Dopamine-Sensitive Anterior Cingulate Cortical Glucose-Monitoring Neurons as Potential Therapeutic Targets for Gustatory and Other Behavior Alterations
Abstract
1. Introduction
2. Materials and Methods
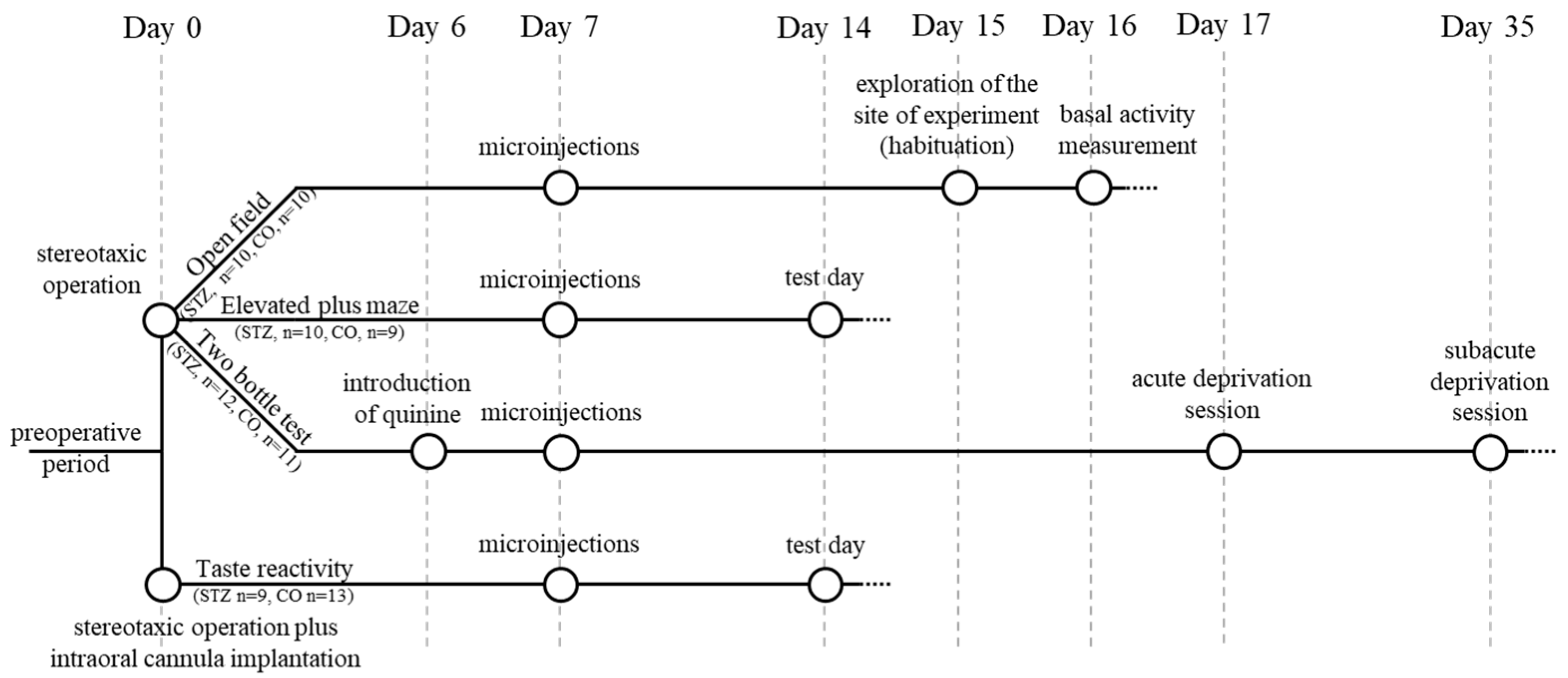
2.1. Experimental Design, Basic Conditions
2.2. Extracellular Single-Neuron Activity Recording, Administration of Neurochemicals, Gustatory Stimulus Delivery
2.3. Behavioral Investigations (Operation; Intracerebral Microinjection; Behavioral Tests)
2.3.1. Stereotaxic Surgery
2.3.2. Microinjection
2.3.3. Behavioral Tests
Open-Field Test
Elevated Plus Maze
Two-Bottle Test
Taste Reactivity Test
2.3.4. Histology
2.3.5. Statistical Analysis of Data
3. Results
3.1. Microelectrophysiology
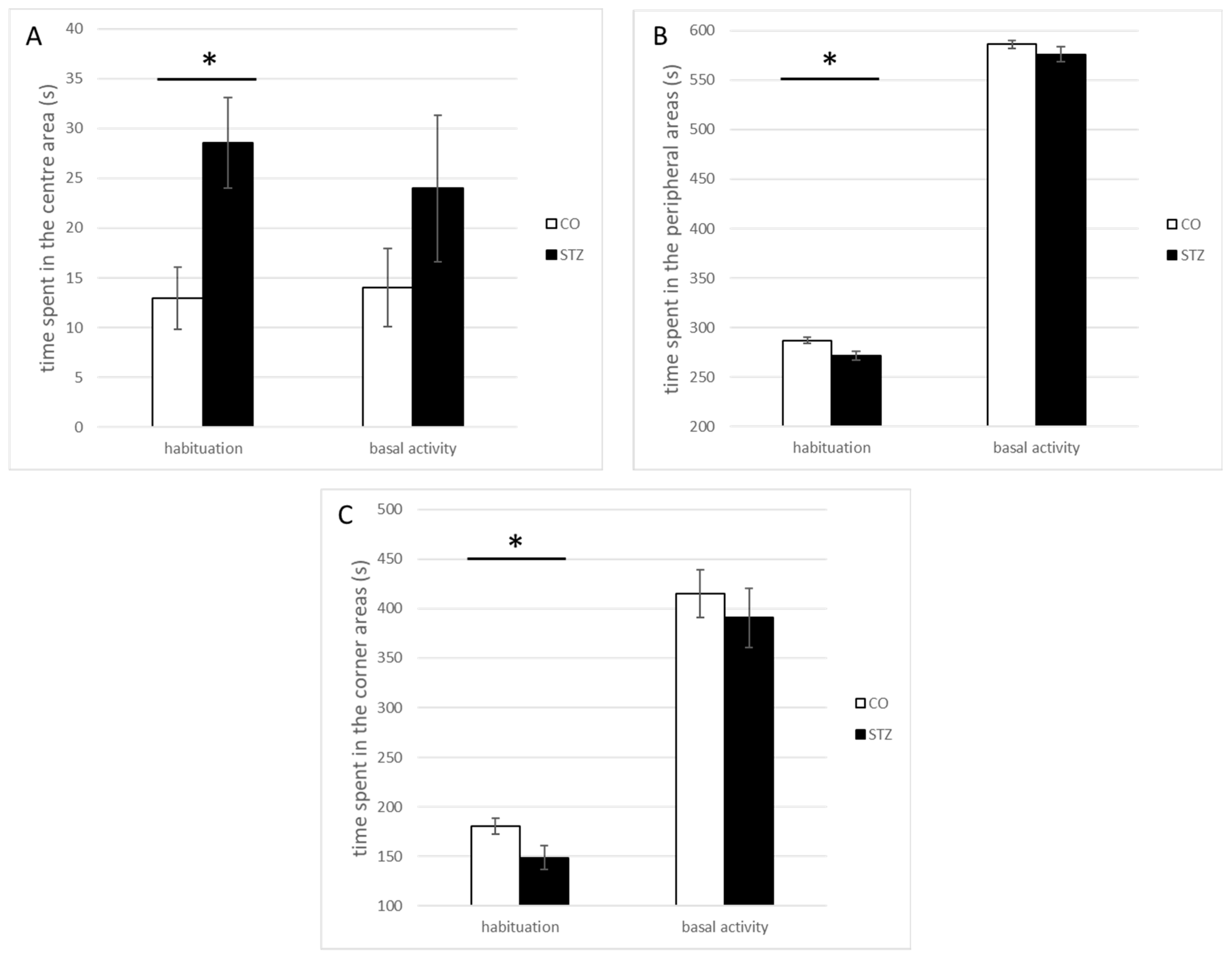
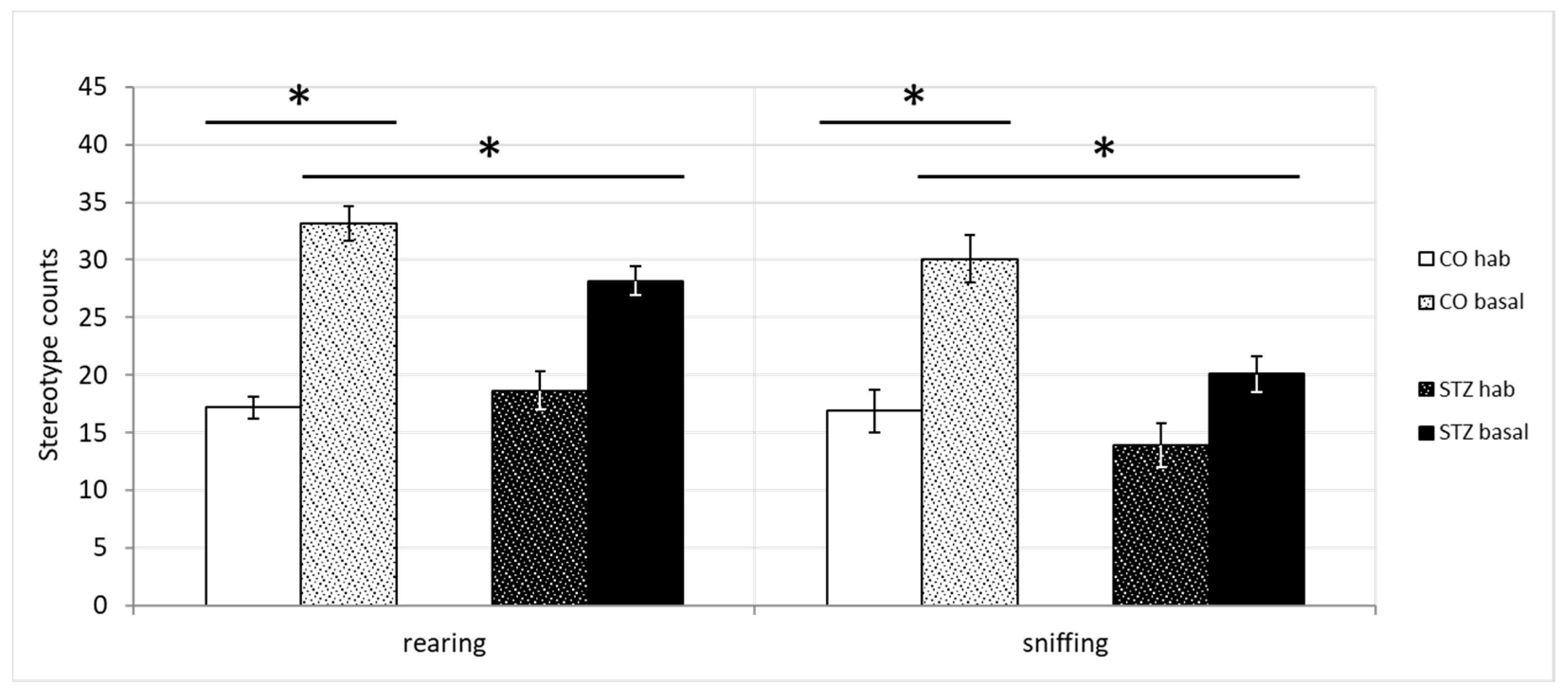
3.2. Open-Field Test
3.3. Elevated Plus Maze Test
3.4. Two-Bottle Test
3.5. Taste Reactivity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Symbol/Abbreviation | Referred to |
| * | statistical significance level of differences at p ≤ 0.05 |
| ** | statistical significance level of differences at p ≤ 0.001 |
| ACC | anterior cingulate cortex |
| CC or cctx | cingulate cortex |
| CO | control |
| DA | dopamine |
| glut | glutamate |
| GLUT2 | type 2 glucose transporter protein |
| GM | glucose-monitoring |
| QHCl | quinine hydrochloride |
| STZ | streptozotocin |
References
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Salbe, A.D.; DelParigi, A.; Pratley, R.E.; Drewnowski, A.; Tataranni, P.A. Taste preferences and body weight changes in an obesity-prone population. Am. J. Clin. Nutr. 2004, 79, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 93, S9-S30. [Google Scholar] [CrossRef]
- Guthrie, R.A.; Guthrie, D.W. Pathophysiology of diabetes mellitus. Crit. Care Nurs. Q. 2004, 27, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Wabitsch, M. Childhood obesity. Curr. Opin. Lipidol. 2011, 22, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Scherneck, S.; Joost, H.G.; Al-Hasani, H. Molecular links between Obesity and Diabetes: “Diabesity”. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; Mdtext.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Marshall, N.B.; Mayer, J. Specificity of gold thioglucose for ventromedial hypothalamic lesions and hyperphagia. Nature 1956, 178, 1399–1400. [Google Scholar] [PubMed]
