Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Molecular Biology
2.3. Cell Culture and Transfection
2.4. G-Protein Signaling Assay
2.5. β-Arrestin 2 Signaling Assay
2.6. Data Analysis and Statistics
3. Results
3.1. β-Arrestin 2 Recruitment of the WT, I212F, and M345R
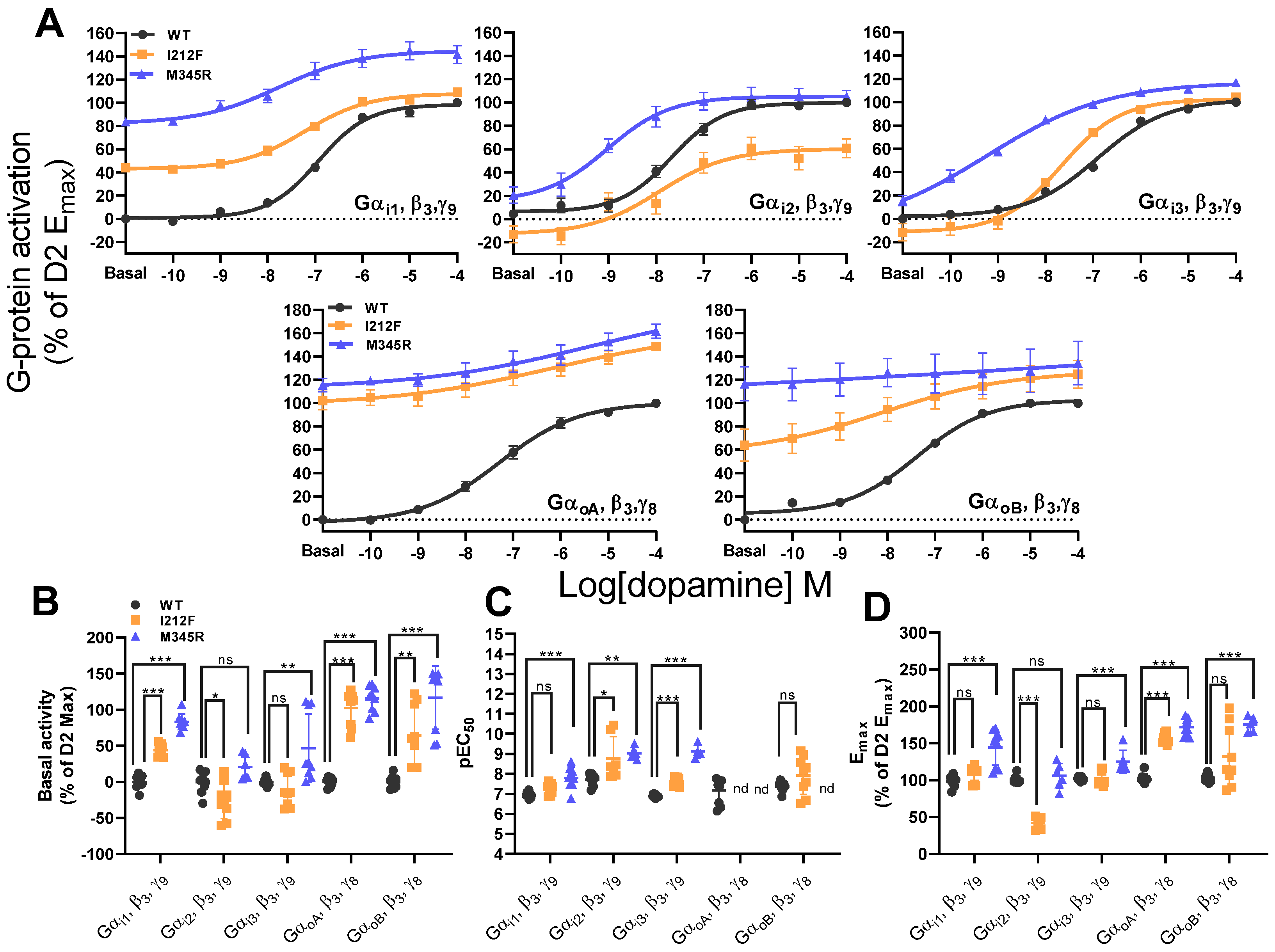
3.2. WT, I212F, and M345R G-Protein Activation
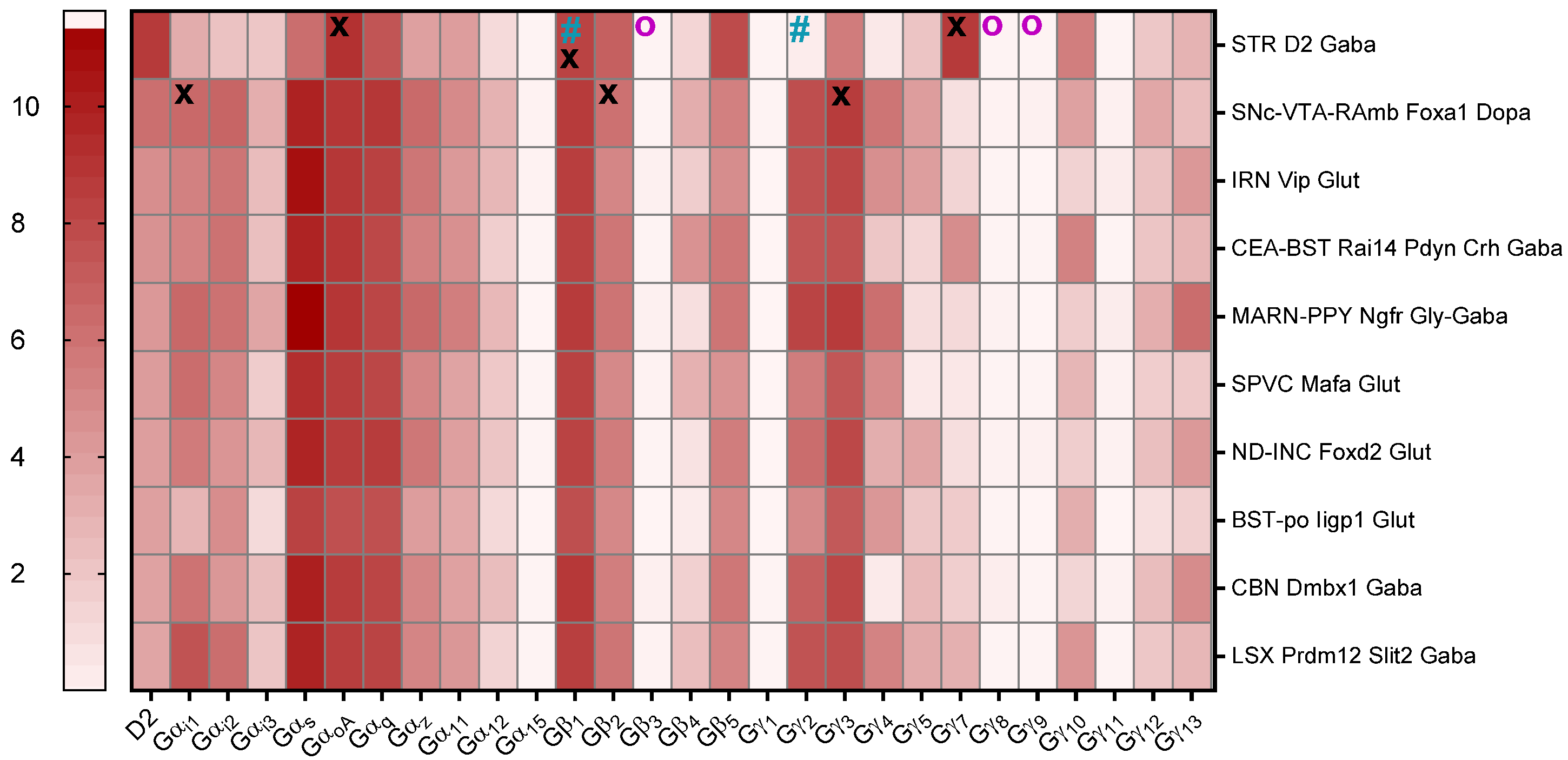
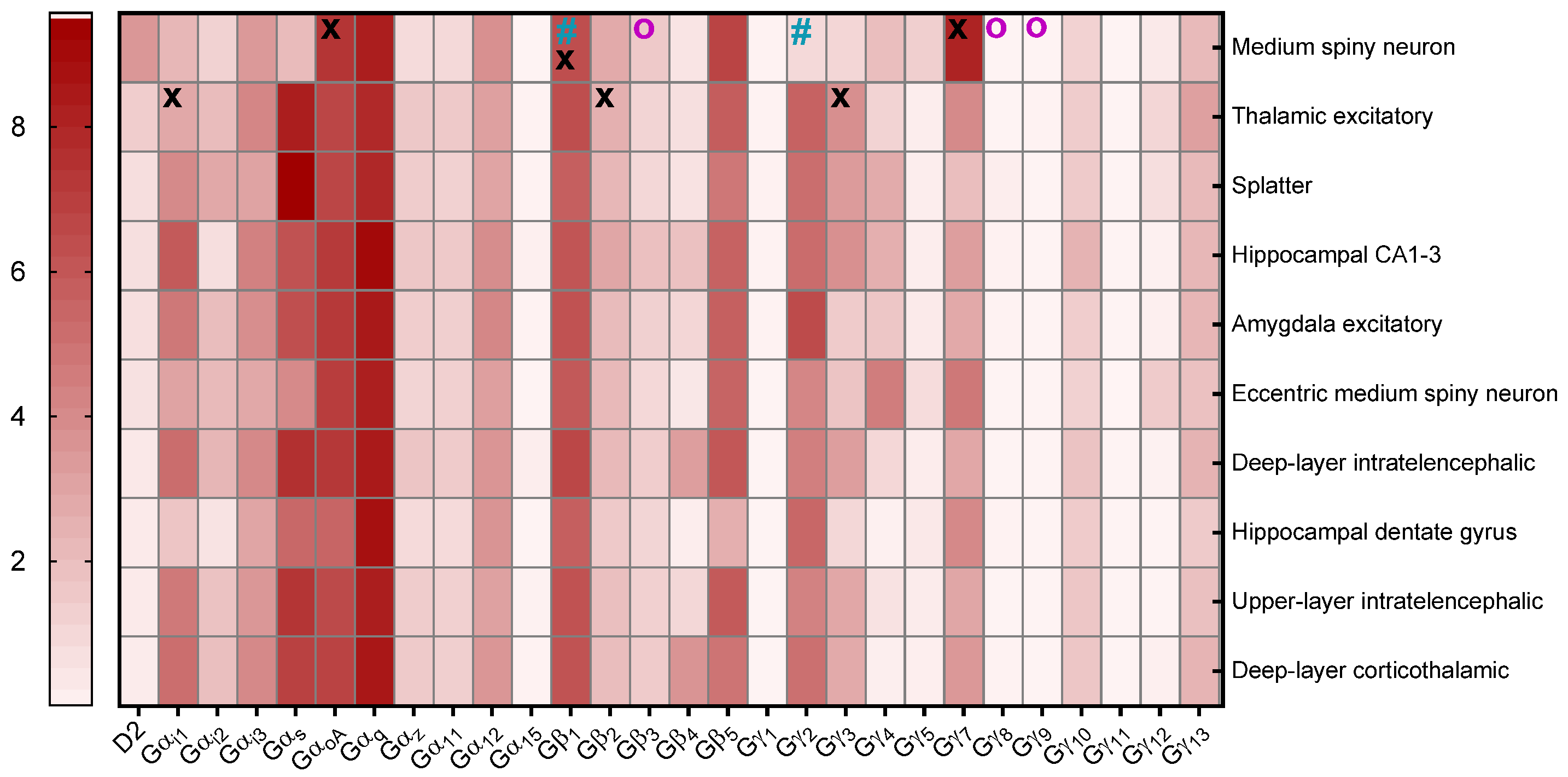
3.3. G-Protein Expression in Mouse and Human Brains
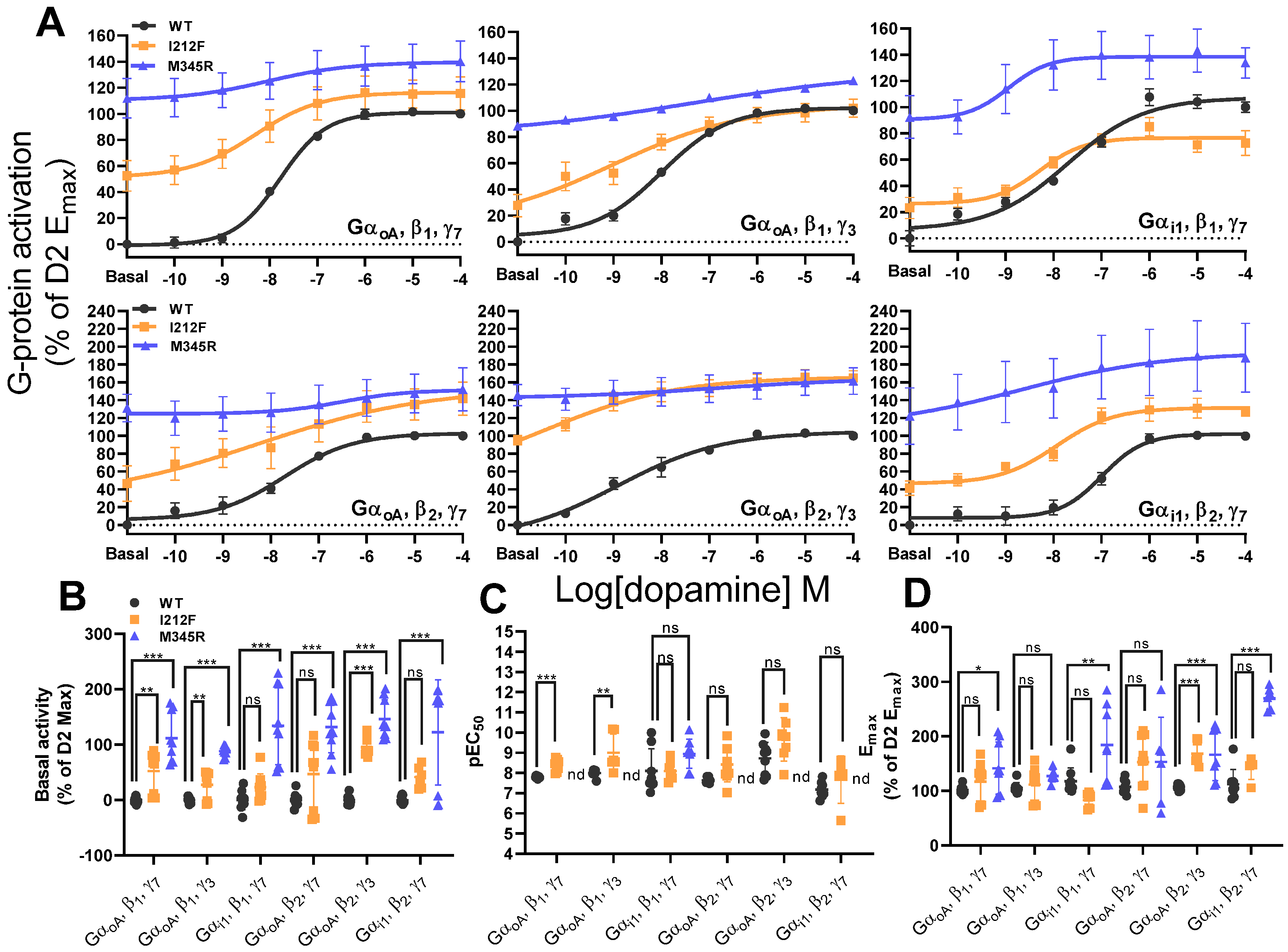
3.4. Effect of I212F and M345R on GαoA and αi1 in Combination with β1 and β2 and γ3 and γ7
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Finkelstein, D.I.; Fukunaga, K. Dopamine D1–D5 Receptors in Brain Nuclei: Implications for Health and Disease. Receptors 2024, 3, 155–181. [Google Scholar] [CrossRef]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms, and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Omble, A.; Kulkarni, K. GPCRs that Rhoar the Guanine nucleotide exchange factors. Small GTPases 2022, 13, 84–99. [Google Scholar] [CrossRef]
- Khan, S.M.; Sleno, R.; Gora, S.; Zylbergold, P.; Laverdure, J.P.; Labbe, J.C.; Miller, G.J.; Hebert, T.E. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol. Rev. 2013, 65, 545–577. [Google Scholar] [CrossRef]
- Senarath, K.; Kankanamge, D.; Samaradivakara, S.; Ratnayake, K.; Tennakoon, M.; Karunarathne, A. Regulation of G Protein betagamma Signaling. Int. Rev. Cell Mol. Biol. 2018, 339, 133–191. [Google Scholar] [CrossRef]
