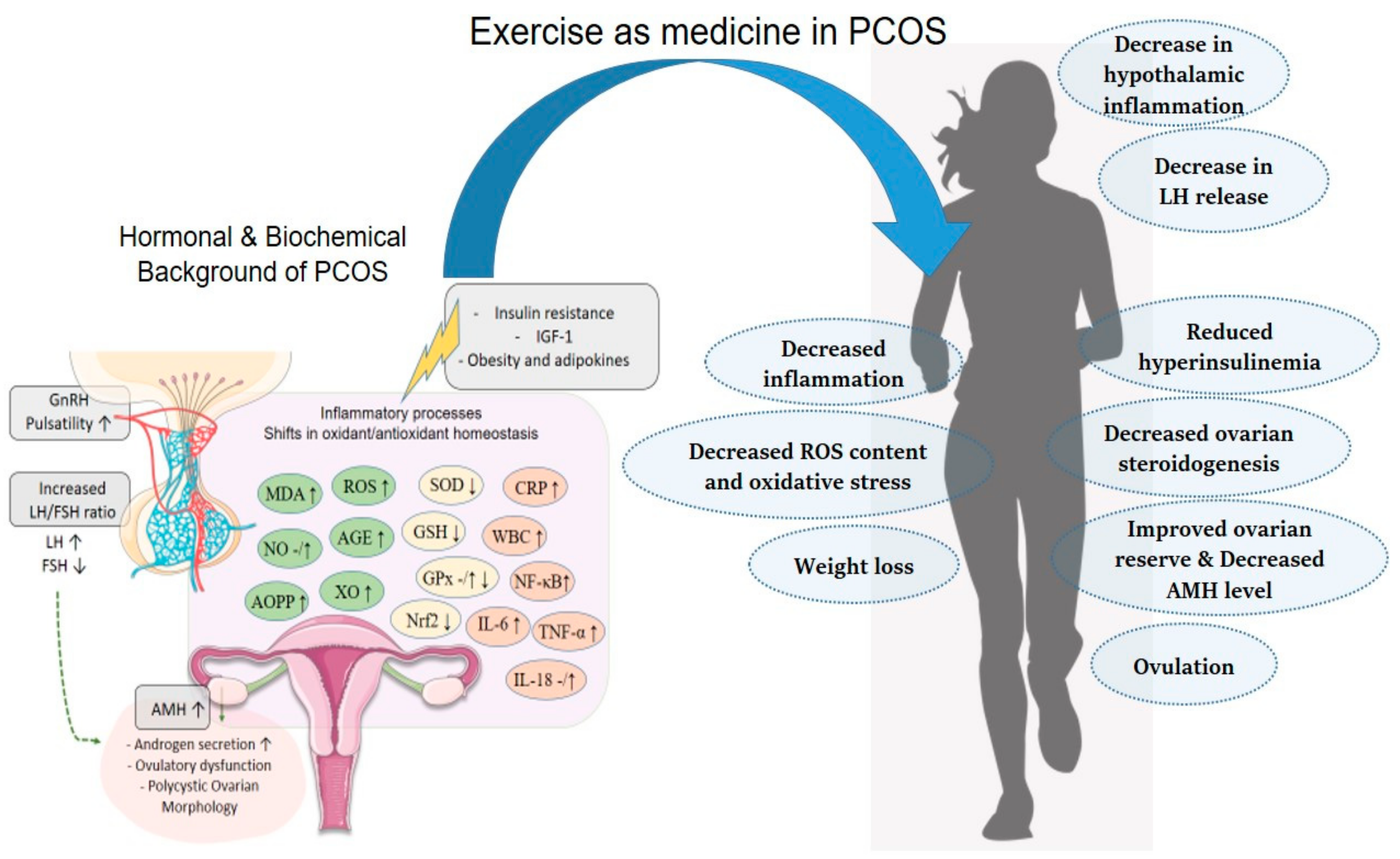
Mechanisms and Target Parameters in Relation to Polycystic Ovary Syndrome and Physical Exercise: Focus on the Master Triad of Hormonal Changes, Oxidative Stress, and Inflammation
Abstract
1. Introduction
Background
2. Hormonal Changes in Patients with PCOS
2.1. Hormones of the HPG Axis and PCOS
2.2. Action of Physical Exercise on the HPG Axis Hormones in PCOS
2.3. Anti-Müllerian Hormone and PCOS
2.4. Effects of Physical Exercise on Anti-Müllerian Hormone in PCOS
2.5. Insulin, Adipokines, and PCOS
2.6. Effects of Physical Exercise on Insulin Sensitivity and Adipokines in PCOS
2.7. IGF-1 and PCOS
2.8. Effects of Physical Exercise on IGF-1 in PCOS
3. Role of Oxidative Stress and Inflammation in PCOS
3.1. Oxidative Stress and Lipid Peroxidation
3.1.1. MDA and PCOS
3.1.2. Nitric Oxide and PCOS
3.1.3. AGEs, AOPPs, and PCOS
3.1.4. Xantin Oxidase in PCOS
3.1.5. Mitochondrial DNA and PCOS
3.2. Effects of Physical Exercise on Oxidative Stress Parameters in PCOS
3.3. Antioxidant Defense Mechanisms
3.3.1. Superoxide Dismutase and PCOS
3.3.2. Glutathione Peroxidase, Glutathione, and PCOS
3.3.3. Nrf2 and PCOS
3.4. Effects of Physical Exercise on Antioxidant Parameters in PCOS
3.5. Inflammation
3.5.1. Interleukins and PCOS
3.5.2. TNF-α and PCOS
3.5.3. NF-κB and PCOS
3.6. Effects of Physical Exercise on Inflammatory Parameters in PCOS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polycystic Ovary Syndrome. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/polycystic-ovary-syndrome (accessed on 26 February 2024).
- Azziz, R.; Kintziger, K.; Li, R.; Laven, J.; Morin-Papunen, L.; Merkin, S.S.; Teede, H.; Yildiz, B.O. Recommendations for epidemiologic and phenotypic research in polycystic ovary syndrome: An androgen excess and PCOS society resource. Hum. Reprod. 2019, 34, 2254–2265. [Google Scholar] [CrossRef]
- Ndefo, U.A.; Eaton, A.; Green, M.R. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. Pharm. Ther. 2013, 38, 336–355. [Google Scholar]
- Garg, A.; Patel, B.; Abbara, A.; Dhillo, W.S. Treatments targeting neuroendocrine dysfunction in polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2022, 97, 156–164. [Google Scholar] [CrossRef]
- Rosenfield, R.L. The Polycystic Ovary Morphology-Polycystic Ovary Syndrome Spectrum. J. Pediatr. Adolesc. Gynecol. 2015, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Chavez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejias, J.; Calvo, M.; Bermudez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef]
- Liu, S.; Navarro, G.; Mauvais-Jarvis, F. Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS ONE 2010, 5, e11302. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Al-Farsi, Y.M.; Al-Khaduri, M.M.; Saleh, J.; Waly, M.I. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int. J. Womens Health 2018, 10, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Enechukwu, C.I.; Onuegbu, A.J.; Olisekodiaka, M.J.; Eleje, G.U.; Ikechebelu, J.I.; Ugboaja, J.O.; Amah, U.K.; Okwara, J.E.; Igwegbe, A.O. Oxidative stress markers and lipid profiles of patients with polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet. Gynecol. Sci. 2019, 62, 335–343. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Waqar, A.; Jain, A.; Joseph, C.; Srivastava, K.; Ochuba, O.; Alkayyali, T.; Ruo, S.W.; Poudel, S. Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate. Cureus 2021, 13, e17887. [Google Scholar] [CrossRef] [PubMed]
