Pathology of Red Blood Cells in Patients with SARS-CoV-2
Abstract
1. Introduction
2. Methods
2.1. Virus
2.2. Patients
- Patients diagnosed with SARS-CoV-2 within positive SARS-CoV-2 qRT-PCR.
- Residents of the National Centre of Infectious Diseases, Ministry of Health, Republic of Armenia.
- Age of patients 18 years and older.
- Negative pregnancy test for females.
- Informed consent is required from the patient or his/her representative to participate in the study.
- The patient should be able to comply with all the requirements of the clinical trial (including home follow-up during isolation).
- Known history of allergy.
- Positive IgG to SARS-CoV-2 was obtained prior to testing.
- Any of the following comorbidities (or any other condition that may interfere with the study): Immunosuppression. Chronic obstructive pulmonary disease. Obesity. Acute or chronic renal insufficiency. History of severe coronary disease. History of cerebrovascular disease. Current neoplasm.
2.3. Clinical Criteria
2.4. Laboratory Measurements
2.5. Blood Smears, Giemsa Staining, and Nucleated Blood Cells Analysis
2.6. Microspectrophotometry
2.7. Statistical Analysis
3. Results
3.1. Basic Characteristics of the Patients
3.2. Laboratory Results
3.3. Routine Blood Test and Erythroblastosis
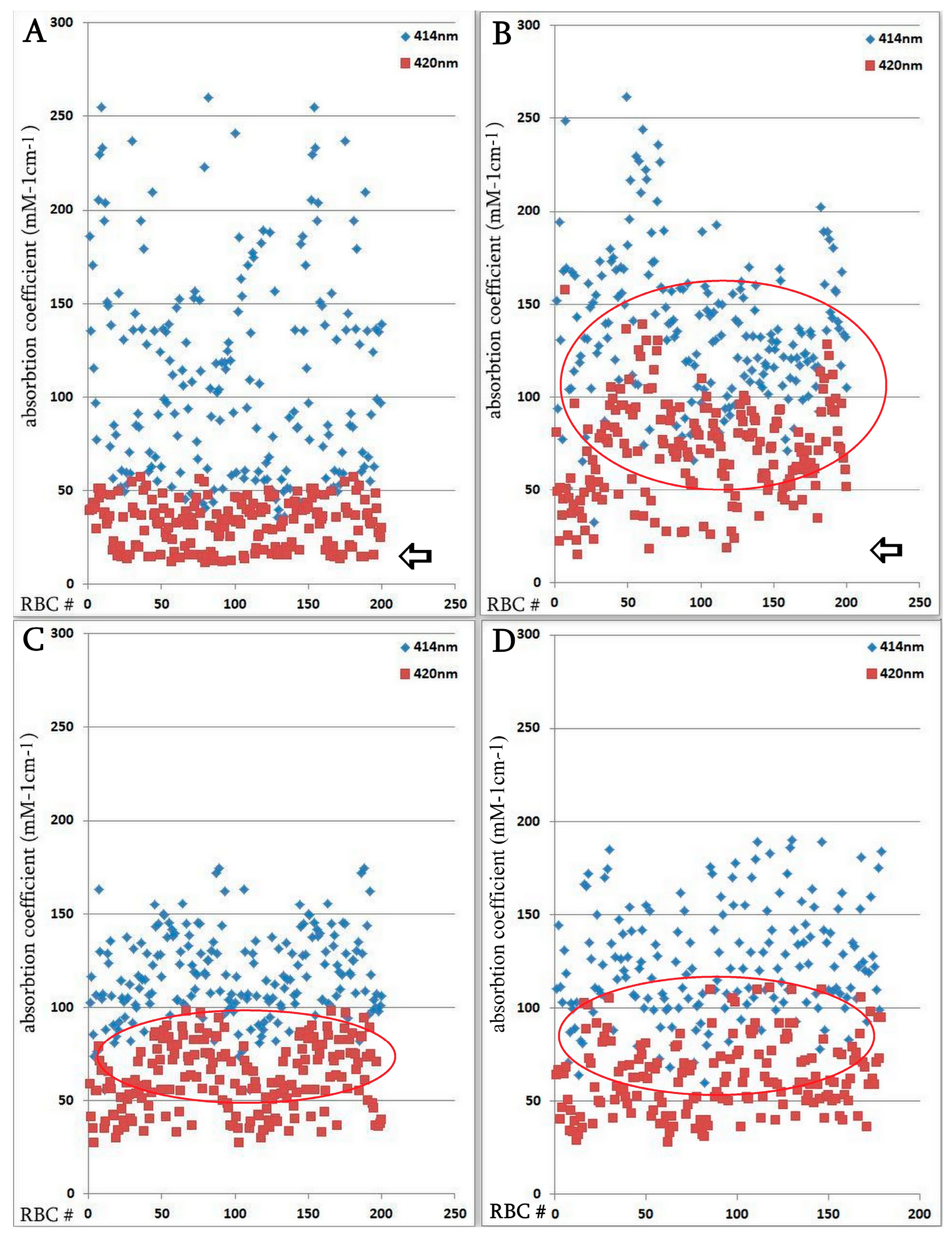
3.4. Influence of Coronavirus Infection on Erythrocyte Parameters, Determined by Microspectrophotometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Zarandi, P.K.; Zinatizadeh, M.R.; Zinatizadeh, M.; Yousefi, M.H.; Rezaei, N. SARS-CoV-2: From the pathogenesis to potential anti-viral treatments. Biomed. Pharmacother. 2021, 137, 111352. [Google Scholar] [CrossRef] [PubMed]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Demeester, S.; Demuyser, T.; Fauconnier, C.; Heestermans, R.; Orlando, C.; Depreter, B.; Jochmans, K. Routine haematology parameters in COVID-19 patients and clinical outcome: A Belgian single-centre study. Int. J. Lab. Hematol. 2020, 42, e252–e255. [Google Scholar] [CrossRef]
- Lee, W.S.; Margolskee, E. Leukoerythroblastosis and plasmacytoid lymphocytes in a child with SARS-CoV-2-associated multisystem inflammatory syndrome. Blood 2020, 136, 914. [Google Scholar] [CrossRef]
- Mitra, A.; Dwyre, D.M.; Schivo, M.; Thompson, G.R., 3rd; Cohen, S.H.; Ku, N.; Graff, J.P. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am. J. Hematol. 2020, 95, 999–1000. [Google Scholar] [CrossRef]
- Avetyan, D.; Hakobyan, S.; Nikoghosyan, M.; Ghukasyan, L.; Khachatryan, G.; Sirunyan, T.; Muradyan, N.; Zakharyan, R.; Chavushyan, A.; Hayrapetyan, V.; et al. Molecular Analysis of SARS-CoV-2 Lineages in Armenia. Viruses 2022, 14, 1074. [Google Scholar] [CrossRef]
