Abstract
Background/Objectives: GFI1-36N represents a single-nucleotide polymorphism (SNP) of the zinc finger protein Growth Factor Independence 1 (GFI1), in which the amino acid serine (S) is replaced by asparagine (N). The presence of the GFI1-36N gene variant is associated with a reduced DNA repair capacity favoring myeloid leukemogenesis and leads to an inferior prognosis of acute myeloid leukemia (AML) patients. However, the underlying reasons for the reduced DNA repair capacity in GFI1-36N leukemic cells are largely unknown. Since we have demonstrated that GFI1 plays an active role in metabolism, in this study, we investigated whether increased levels of reactive oxygen species (ROS) could contribute to the accumulation of genetic damage in GFI1-36N leukemic cells. Methods: We pursued this question in a murine model of human AML by knocking in human GFI1-36S or GFI1-36N variant constructs into the murine Gfi1 gene locus and retrovirally expressing MLL-AF9 to induce AML. Results: Following the isolation of leukemic bone marrow cells, we were able to show that the GFI1-36N SNP in our model is associated with enhanced oxidative phosphorylation (OXPHOS), increased ROS levels, and results in elevated γ-H2AX levels as a marker of DNA double-strand breaks (DSBs). The use of free radical scavengers such as N-acetylcysteine (NAC) and α-tocopherol (αT) reduced ROS-induced DNA damage, particularly in GFI1-36N leukemic cells. Conclusions: We demonstrated that the GFI1-36N variant is associated with extensive metabolic changes that contribute to the accumulation of genetic damage.
1. Introduction
Acute myeloid leukemia (AML) is a malignant disease of the bone marrow (BM) arising from a differentiation block in the early stages of hematopoiesis. As AML is primarily a disease of the elderly, aggressive treatment regimens including chemotherapy and allogeneic stem cell transplantation are often no longer feasible, underlining the importance of developing personalized, targeted therapeutic approaches.
The presence of a polymorphism of the transcription factor Growth Factor Independence 1 (GFI1) gene could provide a possible target for specific AML therapy. GFI1 plays an essential role as a transcriptional repressor during the differentiation of myeloid and lymphoid progenitor cells. In recent years, there has been increasing evidence that GFI1 not only influences hematopoiesis as an epigenetic regulator but is also directly involved in DNA repair [1]. In the coding N-terminal region, GFI1 exhibits a single-nucleotide polymorphism (SNP) in which the amino acid serine (S) in position 36 is replaced by asparagine (N) [2]. Thus, there are two variants of the GFI1 protein: the more common variant called GFI1-36S and the less common variant called GFI1-36N. The GFI1-36N gene variant is present in approximately 7% of the healthy population, indicating its broad distribution. Notably, its prevalence increases to up to 15% among patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), suggesting that the GFI1-36N variant is implicated in the pathogenesis of MDS and AML [2,3,4].
We have already discovered that the leukemia-predisposing function of the GFI1-36N protein variant is caused, among other factors, by reduced DNA repair processes. We have recently shown that murine GFI1-36N-MLL-AF9 cells exhibited increased levels of the cell cycle propagating cyclin-dependent kinases 4 (CDK4) and 6 (CDK6) leading to a faster proliferation of GFI1-36N leukemic cells in vitro [5]. Furthermore, we found that the presence of the GFI1-36N variant impedes both homologous recombination- (HR-) and O6-methylguanine DNA methyltransferase- (MGMT-) directed DNA repair in leukemic cells [6]. This could provide an explanation as to why carriers of the GFI1-36N variant exhibit more genetic alterations.
In addition to direct deficits in DNA repair, reactive oxygen species (ROS) are also known to induce DNA damage. ROS are oxygen radicals such as superoxide anions (O2−) or hydrogen peroxide (H2O2), which are formed in the respiratory chain by NADPH oxidase-catalyzed reactions or by ionizing radiation [7,8]. The radicals can then react with numerous other molecules in the cell, such as lipids or metals, and form new radicals as part of a radical chain reaction. This induces DNA damage on the one hand and impairs DNA repair mechanisms on the other [9].
2. Materials and Methods
2.1. Laboratory Animal Strain
The laboratory mice (Mus musculus) used for the project originate from the C57BL/6 strain of the Jackson Laboratory (Bar Harbor, ME, USA). The findings described in this article refer to hGFI1-36S and hGFI1-36N mice that expressed either the human GFI1-36S or the human GFI1-36N gene variants, respectively, instead of the murine Gfi1. The integration of the human gene into the murine Gfi1 locus was described earlier [10]. Mice expressing the human GFI1 gene variants are referred to as either GFI1-36S or GFI1-36N mice. The Gfi1-expressing mice were purchased from Charles River Laboratories (Wilmington, MA, USA). The GFI1-36S and GFI1-36N mice were bred in the Central Animal Facilities of the University of Münster or the University of Lübeck. The mice were genotyped using polymerase chain reaction (PCR). To avoid infections and ensure high reproducibility the mice used in this study were kept under specific pathogen-free (SPF) conditions (sterilized supplies, controlled environment, health monitoring, and strict protocols for personnel) in individually ventilated cages (IVC). The cages were filled with enrichment materials, and HEPA-filtered air was circulated in a rack system. The mice were fed ad libitum with standard aseptic diets and aseptic water.
2.2. Generation of MLL-AF9 Leukemic Mice
In order to generate leukemic mice, they were BM transplanted with cells featuring an MLL-AF9 fusion gene, which induces leukemogenesis and is associated with a particularly poor prognosis compared to the expression of other fusion genes such as AML1-ETO [11]. In the primary transplantation, lineage-negative (Lin−) BM cells transduced with an MCSV-MLL-AF9-IRES-GFP plasmid were transplanted after the mice had been lethally irradiated (7 + 3 Gy) (Figure S1). In the secondary transplantation, instead of transduced Lin− cells, leukemic cells stored in liquid nitrogen were transplanted into sub-lethally irradiated (3 Gy) mice. The cells were obtained from mice previously diseased with AML in vivo. The exact procedures of primary and secondary transplantation were described previously [6]. To avoid infections after BM transplantation, enrofloxacin (Bayer, Leverkusen, GER) was added to the drinking water of the mice.
2.3. Collection of Leukemic Cells
Leukemic BM cells were washed out of the tibiae and femora. In addition, blood was collected from the right ventricle of the heart after a median sternotomy to perform a differential blood count using a Scil Vet abc hematology analyzer (Scil Animal Care Company, Viernheim, GER). The expression of seven antigens was determined by flow cytometry to distinguish the of AML from other hematologic malignancies and to confirm the blood analyses. The Ter119/APC-B220/PerCP and CD8a/APC-CD4/PerCP stainings were used to exclude acute lymphoblastic leukemia (ALL) or mixed phenotype acute leukemia (MPAL), to which the MLL-AF9 fusion protein predisposes as well [12]. The c-Kit/APC staining and GFP detection were performed for BM cells and heart blood. Following lysis of erythrocytes, 1 × 106 BM cells and the heart blood were centrifuged (336× g, 5 min, 4 °C) in 1 mL FACS buffer. A 10 μL BD Fc Block (BD, Franklin Lakes, NJ, USA) was added to each sample. The samples were vortexed and incubated on ice for 4 min. This was followed by double or single staining using the following antibodies: anti-Mouse CD117 (c-Kit) APC (#105811), anti-Mouse CD4 PerCP (#100537), anti-Mouse CD8a APC (#100711), anti-Mouse Ly-6G/Ly-6C (Gr-1) APC (#108411), anti-Mouse TER-119 APC (#116211), anti-Mouse/Human CD11b PerCP/Cy 5.5 (#101229), anti-Mouse/Human CD45R/B220 PerCP (#103235) (all BioLegend, San Diego, CA, USA). These were diluted in FACS buffer to a concentration of 0.2 μg/mL. An 80 μL of the antibody solution was added to each sample and incubated on ice for 10 min. FACS analysis was performed using the Attune NxT Acoustic Focusing Cytometer running on the Attune NxT V3 software (Thermo Fisher Scientific, Waltham, MA, USA). The outcomes of detecting leukemia with the GFI1-36S-MLL-AF9 or GFI1-36N-MLL-AF9 cells have already been shown in previous publications and are, therefore, not included as a separate figure in this study [5,6].