- Velez, L.M.; Seldin, M.; Motta, A.B. Inflammation and reproductive function in women with polycystic ovary syndromedagger. Biol. Reprod. 2021, 104, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Mizutani, T.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Fujita, M.; Tsuyoshi, H.; Yoshida, Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front. Endocrinol. 2023, 14, 1324429. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, Z.; Zhang, W. The role of macrophages in polycystic ovarian syndrome and its typical pathological features: A narrative review. Biomed. Pharmacother. 2023, 167, 115470. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Butt, M.S.; Saleem, J.; Zakar, R.; Aiman, S.; Khan, M.Z.; Fischer, F. Benefits of physical activity on reproductive health functions among polycystic ovarian syndrome women: A systematic review. BMC Public Health 2023, 23, 882. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef]
- Yang, H.; Di, J.; Pan, J.; Yu, R.; Teng, Y.; Cai, Z.; Deng, X. The Association Between Prolactin and Metabolic Parameters in PCOS Women: A Retrospective Analysis. Front. Endocrinol. 2020, 11, 263. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z. Hypersecretion of basal luteinizing hormone and an increased risk of pregnancy loss among women with polycystic ovary syndrome undergoing controlled ovarian stimulation and intrauterine insemination. Heliyon 2023, 9, e16233. [Google Scholar] [CrossRef]
- Emanuel, R.H.K.; Roberts, J.; Docherty, P.D.; Lunt, H.; Campbell, R.E.; Moller, K. A review of the hormones involved in the endocrine dysfunctions of polycystic ovary syndrome and their interactions. Front. Endocrinol. 2022, 13, 1017468. [Google Scholar] [CrossRef]
- Lee, J.E.; Yoon, S.H.; Kim, H.O.; Min, E.G. Correlation between the serum luteinizing hormone to folliclestimulating hormone ratio and the anti-Mullerian hormone levels in normo-ovulatory women. J. Korean Med. Sci. 2015, 30, 296–300. [Google Scholar] [CrossRef]
- Barlampa, D.; Bompoula, M.S.; Bargiota, A.; Kalantaridou, S.; Mastorakos, G.; Valsamakis, G. Hypothalamic Inflammation as a Potential Pathophysiologic Basis for the Heterogeneity of Clinical, Hormonal, and Metabolic Presentation in PCOS. Nutrients 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.X.; Al-Massadi, O.; Donelan, E.; Lehti, M.; Weber, J.; Ress, C.; Trivedi, C.; Muller, T.D.; Woods, S.C.; Hofmann, S.M. Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol. Behav. 2012, 106, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front. Horm. Res. 2016, 47, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Babaei Bonab, S.; Parvaneh, M. Effect of 12-week of aerobic exercise on hormones and lipid profile status in adolescent girls with polycystic ovary syndrome: A study during COVID-19. Sci. Sports 2023, 38, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Jedel, E.; Labrie, F.; Oden, A.; Holm, G.; Nilsson, L.; Janson, P.O.; Lind, A.K.; Ohlsson, C.; Stener-Victorin, E. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: A randomized controlled trial. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E37–E45. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, L.; Rice, S.; Mason, H.D. Anti-Mullerian hormone and polycystic ovary syndrome: A mountain too high? Reproduction 2010, 139, 825–833. [Google Scholar] [CrossRef]
- Homburg, R.; Crawford, G. The role of AMH in anovulation associated with PCOS: A hypothesis. Hum. Reprod. 2014, 29, 1117–1121. [Google Scholar] [CrossRef]
