Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Determination of Vitamin D
2.3. Determination of Tryptophan and Kynurenine
2.4. Determination of Pro-Inflammatory Cytokines
2.5. Determination of Advanced Oxidation Protein Products
2.6. Determination of SCFAs
2.7. Statistical Analysis
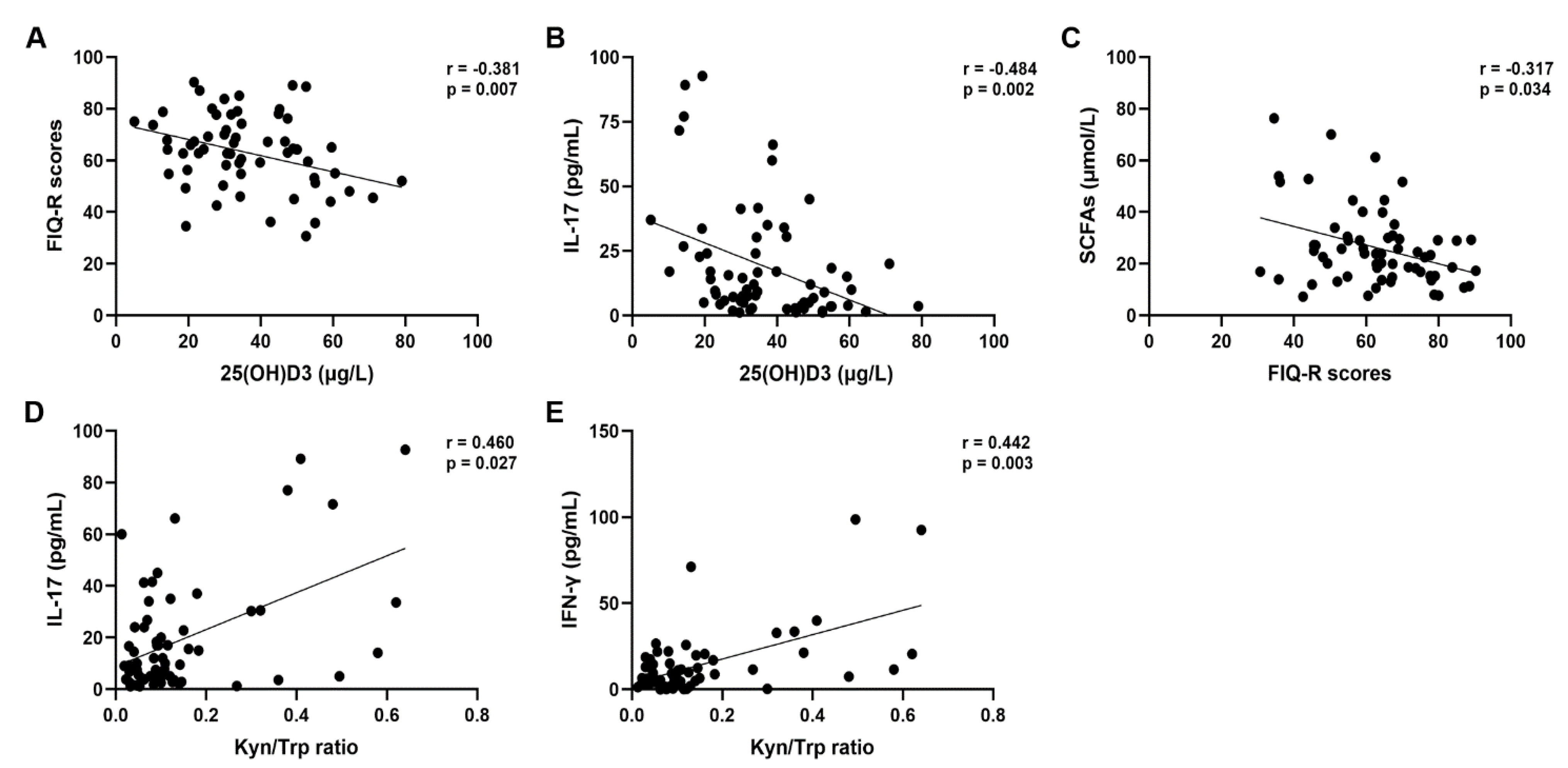
3. Results
3.1. Clusterization of Patients on the Basis of the FIQ-R Scores
3.2. Clusterization of Patients on the Basis of the Vitamin D Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clauw, D.J. Fibromyalgia: A Clinical Review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef]
- De Luca, C.; Scordo, M.G.; Cesareo, E.; Pastore, S.; Mariani, S.; Maiani, G.; Stancato, A.; Loreti, B.; Valacchi, G.; Lubrano, C.; et al. Biological Definition of Multiple Chemical Sensitivity from Redox State and Cytokine Profiling and Not from Polymorphisms of Xenobiotic-Metabolizing Enzymes. Toxicol. Appl. Pharmacol. 2010, 248, 285–292. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Gugliandolo, A.; Calabrò, C.; Currò, M.; Ientile, R.; Raskovic, D.; Korkina, L.; Caccamo, D. Role of Polymorphisms of Inducible Nitric Oxide Synthase and Endothelial Nitric Oxide Synthase in Idiopathic Environmental Intolerances. Mediat. Inflamm. 2015, 2015, 6–9. [Google Scholar] [CrossRef]
- Guggino, G.; Schinocca, C.; Lo Pizzo, M.; Di Liberto, D.; Garbo, D.; Raimondo, S.; Alessandro, R.; Brighina, F.; Ruscitti, P.; Giacomelli, R.; et al. T Helper 1 Response Is Correlated with Widespread Pain, Fatigue, Sleeping Disorders and the Quality of Life in Patients with Fibromyalgia and Is Modulated by Hyperbaric Oxygen Therapy. Clin. Exp. Rheumatol. 2019, 37, 81–89. [Google Scholar]
- Garofalo, C.; Cristiani, C.M.; Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Viglietto, G.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; et al. Fibromyalgia and Irritable Bowel Syndrome Interaction: A Possible Role for Gut Microbiota and Gut-Brain Axis. Biomedicines 2023, 11, 1701. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Atzeni, F.; Gorla, R.; Kosek, E.; Choy, E.H.; Bazzichi, L.; Häuser, W.; Ablin, J.N.; Aloush, V.; et al. Fibromyalgia position paper. Clin. Exp. Rheumatol. 2021, 39, 186–193. [Google Scholar] [CrossRef]
- Rus, A.; Robles-Fernandez, I.; Martinez-Gonzalez, L.J.; Carmona, R.; Alvarez-Cubero, M.J. Influence of Oxidative Stress-Related Genes on Susceptibility to Fibromyalgia. Nurs. Res. 2021, 70, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Dagostino, C.; Malafoglia, V.; Lauro, F.; Giancotti, L.A.; Spila, A.; Proietti, S.; Ventrice, D.; Rizzo, M.; Gliozzi, M.; et al. Protective Effect of Antioxidants in Nitric Oxide/Cox-2 Interaction during Inflammatory Pain: The Role of Nitration. Antioxidants 2020, 9, 1284. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant Modulation of Sirtuin 3 during Acute Inflammatory Pain: The ROS Control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota–Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef]
