Differential Role of Factor XIII in Acute Myocardial Infarction and Ischemic Stroke
Abstract
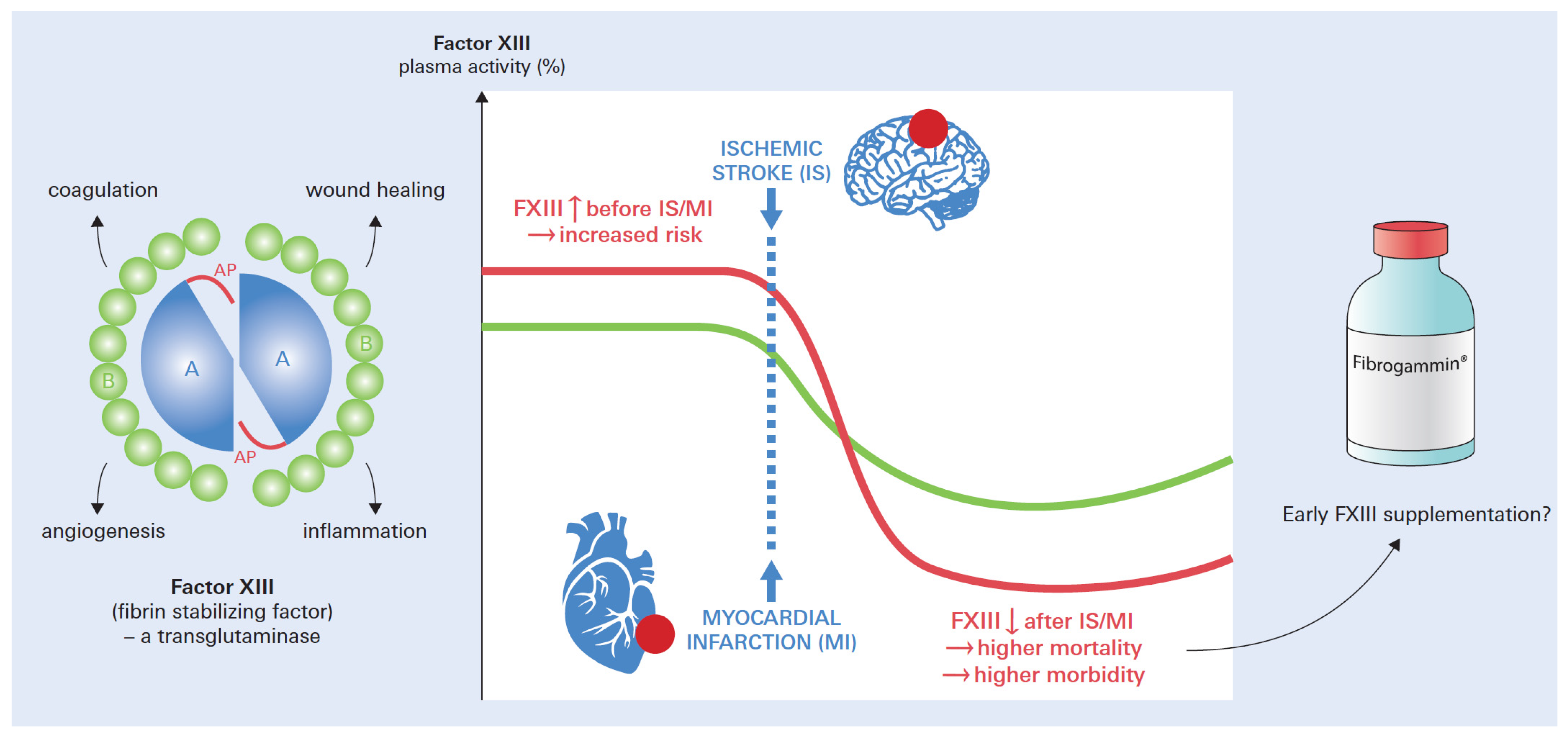
1. Introduction
2. Pathophysiology—Similarities and Differences
3. Coagulation Factor XIII
4. Factor XIII Deficiencies—Congenital vs. Acquired
5. Plasma vs. Tissue Quantification of FXIII Activity
6. Factor XIII in Myocardial Infarction
6.1. FXIII and Risk of Myocardial Infarction
6.2. FXIII Dynamics in Myocardial Infarction
6.3. Prognostic Significance of FXIII after Myocardial Infarction
6.4. Val34Leu FXIII Polymorphism in Myocardial Infarction
6.5. Role of His95Arg FXIII Polymorphism in Myocardial Infarction
6.6. Diagnostic Considerations
6.7. Therapeutic Considerations
7. Factor XIII in Ischemic Stroke
7.1. FXIII in Ischemic Stroke
7.2. Genetic Variants of FXIII in Ischemic Stroke
7.3. Val34Leu Polymorphism
7.4. FXIII Polymorphisms and FXIIIB
7.5. FXIII Polymorphisms and Pediatric Strokes
7.6. FXIII Polymorphisms and Outcome
7.7. FXIII Levels and Outcome
7.8. Imaging of FXIII in Ischemic Stroke
7.9. Therapeutic Implications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dauerman, H.L.; Ibanez, B. The Edge of Time in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1871–1874. [Google Scholar] [CrossRef]
- Murphy, A.; Goldberg, S. Mechanical Complications of Myocardial Infarction. Am. J. Med. 2022, 135, 1401–1409. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef]
- Shi, D.Y.; Wang, S.J. Advances of Coagulation Factor XIII. Chin. Med. J. 2017, 130, 219–223. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Kar, S. Factor XIIIa inhibitors as potential novel drugs for venous thromboembolism. Eur. J. Med. Chem. 2020, 200, 112442. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komaromi, I.; Katona, E. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, A.H.; Haramura, G.; Bagoly, Z.; Muszbek, L. The combined effect of fibrin formation and factor XIII A subunit Val34Leu polymorphism on the activation of factor XIII in whole plasma. Biochim. Biophys. Acta 2006, 1764, 1420–1423. [Google Scholar] [CrossRef]
- Muszbek, L.; Ariens, R.A.; Ichinose, A.; on behalf of the ISTH SSC subcommittee on factor XIII. Factor XIII: Recommended terms and abbreviations. J. Thromb. Haemost. 2007, 5, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin. Thromb. Hemost. 2016, 42, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Komaromi, I.; Bagoly, Z.; Muszbek, L. Factor XIII: Novel structural and functional aspects. J. Thromb. Haemost. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Marx, G.; Korner, G.; Mou, X.; Gorodetsky, R. Packaging zinc, fibrinogen, and factor XIII in platelet alpha-granules. J. Cell Physiol. 1993, 156, 437–442. [Google Scholar] [CrossRef]
- Katona, E.E.; Ajzner, E.; Toth, K.; Karpati, L.; Muszbek, L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J. Immunol. Methods 2001, 258, 127–135. [Google Scholar] [CrossRef]
- Muszbek, L.; Haramura, G.; Polgar, J. Transformation of cellular factor XIII into an active zymogen transglutaminase in thrombin-stimulated platelets. Thromb. Haemost. 1995, 73, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Yee, V.C.; Hevessy, Z. Blood coagulation factor XIII: Structure and function. Thromb. Res. 1999, 94, 271–305. [Google Scholar] [CrossRef]
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Nature’s biological glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef]