2.4. Generation of GFI1-36N-K562 Cells
Human K562 cells were sourced from the Leibniz Institute DSMZ (Braunschweig, GER) and cultured in RPMI medium supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 environment. For generation of GFI1-36N cells, two lentiviral plasmids were utilized. The Lenti-hyeA3A-BE4max plasmid, a modification of Lenti-117G-hyeA3A-BE4max (#157946, Addgene, Watertown, MA, USA), was combined with tet-pLKO-sgRNA-puro (#10432, Addgene). Lentiviral production and transduction followed previously described methods [13,14,15]. The infected K562 cells were selected via GFP sorting and puromycin treatment. Single-cell clones were obtained by limiting dilution in 96-well plates, expanded for 14 d, and subjected to genomic DNA sequencing, as described before [5].
2.5. Quantification of DNA Double-Strand Breaks (DSBs)
To test DNA repair after ROS inhibition, 250 × 103 leukemic cells were treated for 48 h with either 10 mM N-acetylcysteine (NAC) or 30 μL α-tocopherol (αT). The same proportions of ddH2O or DMSO were added to the untreated control. To induce DNA damage, the cells were then irradiated with 3 Gy in a Multi Rad225 irradiation system (Precision X-ray, Madison, CT, USA) and incubated for 30, 60, and 120 min. Cells were centrifuged with FACS buffer (395× g, 4 min, 4 °C), the supernatant was removed and 100 μL BD Cytofix/Cytoperm buffer (BD, Franklin Lakes, NJ, USA) was added. Samples were vortexed and incubated on ice for 25 min. The cells were then washed in 1 mL BD Perm/Wash buffer (BD) and centrifuged (395× g, 4 min, 4 °C). The conjugated γ-H2AX antibody anti-Mouse H2A.X APC (#sc-517336, BioLegend) was diluted to 1:100 in FACS buffer. In total, 100 μL of the antibody dilution was added to each sample. The sample was vortexed and incubated at room temperature (RT) for 1 h protected from light. The cells were then washed twice in phosphate-buffered saline (PBS). Finally, 300 μL FACS buffer was added to each sample. The samples were subsequently analyzed in the Attune NxT Acoustic Focusing Cytometer using Attune NxT V3 software (Thermo Fisher Scientific).
2.6. Seahorse Experiments
Seahorse Mito Stress test and Glycolysis Stress test were performed following the same procedure and the same drug concentrations as previously described by our group [16,17].
2.7. Mitochondrial Membrane Potential (MMP), Mitochondrial Mass (Mito-Mass), and ROS Measurement
To assess MMP and ROS levels, 0.25 × 106 cells were resuspended in prewarmed (37 °C) cell culture media and stained with either 50 nM tetramethylrhodamine methyl ester perchlorate (TMRE) (#ab113852, Abcam, Cambridge, UK) or 5 μM CellROX Deep Red (#C10422, Thermo Fisher Scientific). The cells were incubated for 20 min at 37 °C in a CO2 incubator, followed by direct measurement using flow cytometry. For negative control in TMRE staining, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (#ab120081, Abcam) was used. Mito-mass was analyzed by resuspending 0.25 × 106 cells in prewarmed (37 °C) cell culture media without fetal calf serum (FCS), followed by staining with 50 nM MitoTracker Deep Red (#M22426, Thermo Fisher Scientific). After 20 min of incubation at 37 °C in a CO2 incubator, the cells were assessed using flow cytometry as described earlier [16,17].
2.8. Glucose Consumption and Lactate Secretion
Glucose consumption and lactate secretion were measured using an enzyme-based colorimetric kit (Biovision, San Francisco, CA, USA) following the manufacturer’s instructions.
2.9. Mass Spectrometry (MS)
MS was conducted according to the same protocol we followed before and is, therefore, not explicitly addressed in this manuscript [6].
3. Results
3.1. Altered Mitochondrial Metabolism in GFI1-36N Leukemic Cells
In previous work, our group showed that reduced GFI1 expression in primary murine AML cells leads to an increased rate of oxidative phosphorylation (OXPHOS) in vitro as well as in vivo [17]. Since it is already known that GFI1-36N cells and cells exhibiting low GFI1 expression both show deficient homologous recombination (HR) activity with unimpaired non-homologous end joining (NHEJ), the question arose as to whether similar changes in metabolism are also present in GFI1-36N leukemic cells [1,6].
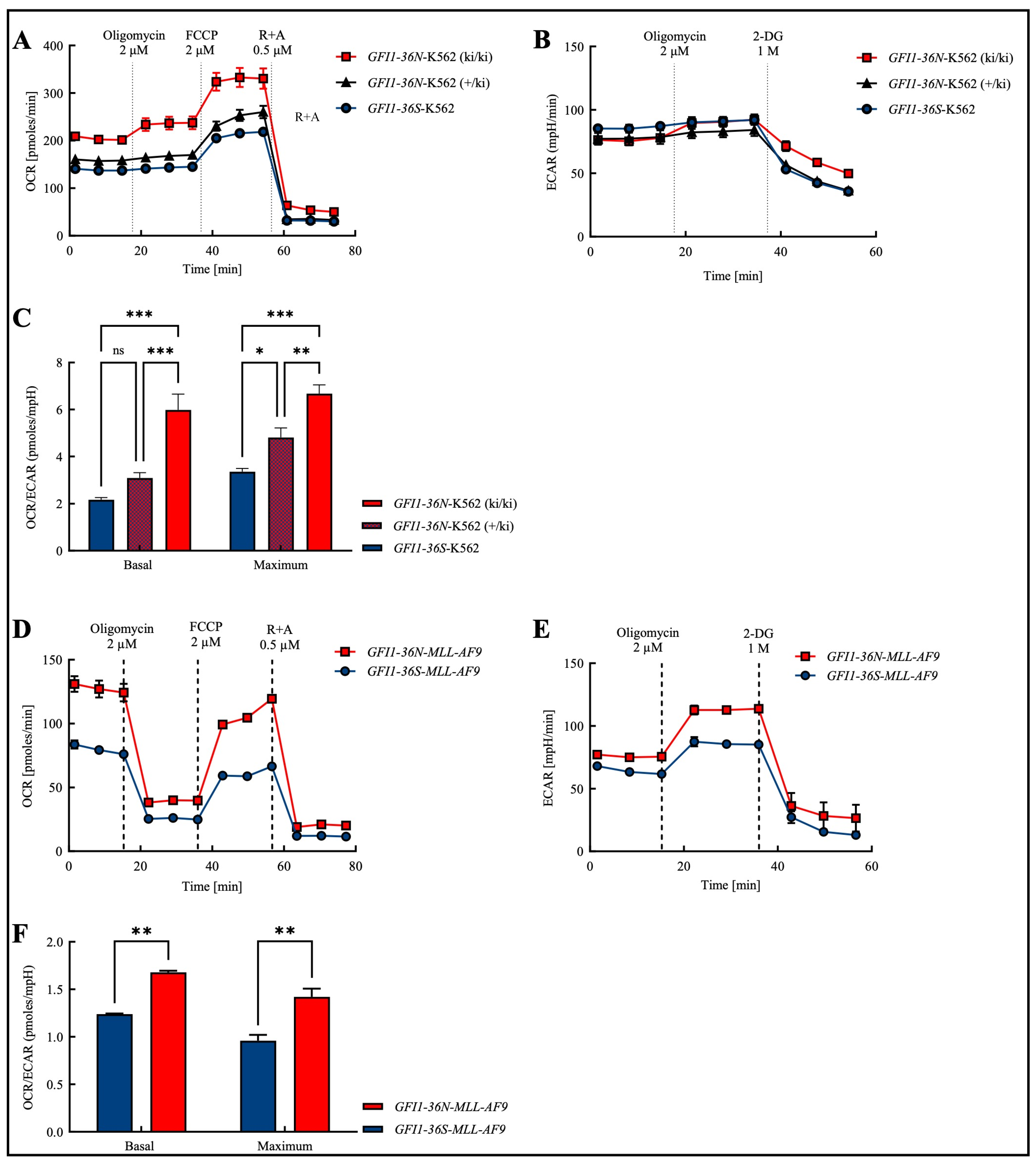
In order to obtain a cross-entity assessment that is not only focused on AML and to obtain an initial overview of the effects of the polymorphism, we addressed this question in the human myeloid leukemia cell line K562, in which we knocked in a GFI1-36N variant. As expected, we found that the presence of the GFI1 protein variant leads to an increase in the basal and maximal levels of OXPHOS (Figure 1A). In addition to mitochondrial OXPHOS, ATP generation also occurs non-mitochondrially through glycolysis [18]. Consequently, we further measured the glycolysis rate using Seahorse glycostress assays. Since glycolysis releases lactate as a by-product, the surrounding medium is acidified, leading to an increase in the ECAR (extracellular acidification rate). However, similar to GFI1-knock-down (GFI1-KD) cells, K562-GFI1-36N cells displayed no significant changes in ECAR (Figure 1B).