- Malafoglia, V.; Ilari, S.; Gioia, C.; Vitiello, L.; Tenti, M.; Iannuccelli, C.; Cristiani, C.M.; Garofalo, C.; Passacatini, L.C.; Viglietto, G.; et al. An Observational Study on Chronic Pain Biomarkers in Fibromyalgia and Osteoarthritis Patients: Which Role for Mu Opioid Receptor’s Expression on NK Cells? Biomedicines 2023, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut Microbiome and Serum Metabolome Analyses Identify Molecular Biomarkers and Altered Glutamate Metabolism in Fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Malatji, B.G.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Meyer, H.; van Reenen, M.; Reinecke, C.J. The GC-MS Metabolomics Signature in Patients with Fibromyalgia Syndrome Directs to Dysbiosis as an Aspect Contributing Factor of FMS Pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered Microbiome Composition in Individuals with Fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Visalli, V.; Colica, C.; Nisticò, R.; Palma, E.; Costa, N.; Rotiroti, D.; Nisticò, G.; Mollace, V. The Effect of Inflammatory Stimuli on NMDA-Related Activation of Glutamine Synthase in Human Cultured Astroglial Cells. Neurosci. Lett. 2005, 373, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Doyle, T.; Dagostino, C.; Bryant, L.; Chen, Z.; Watkins, L.R.; Ryerse, J.; Bieberich, E.; Neumman, W.; Salvemini, D. Counter-Regulation of Opioid Analgesia by Glial-Derived Bioactive Sphingolipids. J. Neurosci. 2010, 30, 15400–15408. [Google Scholar] [CrossRef]
- Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. Int. J. Mol. Sci. 2020, 21, 1499. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, A.; Reyes-Long, S.; Roldan-Valadez, E.; González-Torres, M.; Bonilla-Jaime, H.; Bandala, C.; Avila-Luna, A.; Bueno-Nava, A.; Cabrera-Ruiz, E.; Sanchez-Aparicio, P.; et al. Association of the Serotonin and Kynurenine Pathways as Possible Therapeutic Targets to Modulate Pain in Patients with Fibromyalgia. Pharmaceuticals 2024, 17, 1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.T.; Kang, J. Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia. Int. J. Environ. Res. Public. Health 2023, 20, 3183. [Google Scholar] [CrossRef]