- Dickneite, G.; Herwald, H.; Korte, W.; Allanore, Y.; Denton, C.P.; Matucci Cerinic, M. Coagulation factor XIII: A multifunctional transglutaminase with clinical potential in a range of conditions. Thromb. Haemost. 2015, 113, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Mangla, A.; Hamad, H.; Kumar, A. Factor XIII Deficiency. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hsieh, L.; Nugent, D. Factor XIII deficiency. Haemophilia 2008, 14, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Singh, S.; Ramaraje Urs, S.U.; Oldenburg, J.; Biswas, A. Genetic landscape in coagulation factor XIII associated defects—Advances in coagulation and beyond. Blood Rev. 2023, 59, 101032. [Google Scholar] [CrossRef]
- Biswas, A.; Ivaskevicius, V.; Thomas, A.; Oldenburg, J. Coagulation factor XIII deficiency. Diagnosis, prevalence and management of inherited and acquired forms. Hamostaseologie 2014, 34, 160–166. [Google Scholar] [CrossRef]
- Ivaskevicius, V.; Seitz, R.; Kohler, H.P.; Schroeder, V.; Muszbek, L.; Ariens, R.A.; Seifried, E.; Oldenburg, J.; Study, G. International registry on factor XIII deficiency: A basis formed mostly on European data. Thromb. Haemost. 2007, 97, 914–921. [Google Scholar]
- Yan, M.T.S.; Rydz, N.; Goodyear, D.; Sholzberg, M. Acquired factor XIII deficiency: A review. Transfus. Apher. Sci. 2018, 57, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Duque, P.; Chasco-Ganuza, M.; Ortuzar, A.; Almaraz, C.; Terradillos, E.; Perez-Rus, G.; Pascual, C. Acquired FXIII Deficiency is Associated with High Morbidity. Thromb. Haemost. 2022, 122, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Katona, E.; Penzes, K.; Molnar, E.; Muszbek, L. Measurement of factor XIII activity in plasma. Clin. Chem. Lab. Med. 2012, 50, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bereczky, Z.; Cohan, N.; Muszbek, L. Factor XIII Deficiency. Semin. Thromb. Hemost. 2009, 35, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Aikawa, E.; Figueiredo, J.L.; Stangenberg, L.; van den Borne, S.W.; Blankesteijn, W.M.; Sosnovik, D.E.; Jaffer, F.A.; Tung, C.H.; Weissleder, R. Transglutaminase activity in acute infarcts predicts healing outcome and left ventricular remodelling: Implications for FXIII therapy and antithrombin use in myocardial infarction. Eur. Heart J. 2008, 29, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H. MR in mouse models of cardiac disease. NMR Biomed. 2007, 20, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Shan, L. GKVLAK-(Gadolinium-(1,4,7,10-tetraazacyclododecane-1,4,7-tris(acetic acid t-butyl ester)-10-acetic acid monoamide))-GGGGTVQQEL. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Tung, C.H.; Ho, N.H.; Zeng, Q.; Tang, Y.; Jaffer, F.A.; Reed, G.L.; Weissleder, R. Novel factor XIII probes for blood coagulation imaging. Chembiochem 2003, 4, 897–899. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Keliher, E.J.; Heidt, T.; Leuschner, F.; Truelove, J.; Sena, B.F.; Gorbatov, R.; Iwamoto, Y.; Dutta, P.; Wojtkiewicz, G.; et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013, 127, 2038–2046. [Google Scholar] [CrossRef]
- Shan, L. GKVLAK-(IRIS Blue-(1,4,7,10-tetraazacyclododecane-1,4,7-tris(acetic acid t-butyl ester)-10-acetic acid monoamide))-GGGGTVQQEL. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Zabczyk, M.; Ariens, R.A.S.; Undas, A. Fibrin clot properties in cardiovascular disease: From basic mechanisms to clinical practice. Cardiovasc. Res. 2023, 119, 94–111. [Google Scholar] [CrossRef]