- Mayer, J. Regulation of energy intake and the body weight: The glucostatic theory and the lipostatic hypothesis. Ann. N. Y. Acad. Sci. 1955, 63, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Oomura, Y. Input-output organization in the hypothalamus relating to food intake behavior. Handb. Hypothal. 1980, 2, 557–620. [Google Scholar]
- Karadi, Z.; Lukats, B.; Papp, S.; Takacs, G.; Egyed, R.; Lenard, L. The central glucose-monitoring neural network: Major protector of the adaptive homeostatic balance for well being of the organism. Int. Congr. Ser. 2004, 1269, 30–33. [Google Scholar] [CrossRef]
- Oomura, Y.; Yoshimatsu, H. Neural network of glucose monitoring system. J. Auton. Nerv. Syst. 1984, 10, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Bruning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Niijima, A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J. Physiol. 1982, 332, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.K.; Chhina, G.S.; Sharma, K.N.; Dua, S.; Singh, B. Activity of Single Neurons in the Hypothalamic Feeding Centers: Effect of Glucose. Am. J. Physiol. 1964, 207, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Oomura, Y.; Kimura, K.; Ooyama, H.; Maeno, T.; Iki, M.; Kuniyoshi, M. Reciprocal Activities of the Ventromedial and Lateral Hypothalamic Areas of Cats. Science 1964, 143, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Oomura, Y.; Ono, T.; Ooyama, H.; Wayner, M.J. Glucose and osmosensitive neurones of the rat hypothalamus. Nature 1969, 222, 282–284. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 1990, 39, 647–652. [Google Scholar] [CrossRef]
- Karadi, Z.; Oomura, Y.; Nishino, H.; Scott, T.R.; Lenard, L.; Aou, S. Responses of lateral hypothalamic glucose-sensitive and glucose-insensitive neurons to chemical stimuli in behaving rhesus monkeys. J. Neurophysiol. 1992, 67, 389–400. [Google Scholar] [CrossRef]
- Arluison, M.; Quignon, M.; Nguyen, P.; Thorens, B.; Leloup, C.; Penicaud, L. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain--an immunohistochemical study. J. Chem. Neuroanat. 2004, 28, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Arluison, M.; Lepetit, N.; Cartier, N.; Marfaing-Jallat, P.; Ferre, P.; Penicaud, L. Glucose transporter 2 (GLUT 2): Expression in specific brain nuclei. Brain Res. 1994, 638, 221–226. [Google Scholar] [CrossRef]
- Nagy, B.; Szabo, I.; Papp, S.; Takacs, G.; Szalay, C.; Karadi, Z. Glucose-monitoring neurons in the mediodorsal prefrontal cortex. Brain Res. 2012, 1444, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Telkes, I.; Szalay, C.; Lenard, L.; Karadi, Z. Deficits of hypothalamic GLUT2 immunolabeling after streptozotocin microinjection into the ventromedial hypothalamic nucleus of the rat. In Proceedings of the 13th Conference of the Hungarian Neuroscience Society, Budapest, Hungary, 20–22 January 2011. [Google Scholar]