- Tang, Y.; Du, J.; Wu, H.; Wang, M.; Liu, S.; Tao, F. Potential Therapeutic Effects of Short-Chain Fatty Acids on Chronic Pain. Curr. Neuropharmacol. 2022, 22, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Shipton, E.E.; Shipton, E.A. Vitamin D Deficiency and Pain: Clinical Evidence of Low Levels of Vitamin D and Supplementation in Chronic Pain States. Pain. Ther. 2015, 4, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, G.; Sanal-Toprak, C.; Yagci, I.; Giray, E.; Kuru-Bektasoglu, P. The Effect of Vitamin D Supplementation on Pain, Quality of Life, and Nerve Conduction Studies in Women with Chronic Widespread Pain. Int. J. Rehabil. Res. 2017, 40, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut Microbiota Interactions with the Immunomodulatory Role of Vitamin D in Normal Individuals. Metabolism. 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Sun, J. Dietary Vitamin D, Vitamin D Receptor, and Microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef]
- Caccamo, D.; Ferlazzo, N.; Currò, M.; Ricca, S.; Ientile, R. Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D. Med. Sci. 2018, 6, 10–15. [Google Scholar] [CrossRef]
- Currò, M.; Visalli, G.; Pellicanò, G.F.; Ferlazzo, N.; Costanzo, M.G.; D’andrea, F.; Caccamo, D.; Nunnari, G.; Ientile, R. Vitamin d Status Modulates Inflammatory Response in Hiv+ Subjects: Evidence for Involvement of Autophagy and Tg2 Expression in Pbmc. Int. J. Mol. Sci. 2020, 21, 7558. [Google Scholar] [CrossRef]
- Currò, M.; Ferlazzo, N.; Costanzo, M.G.; Caccamo, D.; Ientile, R. Vitamin D Status Influences Transcriptional Levels of RANKL and Inflammatory Biomarkers Which Are Associated with Activation of PBMC. Clin. Chim. Acta 2020, 507, 219–223. [Google Scholar] [CrossRef]
- Makrani, A.H.; Afshari, M.; Ghajar, M.; Forooghi, Z.; Moosazadeh, M. Vitamin D and Fibromyalgia: A Meta-Analysis. Korean J. Pain. 2017, 30, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; DI Carlo, M.; Farah, S.; Atzeni, F.; Buskila, D.; Ablin, J.N.; Häuser, W.; Sarzi-Puttini, P. Comment on: Diagnosis of Fibromyalgia: Comparison of the 2011/2016 ACR and AAPT Criteria and Validation of the Modified Fibromyalgia Assessment Status: Reply. Rheumatology 2020, 59, E81. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Saija, C.; Naccari, F.; Ientile, R.; Di Mauro, D.; Trimarchi, F.; Caccamo, D. Saliva Testing as Noninvasive Way for Monitoring Exercise-Dependent Response in Teenage Elite Water Polo Players: A Cohort Study. Medicine 2021, 100, E27847. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Buzzanca, C.; Chiaia, V.; Mondello, M.; Cacciola, F.; Caccamo, D.; Mondello, L. Measurement of Short-Chain Fatty Acids in Human Plasma by Means of Fast Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2024, 1235, 124044. [Google Scholar] [CrossRef]
- Geisler, S.; Mayersbach, P.; Becker, K.; Schennach, H.; Fuchs, D.; Gostner, J.M. Serum Tryptophan, Kynurenine, Phenylalanine, Tyrosine and Neopterin Concentrations in 100 Healthy Blood Donors. Pteridines 2015, 26, 31–36. [Google Scholar] [CrossRef]