- Dupre, D.J.; Robitaille, M.; Rebois, R.V.; Hebert, T.E. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 31–56. [Google Scholar] [CrossRef]
- Hildebrandt, J.D.; Codina, J.; Risinger, R.; Birnbaumer, L. Identification of a gamma subunit associated with the adenylyl cyclase regulatory proteins Ns and Ni. J. Biol. Chem. 1984, 259, 2039–2042. [Google Scholar] [CrossRef]
- Chung, K.Y. Structural Aspects of GPCR-G Protein Coupling. Toxicol. Res. 2013, 29, 149–155. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7. [Google Scholar] [CrossRef]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.-L.; Hu, L.; Lönnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic diversity of cell types across the adult human brain. Science 2023, 382, eadd7046. [Google Scholar] [CrossRef]
- Pitcher, J.A.; Freedman, N.J.; Lefkowitz, R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998, 67, 653–692. [Google Scholar] [CrossRef] [PubMed]
- Mushegian, A.; Gurevich, V.V.; Gurevich, E.V. The origin and evolution of G protein-coupled receptor kinases. PLoS ONE 2012, 7, e33806. [Google Scholar] [CrossRef]
- Smith, J.S.; Rajagopal, S. The beta-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors. J. Biol. Chem. 2016, 291, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. G Protein-Coupled Receptor Signaling Through beta-Arrestin-Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Gurevich, V.V.; Gurevich, E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006, 110, 465–502. [Google Scholar] [CrossRef]