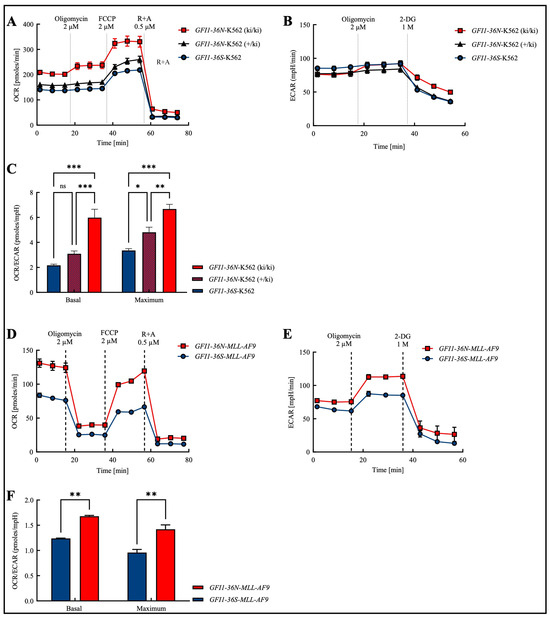
Figure 1.
Increased OCR/ECAR ratio in the presence of the GFI1-36N SNP in both the human chronic myeloid leukemia (CML) cell line K562 and in the murine model of human acute myeloid leukemia (AML). (A): Oxygen consumption rate (OCR) was highest in GFI1-36N-K562 (ki/ki) cells while (B): extracellular acidification rate (ECAR) remained unchanged between GFI1-36N (ki/ki), GFI1-36N (+/ki), and GFI1-36S cells. (C): Increased OCR/ECAR ratio in GFI1-36N-K562 cells. (D): OCR and (E): ECAR were both increased in GFI1-36N-MLL-AF9 cells compared to GFI1-36S-MLL-AF9 controls. (F): Elevated OCR/ECAR ratio in GFI1-36N-MLL-AF9 cells. FCCP: carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; R + A: rotenone + antimycin A; 2-DG: 2-deoxyglucose. Mean ± SEM; ns: not significant; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; n = 3–4.
Next, we investigated whether we could extend those findings obtained in the cell line to a murine model of human AML. Therefore, we tested OCR (oxygen consumption rate) and ECAR in murine GFI1-36S- and GFI1-36N-MLL-AF9 cells and found both parameters upregulated in the presence of the SNP (Figure 1D,E).
In both, human K562 and murine MLL-AF9 cells, we observed that the basal and maximal OCR/ECAR ratios were increased in the presence of the GFI1-36N gene variant (Figure 1C,F). In K562 cells, we further showed that the OCR/ECAR ratio in heterozygous K562-GFI1-36N cells (+/ki) was higher than in homozygous K562-GFI1-36S cells and that the ratio in homozygous K562-GFI1-36N cells (ki/ki) was again increased compared to heterozygous K562-GFI1-36N cells (Figure 1C). These virtually identical results in the human cell line and murine cells prompted us to perform further experiments with GFI1-36N-MLL-AF9 cells.
3.2. Increased ROS and MMP Levels in GFI1-36N-MLL-AF9 Cells
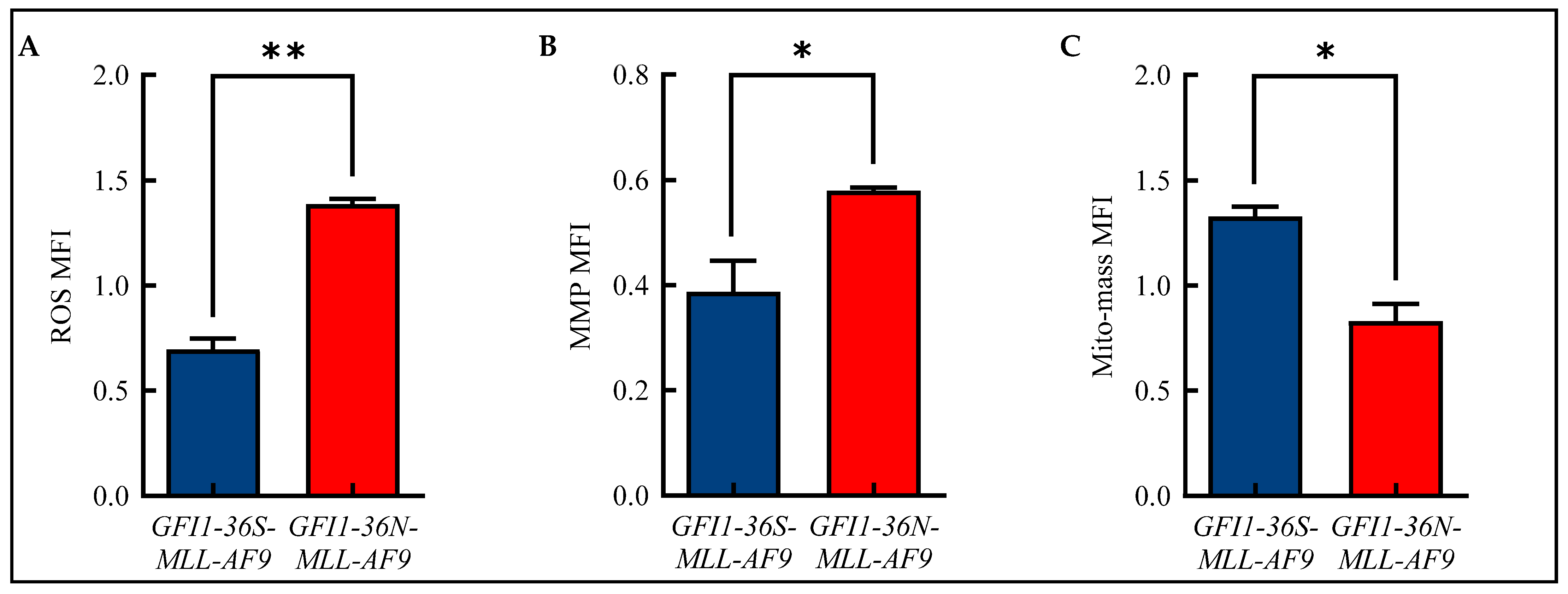
Alterations in mitochondrial metabolism are closely linked to the formation of ROS [19]. Therefore, we analyzed ROS in our murine model of human AML using flow cytometry and were able to show that GFI1-36N leukemic cells displayed significantly increased ROS levels compared to GFI1-36S-MLL-AF9 cells (Figure 2A and Figure S2A). We also demonstrated that GFI1-36N-MLL-AF9 cells exhibited increased MMP (Figure 2B and Figure S2B), but that the mito-mass was—contrary to expectations—decreased (Figure 2C and Figure S2C).