- Kiranmayee, D.; Praveena, T.; Himabindu, Y.; Sriharibabu, M.; Kavya, K.; Mahalakshmi, M. The Effect of Moderate Physical Activity on Ovarian Reserve Markers in Reproductive Age Women Below and Above 30 Years. J. Hum. Reprod. Sci. 2017, 10, 44–48. [Google Scholar]
- Moran, L.J.; Harrison, C.L.; Hutchison, S.K.; Stepto, N.K.; Strauss, B.J.; Teede, H.J. Exercise decreases anti-mullerian hormone in anovulatory overweight women with polycystic ovary syndrome: A pilot study. Horm. Metab. Res. 2011, 43, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Al-Eisa, E.; Gabr, S.A.; Alghadir, A.H. Effects of supervised aerobic training on the levels of anti-Mullerian hormone and adiposity measures in women with normo-ovulatory and polycystic ovary syndrome. J. Pak. Med. Assoc. 2017, 67, 499–507. [Google Scholar] [PubMed]
- Wu, X.; Wu, H.; Sun, W.; Wang, C. Improvement of anti-Mullerian hormone and oxidative stress through regular exercise in Chinese women with polycystic ovary syndrome. Hormones 2021, 20, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Cadagan, D.; Khan, R.; Amer, S. Thecal cell sensitivity to luteinizing hormone and insulin in polycystic ovarian syndrome. Reprod. Biol. 2016, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Munir, I.; Yen, H.W.; Geller, D.H.; Torbati, D.; Bierden, R.M.; Weitsman, S.R.; Agarwal, S.K.; Magoffin, D.A. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 2004, 145, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Schuler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Polak, K.; Czyzyk, A.; Simoncini, T.; Meczekalski, B. New markers of insulin resistance in polycystic ovary syndrome. J. Endocrinol. Investig. 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Woodward, A.; Klonizakis, M.; Broom, D. Exercise and Polycystic Ovary Syndrome. Adv. Exp. Med. Biol. 2020, 1228, 123–136. [Google Scholar] [CrossRef]
- Stepto, N.K.; Hiam, D.; Gibson-Helm, M.; Cassar, S.; Harrison, C.L.; Hutchison, S.K.; Joham, A.E.; Canny, B.J.; Moreno-Asso, A.; Strauss, B.J.; et al. Exercise and insulin resistance in PCOS: Muscle insulin signalling and fibrosis. Endocr. Connect. 2020, 9, 346–359. [Google Scholar] [CrossRef]
- Hansen, S.L.; Bojsen-Moller, K.N.; Lundsgaard, A.M.; Hendrich, F.L.; Nilas, L.; Sjoberg, K.A.; Hingst, J.R.; Serup, A.K.; Olguin, C.H.; Carl, C.S.; et al. Mechanisms Underlying Absent Training-Induced Improvement in Insulin Action in Lean, Hyperandrogenic Women With Polycystic Ovary Syndrome. Diabetes 2020, 69, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Dantas, W.S.; Marcondes, J.A.; Shinjo, S.K.; Perandini, L.A.; Zambelli, V.O.; Neves, W.D.; Barcellos, C.R.; Rocha, M.P.; Yance Vdos, R.; Pereira, R.T.; et al. GLUT4 translocation is not impaired after acute exercise in skeletal muscle of women with obesity and polycystic ovary syndrome. Obesity 2015, 23, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; Lamonte, M.J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sports Med. 2013, 43, 243–256. [Google Scholar] [CrossRef]
- Tersigni, C.; Di Nicuolo, F.; D’Ippolito, S.; Veglia, M.; Castellucci, M.; Di Simone, N. Adipokines: New emerging roles in fertility and reproduction. Obstet. Gynecol. Surv. 2011, 66, 47–63. [Google Scholar] [CrossRef]
