Liver Elastography for Liver Fibrosis Stratification: A Comparison of Three Techniques in a Biopsy-Controlled MASLD Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Two-Dimensional Shear Wave Elastography
2.3. Transient Elastography
2.4. S-Shearwave Elastography
2.5. Liver Biopsy and Histopathologic Evaluation
2.6. Statistical Analysis
3. Results
3.1. Clinical, Histological, and Ultrasound Characteristics of the Study Population
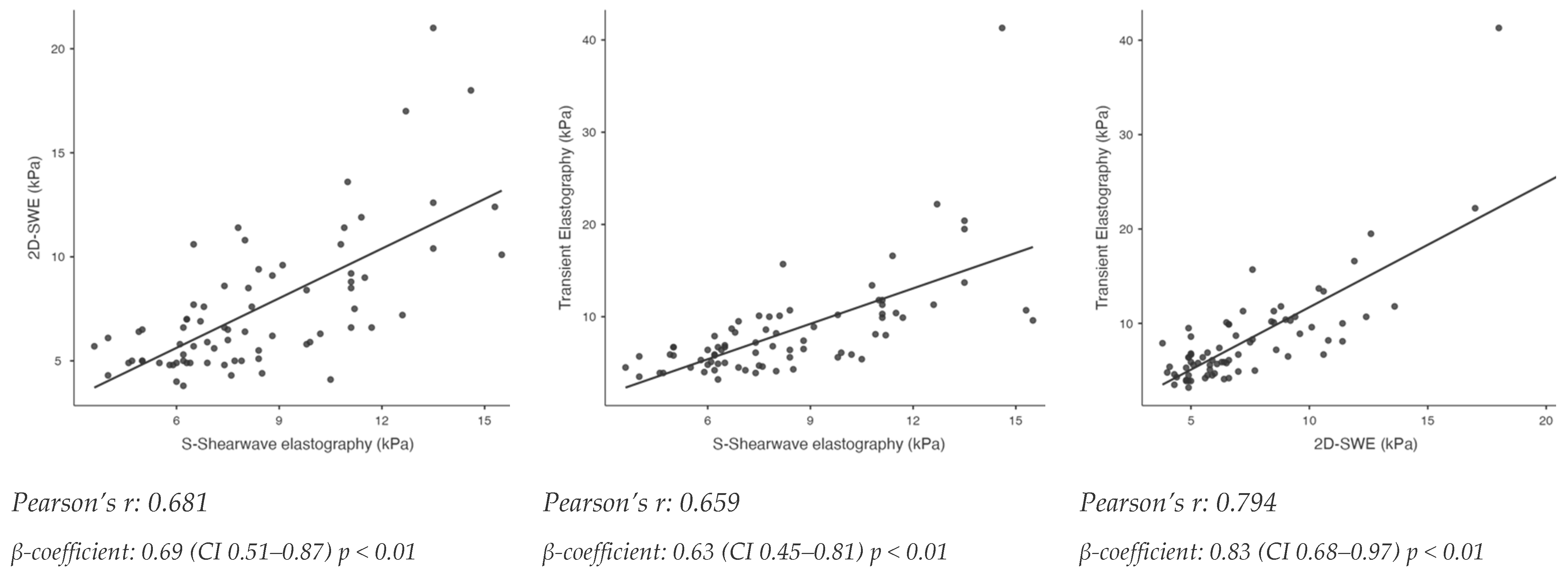
3.2. Relationship Between S-Shearwave Elastography, 2D-Shear Wave Elastography and Transient Elastography and Association to Histological Liver Fibrosis
3.3. Performances of S-Shearwave Elastography, 2D-Shear Wave Elastography and Transient Elastography for Detection of Significant Fibrosis (F ≥ 2) and Advanced Fibrosis (F ≥ 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Bureau, C.; Stefanescu, H.; Augustin, S.; Ney, M.; Blasco, H.; Procopet, B.; Bosch, J.; Genesca, J.; Berzigotti, A.; et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology 2016, 64, 2173–2184. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bissell, D.M. Noninvasive measures of liver fibrosis. Hepatology 2006, 43 (Suppl. 1), S113–S120. [Google Scholar] [CrossRef]
- Papastergiou, V.; Tsochatzis, E.; Burroughs, A.K. Non-invasive assessment of liver fibrosis. Ann. Gastroenterol. 2012, 25, 218–231. [Google Scholar] [PubMed]
- Kaposi, P.N.; Unger, Z.; Fejér, B.; Kucsa, A.; Tóth, A.; Folhoffer, A.; Szalay, F.; Bérczi, V. Interobserver agreement and diagnostic accuracy of shearwave elastography for the staging of hepatitis C virus-associated liver fibrosis. J. Clin. Ultrasound JCU 2020, 48, 67–74. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, J.M.; Joo, I.; Yoon, J.H. Assessment of liver fibrosis using 2-dimensional shear wave elastography: A prospective study of intra- and inter-observer repeatability and comparison with point shear wave elastography. Ultrasonography 2020, 39, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Kim, S.G.; Jang, J.Y.; Yoo, J.-J.; Jeong, S.W.; Kim, Y.S.; Kim, B.S. Two-dimensional shear wave elastography for assessing liver fibrosis in patients with chronic liver disease: A prospective cohort study. Korean J. Intern. Med. 2021, 37, 285–293. [Google Scholar] [CrossRef]