- Avissar, S.; Schreiber, G. The involvement of G proteins and regulators of receptor-G protein coupling in the pathophysiology, diagnosis and treatment of mood disorders. Clin. Chim. Acta 2006, 366, 37–47. [Google Scholar] [CrossRef]
- Kostiou, V.D.; Theodoropoulou, M.C.; Hamodrakas, S.J. GprotPRED: Annotation of Galpha, Gbeta and Ggamma subunits of G-proteins using profile Hidden Markov Models (pHMMs) and application to proteomes. Biochim. Biophys. Acta 2016, 1864, 435–440. [Google Scholar] [CrossRef]
- Kee, T.R.; Khan, S.A.; Neidhart, M.B.; Masters, B.M.; Zhao, V.K.; Kim, Y.K.; McGill Percy, K.C.; Woo, J.A. The multifaceted functions of beta-arrestins and their therapeutic potential in neurodegenerative diseases. Exp. Mol. Med. 2024, 56, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Arachchige, A. Depletion of dopamine in Parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Noth, J.; Thompson, P.D.; Bollen, E.L.; Curra, A.; Deuschl, G.; van Dijk, J.G.; Topper, R.; Schwarz, M.; Roos, R.A. Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov. Disord. 1999, 14, 398–403. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Matsushima, A.; Pineda, S.S.; Crittenden, J.R.; Lee, H.; Galani, K.; Mantero, J.; Tombaugh, G.; Kellis, M.; Heiman, M.; Graybiel, A.M. Transcriptional vulnerabilities of striatal neurons in human and rodent models of Huntington’s disease. Nat. Commun. 2023, 14, 282. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Murphy, K.P.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef]