- Kapolowicz, M.R.; Thompson, L.T. Plasticity in Limbic Regions at Early Time Points in Experimental Models of Tinnitus. Front. Syst. Neurosci. 2019, 13, 88. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Parkinson, J.A.; Marbini, H.D.; Toner, A.J.; Bussey, T.J.; Robbins, T.W.; Everitt, B.J. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav. Neurosci. 2003, 117, 566–587. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Kanoski, S.E. Homeostatic and non-homeostatic controls of feeding behavior: Distinct vs. common neural systems. Physiol. Behav. 2018, 193, 223–231. [Google Scholar] [CrossRef]
- Ernst, J.; Hock, A.; Henning, A.; Seifritz, E.; Boeker, H.; Grimm, S. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol. Psychiatry 2017, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Takeuch, K.; Matsumoto, T.; Kamimura, K.; Hamada, R.; Nakamura, K.; Kato, N. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psychiatry Res. 2007, 154, 49–58. [Google Scholar] [CrossRef]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain J. Neurol. 1995, 118 Pt 1, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Whalen, P.J.; Pitman, R.K.; Bush, G.; Macklin, M.L.; Lasko, N.B.; Orr, S.P.; McInerney, S.C.; Rauch, S.L. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry 2001, 50, 932–942. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Hayman, L.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Monosov, I.E.; Haber, S.N.; Leuthardt, E.C.; Jezzini, A. Anterior Cingulate Cortex and the Control of Dynamic Behavior in Primates. Curr. Biol. 2020, 30, R1442–R1454. [Google Scholar] [CrossRef]
- Apps, M.A.; Ramnani, N. The anterior cingulate gyrus signals the net value of others’ rewards. J. Neurosci. 2014, 34, 6190–6200. [Google Scholar] [CrossRef]
- Lerner, T.N.; Holloway, A.L.; Seiler, J.L. Dopamine, Updated: Reward Prediction Error and Beyond. Curr. Opin. Neurobiol. 2021, 67, 123–130. [Google Scholar] [CrossRef]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef]
- Juarez Olguin, H.; Calderon Guzman, D.; Hernandez Garcia, E.; Barragan Mejia, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef] [PubMed]