- Francis, C.W.; Connaghan, D.G.; Scott, W.L.; Marder, V.J. Increased plasma concentration of cross-linked fibrin polymers in acute myocardial infarction. Circulation 1987, 75, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; Heymans, S. Factor XIII: The cement of the heart after myocardial infarction? Eur. Heart J. 2008, 29, 427–428. [Google Scholar] [CrossRef]
- Balogh, L.; Katona, E.; Mezei, Z.A.; Kallai, J.; Gindele, R.; Edes, I.; Muszbek, L.; Papp, Z.; Bereczky, Z. Effect of factor XIII levels and polymorphisms on the risk of myocardial infarction in young patients. Mol. Cell Biochem. 2018, 448, 199–209. [Google Scholar] [CrossRef]
- Bereczky, Z.; Balogh, E.; Katona, E.; Czuriga, I.; Edes, I.; Muszbek, L. Elevated factor XIII level and the risk of myocardial infarction in women. Haematologica 2007, 92, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.P.; Schmeisser, G.; Schaffter, A.; Kanuri, S.; Owens, J.; Maatman, B.; Sinha, A.; von der Lohe, E.; Breall, J.A. Prediction of Ischemic Events after Percutaneous Coronary Intervention: Thrombelastography Profiles and Factor XIIIa Activity. TH Open 2018, 2, e173–e181. [Google Scholar] [CrossRef]
- Kohler, H.P.; Ariens, R.A.; Catto, A.J.; Carter, A.M.; Miller, G.J.; Cooper, J.A.; Mansfield, M.W.; Standeven, K.F.; Grant, P.J. Factor XIII A-subunit concentration predicts outcome in stroke subjects and vascular outcome in healthy, middle-aged men. Br. J. Haematol. 2002, 118, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.; Mansfield, M.W.; Grant, P.J. Coagulation factor XIII and cardiovascular disease in UK Asian patients undergoing coronary angiography. Thromb. Haemost. 2001, 85, 408–411. [Google Scholar] [PubMed]
- Girolami, A.; Ferrari, S.; Sambado, L.; Peroni, E.; Cosi, E. Myocardial infarctions and other acute coronary syndromes in rare congenital bleeding disorders: A critical analysis of all reported cases. Clin. Appl. Thromb. Hemost. 2015, 21, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Girolami, A.; Ruzzon, E.; Tezza, F.; Scandellari, R.; Vettore, S.; Girolami, B. Arterial and venous thrombosis in rare congenital bleeding disorders: A critical review. Haemophilia 2006, 12, 345–351. [Google Scholar] [CrossRef]
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132. [Google Scholar] [CrossRef]
- Frey, A.; Gassenmaier, T.; Hofmann, U.; Schmitt, D.; Fette, G.; Marx, A.; Herterich, S.; Boivin-Jahns, V.; Ertl, G.; Bley, T.; et al. Coagulation factor XIII activity predicts left ventricular remodelling after acute myocardial infarction. ESC Heart Fail 2020, 7, 2354–2364. [Google Scholar] [CrossRef]
- Alkjaersig, N.; Fletcher, A.P.; Lewis, M.; Ittyerah, R. Reduction of coagulation factor XIII concentration in patients with myocardial infarction, cerebral infarction, and other thromboembolic disorders. Thromb. Haemost. 1977, 38, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bjerre Knudsen, J.; Gormsen, J.; Skagen, K.; Amtorp, O. Changes in Platelet Functions, Coagulation and Fibrinolysis in Uncomplicated Cases of Acute Myocardial Infarction. Thromb. Haemost. 1979, 42, 1513–1522. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Sosnovik, D.E.; Waterman, P.; Swirski, F.K.; Pande, A.N.; Aikawa, E.; Figueiredo, J.L.; Pittet, M.J.; Weissleder, R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ. Res. 2007, 100, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Hu, K.; Frantz, S.; Jaffer, F.A.; Tung, C.H.; Hiller, K.H.; Voll, S.; Nordbeck, P.; Sosnovik, D.; Gattenlohner, S.; et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 2006, 113, 1196–1202. [Google Scholar] [CrossRef]