- Moritz, A.E.; Free, R.B.; Sibley, D.R. Advances and challenges in the search for D(2) and D(3) dopamine receptor-selective compounds. Cell Signal 2018, 41, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, D.; Garcia-Nafria, J.; Chan, A.E.; Shinde, U.; Neve, K.A. Comparison of the function of two novel human dopamine D2 receptor variants identifies a likely mechanism for their pathogenicity. Biochem. Pharmacol. 2024, 228, 116228. [Google Scholar] [CrossRef] [PubMed]
- Mencacci, N.E.; Steel, D.; Magrinelli, F.; Hsu, J.; Keller Sarmiento, I.J.; Troncoso Schifferli, M.; Munoz, D.; Stefanis, L.; Lubbe, S.J.; Wood, N.W.; et al. Childhood-Onset Chorea Caused by a Recurrent De Novo DRD2 Variant. Mov. Disord. 2021, 36, 1472–1473. [Google Scholar] [CrossRef] [PubMed]
- van der Weijden, M.C.M.; Rodriguez-Contreras, D.; Delnooz, C.C.S.; Robinson, B.G.; Condon, A.F.; Kielhold, M.L.; Stormezand, G.N.; Ma, K.Y.; Dufke, C.; Williams, J.T.; et al. A Gain-of-Function Variant in Dopamine D2 Receptor and Progressive Chorea and Dystonia Phenotype. Mov. Disord. 2021, 36, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, D.; Condon, A.F.; Buck, D.C.; Asad, N.; Dore, T.M.; Verbeek, D.S.; Tijssen, M.A.J.; Shinde, U.; Williams, J.T.; Neve, K.A. Signaling-Biased and Constitutively Active Dopamine D2 Receptor Variant. ACS Chem. Neurosci. 