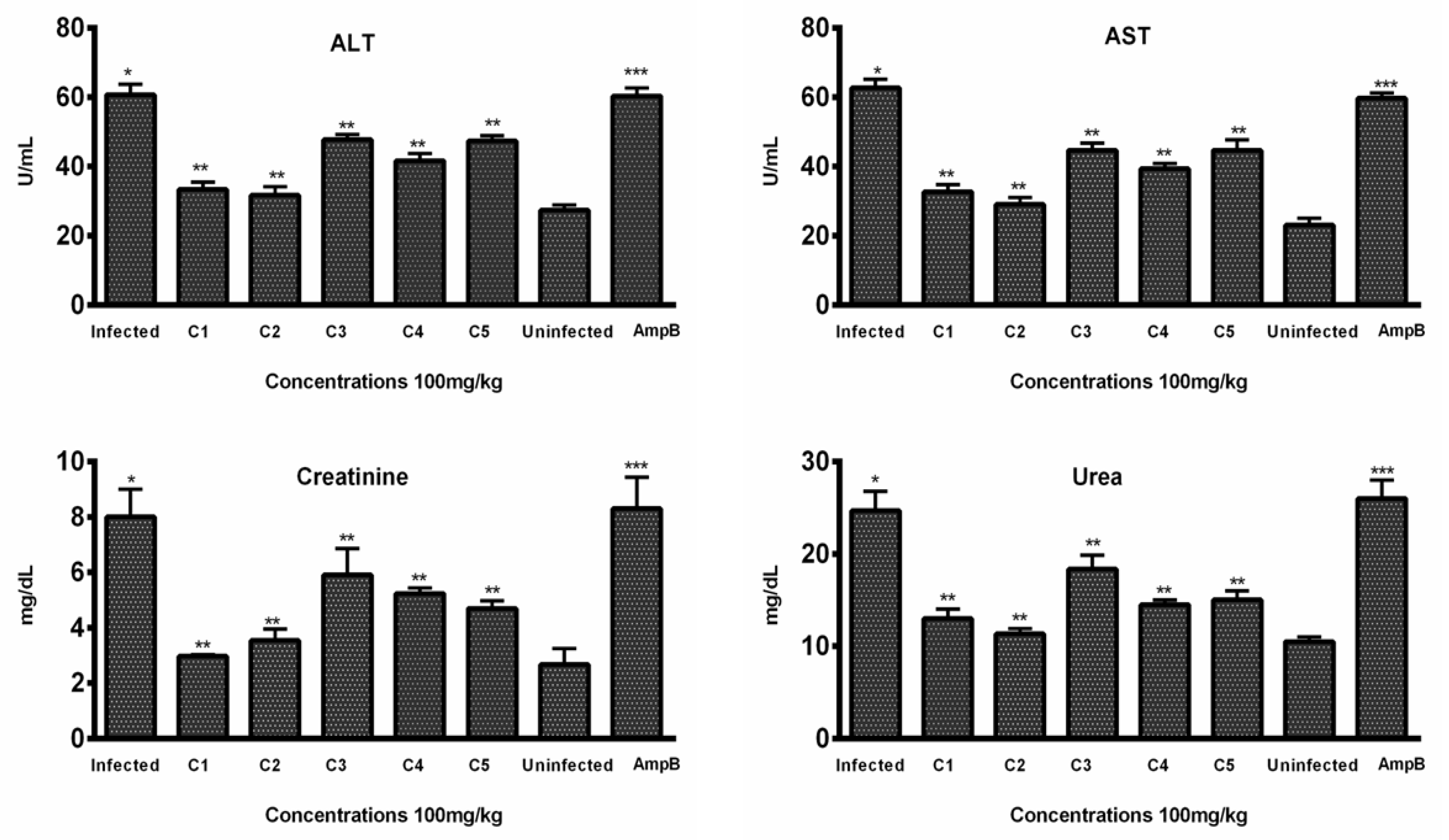
3.3.2. Hepatic Toxicity Evaluation by Enzymatic Markers
3.3.3. Renal Toxicity Evaluation by Blood Urea and Creatinine
3.4. In Vivo Anti-Inflammatory Assay
3.5. In Vivo Antipyretic Assay
3.6. In Vivo Analgesic Activity
3.7. In Vivo Antisedative Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, N.E.; Jimam, N.S.; Goh, K.W.; Tan, C.S.; Ming, L.C. Economic burdens of uncomplicated malaria in primary health care (PHC) facilities of Plateau State, Nigeria: Patients’ perspectives. Int. J. Environ. Res. Public Health 2023, 20, 1093. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.S.; Khare, S.; Kumar, A.B.; Supek, F.; Buchynskyy, A.; Mathison, C.J.; Chennamaneni, N.K.; Pendem, N.; Buckner, F.S.; Gelb, M.H. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 2014, 114, 11305–11347. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Iqbal, W.; Iram, U.; Nisar, S.; Musa, N.; Alam, A.; Khan, M.; Ullah, B.; Ullah, M.; Ali, I. Epidemiology and clinical features of cutaneous leishmaniasis in Khyber Pakhtunkhwa, Pakistan. Braz. J. Biol. 2022, 84, e249124. [Google Scholar] [CrossRef]
- Reis, T.A.; Oliveira-da-Silva, J.A.; Tavares, G.S.; Mendonça, D.V.; Freitas, C.S.; Costa, R.R.; Lage, D.P.; Martins, V.T.; Machado, A.S.; Ramos, F.F. Ivermectin presents effective and selective antileishmanial activity in vitro and in vivo against Leishmania infantum and is therapeutic against visceral leishmaniasis. Exp. Parasitol. 2021, 221, 108059. [Google Scholar] [CrossRef] [PubMed]
- Afghan, A.K.; Kassi, M.; Kasi, P.M.; Ayub, A.; Kakar, N.; Marri, S.M. Clinical manifestations and distribution of cutaneous leishmaniasis in Pakistan. J. Trop. Med. 2011, 2011, 359145. [Google Scholar] [CrossRef]
- Serban, G. 2-Amino-1,3,4-thiadiazoles as prospective agents in trypanosomiasis and other parasitoses. Acta Pharm. 2020, 70, 259–290. [Google Scholar] [CrossRef]
- Foye, W.O. Foye’s Principles of Medicinal Chemistry; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Grumezescu, A.M. Nano-and Microscale Drug Delivery Systems: Design and Fabrication; William Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Monzote, L.; Montalvo, A.M.; Fonseca, L.; Pérez, R.; Suárez, M.; Rodríguez, H. In vitro activities of thiadiazine derivatives against Leishmania amazonensis. Arzneimittelforschung 2005, 55, 232–238. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro-and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Joule, J.A. Natural products containing nitrogen heterocycles—Some highlights 1990–2015. Adv. Heterocycl. Chem. 2016, 119, 81–106. [Google Scholar]
- Diaba, F.; Montiel, J.A.; Serban, G.; Bonjoch, J. Synthesis of normorphans through an efficient intramolecular carbamoylation of ketones. Org. Lett. 2015, 17, 3860–3863. [Google Scholar] [CrossRef] [PubMed]