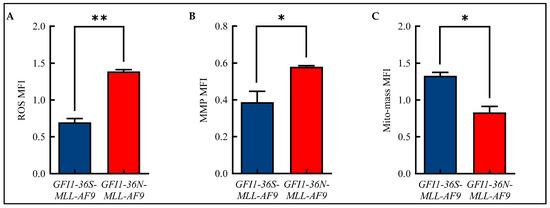
Figure 2.
Alterations in mitochondrial metabolism. Increased (A): reactive oxygen species (ROS) and (B): mitochondrial membrane potential (MMP) with (C): decreased mitochondrial mass (mito-mass) in GFI1-36N-MLL-AF9 cells. Mean ± SEM; * p ≤ 0.05; ** p ≤ 0.01; n = 3.
3.3. ROS-Induced DNA Damage in GFI1-36N-MLL-AF9 Cells
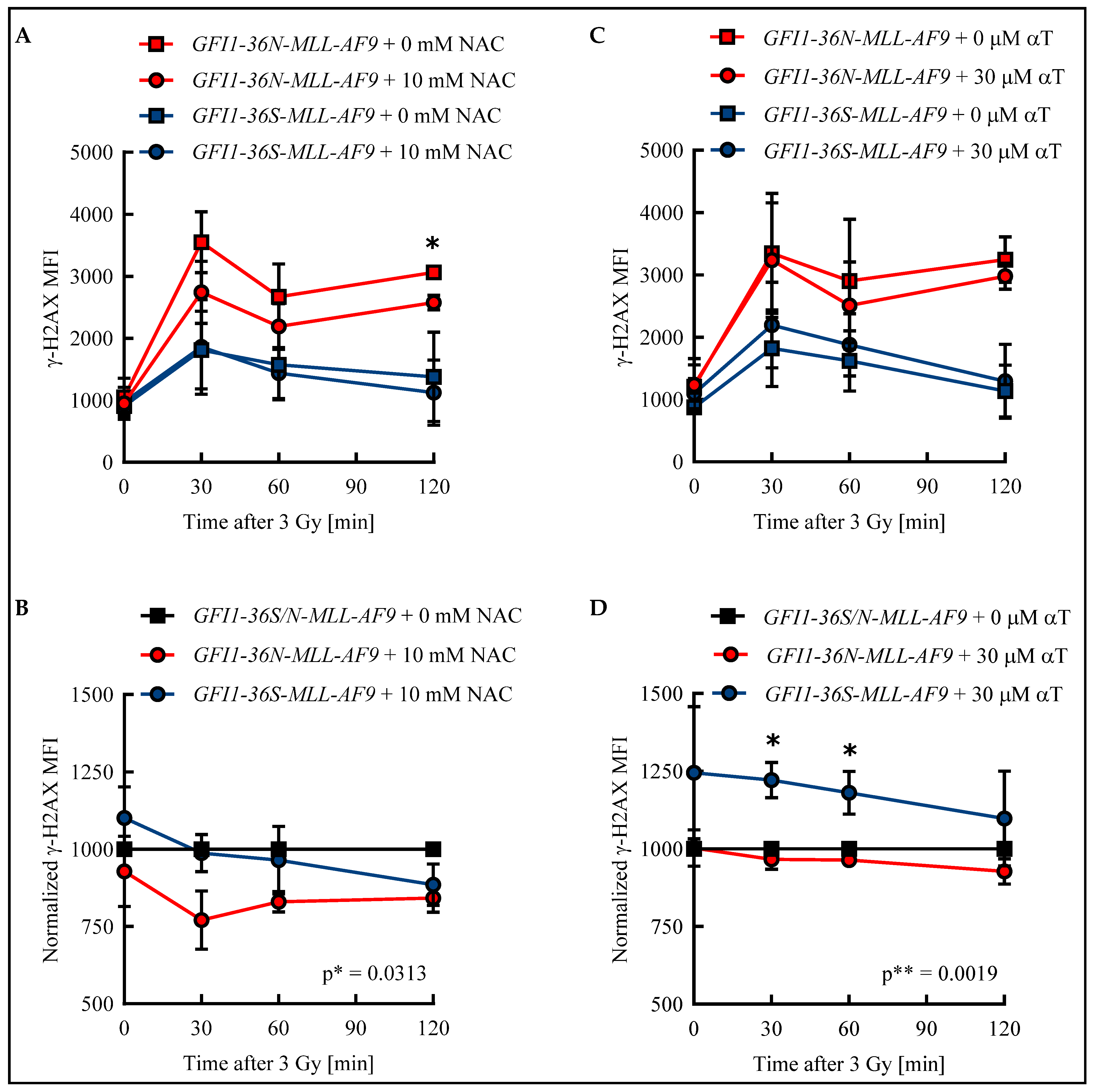
Apart from the increased ROS level described here, we found in previous work that cells harboring the GFI1-36N variant accumulate more genetic damage [6]. ROS can cause DNA DSBs and thus provide a possible explanation for the genetic instability of GFI1-36N cells [9]. To test whether an elevated ROS level results in an increased amount of DSBs in GFI1-36N leukemic cells and impairs their repair, GFI1-36S and GFI1-36N leukemic cells were treated with either the ROS inhibitor NAC or the ROS inhibitor αT. The number of DSBs was quantified by determining the γ-H2AX mean fluorescence intensity (MFI) in an unirradiated control and after irradiation.
In both leukemic GFI1-36S and GFI1-36N cells, the maximum number of DSBs was detected 30 min after irradiation. While in GFI1-36S leukemic cells the γ-H2AX MFI decreased at subsequent time points, it remained at a constant high level in GFI1-36N leukemic cells (Figure 3A,C). The γ-H2AX level was higher in the untreated GFI1-36N cells with a maximum MFI of 3548 ± 492 than in the untreated GFI1-36S cells with a maximum of 1811 ± 627 (Figure 3A,C). The γ-H2AX MFI of NAC-treated GFI1-36N cells was also increased compared to that of treated GFI1-36S cells (Figure 3A). Strikingly, 10 mM NAC was only able to reduce γ-H2AX MFI in the leukemic GFI1-36N cells, whereas GFI1-36S cells were not affected by NAC treatment. Consequently, the γ-H2AX MFI normalized to untreated cells was significantly lower in GFI1-36N cells than in GFI1-36S cells (Figure 3B). A similar pattern was seen regarding the number of GFI1-36N-γ-H2AX+ cells that could be reduced by NAC (Figure S3A,B). The leukemic GFI1-36S cells treated with 30 μM αT had a higher γ-H2AX intensity than the untreated GFI1-36S cells at all time points (Figure 3C). The treated GFI1-36N cells exhibited slightly decreased γ-H2AX intensity compared to the untreated GFI1-36N cells (Figure 3C). After normalization of the γ-H2AX MFI of treated GFI1-36S and GFI1-36N cells to the untreated control, GFI1-36N leukemic cells were found to have a significantly lower γ-H2AX level (Figure 3D). However, it should be noted that the significant difference in γ-H2AX MFI between GFI1-36S-MLL-AF9 and GFI1-36N-MLL-AF9 cells was only due to the slightly increased MFI in GFI1-36S cells. Accordingly, αT did not have as pronounced an effect on the reduction of GFI1-36N-γ-H2AX+ cells as NAC (Figure S3C,D).
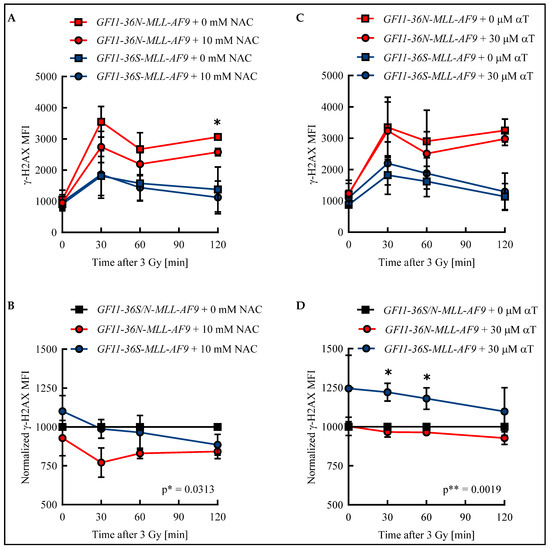
Figure 3.