- He, R.; Liu, C.; Grgurevic, I.; Guo, Y.; Xu, H.; Liu, J.; Liu, Y.; Wang, X.; Shi, H.; Madir, A.; et al. Validation of Baveno VII criteria for clinically significant portal hypertension by two-dimensional shear wave elastography. Hepatol. Int. 2024, 18, 1020–1028. [Google Scholar] [CrossRef]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Duarte-Rojo, A.; Patel, K.; Asrani, S.K.; Alsawas, M.; Dranoff, J.A.; Fiel, M.I.; Murad, M.H.; Leung, D.H.; Levine, D.; et al. AASLD Practice Guideline on imaging-based noninvasive liver disease assessment of hepatic fibrosis and steatosis. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Auteri, G.; Sansone, V.; Bartoletti, D.; Di Pietro, C.; Sutto, E.; Mazzoni, C.; Vianelli, N.; Cavo, M.; Piscaglia, F.; Palandri, F. Spleen and Liver Fibrosis Is Associated to Treatment Response and Prognosis in Philadelphia-Negative Chronic Myeloproliferative Neoplasms. Blood 2021, 138 (Suppl. 1), 3626. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Foucquier, J.; Younossi, Z.M.; Harrison, S.A.; Newsome, P.N.; Chan, W.-K.; Yilmaz, Y.; De Ledinghen, V.; Costentin, C.; Zheng, M.-H.; et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J. Hepatol. 2022, 78, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Barr, R.G.; Berzigotti, A.; Sporea, I.; Wong, V.W.; Reiberger, T.; Karlas, T.; Thiele, M.; Cardoso, A.C.; Ayonrinde, O.T.; et al. WFUMB Guideline/Guidance on Liver Multiparametric Ultrasound: Part 1. Update to 2018 Guidelines on Liver Ultrasound Elastography. Ultrasound Med. Biol. 2024, 50, 1071–1087. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Herrmann, E.; De Lédinghen, V.; Cassinotto, C.; Chu, W.C.W.; Leung, V.Y.-F.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, E.A.; Mózes, F.E.; Jayaswal, A.N.A.; Zafarmand, M.H.; Vali, Y.; Lee, J.A.; Levick, C.K.; Young, L.A.J.; Palaniyappan, N.; Liu, C.-H.; et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2021, 75, 770–785. [Google Scholar] [CrossRef] [PubMed]


| Entire Cohort | F < 3 | F ≥ 3 | p Value * | |
| n = 75 | n = 52 | n = 23 | ||
| Gender (male) | 42 (56%) | 31 (59.6%) | 11 (47.8%) | 0.34 |
| Age (years) | 52 (44–56) | 50 (39–55) | 54 (51–58) | 0.01 |
| BMI (kg/m2) | 31.1 (28.1–37.8) | 30.4 (27.7–35.4) | 33.6 (29.1–40.7) | 0.07 |
| Obesity | 41 (54.7%) | 26 (50%) | 15 (65.2%) | 0.22 |
| Diabetes | 21 (28%) | 10 (19.2%) | 11 (47.8%) | 0.01 |
| Hypertension | 30 (40%) | 13 (25%) | 17 (73.9%) | <0.01 |
| Biochemical Profile | ||||
| Platelet count (×106/mL) | 233 (187–268) | 239 (202–281) | 198 (165–238) | <0.01 |
| AST (IU/L) | 34 (24–50) | 33 (24–45) | 42 (24–52) | 0.94 |
| ALT (IU/L) | 51 (29–72) | 51 (28–75) | 51 (30–61) | 0.47 |
| GGT (IU/L) | 65 (29–100) | 47 (30–92) | 66 (30–124) | 0.85 |
| ALP (IU/L) | 66 (53–88) | 64 (41–87) | 70 (57–89) | 0.95 |
| Albumin (g/L) | 41 (38–43) | 42 (41–43) | 38 (37–39) | <0.01 |
| Triglycerides (mg/dL) | 140 (93–89) | 142 (96–189) | 133 (90–187) | 0.92 |
| Total Cholesterol (mg/dL) | 199 (173–221) | 204 (177–229) | 184 (165–208) | 0.02 |