- de Souza, H.C.D.; Philbois, S.V.; de Paula Facioli, T.; Ferriani, R.A.; Gastaldi, A.C. Aerobic physical training impact on adipokines in women with polycystic ovary syndrome—Effects of body fat percentage. Arch. Endocrinol. Metab. 2022, 66, 837–847. [Google Scholar] [CrossRef]
- Shele, G.; Genkil, J.; Speelman, D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J. Funct. Morphol. Kinesiol. 2020, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Fu, S.; Lv, H. Association of Insulin-Like Growth Factor-1 With Polycystic Ovarian Syndrome: A Systematic Review and Meta-analysis. Endocr. Pract. 2023, 29, 388–397. [Google Scholar] [CrossRef]
- Premoli, A.C.; Santana, L.F.; Ferriani, R.A.; Moura, M.D.; De Sa, M.F.; Reis, R.M. Growth hormone secretion and insulin-like growth factor-1 are related to hyperandrogenism in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N.; Thuesen, B.; Jorgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The association between IGF-I and insulin resistance: A general population study in Danish adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Frystyk, J.; Moller, N.; Dall, R.; Chen, J.W.; Nielsen, S.C.; Christiansen, J.S.; Jorgensen, J.O.; Flyvbjerg, A. The effect of submaximal exercise on immuno- and bioassayable IGF-I activity in patients with GH-deficiency and healthy subjects. Growth Horm. IGF Res. 2005, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gulick, C.N.; Peddie, M.C.; Jowett, T.; Hackney, A.C.; Rehrer, N.J. Exercise, Dietary Protein, and Combined Effect on IGF-1. Int. J. Sci. Res. Methodol. 2020, 16, 61–77. [Google Scholar]
- Stener-Victorin, E.; Jedel, E.; Janson, P.O.; Sverrisdottir, Y.B. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R387–R395. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapalowska-Chwyc, M.; Drozd, A.; Maciejewska, D.; Starczewski, A.; Wysokinski, P.; Stachowska, E. Changes in the IGF-1 and TNF-alpha synthesis pathways before and after three-month reduction diet with low glicemic index in women with PCOS. Ginekol. Pol. 2018, 89, 295–303. [Google Scholar] [CrossRef]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramirez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Kuscu, N.K.; Var, A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2009, 88, 612–617. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, W.Y.; Liao, H.; Wang, C.F.; Sun, Y. The effects of oxidative stress to PCOS. Sichuan Da Xue Xue Bao Yi Xue Ban 2008, 39, 421–423. [Google Scholar] [PubMed]
- Awonuga, A.O.; Camp, O.G.; Abu-Soud, H.M. A review of nitric oxide and oxidative stress in typical ovulatory women and in the pathogenesis of ovulatory dysfunction in PCOS. Reprod. Biol. Endocrinol. 2023, 21, 111. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Novel Insights on the Role of Nitric Oxide in the Ovary: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 980. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Cakmak, M.; Kiran, R.T.; Sahin, I. Oxidative Stress Status, Metabolic Profile and Cardiovascular Risk Factors in Patients with Polycystic Ovary Syndrome. Med-Science 2012, 1, 27–34. [Google Scholar] [CrossRef]
- Unoki, H.; Yamagishi, S. Advanced glycation end products and insulin resistance. Curr. Pharm. Des. 2008, 14, 987–989. [Google Scholar] [CrossRef]
- Lin, P.H.; Chang, C.C.; Wu, K.H.; Shih, C.K.; Chiang, W.; Chen, H.Y.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M.; et al. Dietary Glycotoxins, Advanced Glycation End Products, Inhibit Cell Proliferation and Progesterone Secretion in Ovarian Granulosa Cells and Mimic PCOS-like Symptoms. Biomolecules 2019, 9, 327. [Google Scholar] [CrossRef]