- Avuloğlu-Yılmaz, E.; Yüzbaşıoğlu, D.; Özçelik, A.B.; Ersan, S.; Ünal, F. Evaluation of genotoxic effects of 3-methyl-5-(4-carboxycyclohexylmethyl)-tetrahydro-2H-1,3 5-thiadiazine-2-thione on human peripheral lymphocytes. Pharm. Biol. 2017, 55, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, X.; Yan, J.; Wang, A.; Wang, M.; Chen, M.; Yang, C.; Song, Y. Design and synthesis of novel 2-(6-thioxo-1,3,5-thiadiazinan-3-yl)-N′-phenylacethydrazide derivatives as potential fungicides. Mol. Divers. 2019, 23, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ozçelik, A.B.; Ersan, S.; Ural, A.U.; Ozkan, S.; Ertan, M. Synthesis of 3-substituted-5-(4-carb oxycyclohexylmethyl)-tetrahydro-2H-1,3,5-thiadiazine-2-thione derivatives as antifibrinolytic and antimicrobial agents. Arzneim.-Forsch. 2007, 57, 554–559. [Google Scholar]
- Sever, B.; Altıntop, M.D.; Kuş, G.; Özkurt, M.; Özdemir, A.; Kaplancıklı, Z.A. Indomethacin based new triazolothiadiazine derivatives: Synthesis, evaluation of their anticancer effects on T98 human glioma cell line related to COX-2 inhibition and docking studies. Eur. J. Med. Chem. 2016, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Varshney, H.; Rauf, A.; Sherwani, A.; Owais, M. Synthesis and anticancer activity of long chain substituted 1,3,4-oxadiazol-2-thione, 1,2,4-triazol-3-thione and 1,2,4-triazolo [3,4-b]-1,3,4-thiadiazine derivatives. Arab. J. Chem. 2017, 10, S3347–S3357. [Google Scholar] [CrossRef]
- Arshad, N.; Hashim, J.; Minhas, M.A.; Aslam, J.; Ashraf, T.; Hamid, S.Z.; Iqbal, T.; Javed, S. New series of 3,5-disubstituted tetrahydro-2H-1,3,5-thiadiazine thione (THTT) derivatives: Synthesis and potent antileishmanial activity. Bioorg. Med. Chem. Lett. 2018, 28, 3251–3254. [Google Scholar] [CrossRef] [PubMed]
- Tamanna; Fu, C.; Qadir, M.; Shah, M.I.A.; Shtaiwi, A.; Khan, R.; Khan, S.U.; Htar, T.T.; Zada, A.; Lodhi, M.A. Thiadiazine thione derivatives as anti-leishmanial agents: Synthesis, biological evaluation, structure activity relationship, ADMET, molecular docking and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2024, 42, 7758–7772. [Google Scholar]
- Hayat, O.; Ullah, N.; Sirajuddin, M.; Giardini, M.A.; Nguyen, J.V.; Francisco, K.R.; Liu, L.J.; Sun, Y.U.; Maurya, S.; McGrosso, D. A Broad Spectrum Antiparasitic Activity of Organotin (IV) Derivatives and Its Untargeted Proteomic Profiling Using Leishmania donovani. Pathogens 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Subhan, F.; Islam, N.U.; Shahid, M.; Rahman, F.U.; Sewell, R.D. Gabapentin and its salicylaldehyde derivative alleviate allodynia and hypoalgesia in a cisplatin-induced neuropathic pain model. Eur. J. Pharmacol. 2017, 814, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Akhtar, R.; Anwar, F.; Shah, M.A.; Chaudary, Z.; Ayaz, M.; Ahmad, B. Neuroprotective potential of Malva neglecta is mediated via down-regulation of cholinesterase and modulation of oxidative stress markers. Metab. Brain Dis. 2021, 36, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Ali, G.; Rashid, U.; Khan, R.; Jan, M.S.; Ullah, R.; Ahmad, S.; Abbasi, S.W.; Khalil, A.A.K.; Sewell, R.E. Mechanistic evaluation of a novel cyclohexenone derivative’s functionality against nociception and inflammation: An in-vitro, in-vivo and in-silico approach. Eur. J. Pharmacol. 2021, 902, 174091. [Google Scholar] [CrossRef]