- Lionetto, L.; Ulivieri, M.; Capi, M.; De Bernardini, D.; Fazio, F.; Petrucca, A.; Pomes, L.M.; De Luca, O.; Gentile, G.; Casolla, B.; et al. Increased Kynurenine-to-Tryptophan Ratio in the Serum of Patients Infected with SARS-CoV2: An Observational Cohort Study. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166042. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef]
- Jiang, B.C.; Liu, T.; Gao, Y.J. Chemokines in Chronic Pain: Cellular and Molecular Mechanisms and Therapeutic Potential. Pharmacol. Ther. 2020, 212, 107581. [Google Scholar] [CrossRef] [PubMed]
- Bäckryd, E.; Tanum, L.; Lind, A.L.; Larsson, A.; Gordh, T. Evidence of Both Systemic Inflammation and Neuroinflammation in Fibromyalgia Patients, as Assessed by a Multiplex Protein Panel Applied to the Cerebrospinal Fluid and to Plasma. J. Pain. Res. 2017, 10, 515–525. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Muscoli, C.; Dagostino, C.; Ilari, S.; Lauro, F.; Gliozzi, M.; Bardhi, E.; Palma, E.; Mollace, V.; Salvemini, D. Posttranslational Nitration of Tyrosine Residues Modulates Glutamate Transmission and Contributes to N-Methyl-D-Aspartate-Mediated Thermal Hyperalgesia. Mediators Inflamm. 2013, 2013, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Palma, E.; Tomino, C.; Fini, M.; Raffaeli, W.; et al. Tantali Fibromyalgic Supplicium: Is There Any Relief with the Antidepressant Employment? A Systematic Review. Pharmacol. Res. 2022, 186, 106547. [Google Scholar] [CrossRef] [PubMed]
- Tenti, M.; Raffaeli, W.; Malafoglia, V.; Paroli, M.; Ilari, S.; Muscoli, C.; Fraccaroli, E.; Bongiovanni, S.; Gioia, C.; Iannuccelli, C.; et al. Common-Sense Model of Self-Regulation to Cluster Fibromyalgia Patients: Results from a Cross-Sectional Study in Italy. Clin. Exp. Rheumatol. 2022, 40, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Nagi, K.; Thillaiappan, N.B.; Sukumaran, V.K.; Akhtar, S. Vitamin D and Its Potential Interplay With Pain Signaling Pathways. Front. Immunol. 2020, 11, 820. [Google Scholar] [CrossRef]
- Xu, H.W.; Zhang, S.B.; Yi, Y.Y.; Chen, H.; Hu, T.; Wang, S.J.; Wu, D.S. Relationship between Vitamin D and Nonspecific Low Back Pain May Be Mediated by Inflammatory Markers. Pain. Physician 2021, 24, E1015–E1023. [Google Scholar] [PubMed]
- Johnson, C.R.; Thacher, T.D. Vitamin D: Immune Function, Inflammation, Infections and Auto-Immunity. Paediatr. Int. Child. Health 2023, 43, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.; Alyahya, K.O.; Raghupathy, R. Association between Levels of Vitamin D and Inflammatory Markers in Healthy Women. J. Inflamm. Res. 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Haddad, H.W.; Mallepalli, N.R.; Scheinuk, J.E.; Bhargava, P.; Cornett, E.M.; Urits, I.; Kaye, A.D. The Role of Nutrient Supplementation in the Management of Chronic Pain in Fibromyalgia: A Narrative Review. Pain. Ther. 2021, 10, 827–848. [Google Scholar] [CrossRef]