Radical scavengers N-acetylcysteine (NAC) and α-tocopherol (αT) reduced DNA damage in GFI1-36N leukemic cells. Leukemic bone marrow (BM) cells were incubated for 48 h with (A,B): 10 mM NAC or H2O or with (C,D): 30 μM αT or DMSO. After irradiation of the cells with 3 Gy, γ-H2AX MFI was determined by flow cytometry in GFI1-36S and GFI1-36N leukemic cells. B and D: After normalization to data from the untreated control, treated GFI1-36N leukemic cells showed fewer DNA double-strand breaks (DSBs) than GFI1-36S leukemic controls. Mean ± SEM; * p ≤ 0.05; ** p ≤ 0.01; n = 3.
3.4. GFI1-36N-MLL-AF9 Cells Exhibit Pro-Oncogenic Metabolic Changes
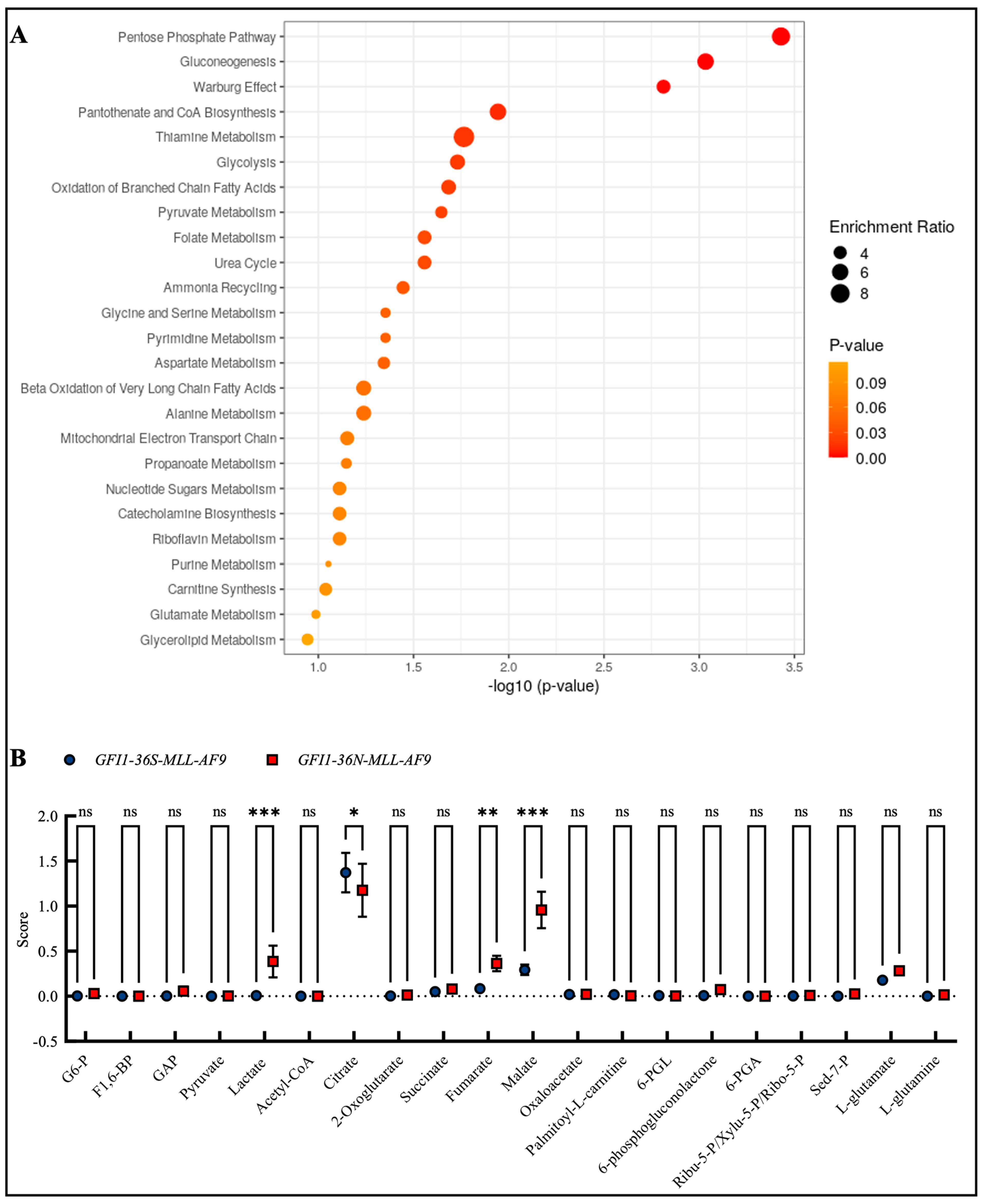
Due to the changes in mitochondrial metabolism and the fact that, as previously described, GFI1-KD cells show extensive alterations in metabolism, we were next interested in whether such changes could also be found in the presence of the GFI1-36N variant. Therefore, we screened the enrichment of different metabolites in GFI1-36N-MLL-AF9 cells by mass spectrometry. We found the highest enrichment in the metabolites of the pentose phosphate pathway, gluconeogenesis, and the Warburg effect (Figure 4A,B), as well as in DNA repair, cell cycle progression and multiple further pathways that we already addressed in previous work (Figure S4A,B). In line with this, we discovered that GFI1-36N leukemic cells exhibited increased glucose consumption and lactate secretion (Figure S5A,B).
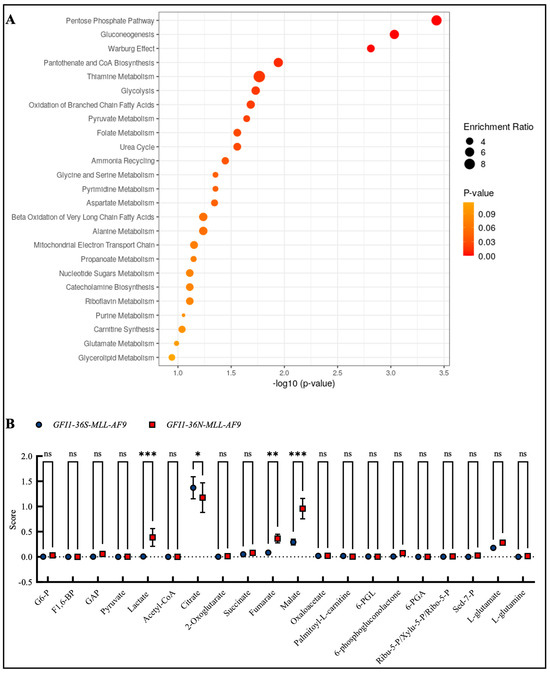
Figure 4.
Mass spectrometry discovered enrichment of pro-oncogenic metabolites in GFI1-36N leukemic cells. (A): Top 25 upregulated metabolite sets and (B): upregulated metabolites in GFI1-36N-MLL-AF9 cells. Mean ± SEM; ns: not significant; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; n = 2–3.