- Kamil, M.; Fatima, A.; Ullah, S.; Ali, G.; Khan, R.; Ismail, N.; Qayum, M.; Irimie, M.; Dinu, C.G.; Ahmedah, H.T. Toxicological evaluation of novel cyclohexenone derivative in an animal model through histopathological and biochemical techniques. Toxics 2021, 9, 119. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ghaffarifar, F.; Dalimi, A.; Sadraei, J. In-vitro and in-vivo antileishmanial activity of a compound derived of platinum, oxaliplatin, against Leishmania major. Iran. J. Pharm. Res. IJPR 2019, 18, 2028. [Google Scholar] [PubMed]
- Nahrevanian, H.; Farahmand, M.; Aghighi, Z.; Assmar, M.; Amirkhani, A. Pharmacological evaluation of anti-leishmanial activity by in vivo nitric oxide modulation in Balb/c mice infected with Leishmania major MRHO/IR/75/ER: An Iranian strain of cutaneous leishmaniasis. Exp. Parasitol. 2007, 116, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ezatpour, B.; Saedi Dezaki, E.; Mahmoudvand, H.; Azadpour, M.; Ezzatkhah, F. In vitro and in vivo antileishmanial effects of Pistacia khinjuk against Leishmania tropica and Leishmania major. Evid. -Based Complement. Altern. Med. 2015, 2015, 149707. [Google Scholar] [CrossRef]
- Mendonça, D.V.; Lage, L.M.; Lage, D.P.; Chávez-Fumagalli, M.A.; Ludolf, F.; Roatt, B.M.; Menezes-Souza, D.; Faraco, A.A.; Castilho, R.O.; Tavares, C.A. Poloxamer 407 (Pluronic® F127)-based polymeric micelles for amphotericin B: In vitro biological activity, toxicity and in vivo therapeutic efficacy against murine tegumentary leishmaniasis. Exp. Parasitol. 2016, 169, 34–42. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A.; Alkreathy, H.M. Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. Food Nutr. Res. 2015, 59, 28438. [Google Scholar] [CrossRef] [PubMed]
- AlSaffar, R.M.; Rashid, S.; Ahmad, S.B.; Rehman, M.U.; Hussain, I.; Parvaiz Ahmad, S.; Ganaie, M.A. D-limonene (5 (one-methyl-four-[1-methylethenyl]) cyclohexane) diminishes CCl4-induced cardiac toxicity by alleviating oxidative stress, inflammatory and cardiac markers. Redox Rep. 2022, 27, 92–99. [Google Scholar] [CrossRef]
- Jan, M.S.; Ahmad, S.; Hussain, F.; Ahmad, A.; Mahmood, F.; Rashid, U.; Ullah, F.; Ayaz, M.; Sadiq, A. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. Eur. J. Med. Chem. 2020, 186, 111863. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Khalili, H.; Fatemi, I.; Malayeri, A.; Siahpoosh, A.; Goudarzi, M. Zingerone mitigates carrageenan-induced inflammation through antioxidant and anti-inflammatory activities. Inflammation 2021, 44, 186–193. [Google Scholar] [CrossRef]