- Hyderali, B.N.; Mala, K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 15–22. [Google Scholar] [CrossRef]
- Isik, H.; Aynioglu, O.; Timur, H.; Sahbaz, A.; Harma, M.; Can, M.; Guven, B.; Alptekin, H.; Kokturk, F. Is Xanthine oxidase activity in polycystic ovary syndrome associated with inflammatory and cardiovascular risk factors? J. Reprod. Immunol. 2016, 116, 98–103. [Google Scholar] [CrossRef]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Ciebiera, M.; Hennig, E.E. Polycystic Ovary Syndrome and Oxidative Stress-From Bench to Bedside. Int. J. Mol. Sci. 2023, 24, 14126. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, D.J.; Lee, H.S.; Kim, T.J.; Kim, M.H.; Jeong, H.J.; Im, J.A.; Lee, D.C.; Lee, J.W. Mitochondrial DNA copy number in peripheral blood in polycystic ovary syndrome. Metabolism 2011, 60, 1677–1682. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Zhu, X.; Wei, Y.; Zhao, W.; Si, S.; Li, Y. A Mitochondrial Perspective on Noncommunicable Diseases. Biomedicines 2023, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Malamouli, M.; Levinger, I.; McAinch, A.J.; Trewin, A.J.; Rodgers, R.J.; Moreno-Asso, A. The mitochondrial profile in women with polycystic ovary syndrome: Impact of exercise. J. Mol. Endocrinol. 2022, 68, R11–R23. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Seleem, A.K.; El Refaeey, A.A.; Shaalan, D.; Sherbiny, Y.; Badawy, A. Superoxide dismutase in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2014, 31, 499–504. [Google Scholar] [CrossRef]
- Sabuncu, T.; Vural, H.; Harma, M.; Harma, M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin. Biochem. 2001, 34, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bizon, A.; Tchorz, A.; Madej, P.; Lesniewski, M.; Wojtowicz, M.; Piwowar, A.; Franik, G. The Activity of Superoxide Dismutase, Its Relationship with the Concentration of Zinc and Copper and the Prevalence of rs2070424 Superoxide Dismutase Gene in Women with Polycystic Ovary Syndrome-Preliminary Study. J. Clin. Med. 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Uckan, K.; Demir, H.; Turan, K.; Sarikaya, E.; Demir, C. Role of Oxidative Stress in Obese and Nonobese PCOS Patients. Int. J. Clin. Pract. 2022, 2022, 4579831. [Google Scholar] [CrossRef] [PubMed]
- Chelchowska, M.; Jurczewska, J.; Gajewska, J.; Mazur, J.; Szostak-Wegierek, D.; Rudnicka, E.; Ambroszkiewicz, J. Antioxidant Defense Expressed as Glutathione Status and Keap1-Nrf2 System Action in Relation to Anthropometric Parameters and Body Composition in Young Women with Polycystic Ovary Syndrome. Antioxidants 2023, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, Z.; Aleyasin, A.; Eslami, M.; Nekoonam, S.; Zendedel, A.; Bahramrezaie, M.; Amidi, F. Quercetin protects human granulosa cells against oxidative stress via thioredoxin system. Reprod. Biol. 2019, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, N.; Zeng, Z.; Tang, L.; Zhao, S.; Zhou, F.; Zhou, L.; Xia, W.; Zhu, C.; Rao, M. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol. Hum. Reprod. 2021, 27, gaaa081. [Google Scholar] [CrossRef]