2021, 12, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Jeanneteau, F.; Diaz, J.; Sokoloff, P.; Griffon, N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol. Biol. Cell 2004, 15, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Nafria, J.; Watson, J.F.; Greger, I.H. IVA cloning: A single-tube universal cloning system exploiting bacterial In Vivo Assembly. Sci. Rep. 2016, 6, 27459. [Google Scholar] [CrossRef] [PubMed]
- Barnea, G.; Strapps, W.; Herrada, G.; Berman, Y.; Ong, J.; Kloss, B.; Axel, R.; Lee, K.J. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA 2008, 105, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, W.K.; Sassano, M.F.; Huang, X.P.; Lansu, K.; McCorvy, J.D.; Giguere, P.M.; Sciaky, N.; Roth, B.L. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.H.J.; DiBerto, J.F.; English, J.G.; Glaudin, A.M.; Krumm, B.E.; Slocum, S.T.; Che, T.; Gavin, A.C.; McCorvy, J.D.; Roth, B.L.; et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 2020, 16, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.J.; St-Pierre, F.; Gong, Y.; Marshall, J.D.; Cranfill, P.J.; Baird, M.A.; McKeown, M.R.; Wiedenmann, J.; Davidson, M.W.; Schnitzer, M.J.; et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 2012, 9, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; van Velthoven, C.T.J.; Kunst, M.; Zhang, M.; McMillen, D.; Lee, C.; Jung, W.; Goldy, J.; Abdelhak, A.; Aitken, M.; et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 2023, 624, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Allen Institute for Brain Science (2004). Allen Mouse Brain Atlas [Dataset]. Allen Institute for Brain Science (2011). Available online: https://mouse.brain-map.org (accessed on 25 December 2024).