- Metowogo, K.; Agbonon, A.; Eklu-Gadegbeku, K.; Aklikokou, A.; Gbeassor, M. Anti-ulcer and anti-inflammatory effects of hydroalcohol extract of Aloe buettneri A. Berger (Lilliaceae). Trop. J. Pharm. Res. 2008, 7, 907–912. [Google Scholar] [CrossRef][Green Version]
- Alam, F.; Dey, B.K. Synthesis of Pharmaceutically Important 1,3,4-Thiadiazole Derivatives as Analgesic and Antipyretic Agents. World J. Pharm. Res. 2015, 4, 1303–1324. [Google Scholar]
- Muhammad, N.; Saeed, M.; Khan, H.; Haq, I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J. Nat. Med. 2013, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- El-Shorbagi, A.-N.; El-Naggar, M.; Tarazi, H.; Chaudhary, S.; Abdu-Allah, H.; Hersi, F.; Omar, H. Bis-(5-substituted-2-thiono-1,3,5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multicomponent process. Med. Chem. Res. 2018, 27, 1103–1110. [Google Scholar] [CrossRef]
- El-Shorbagi, A.N. New Tetrahydro-2H-1,3,5-thiadiazine-2-thione Derivatives as Potential Antimicrobial Agents. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2000, 333, 281–286. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Shorbagi, A.-N.; Al-Tel, T.H.; Alsalahat, I.M. Targeting γ-aminobutyric acid (GABA) carriers to the brain: Potential relevance as antiepileptic pro-drugs. Med. Chem. 2010, 6, 144–149. [Google Scholar] [CrossRef]
- Vicentini, C.B.; Forlani, G.; Manfrini, M.; Romagnoli, C.; Mares, D. Development of new fungicides against Magnaporthe grisea: Synthesis and biological activity of pyrazolo [3,4-d][1,3] thiazine, pyrazolo [1,5-c][1,3,5] thiadiazine, and pyrazolo [3,4-d] pyrimidine derivatives. J. Agric. Food Chem. 2002, 50, 4839–4845. [Google Scholar] [CrossRef]
- Coro, J.; Atherton, R.; Little, S.; Wharton, H.; Yardley, V.; Alvarez Jr, A.; Súarez, M.; Pérez, R.; Rodríguez, H. Alkyl-linked bis-THTT derivatives as potent in vitro trypanocidal agents. Bioorg. Med. Chem. Lett. 2006, 16, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Tiwari, V.; Tripathi, R.; Srivastava, A.; Chaturvedi, V.; Srivastava, R.; Srivastava, B. Synthesis and antimycobacterial activity of 3,5-disubstituted thiadiazine thiones. Bioorg. Med. Chem. 2003, 11, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Coro, J.; Pérez, R.; Rodríguez, H.; Suárez, M.; Vega, C.; Rolón, M.; Montero, D.; Nogal, J.J.; Gómez-Barrio, A. Synthesis and antiprotozoan evaluation of new alkyl-linked bis (2-thioxo-[1,3,5] thiadiazinan-3-yl) carboxylic acids. Bioorg. Med. Chem. 2005, 13, 3413–3421. [Google Scholar] [CrossRef]
- Ji, X.; Zhong, Z.; Chen, X.; Xing, R.; Liu, S.; Wang, L.; Li, P. Preparation of 1,3,5-thiadiazine-2-thione derivatives of chitosan and their potential antioxidant activity in vitro. Bioorg. Med. Chem. Lett. 2007, 17, 4275–4279. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.B.; Guccione, S.; Giurato, L.; Ciaccio, R.; Mares, D.; Forlani, G. Pyrazole derivatives as photosynthetic electron transport inhibitors: New leads and structure− activity relationship. J. Agric. Food Chem. 2005, 53, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Mottram, J.; Coombs, G. Cysteine proteinases of parasitic protozoa. Parasitol. Today 1990, 6, 270–275. [Google Scholar] [CrossRef]