- Ersoy, S.; Kesiktas, F.N.; Sirin, B.; Bugdayci, D.; Paker, N. The Effect of Vitamin D Treatment on Quality of Life in Patients with Fibromyalgia. Ir. J. Med. Sci. 2024, 193, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.F.; da Rocha Araújo, F.A.G.; da Mota, L.M.H.; Aires, R.B.; de Araujo, R.P. Vitamin D Supplementation Seems to Improve Fibromyalgia Symptoms: Preliminary Results. Isr. Med. Assoc. J. 2018, 20, 379–381. [Google Scholar] [PubMed]
- Altindag, O. Serum Vitamin D Level and Its Relation with Clinical Parameters in Fibromyalgia as a Neuropathic Pain. Orthop. Muscular Syst. 2014, 3, 171. [Google Scholar] [CrossRef]
- Beserra, S.R.; Souza, F.I.S.; Sarni, R.O.S.; de Morais Pereira, M.M. Association Between Low Vitamin D Levels and the Greater Impact of Fibromyalgia. J. Clin. Med. Res. 2020, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Mateos, F.; Valero, C.; Olmos, J.M.; Casanueva, B.; Castillo, J.; Martínez, J.; Hernández, J.L.; González Macías, J. Bone Mass and Vitamin D Levels in Women with a Diagnosis of Fibromyalgia. Osteoporos. Int. 2014, 25, 525–533. [Google Scholar] [CrossRef]
- Okyay, R.; Koçyigit, B.F.; Gürsoy, S. Vitamin D Levels in Women with Fibromyalgia and Relationship between Pain, Tender Point Count and Disease Activity. Acta Medica Mediterr. 2016, 32, 243–247. [Google Scholar] [CrossRef]
- Baygutalp, N. The Relation between Serum Vitamin D Levels and Clinical Findings of Fibromyalgia Syndrome. Dicle Med. J./Dicle Tip. Derg. 2014, 41, 446–450. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the Brain: Genomic and Non-Genomic Actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the Brain: Key Questions for Future Research. J. Steroid Biochem. Mol. Biol. 2015, 148, 305–309. [Google Scholar] [CrossRef]
- Koch, A.; Zacharowski, K.; Boehm, O.; Stevens, M.; Lipfert, P.; von Giesen, H.-J.; Wolf, A.; Freynhagen, R. Nitric Oxide and Pro-Inflammatory Cytokines Correlate with Pain Intensity in Chronic Pain Patients. Inflamm. Res. 2007, 56, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical Correlates of Elevated Serum Concentrations of Cytokines and Autoantibodies in Patients with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Uçeyler, N.; Rogausch, J.P.; Toyka, K.V.; Sommer, C. Differential Expression of Cytokines in Painful and Painless Neuropathies. Neurology 2007, 69, 42–49. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pintó, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and Cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef]