- Drube, J.; Haider, R.S.; Matthees, E.S.F.; Reichel, M.; Zeiner, J.; Fritzwanker, S.; Ziegler, C.; Barz, S.; Klement, L.; Filor, J.; et al. GPCR kinase knockout cells reveal the impact of individual GRKs on arrestin binding and GPCR regulation. Nat. Commun. 2022, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. Biased GPCR signaling: Possible mechanisms and inherent limitations. Pharmacol. Ther. 2020, 211, 107540. [Google Scholar] [CrossRef]
- Wright, S.C.; Avet, C.; Gaitonde, S.A.; Muneta-Arrate, I.; Le Gouill, C.; Hogue, M.; Breton, B.; Koutsilieri, S.; Diez-Alarcia, R.; Heroux, M.; et al. Conformation- and activation-based BRET sensors differentially report on GPCR-G protein coupling. Sci. Signal 2024, 17, eadi4747. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Avet, C.; Normand, C.; Mancini, A.; Inoue, A.; Bouvier, M.; Gloriam, D.E. Common coupling map advances GPCR-G protein selectivity. elife 2022, 11, e74107. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell 2019, 177, 1933–1947.e25. [Google Scholar] [CrossRef]
- Masuho, I.; Ostrovskaya, O.; Kramer, G.M.; Jones, C.D.; Xie, K.; Martemyanov, K.A. Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci. Signal 2015, 8, ra123. [Google Scholar] [CrossRef] [PubMed]
- Von Moo, E.; Harpsoe, K.; Hauser, A.S.; Masuho, I.; Brauner-Osborne, H.; Gloriam, D.E.; Martemyanov, K.A. Ligand-directed bias of G protein signaling at the dopamine D(2) receptor. Cell Chem. Biol. 2022, 29, 226–238e224. [Google Scholar] [CrossRef]
- Kankanamge, D.; Tennakoon, M.; Karunarathne, A.; Gautam, N. G protein gamma subunit, a hidden master regulator of GPCR signaling. J. Biol. Chem. 2022, 298, 102618. [Google Scholar] [CrossRef]
- Masuho, I.; Skamangas, N.K.; Muntean, B.S.; Martemyanov, K.A. Diversity of the Gbetagamma complexes defines spatial and temporal bias of GPCR signaling. Cell Syst. 2021, 12, 324–337e325. [Google Scholar] [CrossRef] [PubMed]
- Rysiewicz, B.; Blasiak, E.; Mystek, P.; Dziedzicka-Wasylewska, M.; Polit, A. Beyond the G protein alpha subunit: Investigating the functional impact of other components of the Galphai(3) heterotrimers. Cell Commun. Signal 2023, 21, 279. [Google Scholar] [CrossRef] [PubMed]
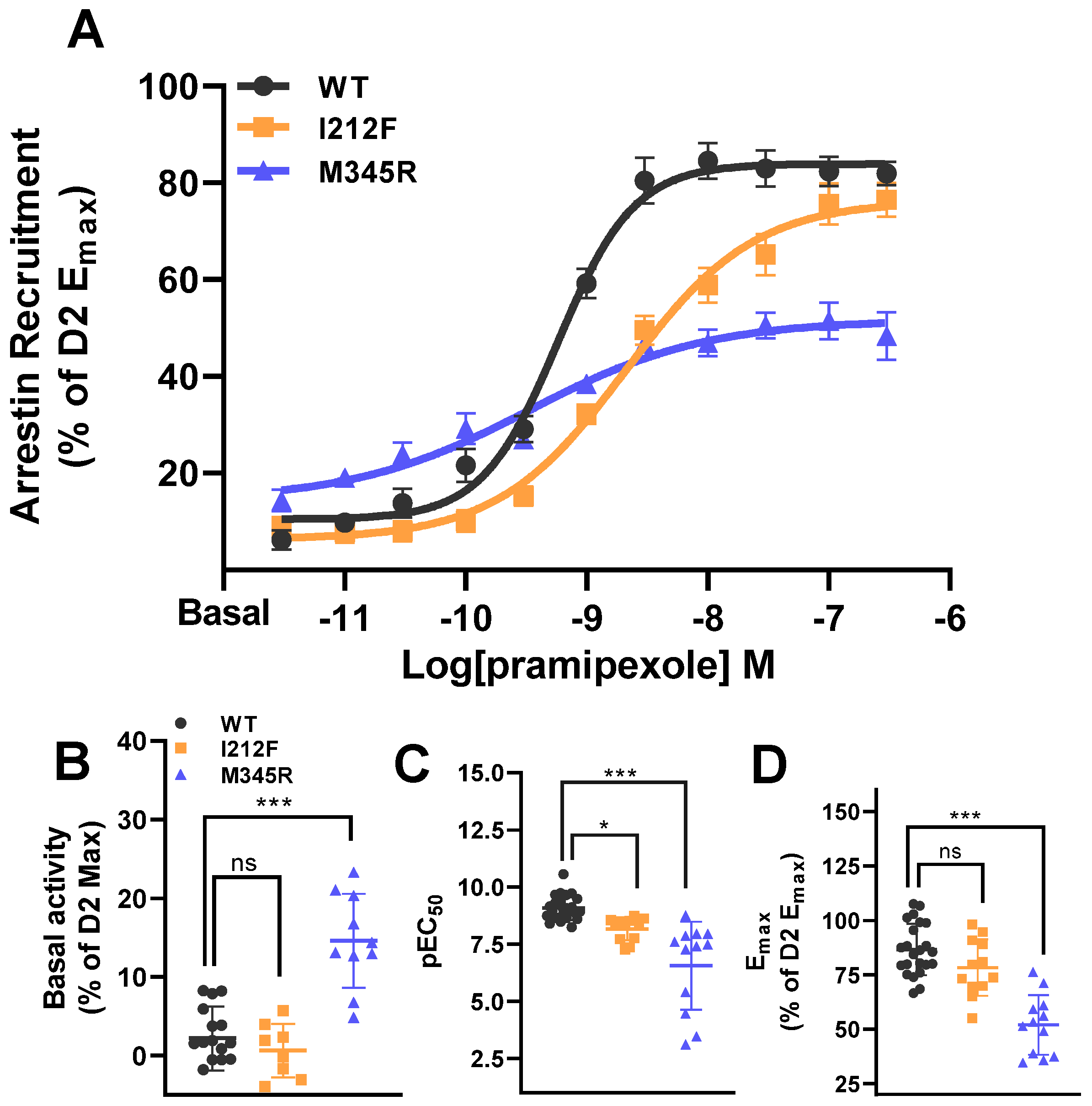
| β-arrestin 2 | WT | I212F | M345R | Anova | n/N | ||
|---|---|---|---|---|---|---|---|
| pEC50 | 9.08 ± 0.10 | 8.17 ± 0.15 | p = 0.036 | 6.56 ± 0.57 | p < 0.001 | F(2,45) = 22.99, p < 0.001 | 9/3 |
| Basal activity | 2.21 ± 1.02 | 0.64 ± 1.21 | p = 0.661 | 14.60 ±1.89 | p < 0.001 | F(2,31) = 28.40, p < 0.001 | 9/3 |