- Rodríguez, H.; Suárez, M.; Albericio, F. Thiadiazines, N, N-heterocycles of biological relevance. Molecules 2012, 17, 7612–7628. [Google Scholar] [CrossRef]
- Yan, J.; Si, W.; Hu, H.; Zhao, X.; Chen, M.; Wang, X. Design, synthesis and antimicrobial activities of novel 1,3,5-thiadiazine-2-thione derivatives containing a 1,3,4-thiadiazole group. PeerJ 2019, 7, e7581. [Google Scholar] [CrossRef]
- Done, C.I. Qualitative and Quantitative Tier 3 Assessment. Polymer 2022, 1327, 41–49. [Google Scholar]
- Rahman, K.; Ali, G.; Khan, R.; Khan, I.; Ali, I.; Mosa, O.F.; Ahmed, A.; Ayaz, M.; Nawaz, A.; Murthy, H.A. Analagesic and anti-inflammatory potentials of a less ulcerogenic thiadiazinethione derivative in animal models: Biochemical and histochemical correlates. Drug Des. Dev. Ther. 2022, 16, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Roy, P.; Singh, V.; Kumar, A.; Singh, R.; Sharma, P. Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in male wistar rats. Int. J. Inflamm. 2012, 2012, 752015. [Google Scholar]
- Chan, F.; Graham, D. Prevention of non-steroidal anti-inflammatory drug gastrointestinal complications–review and recommendations based on risk assessment. Aliment. Pharmacol. Ther. 2004, 19, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Traoré, O.; Diarra, A.S.; Kassogué, O.; Abu, T.; Maïga, A.; Kanté, M. The clinical and endoscopic aspects of peptic ulcers secondary to the use of nonsteroidal anti-inflammatory drugs of various origins. Pan Afr. Med. J. 2021, 38, 170. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Jawaid, S.; Hashim, J.; Ullah, I.; Gul, S.; Aziz, A.; Wadood, A.; Khan, A. Highly potent anti-inflammatory, analgesic and antioxidant activities of 3,5-disubstituted tetrahydro-2H-1,3,5-thiadiazine thiones. Bioorg. Med. Chem. Lett. 2023, 79, 129068. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Ali, G.; Idrees, M.; Muhammad, T.; Kong, I.-K.; Abbas, M.; Shah, M.I.A.; Ahmad, S.; Sewell, R.D.; Ullah, S. Selected thiadiazine-thione derivatives attenuate neuroinflammation in chronic constriction injury induced neuropathy. Front. Mol. Neurosci. 2021, 14, 728128. [Google Scholar] [CrossRef]
- Ullah, S.H.; Khan, A.; Halim, S.A.; Khan, R.; Pan, X.-D.; Ullah, R.; Wadood, A.; Khalid, A.; Abdalla, A.N.; Khogeer, S. Blocking the major inflammatory pathways by newly synthesized thiadiazine derivatives via in-vivo, in-vitro and in-silico mechanism. Bioorg. Chem. 2023, 140, 106760. [Google Scholar]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef]
- Rege, M.G.; Ayanwuyi, L.O.; Zezi, A.U.; Odoma, S. Anti-nociceptive, anti-inflammatory and possible mechanism of anti-nociceptive action of methanol leaf extract of Nymphaea lotus Linn (Nymphaeceae). J. Tradit. Complement. Med. 2021, 11, 123–129. [Google Scholar] [CrossRef]