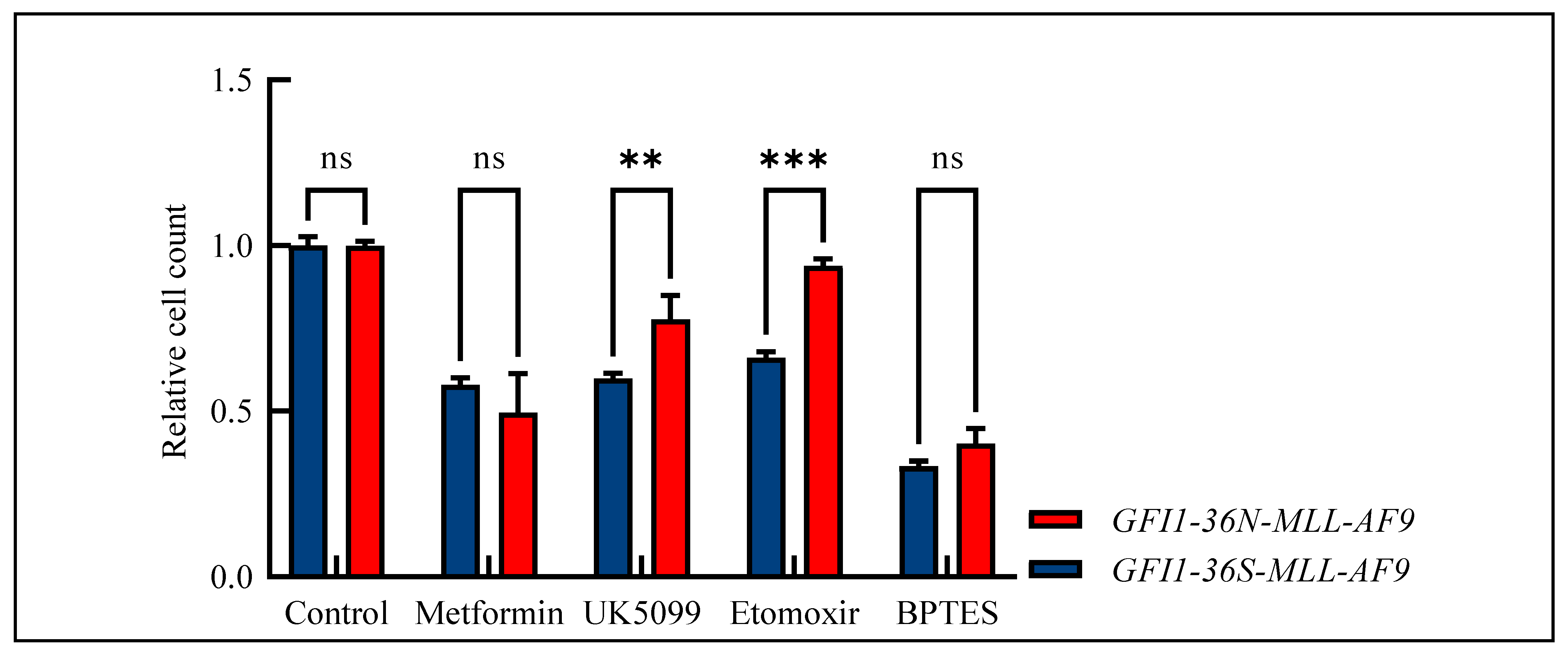
Due to the widespread metabolic changes and the fact that we have already shown that GFI1-KD cells are sensitive to metformin treatment [17], we studied the effect of metformin and three further drugs—UK5099 as mitochondrial pyruvate carrier inhibitor, etomoxir as carnitine palmitoyltransferase-1 inhibitor, and BPTES (bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethylsulfide) as glutaminase inhibitor—in our MLL-AF9 leukemic cells. Indeed, the GFI1-36N-MLL-AF9 leukemic cells responded slightly more favorably to metformin treatment than the GFI1-36S-MLL-AF9 cells. However, these differences did not reach a significant score (Figure 5). Interestingly, we also demonstrated that GFI1-36N leukemic cells were resistant to UK5099, etoxomir, and BPTES as tested by measuring the relative cell count and that they responded significantly poorer to treatment than GFI1-36S-MLL-AF9 cells (Figure 5).
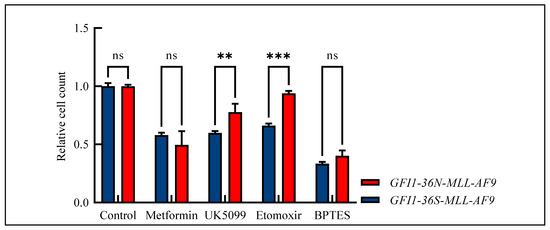
Figure 5.
Relative cell count of GFI1-36S- and GFI1-36N-MLL-AF9 cells after drug treatment normalized to control. GFI1-36N-MLL-AF9 cells did not respond to metformin treatment and showed decreased sensitivity against treatment with UK5099, etoxomir, and BPTES. Mean ± SEM, ns: not significant; ** p ≤ 0.01; *** p ≤ 0.001; n = 3–6.
4. Discussion
ROS are highly reactive molecules that influence cellular processes in a concentration-dependent manner. At low-to-moderate levels, ROS function as signaling molecules, regulating cell proliferation, differentiation, and survival, and are vital for immune defense [20]. They also activate stress-adaptive pathways like Nrf2, enhancing antioxidant defenses [21]. At high levels, ROS induce oxidative damage to proteins, lipids, and DNA causing mutations and chronic inflammation [22,23,24]. Excess ROS contribute to aging, degenerative diseases, and oncogenesis through DNA damage and genomic instability [25,26].
We have shown here that GFI1-36N-expressing leukemic cells are associated with a significantly increased ROS level compared to GFI1-36S leukemic cells. An explanation for this observation has not yet been found—it is possible that the GFI1-36N gene product influences respiratory chain complexes or reduces the activity of antioxidant protective mechanisms. Examples include superoxide dismutase, catalase, peroxidases, and reductases [27].
The aminothiol NAC is a synthetic precursor of intracellular cysteine and glutathione [28]. Glutathione plays an important role in ROS elimination: after superoxide radicals have been converted into hydrogen peroxide by superoxide dismutase, the latter can be split into water and oxygen by catalase or oxidize glutathione (GSH) to glutathione disulfide (GSSG). GSSG is then reduced to glutathione in an NADPH-dependent manner by glutathione reductase. NADPH is provided by glucose-6-phosphate dehydrogenase [29]. An increased GSSG–GSH ratio, therefore, indicates a high ROS level in the cell [30]. This can be reduced by NAC. αT can also reduce ROS-mediated DNA damage by scavenging lipid peroxyl radicals and thus becoming a radical itself, but one that is resonance-stabilized [31]. The free radical can be finally eliminated and αT regenerated via the previously described GSSH–GSH system [29].
As we detected increased ROS levels in GFI1-36N-expressing leukemic cells, this could provide a further explanation for the increased number of mutations in the presence of the GFI1-36N variant. Consequently, minimizing the ROS level by NAC and αT could results in a reduction in the number of DSBs, particularly in GFI1-36N-expressing cells. This hypothesis was investigated by treating leukemic BM cells with NAC or αT and then quantifying the number of DSBs using γ-H2AX MFI assay. As expected, the γ-H2AX MFI of the leukemic GFI1-36N cells in the treated cells before and after irradiation was significantly lower than that of the untreated control. In contrast, the γ-H2AX MFI in leukemic GFI1-36S cells was almost unchanged. The results indicate that genetic damage could be reduced by NAC specifically in GFI1-36N-expressing leukemic cells. This emphasizes that the elevated ROS level in GFI1-36N-MLL-AF9 cells contributes to the accumulation of DNA DSBs and can be attenuated by ROS inhibition. Next, we addressed the question of whether αT as another ROS inhibitor can achieve a similar effect to NAC. The γ-H2AX MFI was slightly decreased in the GFI1-36N-expressing cells following treatment and slightly increased in the GFI1-36S-expressing cells. The results confirm the previously reported NAC results that the number of DSBs in leukemic GFI1-36N cells can be reduced by ROS inhibition. However, the differences between GFI1-36S and GFI1-36N leukemic cells were not as pronounced as in the NAC treatment experiments. Nevertheless, the results show that the DSB-protective effect of αT appears to be more potent in GFI1-36N leukemic cells than in GFI1-36S leukemic cells.