- Inbal, A.; Lubetsky, A.; Krapp, T.; Castel, D.; Shaish, A.; Dickneitte, G.; Modis, L.; Muszbek, L.; Inbal, A. Impaired wound healing in factor XIII deficient mice. Thromb. Haemost. 2005, 94, 432–437. [Google Scholar] [CrossRef]
- Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766. [Google Scholar] [CrossRef] [PubMed]
- Stampe, N.K.; Ottenheijm, M.E.; Drici, L.; Wewer Albrechtsen, N.J.; Nielsen, A.B.; Christoffersen, C.; Warming, P.E.; Engstrom, T.; Winkel, B.G.; Jabbari, R.; et al. Discovery of plasma proteins associated with ventricular fibrillation during first ST-elevation myocardial infarction via proteomics. Eur. Heart J. Acute Cardiovasc. Care 2023, zuad125. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Weissleder, R.; Ertl, G. Does FXIII deficiency impair wound healing after myocardial infarction? PLoS ONE 2006, 1, e48. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.P.; Futers, T.S.; Grant, P.J. Prevalence of three common polymorphisms in the A-subunit gene of factor XIII in patients with coronary artery disease. Thromb. Haemost. 1999, 81, 511–515. [Google Scholar] [CrossRef]
- Shemirani, A.H.; Muszbek, L. Rapid detection of the factor XIII Val34Leu (163 G-->T) polymorphism by real-time PCR using fluorescence resonance energy transfer detection and melting curve analysis. Clin. Chem. Lab. Med. 2004, 42, 877–879. [Google Scholar] [CrossRef]
- Tammen, H.; Mohring, T.; Kellmann, M.; Pich, A.; Kreipe, H.H.; Hess, R. Mass spectrometric phenotyping of Val34Leu polymorphism of blood coagulation factor XIII by differential peptide display. Clin. Chem. 2004, 50, 545–551. [Google Scholar] [CrossRef]
- Kreutz, R.P.; Bitar, A.; Owens, J.; Desta, Z.; Breall, J.A.; von der Lohe, E.; Sinha, A.; Vatta, M.; Nystrom, P.; Jin, Y.; et al. Factor XIII Val34Leu polymorphism and recurrent myocardial infarction in patients with coronary artery disease. J. Thromb. Thrombolysis 2014, 38, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wartiovaara, U.; Mikkola, H.; Szoke, G.; Haramura, G.; Karpati, L.; Balogh, I.; Lassila, R.; Muszbek, L.; Palotie, A. Effect of Val34Leu polymorphism on the activation of the coagulation factor XIII-A. Thromb. Haemost. 2000, 84, 595–600. [Google Scholar] [PubMed]
- de Lange, M.; Snieder, H.; Ariens, R.A.; Spector, T.D.; Grant, P.J. The genetics of haemostasis: A twin study. Lancet 2001, 357, 101–105. [Google Scholar] [CrossRef]
- Heng, C.K.; Lal, S.; Saha, N.; Low, P.S.; Kamboh, M.I. The impact of factor XIIIa V34L polymorphism on plasma factor XIII activity in the Chinese and Asian Indians from Singapore. Hum. Genet. 2004, 114, 186–191. [Google Scholar] [CrossRef]
- Bereczky, Z.; Balogh, E.; Katona, E.; Czuriga, I.; Karpati, L.; Shemirani, A.H.; Edes, I.; Muszbek, L. Decreased factor XIII levels in factor XIII A subunit Leu34 homozygous patients with coronary artery disease. Thromb. Res. 2008, 121, 469–476. [Google Scholar] [CrossRef]
- Kobbervig, C.; Williams, E. FXIII polymorphisms, fibrin clot structure and thrombotic risk. Biophys. Chem. 2004, 112, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Pena, A.; Vignalou, J.B.; Hulot, J.S.; Galier, S.; Cayla, G.; Bellemain-Appaix, A.; Barthelemy, O.; Beygui, F.; Bal-dit-Sollier, C.; et al. FXIII-A Leu34 genetic variant in premature coronary artery disease: A genotype–phenotype case control study. Thromb. Haemost. 2011, 106, 511–520. [Google Scholar] [CrossRef]
- Dunn, E.J.; Ariens, R.A.; de Lange, M.; Snieder, H.; Turney, J.H.; Spector, T.D.; Grant, P.J. Genetics of fibrin clot structure: A twin study. Blood 2004, 103, 1735–1740. [Google Scholar] [CrossRef]