- Avagyan, H.; Hakobyan, S.; Poghosyan, A.; Hakobyan, L.; Abroyan, L.; Karalova, E.; Avetisyan, A.; Sargsyan, M.; Baghdasaryan, B.; Bayramyan, N.; et al. Severe Acute Respiratory Syndrome Coronavirus-2 Delta Variant Study In Vitro and Vivo. Curr. Issues Mol. Biol. 2022, 45, 249–267. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brecher, G.; Bull, B. Ferritin localization on the erythroblast cell membrane and ropheocytosis in hypersiderotic human bone marrows. Blood 1966, 28, 758–769. [Google Scholar] [CrossRef]
- Yuan, K.; Zheng, Y.B.; Wang, Y.J.; Sun, Y.K.; Gong, Y.M.; Huang, Y.T.; Chen, X.; Liu, X.X.; Zhong, Y.; Su, S.Z.; et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: A call to action. Mol. Psychiatry 2022, 27, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, K.; Shiraishi, T.; Kakinuma, K. Microspectrophotometry of nitric oxide-dependent changes in hemoglobin in single red blood cells incubated with stimulated macrophages. J. Biochem. 1997, 122, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 215–220. [Google Scholar] [CrossRef]
- Baccard-Longere, M.; Freymuth, F.; Cointe, D.; Seigneurin, J.M.; Grangeot-Keros, L. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 2001, 8, 429–431. [Google Scholar] [CrossRef]
- Constantino, B.T.; Cogionis, B. Nucleated RBCs—Significance in the Peripheral Blood Film. Lab. Med. 2000, 31, 223–229. [Google Scholar] [CrossRef]
- Liu, W.; Li, H. COVID-19: Captures iron and generates reactive oxygen species to damage the human immune system. Autoimmunity 2021, 54, 213–224. [Google Scholar] [CrossRef]
- Torti, L.; Maffei, L.; Sorrentino, F.; De Fabritiis, P.; Miceli, R.; Abruzzese, E. Impact of SARS-CoV-2 in Hemoglobinopathies with Immune Disfunction and Epidemiology. A Protective Mechanism from Beta Chain Hemoglobin Defects? Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020052. [Google Scholar] [CrossRef]
- Encabo, H.H.; Grey, W.; Garcia-Albornoz, M.; Wood, H.; Ulferts, R.; Aramburu, I.V.; Kulasekararaj, A.G.; Mufti, G.; Papayannopoulos, V.; Beale, R.; et al. Human Erythroid Progenitors Are Directly Infected by SARS-CoV-2: Implications for Emerging Erythropoiesis in Severe COVID-19 Patients. Stem Cell Rep. 2021, 16, 428–436. [Google Scholar] [CrossRef]
- Allegra, A.; Di Gioacchino, M.; Tonacci, A.; Musolino, C.; Gangemi, S. Immunopathology of SARS-CoV-2 infection: Immune cells and mediators, prognostic factors, and immune-therapeutic implications. Int. J. Mol. Sci. 2020, 21, E4782. [Google Scholar] [CrossRef]
- Kosenko, E.; Tikhonova, L.; Alilova, G.; Montoliu, C. Erythrocytes Functionality in SARS-CoV-2 Infection: Potential Link with Alzheimers Disease. Int. J. Mol. Sci. 2023, 24, 5739. [Google Scholar] [CrossRef]