| Emax | 86.60 ± 2.47 | 78.32 ± 3.75 | p = 0.134 | 51.97 ± 3.96 | p < 0.001 | F(2,44) = 29.99, p < 0.001 | 9/3 |
| pEC50 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gαi1, β3, γ9 | Gαi2, β3, γ9 | Gαi3, β3, γ9 | GαoA, β3, γ8 | GαoB, β3, γ8 | n/N | ||||||
| WT | 6.95 ± 0.04 | 7.72 ± 0.09 | 6.90 ± 0.02 | 0.18 ± 0.21 | 7.38 ± 0.08 | 9/3 | |||||
| I212F | 7.27 ± 0.08 | p = 0.122 | 8.76 ± 0.41 | p = 0.010 | 7.63 ± 0.07 | p < 0.001 | nd | - | 7.93 ± 0.31 | p = 0.110 | 9/3 |
| M345R | 7.80 ± 0.18 | p < 0.001 | 9.03 ± 0.11 | p = 0.002 | 9.13 ± 0.18 | p < 0.001 | nd | - | nd | - | 9/3 |
| Anova | F(2,24) = 13.13, p < 0.001 | F(2,19) = 8.70, p = 0.002 | F(2,19) = 161.40, p < 0.001 | - | t-test | ||||||
| Basal activity | |||||||||||
| Gαi1, β3, γ9 | Gαi2, β3, γ9 | Gαi3, β3, γ9 | GαoA, β3, γ8 | GαoB, β3, γ8 | n/N | ||||||
| WT | 0.00 ± 3.16 | 0.00 ± 20.60 | 0.00 ± 1.53 | 0.00 ± 2.01 | 0.00 ± 2.89 | 9/3 | |||||
| I212F | 43.99 ± 2.65 | p < 0.001 | −26.09 ± 8.27 | p = 0.023 | −11.57 ± 7.46 | p = 0.639 | 102.20 ± 7.99 | p < 0.001 | 64.05 ± 13.81 | p = 0.002 | 9/3 |
| M345R | 83.35 ± 3.50 | p < 0.001 | 20.60 ± 7.04 | p = 0.111 | 46.66 ± 15.85 | p = 0.007 | 115.50 ± 5.66 | p < 0.001 | 116.60 ± 14.66 | p < 0.001 | 9/3 |
| Anova | F(2,24) = 178.20, p < 0.001 | F(2,21) = 10.43, p < 0.001 | F(2,24) = 9.22, p = 0.001 | F(2,24) = 119.99, p < 0.001 | F(2,24) = 24.72, p < 0.001 | ||||||
| Emax | |||||||||||
| Gαi1, β3, γ9 | Gαi2, β3, γ9 | Gαi3, β3, γ9 | GαoA, β3, γ8 | GαoB, β3, γ8 | n/N | ||||||
| WT | 98.53 ± 2.42 | 100.90 ± 1.66 | 102.56 ± 0.88 | 102.93 ± 1.97 | 103.32 ± 1.70 | 9/3 | |||||
| I212F | 107.67 ± 3.68 | p = 0.384 | 42.42 ± 3.27 | p < 0.001 | 102.25 ± 3.28 | p = 0.997 | 155.86 ± 2.41 | p < 0.001 | 132.32 ± 13.54 | p = 0.055 | 9/3 |
| M345R | 144.54 ± 8.07 | p < 0.001 | 105.79 ± 6.79 | p = 0.591 | 125.18 ± 6.23 | p < 0.001 | 172.24 ± 3.84 | p < 0.001 | 175.62 ± 4.66 | p < 0.001 | 9/3 |
| Anova | F(2,24) = 21.06, p < 0.001 | F(2,18) = 74.34, p < 0.001 | F(2,21) = 12.31, p < 0.001 | F(2,23) = 161.90, p < 0.001 | F(2,20) = 12.18, p < 0.001 | ||||||
| pEC50 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GαoA, β1, γ7 | GαoA, β1, γ3 | Gαi1, β1, γ7 | GαoA, β2, γ7 | GαoA, β2, γ3 | Gαi1, β2, γ7 | n/N | |||||||
| WT | 7.80 ± 0.02 | 7.99 ± 0.06 | 8.10 ± 0.38 | 7.64 ± 0.06 | 8.72 ± 0.26 | 7.18 ± 0.14 | 9/3 | ||||||
| I212F | 8.40 ± 0.09 | p < 0.001 | 9.00 ± 0.38 | p = 0.011 | 8.09 ± 0.18 | p = 0.999 | 8.42 ± 0.29 | p = 0.050 | 9.65 ± 0.41 | p = 0.415 | 7.67 ± 0.52 | p = 0.289 | 9/3 |
| M345R | nd | nd | 8.95 ± 0.18 | p = 0.091 | nd | nd | nd | 9/3 | |||||