| HDL (mg/dL) | 45 (33–50) | 45 (33–50) | 40 (34–49) | 0.20 |
| FIB4 | 1.12 (0.78–1.77) | 0.92 (0.73–1.37) | 1.52 (1.10–2.32) | 0.03 |
| NFS | −1.31 (−2.59–0.20) | −1.96 (−3.01–−1.18) | 0.20 (−0.85–0.84) | <0.01 |
| Agile3+ | 0.17 (0.06–0.57) | 0.09 (0.05–0.21) | 0.67 (0.52–0.79) | <0.01 |
| Histologic Findings | ||||
| Steatosis Grade | 0.45 | |||
| 1 | 30 (40%) | 23 (44.2%) | 7 (30.4%) | |
| 2 | 25 (33.3%) | 17 (32.7%) | 8 (34.7%) | |
| 3 | 20 (26.7%) | 12 (23.1%) | 8 (34.7%) | |
| Lobular Inflammation Grade | 0.21 | |||
| 0 | 4 (5.3%) | 4 (7.7%) | 0 | |
| 1 | 55 (73.3%) | 39 (75%) | 16 (69.6%) | |
| 2 | 16 (21.3%) | 9 (17.3%) | 7 (30.4%) | |
| 3 | 0 | 0 | 0 | |
| Hepatocyte Ballooning Grade | 0.03 | |||
| 0 | 12 (16%) | 12 (23.1%) | 0 | |
| 1 | 54 (72%) | 35 (67.3%) | 19 (82.6%) | |
| 2 | 9 (12%) | 5 (9.6%) | 4 (17.4%) | |
| Fibrosis Stage | <0.01 | |||
| 0 | 3 (4%) | 3 (5.7%) | 0 | |
| 1 | 29 (38.7%) | 29 (38.4%) | 0 | |
| 2 | 20 (26.7%) | 20 (38.5%) | 0 | |
| 3 | 19 (25.3%) | 0 | 19 (82.6%) | |
| 4 | 4 (5.3%) | 0 | 4 (17.4%) | |
| MASH | 50 (66.6%) | 30 (57.7%) | 20 (86.9%) | 0.01 |
| Elastographic measurements | ||||
| LSM by TE (kPa) | 6.7 (5.2–10.1) | 5.8 (4.6–6.7) | 10.7 (10.1–14.7) | <0.01 |
| S-Shearwave Samsung (kPa) | 7.7 (6.25–10.7) | 6.6 (6.0–7.9) | 11.1 (10.3–13.1) | <0.01 |
| 2D-SWE (kPa) | 6.5 (5.0–8.9) | 5.7 (4.9–6.6) | 9.4 (8.5–11.6) | <0.01 |
| CAP (dB/m) | 317 (259–370) | 316 (244–356) | 340 (277–380) | 0.06 |
| Att.PLUS (dB/cm/mHz) | 0.50 (0.42–0.61) | 0.49 (0.42–0.63) | 0.54 (0.44–0.59) | 0.87 |
| SSP.PLUS (m/s) | 1535 (1508–1565) | 1537 (1500–1570) | 1520 (1510–1552) | 0.60 |
| TAI (dB/cm/MHz) | 0.93 (0.80–1.06) | 0.93 (0.75–1.04) | 0.91 (0.82–1.10) | 0.86 |
| TSI | 96.7 (91.0–102.0) | 96.8 (90.7–102.4) | 96.3 (91.3–102.1) | 0.60 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | p Value | OR (CI 95%) | p Value | |
| Sex (male) | 0.62 (0.23–1.67) | 0.34 | ||
| Age | 1.07 (1.01–1.14) | 0.02 | 1.05 (0.94–1.17) | 0.41 |
| Obesity | 1.87 (0.68–5.18) | 0.22 | ||
| Triglycerides | 1.00 (0.99–1.01) | 0.92 | ||
| Total Cholesterol | 0.98 (0.97–0.99) | 0.03 | ||
| Platelets | 0.98 (0.97–0.99) | <0.01 | 0.98 (0.96–1.01) | 0.10 |
| Diabetes | 3.85 (1.32–11.22) | 0.01 | 3.44 (0.49–24.4) | 0.21 |
| Hypertension | 8.5 (2.76–26.1) | <0.01 | ||
| ALT | 0.99 (0.98–1.01) | 0.48 | ||
| AST | 1.00 (0.98–1.01) | 0.94 | ||
| S-Shearwave Samsung (kPa) | 2.71 (1.75–4.20) | <0.01 | 2.94 (1.69 –5.11) | <0.01 |
| 2D-SWE (kPa) | 1.90 (1.40–2.58) | <0.01 | ||
| LSM by TE (kPa) | 4.18 (1.95–8.95) | <0.01 | ||
| Variable | Cut-Off Value | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | De Long p Value |