- Mawatari, K.; Matsukawa, S.; Yoneyama, Y. Different effects of subunit association upon absorption and circular dichroism spectra of methemoglobin. Biochim. Biophys. Acta 1983, 745, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Spolitak, T.; Hollenberg, P.F.; Ballou, D.P. Oxidative hemoglobin reactions: Applications to drug metabolism. Arch. Biochem. Biophys. 2016, 600, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Gozalbo-Rovira, R.; Gimenez, E.; Latorre, V.; Frances-Gomez, C.; Albert, E.; Buesa, J.; Marina, A.; Blasco, M.L.; Signes-Costa, J.; Rodriguez-Diaz, J.; et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J. Clin. Virol. 2020, 131, 104611. [Google Scholar] [CrossRef] [PubMed]
- Lega, S.; Naviglio, S.; Volpi, S.; Tommasini, A. Recent insight into SARS-CoV2 immunopathology and rationale for potential treatment and preventive strategies in COVID-19. Vaccines 2020, 8, E224. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Taneri, P.E.; Gómez-Ochoa, S.A.; Llanaj, E.; Raguindin, P.F.; Rojas, L.Z.; Roa-Díaz, Z.M.; Salvador, D.; Groothof, D.; Minder, B.; Kopp-Heim, D.; et al. Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 763–773. [Google Scholar] [CrossRef]
- Patel, K.V.; Ferrucci, L.; Ershler, W.B.; Longo, D.L.; Guralnik, J.M. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch. Intern. Med. 2009, 169, 515–523. [Google Scholar] [CrossRef]
- Emans, M.E.; Gaillard, C.A.; Pfister, R.; Tanck, M.W.; Boekholdt, S.M.; Wareham, N.J.; Khaw, K.T. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int. J. Cardiol. 2013, 168, 3550–3555. [Google Scholar] [CrossRef]



| Characteristic | Regular Group (n = 35) | Severe Group (n = 18) | Low Saturation Group (n = 12) | Erythroblastosis Group (n = 9) |
|---|---|---|---|---|
| Age (year) | 61 ± 4.5 | 58 ± 3.3 | 56 ± 4.8 | 57 ± 5.5 |
| Male sex (%, N) | 51.4 (18) | 50.0 (9) | 50.0 (6) | 55.5 (5) |
| Symptoms (%, N) | ||||
| Fever | 80% (28) | 94.5% (17) | 16.7 (2) | 66.7% (6) |
| Cough | 77.1% (27) | 22.2% (4) | 75% (8) | 22.2% (2) |
| Loss of smell/taste | 57.1 (20) | 16.7% (3) | 16.7% (2) | - |
| Shortness of breath | 22.8% (8) | 16.7% (3) | 50% (6) | 22.2% (2) |
| O2 saturation | 92.2 ± 2.8 | 88.5 ± 4.9 | 75.7 ± 5.3 | 91.2 ± 5.1 |
| Anorexia | 8.7% (3) | 5.5% (1) | - | - |
| Diarrhea | 5.7% (2) | 5.5% (1) | - | - |
| Fatigue | 82.9% (29) | 22.2% (4) | 83.3% (10) | 77.8% (7) |
| Myalgia or arthralgia | 28.6% (10) | 16.7% (3) | 25% (3) | 44.4% (4) |
| Coexisting disorder (%, N) | ||||
| Hypertension | 42.9% (15) | 38.8% (7) | 33.3% (4) | 44.4% (4) |
| Diabetes | 31.4% (11) | 27.8% (5) | 25% (3) | 22.2% (2) |
| Coronary heart disease | 8.7% (3) | 5.5% (1) | 8.3% (1) | 22.2% (2) |
| Cerebrovascular disease | - | 5.5% (1) | - | - |
| Chronic renal disease | 2.9% (1) | 5.5% (1) | - | - |
| Malignant tumor | 5.7% (2) | 5.5% (1) | - | - |
| other coexisting chronic disorder | 20% (7) | 11.1% (2) | 8.3% (1) | - |
| Ferritin level (ng/mL) | 87.6–346 | 104.8–2929 * | 59.8–894 | 87–285 |
| CRP (mg/L) | 46.5 (8–81) | 89.3 (21–231) | 47.9 (14–95) | 19.1 (7–72) |
| Blood Cells | Regular Group (n = 35) * | Severe Group (n = 18) ** | Low Saturation Group (n = 12) *** | Erythroblastosis Group (n = 9) |
|---|---|---|---|---|