In summary, our findings reported here demonstrate that the number of DSBs in GFI1-36N leukemic cells could be reduced by the radical scavengers NAC and αT. GFI1-36S leukemic cells were not affected by NAC and only slightly affected by αT. In addition to the reduced effectiveness of DSB repair, which we have already demonstrated in previous work [6], a higher ROS level in GFI1-36N leukemic cells seems to be responsible for the mutations and chromosomal aberrations in the presence of GFI1-36N SNP. The mechanism of why GFI1-36N leukemic cells show an increased ROS level could not be answered in this study. One possible explanation is offered by proteins of the forkhead box (FOXO) family, whose function is GFI1-dependent [32]. The latest data from our group also indicate that lower GFI1 expression increases the OXPHOS rate via upregulation of the FOXO1-MYC axis [17]. FOXO increases the transcription of proteins that have antioxidant effects [33]. The activity of FOXO proteins is controlled post-translationally via phosphorylation and alkylation [34]. It is conceivable that this function is lost in GFI1-36N-MLL-AF9 cells. The resulting loss of the antioxidant effect could explain the higher ROS level and the increased DSBs in GFI1-36N-MLL-AF9 cells. Recent data indicate that the GFI1 paralog GFI1B regulates the mitochondrial respiratory chain during leukemogenesis [16]. This could additionally influence the development of ROS.
In addition to the increased ROS level, we found that GFI1-36N-MLL-AF9 cells and K562-GFI1-36N cells exhibited increased OXPHOS. Interestingly, we have already shown GFI1-KD cells to display increased glycolysis and OXPHOS in vivo and ex vivo [17]. In this study, we showed that the MMP was significantly increased in the presence of the GFI1-36N polymorphism as in the GFI1-KD cells. This could suggest that the increased ROS level is caused by higher mitochondrial activity, but not by an increased number of mitochondria. Overall, these findings indicate similarities between GFI1-36N and GFI1-KD cells. However, unlike in GFI1-KD cells, we did not find a significantly better treatment response to metformin in the cells with the GFI1 variant—although there was a trend towards a better response.
Against our expectations, the mito-mass in GFI1-36N cells was not increased but, on the contrary, reduced. One possible explanation might be that elevated ROS levels can—as described earlier—directly damage mitochondrial components, including proteins, lipids, and mitochondrial DNA, compromising their function [22]. We assume that this damage may impair the electron transport chain (ETC), causing increased electron leakage and further ROS production, establishing a self-sustaining feedback loop. Additionally, mitochondrial damage may lead to hyperpolarization of the mitochondrial membrane, reflected in an elevated MMP. Hyperpolarization could result from impaired ATP synthase activity or defects in the ETC, which cause an abnormal accumulation of protons across the inner mitochondrial membrane [35]. This state exacerbates ROS production as excess electrons prematurely react with oxygen. The observed reduction in mitochondrial mass may result from the activation of quality control mechanisms, such as mitophagy, in response to oxidative damage [36]. Damaged mitochondria are selectively degraded to maintain cellular health, but if the rate of damage surpasses the capacity for mitochondrial biogenesis, the overall mito-mass decreases [37,38]. This smaller mitochondrial population may remain highly active and dysfunctional, sustaining elevated ROS output and MMP despite their reduced numbers. However, it must be emphasized that these considerations are hypotheses that have not yet been experimentally proven, but certainly offer starting points for further research.
Because of the increased amount of ROS and OXPHOS, we screened for further metabolic alterations in the presence of the GFI1-36N variant and observed an upregulation of pathways such as the pentose phosphate pathway and the Warburg effect. Our data indicate that the GFI1-36N SNP drives a metabolic reprogramming that enhances glycolytic and anabolic processes. These findings support a role of the GFI1-36N protein variant in facilitating a metabolic state favorable for rapid cell proliferation [39], which is further corroborated by increased glucose consumption and lactate secretion. Such shifts in metabolism are often associated with aggressive cancer phenotypes by providing energy and biosynthetic precursors required for uncontrolled cell growth and survival [40]. Therefore, we performed a drug screen and observed that GFI1-36N-MLL-AF9 cells exhibited resistance to UK5099, etomoxir, and BPTES, inhibitors of mitochondrial pyruvate transport, fatty acid oxidation, and glutaminase, respectively. This resistance suggests that GFI1-36N leukemic cells may employ alternative metabolic pathways to maintain their proliferation and survival when challenged with drugs targeting mitochondrial metabolism. For instance, the observed enrichment in the pentose phosphate pathway could provide an alternate source of NADPH and ribose-5-phosphate, supporting redox balance and nucleotide synthesis even when pyruvate uptake is inhibited. Such adaptive metabolic flexibility might contribute to therapeutic resistance as well as inferior prognosis and present a significant hurdle in targeting malignant neoplasms with the GFI1-36N variant.
Due to the strong functional similarities between GFI1-36N cells and GFI1-KD cells, it would certainly be interesting in the future to investigate whether the changes in DNA repair that have already been observed in GFI1-36N-MLL-AF9 cells can also be detected in GFI1-KD cells [6]. This could provide targeted therapies for patients with a low GFI1 level, and not only those with the GFI1-36N SNP. On another note, there is considerable interest as to whether the upregulation of the FOXO1-MYC axis, which is regarded as the cause of increased OXPHOS in GFI1-KD cells, is also evident in GFI1-36N cells. This question could not be answered in the context of the present study, but it does offer promising approaches for further research. Moreover, it would be interesting to discover whether such changes occur in other neoplasms to which the GFI1 variant also predisposes [2,3,4].
In summary, the results presented here emphasize that the presence of the GFI1-36N variant in cell lines as well as in a murine model of human AML contributes to DNA damage through alterations in mitochondrial function and metabolism and that this may provide a further explanation for the accumulation of genetic damage and the poorer prognosis of carriers of the GFI1-36N SNP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13010107/s1, Figure S1: Generation of MLL-AF9 leukemic GFI1-36S and GFI1-36N mice by primary bone marrow (BM) transplantation; Figure S2: Flow cytometry discovers alterations in mitochondrial metabolism; Figure S3: γ-H2AX+ GFI1-36S and GFI1-36N cells after treatment with N-acetylcysteine (NAC) or α-tocopherol (αT); Figure S4: Altered pathways in GFI1-36N-MLL-AF9 cells detected by proteomics; Figure S5: Glucose consumption and lactate secretion in MLL-AF9 leukemic cells.
Author Contributions
Conceptualization, J.V., L.L. and C.K.; methodology, J.V., L.L. and C.K.; software, J.V., L.L. and T.H.S.; formal analysis, J.V., L.L, T.H.S., D.F., H.M.M.A. and P.K.P.; investigation, J.V., L.L., T.H.S., D.F., H.M.M.A. and P.K.P.; data curation, J.V., L.L., T.H.S., D.F., H.M.M.A. and P.K.P.; writing—original draft preparation, J.V.; writing—review and editing, J.V., M.K., E.D., B.O., N.v.B. and C.K.; supervision, N.v.B. and C.K.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the German José Carreras Foundation (DJCLS, 17R/2018), the German Cancer Aid (DKH, 70112392), the German Research Foundation (DFG, KH331/2-3), and the Interdisciplinary Center for Clinical Research Münster (IZKF, Kha2/002/20). J.V. received fellowships from the Jürgen Manchot Foundation and the Medical College Münster (MedK).