- Karadi, Z.; Faludi, B.; Lenard, L.; Czurko, A.; Niedetzky, C.; Vida, I.; Nishino, H. Glucose-sensitive neurons of the globus pallidus: II. Complex functional attributes. Brain Res. Bull. 1995, 37, 157–162. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Manaye, K.F. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): Three-dimensional reconstruction in the rat. J. Comp. Neurol. 1993, 331, 297–309. [Google Scholar] [CrossRef]
- Ugrumov, M.V. Hypothalamic neurons fully or partially expressing the dopaminergic phenotype: Development, distribution, functioning and functional significance. A review. Front. Neuroendocrinol. 2024, 75, 101153. [Google Scholar] [CrossRef] [PubMed]
- Lenard, L.; Karadi, Z.; Faludi, B.; Czurko, A.; Niedetzky, C.; Vida, I.; Nishino, H. Glucose-sensitive neurons of the globus pallidus: I. Neurochemical characteristics. Brain Res. Bull. 1995, 37, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Lukats, B.; Takacs, G.; Szalay, C.; Karadi, Z. Glucose-monitoring neurons in the nucleus accumbens. Neuroreport 2007, 18, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Hormay, E.; Csetenyi, B.; Nagy, B.; Lenard, L.; Karadi, Z. Multiple functional attributes of glucose-monitoring neurons in the medial orbitofrontal (ventrolateral prefrontal) cortex. Neurosci. Biobehav. Rev. 2018, 85, 44–53. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, I.E.; Rolls, E.T. Representation in the human brain of food texture and oral fat. J. Neurosci. 2004, 24, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Hormay, E.; Laszlo, B.; Szabo, I.; Ollmann, T.; Nagy, B.; Peczely, L.; Mintal, K.; Karadi, Z. The effect of loss of the glucose-monitoring neurons in the anterior cingulate cortex: Physiologic challenges induce complex feeding-metabolic alterations after local streptozotocin microinjection in rats. Neurosci. Res. 2019, 149, 50–60. [Google Scholar] [CrossRef]
- Fotakos, D.; Hideg, B.; Szabó, I.; Takács, G.; Szalay, C.; Nagy, B.; Karádi, Z. The Effect of Intraoral and Intragastric Administrations of Chemicals on Glucose Monitoring Neurons in the Cingulate Cortex of the Rat. In Proceedings of the Conference Abstract: IBRO International Workshop, Pécs, Hungary, 21–23 January 2010. [Google Scholar]
- Rolls, E.T. The affective and cognitive processing of touch, oral texture, and temperature in the brain. Neurosci. Biobehav. Rev. 2010, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Szalay, C.; Aradi, M.; Schwarcz, A.; Orsi, G.; Perlaki, G.; Nemeth, L.; Hanna, S.; Takacs, G.; Szabo, I.; Bajnok, L.; et al. Gustatory perception alterations in obesity: An fMRI study. Brain Res. 2012, 1473, 131–140. [Google Scholar] [CrossRef]