- Athar, J.; Niazi, Z.R.; Niazi, H.R.; Irfan, H.M.; Ahmad, T.; Shah, K.U.; Khattak, H.; Ali, G.; Khan, R.; Khan, M.J. Evaluation of Analgesic and Anti-inflammatory Effects of 5-amino-3-phenyl-1,3,5-thiadiazine-2-thione in Mice. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Song, M.-X.; Zhang, C.-B.; Deng, X.-Q.; Sun, Z.-G.; Quan, Z.-S. Synthesis and Anticonvulsant Activity Evaluation of 6-phenyl-7H-[1,2,4] triazolo [3,4-b][1,3,4] thiadiazines. Lett. Drug Des. Discov. 2011, 8, 769–773. [Google Scholar] [CrossRef]
- Tolou-Ghamari, Z.; Zare, M.; Habibabadi, J.M.; Najafi, M.R. A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, S81. [Google Scholar]
- Grover, G.; Nath, R.; Bhatia, R.; Akhtar, M.J. Synthetic and therapeutic perspectives of nitrogen containing heterocycles as anti-convulsants. Bioorg. Med. Chem. 2020, 28, 115585. [Google Scholar] [CrossRef]
- Angelova, V.T.; Rangelov, M.; Todorova, N.; Dangalov, M.; Andreeva-Gateva, P.; Kondeva-Burdina, M.; Karabeliov, V.; Shivachev, B.; Tchekalarova, J. Discovery of novel indole-based aroylhydrazones as anticonvulsants: Pharmacophore-based design. Bioorg. Chem. 2019, 90, 103028. [Google Scholar] [CrossRef] [PubMed]


| Group | Dose | Baseline Mean ± SD | Sig. * | Post-Rx Mean ± SD | Sig. ** |
|---|---|---|---|---|---|
| Control | -- | 1.36 ± 0.04 | -- | 2.06 ± 0.02 | -- |
| AMP | 100 mg/kg | 1.42 ± 0.03 *** | 0.006 | 0.44 ± 0.02 *** | <0.001 |
| C1 | 100 mg/kg | 1.31 ± 0.02 | 0.256 | 0.93 ± 0.02 | 0.237 |
| C2 | 100 mg/kg | 1.34 ± 0.02 | 0.992 | 0.73 ± 0.02 *** | 0.005 |
| C3 | 100 mg/kg | 1.40 ± 0.02 | 0.166 | 0.84 ± 0.01 *** | 0.062 |
| C4 | 100 mg/kg | 1.29 ± 0.01 *** | 0.006 | 0.63 ± 0.02 *** | 0.001 |
| C5 | 100 mg/kg | 1.27 ± 0.02 *** | <0.001 | 0.47 ± 0.02 *** | <0.001 |
| C1 | 25 mg/kg | 1.31 ± 0.02 | 0.166 | 1.04 ± 0.02 | 0.555 |
| C2 | 25 mg/kg | 1.32 ± 0.02 | 0.375 | 0.87 ± 0.02 | 0.131 |
| C3 | 25 mg/kg | 1.43 ± 0.02 *** | 0.001 | 1.22 ± 0.16 | 0.805 |
| C4 | 25 mg/kg | 1.24 ± 0.02 *** | <0.001 | 0.84 ± 0.01 *** | 0.07 |
| C5 | 25 mg/kg | 1.24 ± 0.02 *** | <0.001 | 0.77 ± 0.01 *** | 0.023 |
| C1 | 50 mg/kg | 1.32 ± 0.02 | 0.668 | 0.94 ± 0.02 | 0.296 |
| C2 | 50 mg/kg | 1.33 ± 0.02 | 0.805 | 0.76 ± 0.01 *** | 0.023 |
| C3 | 50 mg/kg | 1.44 ± 0.01 *** | <0.001 | 1.02 ± 0.02 | 0.518 |
| C4 | 50 mg/kg | 1.29 ± 0.02 *** | 0.003 | 0.73 ± 0.03 *** | 0.005 |
| C5 | 50 mg/kg | 1.25 ± 0.02 *** | <0.001 | 0.61 ± 0.01 *** | 0.001 |
| Groups | 1 h (Mean ± SEM) | 2 h (Mean ± SEM) | 3 h (Mean ± SEM) | 4 h (Mean ± SEM) | 5 h (Mean ± SEM) |
|---|---|---|---|---|---|
| Control | 8.23 ± 2.01 | 8.98 ± 1.67 | 10.07 ± 3.44 | 11.34 ± 1.47 | 10.89 ± 1.67 |
| Aspirin | 47.02 ± 1.67 * | 49.66 ± 4.02 * | 53.09 ± 3.98 * | 55.47 ± 2.05 * | 56.67 ± 3.43 * |
| C1-25 | 14.76 ± 2.30 * | 19.46 ± 2.67 * | 23.41 ± 2.44 * | 28.23 ± 3.78 * | 29.21 ± 2.09 * |