- Manzoli, A.; Andreotti, F.; Leone, A.M.; Sperti, G.; Zecchi, P.; Di Sciascio, G. Vascular and haemostatic gene polymorphisms associated with non-fatal myocardial infarction: A critical review. Ital. Heart J. 2000, 1, 184–193. [Google Scholar]
- Wang, G.; Zou, Z.; Ji, X.; Ni, Q.; Ma, Z. Factor XIII-A Val34Leu polymorphism might beassociated with myocardial infarction risk: An updated meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 5547–5552. [Google Scholar]
- Jung, J.H.; Song, G.G.; Kim, J.H.; Seo, Y.H.; Choi, S.J. Association of factor XIII Val34Leu polymorphism and coronary artery disease: A meta-analysis. Cardiol. J. 2017, 24, 74–84. [Google Scholar] [CrossRef]
- Shafey, M.; Anderson, J.L.; Scarvelis, D.; Doucette, S.P.; Gagnon, F.; Wells, P.S. Factor XIII Val34Leu variant and the risk of myocardial infarction: A meta-analysis. Thromb. Haemost. 2007, 97, 635–641. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Qiao, Q.; Xu, P.; Fan, B.; Chen, Z. Effect of factor XIII-A Val34Leu polymorphism on myocardial infarction risk: A meta-analysis. Clin. Appl. Thromb. Hemost. 2014, 20, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Endler, G.; Mannhalter, C. Polymorphisms in coagulation factor genes and their impact on arterial and venous thrombosis. Clin. Chim. Acta 2003, 330, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.P.; Schroder, V. Role of coagulation factor XIII in cardio- and cerebrovascular diseases. Hamostaseologie 2002, 22, 53–58. [Google Scholar]
- Sarecka-Hujar, B.; Loboda, D.; Paradowska-Nowakowska, E.; Golba, K.S. Coagulation Factor XIII Val34Leu Polymorphism in the Prediction of Premature Cardiovascular Events-The Results of Two Meta-Analyses. J. Clin. Med. 2022, 11, 3454. [Google Scholar] [CrossRef]
- Voko, Z.; Bereczky, Z.; Katona, E.; Adany, R.; Muszbek, L. Factor XIII Val34Leu variant protects against coronary artery disease. A meta-analysis. Thromb. Haemost. 2007, 97, 458–463. [Google Scholar] [PubMed]
- Bereczky, Z.; Balogh, E.; Katona, E.; Pocsai, Z.; Czuriga, I.; Szeles, G.; Karpati, L.; Adany, R.; Edes, I.; Muszbek, L. Modulation of the risk of coronary sclerosis/myocardial infarction by the interaction between factor XIII subunit A Val34Leu polymorphism and fibrinogen concentration in the high risk Hungarian population. Thromb. Res. 2007, 120, 567–573. [Google Scholar] [CrossRef]
- Mannila, M.N.; Eriksson, P.; Ericsson, C.G.; Hamsten, A.; Silveira, A. Epistatic and pleiotropic effects of polymorphisms in the fibrinogen and coagulation factor XIII genes on plasma fibrinogen concentration, fibrin gel structure and risk of myocardial infarction. Thromb. Haemost. 2006, 95, 420–427. [Google Scholar] [CrossRef]
- Grant, P.J.; Humphries, S.E. Genetic determinants of arterial thrombosis. Baillieres. Best Pract. Res. Clin. Haematol. 1999, 12, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Corral, J.; Roldan, V.; Gonzalez-Conejero, R.; del Rey, M.L.; Sogorb, F.; Lip, G.Y.; Vicente, V. Factor XIII Val34Leu polymorphism modulates the prothrombotic and inflammatory state associated with atrial fibrillation. J. Mol. Cell Cardiol. 2004, 37, 699–704. [Google Scholar] [CrossRef]
- Nair, D.G.; Sunilkumar, P.N.; Sadasivan, C. Modeling of factor XIII activation peptide (28-41) V34L mutant bound to thrombin. J. Biomol. Struct. Dyn. 2008, 26, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.P.; Grant, P.J. Atherothrombotic disease and factor XIII: Lucky for some? Eur. J. Clin. Investig. 2002, 32, 637–639. [Google Scholar] [CrossRef]