Institutional Review Board Statement
The animal experiments conducted as part of this project were approved by the Faculty of Medicine at the University of Münster and by the local authorities. Both an ethics vote (2019-480-f-S) and an animal experiment approval (81-02.04.2019.A440/GFI1 als Ansatz in der Leukämietherapie) are available. The requirements for expertise in animal experimentation of the German Animal Welfare Ordinance (TierSchVersV) were met. The subsequent notification for the project “81-02.04.2019.A440/GFI1 als Ansatz in der Leukämietherapie” was approved by the local authorities.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the employees of the Central Animal Facilities at the University of Münster and the University of Lübeck for taking care of the mice used in the experiments. We thank Hannelore Leuschke and Dagmar Clemens, University of Münster, for genotyping the mice and for their technical assistance. We would also sincerely like to thank Subbaiah Chary Nimmagadda, University of Lübeck, for critically proofreading the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vadnais, C.; Chen, R.; Fraszczak, J.; Yu, Z.; Boulais, J.; Pinder, J.; Frank, D.; Khandanpour, C.; Hébert, J.; Dellaire, G.; et al. GFI1 facilitates efficient DNA repair by regulating PRMT1 dependent methylation of MRE11 and 53BP1. Nat. Commun. 2018, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Khandanpour, C.; Thiede, C.; Valk, P.J.; Sharif-Askari, E.; Nückel, H.; Lohmann, D.; Horsthemke, B.; Siffert, W.; Neubauer, A.; Grzeschik, K.H.; et al. A variant allele of Growth Factor Independence 1 (GFI1) is associated with acute myeloid leukemia. Blood 2010, 115, 2462–2472. [Google Scholar] [CrossRef]
- Hönes, J.M.; Botezatu, L.; Helness, A.; Vadnais, C.; Vassen, L.; Robert, F.; Hergenhan, S.M.; Thivakaran, A.; Schütte, J.; Al-Matary, Y.S.; et al. GFI1 as a novel prognostic and therapeutic factor for AML/MDS. Leukemia 2016, 30, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, L.; Michel, L.C.; Makishima, H.; Schroeder, T.; Germing, U.; Haas, R.; van der Reijden, B.; Marneth, A.E.; Bergevoet, S.M.; Jansen, J.H.; et al. GFI1(36N) as a therapeutic and prognostic marker for myelodysplastic syndrome. Exp. Hematol. 2016, 44, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, J.; Sun, K.; Frank, D.; Neumann, F.; Hüve, J.; Budde, P.M.; Liu, L.; Xie, X.; Patnana, P.K.; Ahmed, H.M.M.; et al. Presence of the GFI1-36N single nucleotide polymorphism enhances the response of MLL-AF9 leukemic cells to CDK4/6 inhibition. Front. Oncol. 2022, 12, 903691. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Patnana, P.K.; Vorwerk, J.; Mao, L.; Gopal, L.M.; Jung, N.; Hennig, T.; Ruhnke, L.; Frenz, J.M.; Kuppusamy, M.; et al. Germ line variant GFI1-36N affects DNA repair and sensitizes AML cells to DNA damage and repair therapy. Blood 2023, 142, 2175–2191. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular stress responses in radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Fiolka, K.; Hertzano, R.; Vassen, L.; Zeng, H.; Hermesh, O.; Avraham, K.B.; Dührsen, U.; Möröy, T. Gfi1 and Gfi1b act equivalently in haematopoiesis, but have distinct, non-overlapping functions in inner ear development. EMBO Rep. 2006, 7, 326–333. [Google Scholar] [CrossRef]
- Zuber, J.; Radtke, I.; Pardee, T.S.; Zhao, Z.; Rappaport, A.R.; Luo, W.; McCurrach, M.E.; Yang, M.M.; Dolan, M.E.; Kogan, S.C.; et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009, 23, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Loberg, M.A.; Eudy, E.; Schwartz, L.S.; Mujica, K.D.; Trowbridge, J.J. Heritable genetic background alters survival and phenotype of Mll-AF9-induced leukemias. Exp. Hematol. 2020, 89, 61–67.e3. [Google Scholar] [CrossRef] [PubMed]
- Marneth, A.E.; Botezatu, L.; Hönes, J.M.; Israël, J.C.L.; Schütte, J.; Vassen, L.; Lams, R.F.; Bergevoet, S.M.; Groothuis, L.; Mandoli, A.; et al. GFI1 is required for RUNX1/ETO positive acute myeloid leukemia. Haematologica 2018, 103, e395–e399. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Seo, H.S.; Zhang, T.; Wang, Y.; Jiang, B.; Li, Q.; Buckley, D.L.; Nabet, B.; Roberts, J.M.; Paulk, J.; et al. MELK is not necessary for the proliferation of basal-like breast cancer cells. Elife 2017, 6, e26693. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Zhu, B.; Wang, L.; Chen, C.; Hong, M.; Huang, Y.; Li, H.; Han, H.; Cai, B.; et al. Increasing the efficiency and targeting range of cytidine base editors through fusion of a single-stranded DNA-binding protein domain. Nat. Cell Biol. 2020, 22, 740–750. [Google Scholar] [CrossRef]
- Liu, L.; Patnana, P.K.; Xie, X.; Frank, D.; Nimmagadda, S.C.; Su, M.; Zhang, D.; Koenig, T.; Rosenbauer, F.; Liebmann, M.; et al. GFI1B acts as a metabolic regulator in hematopoiesis and acute myeloid leukemia. Leukemia 2022, 36, 2196–2207. [Google Scholar] [CrossRef]
- Patnana, P.K.; Liu, L.; Frank, D.; Nimmagadda, S.C.; Behrens, M.; Ahmed, H.; Xie, X.; Liebmann, M.; Wei, L.; Gerdemann, A.; et al. Dose-dependent expression of GFI1 alters metabolism in the haematopoietic progenitors and MLL::AF9-induced leukaemic cells. Br. J. Haematol. 2023, 202, 1033–1048. [Google Scholar] [CrossRef]
- Plitzko, B.; Loesgen, S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio Protoc. 2018, 8, e2850. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets. 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Rassool, F.V.; Gaymes, T.J.; Omidvar, N.; Brady, N.; Beurlet, S.; Pla, M.; Reboul, M.; Lea, N.; Chomienne, C.; Thomas, N.S.; et al. Reactive oxygen species, DNA damage, and error-prone repair: A model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007, 67, 8762–8771. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Collins, L.B.; Chen, T.H.; Herr, N.; Takeda, S.; Sun, W.; Swenberg, J.A.; Nakamura, J. Oxidative stress at low levels can induce clustered DNA lesions leading to NHEJ mediated mutations. Oncotarget 2016, 7, 25377–25390. [Google Scholar] [CrossRef] [PubMed]
- Kaweme, N.M.; Zhou, S.; Changwe, G.J.; Zhou, F. The significant role of redox system in myeloid leukemia: From pathogenesis to therapeutic applications. Biomark. Res. 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Oberley, T.D.; Oberley, L.W. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997, 12, 525–535. [Google Scholar]
- Sun, S.Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010, 9, 109–110. [Google Scholar] [CrossRef]
- Brandt, U. Oxidoreduktasen und reaktive Sauerstoffspezies. In Löffler/Petrides Biochemie und Pathobiochemie; Heinrich, P.C., Müller, M., Graeve, L., Koch, H.G., Eds.; Springer: Heidelberg, Germany, 2022; pp. 329–335. [Google Scholar]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. In Vitro 2014, 28, 1006–1015. [Google Scholar] [CrossRef]
- Zappe, K.; Pointner, A.; Switzeny, O.J.; Magnet, U.; Tomeva, E.; Heller, J.; Mare, G.; Wagner, K.H.; Knasmueller, S.; Haslberger, A.G. Counteraction of oxidative stress by vitamin E affects epigenetic regulation by increasing global methylation and gene expression of MLH1 and DNMT1 dose dependently in Caco-2 cells. Oxid. Med. Cell Longev. 2018, 2018, 3734250. [Google Scholar] [CrossRef]
- Ferber, E.C.; Peck, B.; Delpuech, O.; Bell, G.P.; East, P.; Schulze, A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012, 19, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; Weijzen, S.; de Vries-Smits, A.M.; Saarloos, I.; de Ruiter, N.D.; Bos, J.L.; Burgering, B.M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Wojtala, A.; Suski, J.M.; Szabadkai, G.; Wilczynski, G.; Wlodarczyk, J.; Diogo, C.V.; Oliveira, P.J.; Tauber, J.; et al. Disrupted ATP synthase activity and mitochondrial hyperpolarisation-dependent oxidative stress is associated with p66Shc phosphorylation in fibroblasts of NARP patients. Int. J. Biochem. Cell Biol. 2013, 45, 141–150. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Botezatu, L.; Michel, L.C.; Helness, A.; Vadnais, C.; Makishima, H.; Hönes, J.M.; Robert, F.; Vassen, L.; Thivakaran, A.; Al-Matary, Y.; et al. Epigenetic therapy as a novel approach for GFI136N-associated murine/human AML. Exp. Hematol. 2016, 44, 713–726.e14. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updat. 2018, 38, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).