- Taheri, M.; Hayati Roudbari, N.; Amidi, F.; Parivar, K. The protective effect of sulforaphane against oxidative stress in granulosa cells of patients with polycystic ovary syndrome (PCOS) through activation of AMPK/AKT/NRF2 signaling pathway. Reprod. Biol. 2021, 21, 100563. [Google Scholar] [CrossRef] [PubMed]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Liao, B.; Qiao, J.; Pang, Y. Central Regulation of PCOS: Abnormal Neuronal-Reproductive-Metabolic Circuits in PCOS Pathophysiology. Front. Endocrinol. 2021, 12, 667422. [Google Scholar] [CrossRef]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Tang, R.; Ding, X.; Zhu, J. Kisspeptin and Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 298. [Google Scholar] [CrossRef]
- Shabbir, S.; Khurram, E.; Moorthi, V.S.; Eissa, Y.T.H.; Kamal, M.A.; Butler, A.E. The interplay between androgens and the immune response in polycystic ovary syndrome. J. Transl. Med. 2023, 21, 259. [Google Scholar] [CrossRef]
- Banerjee, S.; Cooney, L.G.; Stanic, A.K. Immune Dysfunction in Polycystic Ovary Syndrome. Immunohorizons 2023, 7, 323–332. [Google Scholar] [CrossRef]
- Ascani, A.; Torstensson, S.; Risal, S.; Lu, H.; Eriksson, G.; Li, C.; Teschl, S.; Menezes, J.; Sandor, K.; Ohlsson, C.; et al. The role of B cells in immune cell activation in polycystic ovary syndrome. eLife 2023, 12, e86454. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, A.; Prabhu, Y.D.; Valsala Gopalakrishnan, A. Role of IL-6 signalling in Polycystic Ovarian Syndrome associated inflammation. J. Reprod. Immunol. 2020, 141, 103155. [Google Scholar] [CrossRef]
- Vasyukova, E.; Zaikova, E.; Kalinina, O.; Gorelova, I.; Pyanova, I.; Bogatyreva, E.; Vasilieva, E.; Grineva, E.; Popova, P. Inflammatory and Anti-Inflammatory Parameters in PCOS Patients Depending on Body Mass Index: A Case-Control Study. Biomedicines 2023, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Sun, Y.; Lv, X.; Zhang, H.; Liu, C.; Dai, S. Interleukin-6 Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0148531. [Google Scholar] [CrossRef] [PubMed]
- Fulghesu, A.M.; Sanna, F.; Uda, S.; Magnini, R.; Portoghese, E.; Batetta, B. IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm. 2011, 2011, 389317. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; Wegman, A.; Koes, B.; Mehta, S.; King, C.A. Serum IL-1RA levels increase from follicular to luteal phase of the ovarian cycle: A pilot study on human female immune responses. PLoS ONE 2020, 15, e0238520. [Google Scholar] [CrossRef]
- Wan, S.; Chen, Q.; Xiang, Y.; Sang, Y.; Tang, M.; Song, Y.; Feng, G.; Ye, B.; Bai, L.; Zhu, Y. Interleukin-1 increases cyclooxygenase-2 expression and prostaglandin E2 production in human granulosa-lutein cell via nuclear factor kappa B/P65 and extracellular signal-regulated kinase 1/2 signaling pathways. Mol. Cell. Endocrinol. 2023, 566–567, 111891. [Google Scholar] [CrossRef]
- Zafari Zangeneh, F.; Naghizadeh, M.M.; Masoumi, M. Polycystic ovary syndrome and circulating inflammatory markers. Int. J. Reprod. Biomed. 2017, 15, 375–382. [Google Scholar] [CrossRef]
- Luotola, K.; Piltonen, T.T.; Puurunen, J.; Tapanainen, J.S. IL-1 receptor antagonist levels are associated with glucose tolerance in polycystic ovary syndrome. Clin. Endocrinol. 2016, 85, 430–435. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, J.; Li, R.; Li, M.Z. Is interleukin-18 associated with polycystic ovary syndrome? Reprod. Biol. Endocrinol. 2011, 9, 7. [Google Scholar] [CrossRef]
- Kabakchieva, P.; Gateva, A.; Velikova, T.; Georgiev, T.; Yamanishi, K.; Okamura, H.; Kamenov, Z. Elevated levels of interleukin-18 are associated with several indices of general and visceral adiposity and insulin resistance in women with polycystic ovary syndrome. Arch. Endocrinol. Metab. 2022, 66, 3–11. [Google Scholar] [CrossRef]