- Wallace, D.J.; Gavin, I.M.; Karpenko, O.; Barkhordar, F.; Gillis, B.S. Cytokine and Chemokine Profiles in Fibromyalgia, Rheumatoid Arthritis and Systemic Lupus Erythematosus: A Potentially Useful Tool in Differential Diagnosis. Rheumatol. Int. 2015, 35, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Galvin, D.A.; C, M. The Role of T-Lymphocytes in Neuropathic Pain Initiation, Development of Chronicity and Treatment. Brain Behav. Immun. Health 2021, 18, 100371. [Google Scholar] [CrossRef]
- Luchting, B.; Rachinger-Adam, B.; Zeitler, J.; Egenberger, L.; Möhnle, P.; Kreth, S.; Azad, S.C. Disrupted TH17/Treg Balance in Patients with Chronic Low Back Pain. PLoS ONE 2014, 9, e104883. [Google Scholar] [CrossRef] [PubMed]
- Luchting, B.; Rachinger-Adam, B.; Heyn, J.; Hinske, L.C.; Kreth, S.; Azad, S.C. Anti-Inflammatory T-Cell Shift in Neuropathic Pain. J. Neuroinflamm. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Azizi, S.V.; Arabi, Z.; Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Larussa, T.; Khatami, F.; Nemati, M. Vitamin D Down-Regulates the Expression of Some Th17 Cell-Related Cytokines, Key Inflammatory Chemokines, and Chemokine Receptors in Experimental Autoimmune Encephalomyelitis. Nutr. Neurosci. 2019, 22, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J. Clin. Med. 2020, 9, 1814. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Barjandi, G.; Louca Jounger, S.; Löfgren, M.; Bileviciute-Ljungar, I.; Kosek, E.; Ernberg, M. Plasma Tryptophan and Kynurenine in Females with Temporomandibular Disorders and Fibromyalgia—An Exploratory Pilot Study. J. Oral. Rehabil. 2020, 47, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Thaker, A.I.; Kanuri, N.; Riehl, T.E.; Rowley, C.W.; Stenson, W.F.; Ciorba, M.A. Serum Analysis of Tryptophan Catabolism Pathway: Correlation with Crohn’s Disease Activity. Inflamm. Bowel Dis. 2012, 18, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO Activation, Inflammation and Musculoskeletal Disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef]
- Filippini, P.; Del Papa, N.; Sambataro, D.; Del Bufalo, A.; Locatelli, F.; Rutella, S. Emerging Concepts on Inhibitors of Indoleamine 2,3-Dioxygenase in Rheumatic Diseases. Curr. Med. Chem. 2012, 19, 5381–5393. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Maes, M. The Role of Indoleamine 2,3-Dioxygenase (IDO) in the Pathophysiology of Interferon-α-Induced Depression. J. Psychiatry Neurosci. 2004, 29, 11–17. [Google Scholar] [PubMed]