- Grabenhorst, F.; Rolls, E.T.; Parris, B.A.; d’Souza, A.A. How the brain represents the reward value of fat in the mouth. Cereb. Cortex 2010, 20, 1082–1091. [Google Scholar] [CrossRef]
- Schneider, K.N.; Sciarillo, X.A.; Nudelman, J.L.; Cheer, J.F.; Roesch, M.R. Anterior Cingulate Cortex Signals Attention in a Social Paradigm that Manipulates Reward and Shock. Curr. Biol. 2020, 30, 3724–3735.e2. [Google Scholar] [CrossRef]
- Bryden, D.W.; Johnson, E.E.; Tobia, S.C.; Kashtelyan, V.; Roesch, M.R. Attention for learning signals in anterior cingulate cortex. J. Neurosci. 2011, 31, 18266–18274. [Google Scholar] [CrossRef]
- Wu, D.; Deng, H.; Xiao, X.; Zuo, Y.; Sun, J.; Wang, Z. Persistent Neuronal Activity in Anterior Cingulate Cortex Correlates with Sustained Attention in Rats Regardless of Sensory Modality. Sci. Rep. 2017, 7, 43101. [Google Scholar] [CrossRef]
- Chudasama, Y.; Daniels, T.E.; Gorrin, D.P.; Rhodes, S.E.; Rudebeck, P.H.; Murray, E.A. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb. Cortex 2013, 23, 2884–2898. [Google Scholar] [CrossRef] [PubMed]
- Heckers, S.; Weiss, A.P.; Deckersbach, T.; Goff, D.C.; Morecraft, R.J.; Bush, G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am. J. Psychiatry 2004, 161, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.; Pascual-Marqui, R.D.; Nitschke, J.B.; Oakes, T.R.; Larson, C.L.; Abercrombie, H.C.; Schaefer, S.M.; Koger, J.V.; Benca, R.M.; Davidson, R.J. Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am. J. Psychiatry 2001, 158, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Ganda, O.P.; Rossini, A.A.; Like, A.A. Studies on streptozotocin diabetes. Diabetes 1976, 25, 595–603. [Google Scholar] [CrossRef]
- Karadi, Z.; Scott, T.R.; Oomura, Y.; Nishino, H.; Aou, S.; Lenard, L. Complex functional attributes of amygdaloid gustatory neurons in the rhesus monkey. Ann. N. Y. Acad. Sci. 1998, 855, 488–492. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. Acad. Sci. Bohemoslov. 2001, 50, 537–546. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition, 3rd ed.; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Lenard, L.; Karadi, Z.; Szabo, I.; Hahn, Z. Pallidal mechanisms in the organizations of feeding and sensorimotor integration. Recent. Dev. Neurobiol. Hung. 1982, 9, 79–113. [Google Scholar]