- Gemmati, D.; Federici, F.; Campo, G.; Tognazzo, S.; Serino, M.L.; De Mattei, M.; Valgimigli, M.; Malagutti, P.; Guardigli, G.; Ferraresi, P.; et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: Effects on survival in myocardial infarction patients. Mol. Med. 2007, 13, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Elmas, E.; Ahmad-Nejad, P.; Weiss, C.; Neumaier, M.; Borggrefe, M. Plasminogen activator inhibitor-1 (PAI-1), toll-like receptor 4 (TLR4), factor II (FII), FXIII and fibrinogen polymorphisms are not associated with the prevalence of sudden death due to ventricular fibrillation during myocardial infarction. Clin. Chem. Lab. Med. 2008, 46, 1329–1331. [Google Scholar] [CrossRef]
- Komanasin, N.; Catto, A.J.; Futers, T.S.; van Hylckama Vlieg, A.; Rosendaal, F.R.; Ariens, R.A. A novel polymorphism in the factor XIII B-subunit (His95Arg): Relationship to subunit dissociation and venous thrombosis. J. Thromb. Haemost. 2005, 3, 2487–2496. [Google Scholar] [CrossRef]
- Reiner, A.P.; Heckbert, S.R.; Vos, H.L.; Ariens, R.A.; Lemaitre, R.N.; Smith, N.L.; Lumley, T.; Rea, T.D.; Hindorff, L.A.; Schellenbaum, G.D.; et al. Genetic variants of coagulation factor XIII, postmenopausal estrogen therapy, and risk of nonfatal myocardial infarction. Blood 2003, 102, 25–30. [Google Scholar] [CrossRef]
- Doggen, C.J.; Reiner, A.P.; Vos, H.L.; Rosendaal, F.R. Two factor XIII gene polymorphisms associated with a structural and functional defect and the risk of myocardial infarction in men. J. Thromb. Haemost. 2003, 1, 2056–2058. [Google Scholar] [CrossRef]
- Jaffer, F.A.; Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Molecular imaging of myocardial infarction. J. Mol. Cell Cardiol. 2006, 41, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Gill, R.; Nussmeier, N.A.; Olsen, P.S.; Andersen, H.F.; Booth, F.V.; Jespersen, C.M. Repletion of factor XIII following cardiopulmonary bypass using a recombinant A-subunit homodimer. A preliminary report. Thromb. Haemost. 2009, 102, 765–771. [Google Scholar] [CrossRef]
- Fraccarollo, D.; Galuppo, P.; Schraut, S.; Kneitz, S.; van Rooijen, N.; Ertl, G.; Bauersachs, J. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension 2008, 51, 905–914. [Google Scholar] [CrossRef]
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation Factor XIIIA (F13A1): Novel Perspectives in Treatment and Pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459. [Google Scholar] [CrossRef]
- Mukherjee, R.; Zavadzkas, J.A.; Saunders, S.M.; McLean, J.E.; Jeffords, L.B.; Beck, C.; Stroud, R.E.; Leone, A.M.; Koval, C.N.; Rivers, W.T.; et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann. Thorac. Surg. 2008, 86, 1268–1276. [Google Scholar] [CrossRef]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Shemirani, A.H.; Katona, E. Factor XIII and atherothrombotic diseases. Semin. Thromb. Hemost. 2010, 36, 18–33. [Google Scholar] [CrossRef]
- Marta-Enguita, J.; Navarro-Oviedo, M.; Machado, F.J.; Bermejo, R.; Aymerich, N.; Herrera, M.; Zandio, B.; Pagola, J.; Juega, J.; Marta-Moreno, J.; et al. Role of factor XIII in ischemic stroke: A key molecule promoting thrombus stabilization and resistance to lysis. J. Thromb. Haemost. 2023. [Google Scholar] [CrossRef]
- Kulkarni, S.; Jackson, S.P. Platelet factor XIII and calpain negatively regulate integrin alphaIIbbeta3 adhesive function and thrombus growth. J. Biol. Chem. 2004, 279, 30697–30706. [Google Scholar] [CrossRef]