- Baldi, S.; Pagliai, G.; Dinu, M.; Di Gloria, L.; Nannini, G.; Curini, L.; Pallecchi, M.; Russo, E.; Niccolai, E.; Danza, G.; et al. Effect of Ancient Khorasan Wheat on Gut Microbiota, Inflammation, and Short-Chain Fatty Acid Production in Patients with Fibromyalgia. World J. Gastroenterol. 2022, 28, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal Colonization Resistance. Immunology 2013, 138, 1–11. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Chen, S.-W.; Ma, Y.-Y.; Zhu, J.; Zuo, S.; Zhang, J.-L.; Chen, Z.-Y.; Chen, G.-W.; Wang, X.; Pan, Y.-S.; Liu, Y.-C.; et al. Protective Effect of 1,25-Dihydroxyvitamin D3 on Ethanol-Induced Intestinal Barrier Injury Both in Vitro and in Vivo. Toxicol. Lett. 2015, 237, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D Deficiency Promotes Epithelial Barrier Dysfunction and Intestinal Inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean ± SD (Range) | Reference Values |
|---|---|---|
| Age (years) | 49.9 ± 12.35 (16–75) | / |
| FIQ-R scores | 64.76 ± 13.67 (30.7–90.3) | ≤30 remission >30 and ≤45 mild >45 and ≤65 moderate >65 severe |
| 1. Physical function | 19.17 ± 4.76 (9–28.7) | 0–30 |
| 2. General health | 12.64 ± 3.99 (4–19) | 0–20 |
| 3. Symptoms | 33.96 ± 7.74 (10–45.5) | 0–50 |
| 25(OH) vitamin D3 (μg/L) | 35.80 ± 15.4 (5–79) | <30 insufficient >30 sufficient |
| IL-1β (pg/mL) | 10.14 ± 11.98 (1.0–64.77) | <15 |
| IL-6 (pg/mL) | 4.30 ± 3.46 (0.98–16.95) | <6 |
| IL-17 (pg/mL) | 20.9 ± 23.97 (1.2–92.7) | <3 |
| IFN-γ (pg/mL) | 13.53 ± 18.71 (0.1–98.7) | <4.2 |
| TNF-α (pg/mL) | 21.81 ± 16.73 (1.09–82.26) | <8.1 |
| AOPP (μM) | 240.75 ± 217.59 (62.49–1235.21) | <100 |
| Trp (μmol/L) | 15.13 ± 9.42 (3.2–48.27) | 15–59 |
| Kyn (μmol/L) | 1.41 ± 0.84 (0.22–5.19) | 0.92–2.68 |
| Kyn/Trp ratio | 0.13 ± 0.14 (0.013–0.64) | <0.0267 |
| SCFAs (μmol/L) | 26.11 ± 15.55 (7.32–76.3) | 51–120 |
| - Acetate | 23.86 ± 15.19 (5.3–72.82) | 50–100 |
| - Propionate | 1.39 ± 0.70 (0.62–4.28) | 0.5–10 |
| - Butyrate | 0.85 ± 0.86 (0.07–5.23) | 0.5–10 |
| Variables | Mild | Moderate | Severe | Reference Values |
|---|---|---|---|---|
| FIQ-R scores | 36.44 ± 5.25 | 56.87 ± 12.91 | 76.44 ± 8.27 | |
| 1. Physical function | 14.74 ± 4.93 | 16.18 ± 3.95 | 22.66 ± 3.54 | 0–30 |
| 2. General health | 7.66 ± 2.08 | 10.06 ± 3.82 | 15.34 ± 2.63 | 0–20 |
| 3. Symptoms | 17.1 ± 5.06 | 31.0 ± 7.82 | 38.43 ± 4.19 | 0–50 |
| Age (years) | 50.8 ± 14.41 | 49.56 ± 14.78 | 50 ± 9.87 | / |
| 25(OH) vitamin D3 (μg/L) | 48.14 ± 18.96 | 33.98 ± 16.21 * | 29.24 ± 9.26 ** | <30 insufficient >30 sufficient |
| IL-1β (pg/mL) | 7.58 ± 3.02 | 12.74 ± 14.32 | 9.34 ± 12.11 | <15 |
| IL-6 (pg/mL) | 6.40 ± 2.51 | 3.95 ± 2.73 | 3.89 ± 3.05 | <6 |
| IL-17 (pg/mL) | 10.67 ± 9.89 | 17.29 ± 20.79 | 22.50 ± 23.10 | <3 |
| IFN-γ (pg/mL) | 7.56 ± 5.03 | 9.17 ± 8.80 | 17.28 ± 24.66 | <4.2 |
| TNF-α (pg/mL) | 17.97 ± 9.40 | 22.18 ± 20.29 | 21.17 ± 21.08 | <8.1 |
| AOPP (μM) | 157.79 ± 70.97 | 210.17 ± 126.01 | 337.8 ± 340.5 | <100 |
| Trp (μmol/L) | 12.33 ± 2.93 | 15.24 ± 10.11 | 16.57 ± 11.40 | 15–59 |