- Laszlo, B.R.; Hormay, E.; Szabo, I.; Mintal, K.; Nagy, B.; Laszlo, K.; Peczely, L.; Ollmann, T.; Lenard, L.; Karadi, Z. Disturbance of taste reactivity and other behavioral alterations after bilateral interleukin-1beta microinjection into the cingulate cortex of the rat. Behav. Brain Res. 2020, 383, 112537. [Google Scholar] [CrossRef]
- Grill, H.J.; Norgren, R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978, 143, 263–279. [Google Scholar] [CrossRef]
- Takacs, G.; Lukats, B.; Papp, S.; Szalay, C.; Karadi, Z. Taste reactivity alterations after IL-1beta microinjection into the ventromedial hypothalamic nucleus of the rat. Neurosci. Res. 2008, 62, 118–122. [Google Scholar] [CrossRef]
- Grabenhorst, F.; Rolls, E.T.; Margot, C.; da Silva, M.A.; Velazco, M.I. How pleasant and unpleasant stimuli combine in different brain regions: Odor mixtures. J. Neurosci. 2007, 27, 13532–13540. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Takacs, G.; Szabo, I.; Lenard, L.; Karadi, Z. Taste reactivity alterations after streptozotocin microinjection into the mediodorsal prefrontal cortex. Behav. Brain Res. 2012, 234, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cano-Garcia, H.; Kshirsagar, R.; Pricci, R.; Teyeb, A.; O’Brien, F.; Saha, S.; Kosmas, P.; Kallos, E. Enhancing the Accuracy of Non-Invasive Glucose Sensing in Aqueous Solutions Using Combined Millimeter Wave and Near Infrared Transmission. Sensors 2021, 21, 3275. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Oomura, Y.; Novin, D.; Grijalva, C.V.; Cooper, P.H. Functional correlations between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucose-sensitive units in rat. Brain Res. 1983, 265, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, H.; Ono, T.; Nishino, H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J. Neurosci. 1988, 8, 3570–3583. [Google Scholar] [CrossRef]
- Fotakos, D.; Hideg, B.; Szabo, I.; Szalay, C.; Takacs, G.; Nagy, B.; Karadi, Z. The effect of gustatory and intragastric chemicals stimulation on glucose-monitoring neurons in the cingulate cortex of the rat. In Proceedings of the 7th FENS Forum of European Neuroscience, Amsterdam, The Netherlands, 3–7 July 2010; p. 195. [Google Scholar]
- Hormay, E.; Csetenyi, B.; Szabo, I.; Nagy, B.; Torda, V.; Tóth, M.; Karadi, Z. Glucose-monitoring neurons of the rat cingulate cortex: Feeding and metabolic significance. In Obesitologia Hungarica; Hungarian Society for the Study of Obesity: Hungary, Budapest, 2015; p. 57. ISSN 1586-7935. Available online: http://elhizastudomany.hu/obesitologia-hungarica-2015-14-supplementum-2-s1-s92/ (accessed on 24 October 2024).