- Dargazanli, C.; Zub, E.; Deverdun, J.; Decourcelle, M.; de Bock, F.; Labreuche, J.; Lefevre, P.H.; Gascou, G.; Derraz, I.; Riquelme Bareiro, C.; et al. Machine Learning Analysis of the Cerebrovascular Thrombi Proteome in Human Ischemic Stroke: An Exploratory Study. Front. Neurol. 2020, 11, 575376. [Google Scholar] [CrossRef]
- Misra, S.; Singh, P.; Nath, M.; Bhalla, D.; Sengupta, S.; Kumar, A.; Pandit, A.K.; Aggarwal, P.; Srivastava, A.K.; Mohania, D.; et al. Blood-based protein biomarkers for the diagnosis of acute stroke: A discovery-based SWATH-MS proteomic approach. Front. Neurol. 2022, 13, 989856. [Google Scholar] [CrossRef] [PubMed]
- Bagoly, Z.; Barath, B.; Orban-Kalmandi, R.; Szegedi, I.; Bogati, R.; Sarkady, F.; Csiba, L.; Katona, E. Incorporation of alpha2-Plasmin Inhibitor into Fibrin Clots and Its Association with the Clinical Outcome of Acute Ischemic Stroke Patients. Biomolecules 2021, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Berthiller, J.; Derex, L.; Trouillas, P.; Diallo, L.; Hanss, M. Post-thrombolysis haemostasis changes after rt-PA treatment in acute cerebral infarct. Correlations with cardioembolic aetiology and outcome. J. Neurol. Sci. 2015, 349, 77–83. [Google Scholar] [CrossRef]
- Williams, F.M.; Carter, A.M.; Hysi, P.G.; Surdulescu, G.; Hodgkiss, D.; Soranzo, N.; Traylor, M.; Bevan, S.; Dichgans, M.; Rothwell, P.M.; et al. Ischemic stroke is associated with the ABO locus: The EuroCLOT study. Ann. Neurol. 2013, 73, 16–31. [Google Scholar] [CrossRef]
- Rubattu, S.; Di Angelantonio, E.; Nitsch, D.; Gigante, B.; Zanda, B.; Stanzione, R.; Evangelista, A.; Pirisi, A.; Rosati, G.; Volpe, M. Polymorphisms in prothrombotic genes and their impact on ischemic stroke in a Sardinian population. Thromb. Haemost. 2005, 93, 1095–1100. [Google Scholar] [CrossRef]
- Wei, L.K.; Griffiths, L.R.; Kooi, C.W.; Irene, L. Meta-Analysis of Factor V, Factor VII, Factor XII, and Factor XIII-A Gene Polymorphisms and Ischemic Stroke. Medicina 2019, 55, 101. [Google Scholar] [CrossRef]
- Pruissen, D.M.; Slooter, A.J.; Rosendaal, F.R.; van der Graaf, Y.; Algra, A. Coagulation factor XIII gene variation, oral contraceptives, and risk of ischemic stroke. Blood 2008, 111, 1282–1286. [Google Scholar] [CrossRef][Green Version]
- Landau, M.B.; Renni, M.S.; Zalis, M.G.; Spector, N.; Gadelha, T. Coagulation factor XIII Tyr204Phe gene variant and the risk of ischemic stroke. J. Thromb. Haemost. 2013, 11, 1426–1427. [Google Scholar] [CrossRef]
- de Lange, M.; Andrew, T.; Snieder, H.; Ge, D.; Futers, T.S.; Standeven, K.; Spector, T.D.; Grant, P.J.; Ariens, R.A. Joint linkage and association of six single-nucleotide polymorphisms in the factor XIII-A subunit gene point to V34L as the main functional locus. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, A.H.; Antalfi, B.; Pongracz, E.; Mezei, Z.A.; Bereczky, Z.; Csiki, Z. Factor XIII-A subunit Val34Leu polymorphism in fatal atherothrombotic ischemic stroke. Blood Coagul. Fibrinolysis 2014, 25, 364–368. [Google Scholar] [CrossRef]
- Bagoly, Z.; Koncz, Z.; Harsfalvi, J.; Muszbek, L. Factor XIII, clot structure, thrombosis. Thromb. Res. 2012, 129, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, A.H.; Pongracz, E.; Antalfi, B.; Adany, R.; Muszbek, L. Factor XIII A subunit Val34Leu polymorphism in patients suffering atherothrombotic ischemic stroke. Thromb. Res. 2010, 126, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, S.; Erdem, H.B.; Sahin, I.; Ozel, L.; Ozdemir, G.; Eroz, R.; Tatar, A. Correlation with Platelet Parameters and Genetic Markers of Thrombophilia Panel (Factor II g.20210G>A, Factor V Leiden, MTHFR (C677T, A1298C), PAI-1, beta-Fibrinogen, Factor XIIIA (V34L), Glycoprotein IIIa (L33P)) in Ischemic Strokes. Neuromolecular. Med. 2016, 18, 170–176. [Google Scholar] [CrossRef]