| C1-50 | 26.56 ± 3.09 * | 30.47 ± 3.43 * | 34.52 ± 1.98 * | 39.11 ± 2.50 * | 40.52 ± 3.86 * |
| C1-100 | 53.36 ± 1.00 * | 53.00 ± 4.17 * | 60.66 ± 2.08 * | 62.30 ± 1.52 * | 63.66 ± 2.08 * |
| C2-25 | 10.78 ± 2.03 * | 13.76 ± 2.56 * | 17.92 ± 1.67 * | 26.19 ± 3.67 * | 33.11 ± 1.56 * |
| C2-50 | 23.31 ± 1.89 * | 24.98 ± 2.89 * | 29.47 ± 2.23 * | 35.71 ± 1.98 * | 41.54 ± 1.09 * |
| C2-100 | 31.67 ± 2.52 * | 35.66 ± 3.51 * | 40.66 ± 2.08 * | 45.66 ± 2.51 * | 52.33 ± 1.52 * |
| C3-25 | 22.45 ± 3.34 * | 23.67 ± 3.78 * | 26.11 ± 2.67 * | 34.45 ± 1.47 * | 38.13 ± 2.67 * |
| C3-50 | 31.67 ± 2.46 * | 30.16 ± 4.67 * | 37.67 ± 3.67 * | 43.65 ± 2.08 * | 49.21 ± 4.12 * |
| C3-100 | 40.67 ± 4.09 * | 41.66 ± 4.51 * | 48.00 ± 3.00 * | 52.00 ± 2.01 * | 52.66 ± 1.15 * |
| C4-25 | 16.34 ± 3.10 * | 21.30 ± 2.56 * | 22.67 ± 1.59 * | 30.02 ± 4.02 * | 37.23 ± 2.98 * |
| C4-50 | 28.56 ± 2.56 * | 32.12 ± 4.22 * | 36.11 ± 2.06 * | 38.60 ± 3.67 * | 48.31 ± 4.11 * |
| C4-100 | 36.66 ± 2.61 * | 40.33 ± 3.51 * | 45.66 ± 2.51 * | 49.00 ± 3.09 * | 56.00 ± 2.08 * |
| C5-25 | 19.43 ± 1.89 * | 25.05 ± 3.78 * | 27.41 ± 1.56 * | 32.08 ± 2.49 * | 42.31 ± 2.65 * |
| C5-50 | 30.67 ± 2.32 * | 34.90 ± 4.10 * | 38.39 ± 2.67 * | 43.11 ± 1.67 * | 52.33 ± 2.53 * |
| C5-100 | 40.33 ± 2.51 * | 43.00 ± 3.67 * | 48.33 ± 0.57 * | 52.33 ± 2.51 * | 54.08 ± 4.12 * |
| Treatment | Dose | Normal (A) Mean ± SD | 24 h (B) Mean ± SD | 1 h (°C) Mean ± SD | 2 h (°C) Mean ± SD | 3 h (°C) Mean ± SD | 4 h (°C) Mean ± SD | 5 h (°C) Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Normal Saline | 10 mL/kg | 36.67 ± 0.41 | 39.00 ± 0.19 | 38.00 ± 0.18 | 38.67 ± 0.37 | 39.33 ± 0.27 | 38.33 ± 0.14 | 38.00 ± 0.23 |
| Aspirin | 100 mg/kg | 36.67 ±0.32 | 38.67 ± 0.49 | 37.67 ± 0.39 | 37.33 ± 0.23 * | 37.67 ± 0.56 * | 37.67 ± 0.17 * | 37.67 ± 0.14 |
| C1 | 25 mg/kg | 36.33 ± 0.15 | 39.33 ± 0.23 | 38.67 ± 0.26 | 38.67 ± 0.41 | 38.67 ± 0.27 * | 38.67 ± 0.24 | 38.67 ± 0.40 * |
| C1 | 50 mg/kg | 36.00 ± 0.51 * | 39.00 ± 0.25 | 38.67 ± 0.29 | 38.33 ± 0.38 | 38.00 ± 0.37 * | 38.33 ± 0.24 | 38.00 ± 0.00 |
| C1 | 100 mg/kg | 36.00 ± 0.22 * | 38.67 ± 0.37 | 37.67 ± 0.17 | 37.67 ± 0.14 * | 38.67 ± 0.43 * | 37.67 ± 0.21 * | 37.23 ± 0.19 * |
| C2 | 25 mg/kg | 36.45 ± 0.23 | 38.33 ± 0.44 * | 38.00 ± 0.31 | 38.33 ± 0.45 | 38.33 ± 0.44 * | 39.00 ± 0.41 * | 38.67 ± 0.14 * |
| C2 | 50 mg/kg | 36.67 ± 0.34 | 39.33 ± 0.27 | 38.67 ± 0.21 | 38.67 ± 0.21 | 38.67 ± 0.17 * | 38.67 ± 0.14 | 38.67 ± 0.24 * |
| C2 | 100 mg/kg | 36.30 ± 0.28 | 38.67 ± 0.49 | 37.67 ± 0.30 | 37.33 ± 0.36 * | 37.67 ± 0.56 * | 37.67 ± 0.16 * | 37.67 ± 0.04 |
| C3 | 25 mg/kg | 36.00 ± 0.33 * | 38.75 ± 0.18 | 38.67 ± 0.20 | 38.33 ± 0.40 | 38.00 ± 0.57 * | 38.33 ± 0.14 | 38.00 ± 0.00 |
| C3 | 50 mg/kg | 36.00 ± 0.19 * | 38.09 ± 0.22 * | 37.67 ± 0.48 | 37.67 ± 0.51 * | 38.67 ± 0.27 * | 38.01 ± 0.47 | 37.81 ± 0.19 |