- Neafsey, E.J.; Terreberry, R.R.; Hurley, K.M.; Ruit, K.G.; Frysztak, R.J. Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In Neurobiology of Cingulate Cortex and Limbic Thalamus; Springer: Berlin/Heidelberg, Germany, 1993; pp. 206–223. [Google Scholar]
- Binder, M.D.; Hirokawa, N.; Windhorst, U. Encyclopedia of Neuroscience, 1st ed.; Windhorst, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 125, pp. 721–726. [Google Scholar]
- Bush, G.; Vogt, B.A.; Holmes, J.; Dale, A.M.; Greve, D.; Jenike, M.A.; Rosen, B.R. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. USA 2002, 99, 523–528. [Google Scholar] [CrossRef]
- Brockett, A.T.; Tennyson, S.S.; deBettencourt, C.A.; Gaye, F.; Roesch, M.R. Anterior cingulate cortex is necessary for adaptation of action plans. Proc. Natl. Acad. Sci. USA 2020, 117, 6196–6204. [Google Scholar] [CrossRef]
- Gaspar, P.; Berger, B.; Febvret, A.; Vigny, A.; Henry, J.P. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J. Comp. Neurol. 1989, 279, 249–271. [Google Scholar] [CrossRef]
- Crino, P.B.; Morrison, J.H.; Hof, P.R. Monoaminergic innervation of cingulate cortex. In Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook; Springer: Berlin/Heidelberg, Germany, 1993; pp. 285–310. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Baik, J.H. Dopaminergic Control of the Feeding Circuit. Endocrinol. Metab. 2021, 36, 229–239. [Google Scholar] [CrossRef]
- Thanarajah, S.E.; Backes, H.; DiFeliceantonio, A.G.; Albus, K.; Cremer, A.L.; Hanssen, R.; Lippert, R.N.; Cornely, O.A.; Small, D.M.; Bruning, J.C.; et al. Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metab. 2019, 29, 695–706.e694. [Google Scholar] [CrossRef]
- Yu, Y.; Miller, R.; Groth, S.W. A literature review of dopamine in binge eating. J. Eat. Disord. 2022, 10, 11. [Google Scholar] [CrossRef]
- Mura, E.; Taruno, A.; Yagi, M.; Yokota, K.; Hayashi, Y. Innate and acquired tolerance to bitter stimuli in mice. PLoS ONE 2018, 13, e0210032. [Google Scholar] [CrossRef]
- Fontanini, A.; Grossman, S.E.; Figueroa, J.A.; Katz, D.B. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J. Neurosci. 2009, 29, 2486–2495. [Google Scholar] [CrossRef]
- Staszko, S.M.; Boughter, J.D., Jr.; Fletcher, M.L. Taste coding strategies in insular cortex. Exp. Biol. Med. 2020, 245, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Jezzini, A.; Mazzucato, L.; La Camera, G.; Fontanini, A. Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J. Neurosci. 2013, 33, 18966–18978. [Google Scholar] [CrossRef] [PubMed]
- Boughter, J.D., Jr.; Lu, L.; Saites, L.N.; Tokita, K. Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus. Brain Res. 2019, 1714, 99–110. [Google Scholar] [CrossRef]
- Rolls, E.T. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol. Hung. 2008, 95, 131–164. [Google Scholar] [CrossRef] [PubMed]
- Berta, B.; Kertes, E.; Peczely, L.; Ollmann, T.; Laszlo, K.; Galosi, R.; Kallai, V.; Petyko, Z.; Zagoracz, O.; Kovacs, A.; et al. Ventromedial prefrontal cortex is involved in preference and hedonic evaluation of tastes. Behav. Brain Res. 2019, 367, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.D.; Christmas, D.; Eljamel, M.S.; Matthews, K. Anterior cingulotomy for major depression: Clinical outcome and relationship to lesion characteristics. Biol. Psychiatry 2008, 63, 670–677. [Google Scholar] [CrossRef]