- Kaya, C.; Pabuccu, R.; Berker, B.; Satiroglu, H. Plasma interleukin-18 levels are increased in the polycystic ovary syndrome: Relationship of carotid intima-media wall thickness and cardiovascular risk factors. Fertil. Steril. 2010, 93, 1200–1207. [Google Scholar] [CrossRef]
- Lindholm, A.; Blomquist, C.; Bixo, M.; Dahlbom, I.; Hansson, T.; Sundstrom Poromaa, I.; Buren, J. No difference in markers of adipose tissue inflammation between overweight women with polycystic ovary syndrome and weight-matched controls. Hum. Reprod. 2011, 26, 1478–1485. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Thathapudi, S.; Kodati, V.; Erukkambattu, J.; Katragadda, A.; Addepally, U.; Hasan, Q. Tumor necrosis factor-alpha and polycystic ovarian syndrome: A clinical, biochemical, and molecular genetic study. Genet. Test. Mol. Biomark. 2014, 18, 605–609. [Google Scholar] [CrossRef]
- Gao, L.; Gu, Y.; Yin, X. High Serum Tumor Necrosis Factor-Alpha Levels in Women with Polycystic Ovary Syndrome: A Meta-Analysis. PLoS ONE 2016, 11, e0164021. [Google Scholar] [CrossRef]
- Orostica, L.; Astorga, I.; Plaza-Parrochia, F.; Vera, C.; Garcia, V.; Carvajal, R.; Gabler, F.; Romero, C.; Vega, M. Proinflammatory environment and role of TNF-alpha in endometrial function of obese women having polycystic ovarian syndrome. Int. J. Obes. 2016, 40, 1715–1722. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)-Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef]
- Gonzalez, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1508–1512. [Google Scholar] [CrossRef]
- Elbandrawy, A.M.; Yousef, A.M.; Morgan, E.N.; Ewais, N.F.; Eid, M.M.; Elkholi, S.M.; Abdelbasset, W.K. Effect of aerobic exercise on inflammatory markers in polycystic ovary syndrome: A randomized controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3506–3513. [Google Scholar] [CrossRef]
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Castillo, M.C.; Reighard, A.E.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, C.E.; Börzsei, D.; Hoffmann, A.; Varga, C.; Szabó, R. Mechanisms and Target Parameters in Relation to Polycystic Ovary Syndrome and Physical Exercise: Focus on the Master Triad of Hormonal Changes, Oxidative Stress, and Inflammation. Biomedicines 2024, 12, 560. https://doi.org/10.3390/biomedicines12030560
Lőrincz CE, Börzsei D, Hoffmann A, Varga C, Szabó R. Mechanisms and Target Parameters in Relation to Polycystic Ovary Syndrome and Physical Exercise: Focus on the Master Triad of Hormonal Changes, Oxidative Stress, and Inflammation. Biomedicines. 2024; 12(3):560. https://doi.org/10.3390/biomedicines12030560
Chicago/Turabian StyleLőrincz, Csanád Endre, Denise Börzsei, Alexandra Hoffmann, Csaba Varga, and Renáta Szabó. 2024. "Mechanisms and Target Parameters in Relation to Polycystic Ovary Syndrome and Physical Exercise: Focus on the Master Triad of Hormonal Changes, Oxidative Stress, and Inflammation" Biomedicines 12, no. 3: 560. https://doi.org/10.3390/biomedicines12030560
APA StyleLőrincz, C. E., Börzsei, D., Hoffmann, A., Varga, C., & Szabó, R. (2024). Mechanisms and Target Parameters in Relation to Polycystic Ovary Syndrome and Physical Exercise: Focus on the Master Triad of Hormonal Changes, Oxidative Stress, and Inflammation. Biomedicines, 12(3), 560. https://doi.org/10.3390/biomedicines12030560