| C3 | 100 mg/kg | 36.67 ± 0.26 | 38.33 ± 0.10 * | 38.00 ± 0.17 | 38.33 ± 0.40 | 38.33 ± 0.14 * | 38.00 ± 0.19 | 36.67 ± 0.34 * |
| C4 | 25 mg/kg | 36.67 ± 0.49 | 38.67 ± 0.25 | 37.67 ± 0.31 | 37.33 ± 0.11 * | 37.67 ± 0.16 * | 37.67 ± 0.17 * | 37.67 ± 0.47 |
| C4 | 50 mg/kg | 36.35 ± 0.20 | 39.33 ± 0.29 | 38.67 ± 0.18 | 38.67 ± 0.41 | 38.67 ± 0.37 * | 38.67 ± 0.24 | 38.67 ± 0.44 * |
| C4 | 100 mg/kg | 36.15 ± 0.52 * | 39.00 ± 0.17 | 38.67 ± 0.13 | 38.33 ± 0.32 | 38.00 ± 0.27 * | 37.33 ± 0.14 * | 37.00 ± 0.00 * |
| C5 | 25 mg/kg | 36.04 ± 0.24 * | 38.67 ± 0.11 | 38.33 ± 0.18 | 37.67 ± 0.38 | 38.67 ± 0.24 * | 38.67 ± 0.09 | 37.67 ± 0.29 |
| C5 | 50 mg/kg | 36.67 ± 0.33 | 38.33 ± 0.43 * | 38.00 ± 0.15 | 38.33 ± 0.17 | 38.33 ± 0.14 * | 37.00 ± 0.26 * | 37.75 ± 0.19 |
| C5 | 100 mg/kg | 36.33 ± 0.48 | 38.23 ± 0.44 * | 37.67 ± 0.27 | 38.33 ± 0.19 * | 38.33 ± 0.24 * | 37.33 ± 0.14 * | 37.33 ± 0.34 * |
| Groups | Line Crossed | Sig. * |
|---|---|---|
| Mean ± SD | ||
| Control | 131 ± 2 | Ref. |
| Diazepam | 13 ± 1 ** | <0.001 |
| C1-100 | 50 ± 1 ** | 0.007 |
| C1-50 | 74 ± 2 ** | 0.05 |
| C1-25 | 94 ± 2 | 0.773 |
| C2-100 | 55 ± 1 ** | 0.013 |
| C2-50 | 78 ± 2 | 0.097 |
| C2-25 | 81 ± 2 | 0.222 |
| C3-100 | 65 ± 1 ** | 0.026 |
| C3-50 | 81 ± 2 | 0.278 |
| C3-25 | 86 ± 1 | 0.458 |
| C4-100 | 34 ± 2 ** | 0.001 |
| C4-50 | 47 ± 2 ** | 0.003 |
| C4-25 | 79 ± 2 | 0.138 |
| C5-100 | 20 ± 1 ** | <0.001 |
| C5-50 | 34 ± 1 ** | 0.001 |
| C5-25 | 92 ± 1 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, S.; Sarwar, N.; Ali, H.; Rafiullah; Khan, R.; Ahmad, A.; Ullah, A.; Soonmin, H.; Ullah, N. Targeting Cutaneous Leishmaniasis with Thiadiazine Thione Derivatives: An In Vivo Study of Its Anti-Inflammatory, Anti-Pyretic, Anti-Nociceptive, and Anti-Sedative Properties. Biomedicines 2025, 13, 93. https://doi.org/10.3390/biomedicines13010093
Sarwar S, Sarwar N, Ali H, Rafiullah, Khan R, Ahmad A, Ullah A, Soonmin H, Ullah N. Targeting Cutaneous Leishmaniasis with Thiadiazine Thione Derivatives: An In Vivo Study of Its Anti-Inflammatory, Anti-Pyretic, Anti-Nociceptive, and Anti-Sedative Properties. Biomedicines. 2025; 13(1):93. https://doi.org/10.3390/biomedicines13010093
Chicago/Turabian StyleSarwar, Sarah, Nadia Sarwar, Haleema Ali, Rafiullah, Rasool Khan, Ajaz Ahmad, Amin Ullah, Ho Soonmin, and Nazif Ullah. 2025. "Targeting Cutaneous Leishmaniasis with Thiadiazine Thione Derivatives: An In Vivo Study of Its Anti-Inflammatory, Anti-Pyretic, Anti-Nociceptive, and Anti-Sedative Properties" Biomedicines 13, no. 1: 93. https://doi.org/10.3390/biomedicines13010093
APA StyleSarwar, S., Sarwar, N., Ali, H., Rafiullah, Khan, R., Ahmad, A., Ullah, A., Soonmin, H., & Ullah, N. (2025). Targeting Cutaneous Leishmaniasis with Thiadiazine Thione Derivatives: An In Vivo Study of Its Anti-Inflammatory, Anti-Pyretic, Anti-Nociceptive, and Anti-Sedative Properties. Biomedicines, 13(1), 93. https://doi.org/10.3390/biomedicines13010093