| Basophilic erythroblast | - | 0.1 | - | 0.7 |
| Polychromatophilic erythroblast | - | 0.1 | - | 0.7 |
| Acidophilic erythroblast | 0.1 | 0.3 | 0.1 | 0.1 |
| Lymphoblast | 1.8 ± 1.4 | 2.5 | 1.4 | 2.1 |
| Lymphocyte | 22.0 ± 6.4 | 25.3 | 28.8 | 28.5 |
| Lymphocyte aberrant | 1.1 ± 2.1 | 0.4 | 1.4 | 1.8 |
| Monoblast | 0.4 ± 0.7 | 0.3 | 0.4 | 0.7 |
| Monocyte | 2.1 ± 1.4 | 2.6 | 2.6 | 1.6 |
| Myeloid cell | 2.8 ± 2.5 | 1.6 | 3.7 | 1.3 |
| Metamyelocyte | 14.7 ± 7.4 | 16.3 | 17.7 | 12.2 |
| Band neutrophil | 39.9 ± 9.6 | 36.8 | 34.4 | 39.1 |
| Segmented neutrophil | 12.3 ± 1.1 | 10.9 | 7.7 | 8.2 |
| Pathological neutrophil | 0.8 ± 0.9 | 0.7 | 0.7 | 1.6 |
| Eosinophil | 0.4 ± 0.2 | 0.9 | 0.2 | 0.5 |
| Basophil | 0.1 ± 0.2 | 0.1 | 0.0 | 0.1 |
| Destructed cells | 1.3 ± 1.2 | 1.1 | 1.0 | 0.9 |
| Disease Severity | Erythroblastosis | Ferritin | CRP | Increased Hb Absorption on 420 nm (%) | Anisocytosis (%) | Decreased Hb in Erythrocyte (%) | |
|---|---|---|---|---|---|---|---|
| disease severity | 1.000 | 0.316 | 1.000 * | 1.000 * | 0.800 | 1.000 * | −0.105 |
| Erythroblastosis | 0.316 | 1.000 | 0.316 | 0.316 | 0.632 | 0.316 | 0.833 |
| ferritin levels in serum | 1.000 * | 0.316 | 1.000 | 1.000 * | 0.800 ** | 1.000 * | −0.105 |
| CRP levels in serum | 1.000 * | 0.316 | 1.000 * | 1.000 | 0.800 ** | 1.000 * | −0.105 |
| increase in Hb absorption on 420 nm (%) | 0.800 ** | 0.632 | 0.800 ** | 0.800 ** | 1.000 | 0.800 ** | 0.105 |
| anisocytosis (%) | 1.000 * | 0.316 | 1.000 * | 1.000 * | 0.800 ** | 1.000 | −0.105 |
| decreased Hb amount in erythrocyte (%) | −0.105 | 0.833 | −0.105 | −0.105 | 0.105 | −0.105 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakobyan, S.; Hakobyan, L.; Abroyan, L.; Avetisyan, A.; Avagyan, H.; Bayramyan, N.; Niazyan, L.; Davidyants, M.; Sargsyan, K.; Ghalechyan, T.; et al. Pathology of Red Blood Cells in Patients with SARS-CoV-2. Biomedicines 2025, 13, 191. https://doi.org/10.3390/biomedicines13010191
Hakobyan S, Hakobyan L, Abroyan L, Avetisyan A, Avagyan H, Bayramyan N, Niazyan L, Davidyants M, Sargsyan K, Ghalechyan T, et al. Pathology of Red Blood Cells in Patients with SARS-CoV-2. Biomedicines. 2025; 13(1):191. https://doi.org/10.3390/biomedicines13010191
Chicago/Turabian StyleHakobyan, Sona, Lina Hakobyan, Liana Abroyan, Aida Avetisyan, Hranush Avagyan, Nane Bayramyan, Lyudmila Niazyan, Mher Davidyants, Knarik Sargsyan, Tehmine Ghalechyan, and et al. 2025. "Pathology of Red Blood Cells in Patients with SARS-CoV-2" Biomedicines 13, no. 1: 191. https://doi.org/10.3390/biomedicines13010191
APA StyleHakobyan, S., Hakobyan, L., Abroyan, L., Avetisyan, A., Avagyan, H., Bayramyan, N., Niazyan, L., Davidyants, M., Sargsyan, K., Ghalechyan, T., Semerjyan, A., Karalova, E., & Karalyan, Z. (2025). Pathology of Red Blood Cells in Patients with SARS-CoV-2. Biomedicines, 13(1), 191. https://doi.org/10.3390/biomedicines13010191