| Anova | t-test | t-test | F(2,20) = 2.84, p = 0.082 | t-test | t-test | t-test | |||||||
| Basal activity | |||||||||||||
| GαoA, β1, γ7 | GαoA, β1, γ3 | Gαi1, β1, γ7 | GαoA, β2, γ7 | GαoA, β2, γ3 | Gαi1, β2, γ7 | n/N | |||||||
| WT | 0.00 ± 1.86 | 0.00 ± 1.71 | 0.00 ± 5.80 | 0.00 ± 4.08 | 0.00 ± 2.89 | 0.00 ± 2.23 | 9/3 | ||||||
| I212F | 52.37 ± 11.81 | p = 0.005 | 27.64 ± 8.48 | p = 0.002 | 23.08 ± 8.07 | p = 0.440 | 46.82 ± 19.93 | p = 0.062 | 95.13 ± 5.33 | p < 0.001 | 41.62 ± 7.92 | p = 0.334 | 9/3 |
| M345R | 111.87 ± 15.14 | p < 0.001 | 88.30 ± 3.16 | p < 0.001 | 133.67 ± 23.27 | p < 0.001 | 131.34 ± 15.34 | p < 0.001 | 145.76 ± 11.83 | p < 0.001 | 122.23 ± 31.67 | p < 0.001 | 9/3 |
| Anova | F(2,24) = 25.26, p < 0.001 | F(2,24) = 72.21, p < 0.001 | F(2,24) = 23.91, p < 0.001 | F(2,24) = 20.48, p < 0.001 | F(2,24) = 93.01, p < 0.001 | F(2,21) = 9.72, p < 0.001 | |||||||
| Emax | |||||||||||||
| GαoA, β1, γ7 | GαoA, β1, γ3 | Gαi1, β1, γ7 | GαoA, β2, γ7 | GαoA, β2, γ3 | Gαi1, β2, γ7 | n/N | |||||||
| WT | 101.38 ± 2.46 | 105.43 ± 3.25 | 117.75 ± 7.99 | 107.36 ± 4.07 | 107.50 ± 1.82 | 112.01 ± 8.96 | 9/3 | ||||||
| I212F | 117.21 ± 11.89 | p = 0.532 | 111.57 ± 10.26 | p = 0.753 | 83.23 ± 4.85 | p = 0.194 | 152.83 ± 16.34 | p = 0.125 | 168.10 ± 6.70 | p < 0.001 | 141.98 ± 9.34 | p = 0.068 | 9/3 |
| M345R | 141.65 ± 15.92 | p = 0.039 | 127.29 ± 4.23 | p = 0.081 | 184.23 ± 24.78 | p = 0.008 | 153.16 ± 33.17 | p = 0.176 | 166.43 ± 18.07 | p < 0.001 | 269.70 ± 8.12 | p < 0.001 | 9/3 |
| Anova | F(2,21) = 3.08, p = 0.065 | F(2,22) = 2.36, p = 0.118 | F(2,22) = 11.54, p < 0.001 | F(2,21) = 2.30, p = 0.125 | F(2,22) = 13.22, p < 0.001 | F(2,17) = 83.76, p < 0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebrügge, N.; Trovato, O.; Praschberger, R.; Lieb, A. Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations. Biomedicines 2025, 13, 46. https://doi.org/10.3390/biomedicines13010046
Niebrügge N, Trovato O, Praschberger R, Lieb A. Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations. Biomedicines. 2025; 13(1):46. https://doi.org/10.3390/biomedicines13010046
Chicago/Turabian StyleNiebrügge, Nele, Olga Trovato, Roman Praschberger, and Andreas Lieb. 2025. "Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations" Biomedicines 13, no. 1: 46. https://doi.org/10.3390/biomedicines13010046
APA StyleNiebrügge, N., Trovato, O., Praschberger, R., & Lieb, A. (2025). Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations. Biomedicines, 13(1), 46. https://doi.org/10.3390/biomedicines13010046