- Vogt, B.A.; Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 2014, 219, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Foltz, E.L.; White, L.E., Jr. Pain “relief” by frontal cingulumotomy. J. Neurosurg. 1962, 19, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, G. Cingulotomy for Depression and OCD. In Textbook of Stereotactic and Functional Neurosurgery; Springer: Berlin/Heidelberg, Germany, 2009; pp. 2887–2896. [Google Scholar]
- Seamans, J.K.; Floresco, S.B. Event-based control of autonomic and emotional states by the anterior cingulate cortex. Neurosci. Biobehav. Rev. 2022, 133, 104503. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Lighthill, J.A. Selective Anterior Cingulotomy: A Psychosurgical Evaluation. J. Neurosurg. 1968, 29, 513. [Google Scholar] [CrossRef]
- Kistenmacher, A.; Manneck, S.; Wardzinski, E.K.; Martens, J.C.; Gohla, G.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Persistent blood glucose reduction upon repeated transcranial electric stimulation in men. Brain Stimul. 2017, 10, 780–786. [Google Scholar] [CrossRef]
- de Ceballos, M.L.; Kofalvi, A. Boosting brain glucose metabolism to fight neurodegeneration? Oncotarget 2017, 8, 14273–14274. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Purkayastha, S.; Yan, J.; Cai, D. Control of obesity and glucose intolerance via building neural stem cells in the hypothalamus. Mol. Metab. 2014, 3, 313–324. [Google Scholar] [CrossRef]
- Miller, M.W.; Powrozek, T.A.; Vogt, B.A. Dopamine Systems in the Cingulate Gyrus: Organization, Development, and Neurotoxic Vulnerability. In Cingulate Neurobiology and Disease; Vogt, B.A., Ed.; Oxford University Press: Oxford, UK, 2009; pp. 163–188. [Google Scholar]










| Dopamine | |||
|---|---|---|---|
| ± | ø | Total | |
| GM | 7 | 8 | 15 |
| GIS | 10 | 67 | 77 |
| Total | 17 | 75 | 92 |
| ±: excitatory or inhibitory response | |||
| ø: no response | χ(1) = 9.454, p < 0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hormay, E.; László, B.; Szabó, I.; Mintál, K.; Berta, B.; Ollmann, T.; Péczely, L.; Nagy, B.; Tóth, A.; László, K.; et al. Dopamine-Sensitive Anterior Cingulate Cortical Glucose-Monitoring Neurons as Potential Therapeutic Targets for Gustatory and Other Behavior Alterations. Biomedicines 2024, 12, 2803. https://doi.org/10.3390/biomedicines12122803
Hormay E, László B, Szabó I, Mintál K, Berta B, Ollmann T, Péczely L, Nagy B, Tóth A, László K, et al. Dopamine-Sensitive Anterior Cingulate Cortical Glucose-Monitoring Neurons as Potential Therapeutic Targets for Gustatory and Other Behavior Alterations. Biomedicines. 2024; 12(12):2803. https://doi.org/10.3390/biomedicines12122803
Chicago/Turabian StyleHormay, Edina, Bettina László, István Szabó, Kitti Mintál, Beáta Berta, Tamás Ollmann, László Péczely, Bernadett Nagy, Attila Tóth, Kristóf László, and et al. 2024. "Dopamine-Sensitive Anterior Cingulate Cortical Glucose-Monitoring Neurons as Potential Therapeutic Targets for Gustatory and Other Behavior Alterations" Biomedicines 12, no. 12: 2803. https://doi.org/10.3390/biomedicines12122803
APA StyleHormay, E., László, B., Szabó, I., Mintál, K., Berta, B., Ollmann, T., Péczely, L., Nagy, B., Tóth, A., László, K., Lénárd, L., & Karádi, Z. (2024). Dopamine-Sensitive Anterior Cingulate Cortical Glucose-Monitoring Neurons as Potential Therapeutic Targets for Gustatory and Other Behavior Alterations. Biomedicines, 12(12), 2803. https://doi.org/10.3390/biomedicines12122803