|---|---|---|---|---|---|---|---|
| F ≥ 2 | |||||||
| LS by TE (kPa) | 8.0 | 0.813 (0.715–0.910) | 62.7 | 97.5 | 87.1 | 63.4 | Ref |
| S-Shearwave Samsung (kPa) | 7.9 | 0.810 (0.706–0.913) | 74.4 | 87.5 | 88.9 | 71.8 | 0.95 |
| 2D-SWE (kPa) | 7.2 | 0.703 (0.584–0.822) | 55.8 | 84.4 | 82.7 | 58.7 | 0.01 |
| FIB4 | 1.25 | 0.661 (0.537–0.785) | 51.1 | 78.1 | 75.8 | 54.3 | 0.02 |
| NFS | −0.94 | 0.729 (0.609–0.848) | 60.5 | 84.4 | 83.9 | 61.4 | 0.13 |
| F ≥ 3 | |||||||
| LS by TE (kPa) | 8.9 | 0.979 (0.950–1.000) | 95.6 | 94.2 | 88.0 | 98.0 | Ref |
| 8.0 | 100 | 84.6 | 74.2 | 100 | |||
| 12.0 | 39.1 | 98.0 | 90.0 | 78.4 | |||
| S-Shearwave Samsung (kPa) | 8.1 | 0.942 (0.893–0.991) | 95.6 | 78.8 | 66.6 | 97.6 | 0.16 |
| 2D-SWE (kPa) | 7.6 | 0.906 (0.839–0.971) | 82.6 | 84.6 | 70.4 | 91.6 | 0.01 |
| FIB4 | 0.85 | 0.768 (0.659–0.876) | 100 | 46.1 | 45.1 | 100 | <0.01 |
| NFS | −0.94 | 0.833 (0.741–0.924) | 86.9 | 78.8 | 64.5 | 93.2 | <0.01 |
| Agile3+ | 0.24 | 0.944 (0.897–0.991) | 100 | 78.8 | 67.6 | 100 | 0.11 |
| ≤0.451 | 82.6 | 86.5 | 73.1 | 91.8 | |||
| ≥0.679 | 47.8 | 98.8 | 91.6 | 80.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, A.; Esposto, G.; Ainora, M.E.; Mignini, I.; Borriello, R.; Galasso, L.; Paratore, M.; Giustiniani, M.C.; Riccardi, L.; Garcovich, M.; et al. Liver Elastography for Liver Fibrosis Stratification: A Comparison of Three Techniques in a Biopsy-Controlled MASLD Cohort. Biomedicines 2025, 13, 138. https://doi.org/10.3390/biomedicines13010138
Liguori A, Esposto G, Ainora ME, Mignini I, Borriello R, Galasso L, Paratore M, Giustiniani MC, Riccardi L, Garcovich M, et al. Liver Elastography for Liver Fibrosis Stratification: A Comparison of Three Techniques in a Biopsy-Controlled MASLD Cohort. Biomedicines. 2025; 13(1):138. https://doi.org/10.3390/biomedicines13010138
Chicago/Turabian StyleLiguori, Antonio, Giorgio Esposto, Maria Elena Ainora, Irene Mignini, Raffaele Borriello, Linda Galasso, Mattia Paratore, Maria Cristina Giustiniani, Laura Riccardi, Matteo Garcovich, and et al. 2025. "Liver Elastography for Liver Fibrosis Stratification: A Comparison of Three Techniques in a Biopsy-Controlled MASLD Cohort" Biomedicines 13, no. 1: 138. https://doi.org/10.3390/biomedicines13010138
APA StyleLiguori, A., Esposto, G., Ainora, M. E., Mignini, I., Borriello, R., Galasso, L., Paratore, M., Giustiniani, M. C., Riccardi, L., Garcovich, M., Gasbarrini, A., Miele, L., & Zocco, M. A. (2025). Liver Elastography for Liver Fibrosis Stratification: A Comparison of Three Techniques in a Biopsy-Controlled MASLD Cohort. Biomedicines, 13(1), 138. https://doi.org/10.3390/biomedicines13010138