| Kyn (μmol/L) | 0.69 ± 0.45 | 1.49 ± 1.21 | 1.37 ± 0.55 | 0.92–2.68 |
| Kyn/Trp ratio | 0.05 ± 0.02 | 0.12 ± 0.13 | 0.13 ± 0.12 | <0.0267 |
| SCFAs (μmol/L) | 37.22 ± 26.40 | 26.86 ± 11.63 | 23.23 ± 11.69 | 51–120 |
| - Acetate | 35.27 ± 25.53 | 24.62 ± 11.20 | 20.93 ± 11.49 | 50–100 |
| - Propionate | 1.50 ± 1.10 | 1.35 ± 0.52 | 1.32 ± 0.56 | 0.5–10 |
| - Butyrate | 0.44 ± 0.22 | 0.89 ± 0.81 | 0.98 ± 1.14 | 0.5–10 |
| Variables | Insufficient | Sufficient | Reference Values |
|---|---|---|---|
| 25(OH)vitaminD3 (μg/L) | 20.73 ± 6.53 | 44.74 ± 11.88 | |
| Age (years) | 48.81 ± 13.50 | 50.55 ± 11.96 | / |
| FIQ-R scores | 68.65 ± 13.44 | 61.30 ± 12.79 * | ≤30 remission >30 and ≤45 mild >45 and ≤65 moderate >65 severe |
| 1. Physical function | 20.29 ± 5.49 | 18.17 ± 3.95 * | 0–30 |
| 2. General health | 13.04 ± 4.03 | 12.10 ± 3.88 | 0–20 |
| 3. Symptoms | 35.33 ± 7.46 | 32.56 ± 7.64 | 0–50 |
| IL-1β (pg/mL) | 11.43 ± 12.69 | 9.25 ± 11.19 | <15 |
| IL-6 (pg/mL) | 4.89 ± 4.34 | 3.67 ± 2.70 | <6 |
| IL-17 (pg/mL) | 29.77 ± 28.25 | 12.93 ± 15.16 * | <3 |
| IFN-γ (pg/mL) | 15.09 ± 21.18 | 10.98 ± 13.27 | <4.2 |
| TNF-α (pg/mL) | 19.57 ± 18.56 | 22.44 ± 13.26 | <8.1 |
| AOPP (μM) | 341.08 ± 355.34 | 223.21 ± 134.91 | <100 |
| Trp (μmol/L) | 16.91 ± 8.54 | 16.15 ± 9.75 | 15–59 |
| Kyn (μmol/L) | 1.78 ± 1.17 | 1.15 ± 0.61 * | 0.92–2.68 |
| Kyn/Trp ratio | 0.15 ± 0.16 | 0.08 ± 0.04 | <0.0267 |
| SCFAs (μmol/L) | 25.35 ± 15.10 | 26.85 ± 13.74 | 51–120 |
| - Acetate | 22.83 ± 14.50 | 24.79 ± 13.50 | 50–100 |
| - Propionate | 1.34 ± 0.60 | 1.34 ± 0.50 | 0.5–10 |
| - Butyrate | 1.17 ± 1.15 | 0.71 ± 0.67 | 0.5–10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saija, C.; Bertuccio, M.P.; Scoglio, A.; Macaione, V.; Cacciola, F.; Micalizzi, G.; Caccamo, D.; Muscoli, C.; Currò, M. Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain. Biomedicines 2025, 13, 139. https://doi.org/10.3390/biomedicines13010139
Saija C, Bertuccio MP, Scoglio A, Macaione V, Cacciola F, Micalizzi G, Caccamo D, Muscoli C, Currò M. Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain. Biomedicines. 2025; 13(1):139. https://doi.org/10.3390/biomedicines13010139
Chicago/Turabian StyleSaija, Caterina, Maria Paola Bertuccio, Alberto Scoglio, Vincenzo Macaione, Francesco Cacciola, Giuseppe Micalizzi, Daniela Caccamo, Carolina Muscoli, and Monica Currò. 2025. "Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain" Biomedicines 13, no. 1: 139. https://doi.org/10.3390/biomedicines13010139
APA StyleSaija, C., Bertuccio, M. P., Scoglio, A., Macaione, V., Cacciola, F., Micalizzi, G., Caccamo, D., Muscoli, C., & Currò, M. (2025). Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain. Biomedicines, 13(1), 139. https://doi.org/10.3390/biomedicines13010139