- Smith, N.L.; Bis, J.C.; Biagiotti, S.; Rice, K.; Lumley, T.; Kooperberg, C.; Wiggins, K.L.; Heckbert, S.R.; Psaty, B.M. Variation in 24 hemostatic genes and associations with non-fatal myocardial infarction and ischemic stroke. J. Thromb. Haemost. 2008, 6, 45–53. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Yin, Y.; Pi, Y.; Yang, Q.; Gao, C.; Fang, C.; Wang, J.; Li, J. Lack of evidence for association between factor XIII-A Val34Leu polymorphism and ischemic stroke: A meta-analysis of 8800 subjects. Thromb. Res. 2012, 130, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Moskau, S.; Smolka, K.; Semmler, A.; Schweichel, D.; Harbrecht, U.; Muller, J.; Pohl, C.; Klockgether, T.; Linnebank, M. Common genetic coagulation variants are not associated with ischemic stroke in a case-control study. Neurol. Res. 2010, 32, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.P.; Hingorani, A.D.; Bautista, L.E.; Sharma, P. Meta-analysis of genetic studies in ischemic stroke: Thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch. Neurol. 2004, 61, 1652–1661. [Google Scholar] [CrossRef]
- Ranellou, K.; Paraskeva, A.; Kyriazopoulos, P.; Batistatou, A.; Evangelou, A.; El-Aly, M.; Zis, P.; Tavernarakis, A.; Charalabopoulos, K. Polymorphisms in prothrombotic genes in young stroke patients in Greece: A case-controlled study. Blood Coagul. Fibrinolysis 2015, 26, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Kamberi, B.; Kamberi, F.; Spiroski, M. Vascular Genetic Variants and Ischemic Stroke Susceptibility in Albanians from the Republic of Macedonia. Open Access Maced. J. Med. Sci. 2016, 4, 556–564. [Google Scholar] [CrossRef]
- Siegerink, B.; Maino, A.; Algra, A.; Rosendaal, F.R. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. J. Thromb. Haemost. 2015, 13, 1568–1575. [Google Scholar] [CrossRef]
- Salomi, B.S.B.; Solomon, R.; Turaka, V.P.; Aaron, S.; Christudass, C.S. Cryptogenic Stroke in the Young: Role of Candidate Gene Polymorphisms in Indian Patients with Ischemic Etiology. Neurol. India 2021, 69, 1655–1662. [Google Scholar] [CrossRef]
- Huang, Y.; Ballinger, D.G.; Stokowski, R.; Beilharz, E.; Robinson, J.G.; Liu, S.; Robinson, R.D.; Henderson, V.W.; Rossouw, J.E.; Prentice, R.L. Exploring the interaction between SNP genotype and postmenopausal hormone therapy effects on stroke risk. Genome. Med. 2012, 4, 57. [Google Scholar] [CrossRef]
- Hanscombe, K.B.; Traylor, M.; Hysi, P.G.; Bevan, S.; Dichgans, M.; Rothwell, P.M.; Worrall, B.B.; Seshadri, S.; Sudlow, C.; Consortium, M.; et al. Genetic Factors Influencing Coagulation Factor XIII B-Subunit Contribute to Risk of Ischemic Stroke. Stroke 2015, 46, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Kain, K.; Bamford, J.; Bavington, J.; Young, J.; Catto, A.J. Factor XIII–circulating levels and Val34Leu polymorphism in relatives of South Asian patients with ischemic stroke. J. Thromb. Haemost. 2005, 3, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Pruissen, D.M.; Kappelle, L.J.; Rosendaal, F.R.; Algra, A.; Group, S.S. Prothrombotic gene variation in patients with large and small vessel disease. Neuroepidemiology 2008, 31, 89–92. [Google Scholar] [CrossRef]
- Coen Herak, D.; Lenicek Krleza, J.; Radic Antolic, M.; Horvat, I.; Djuranovic, V.; Zrinski Topic, R.; Zadro, R. Association of Polymorphisms in Coagulation Factor Genes and Enzymes of Homocysteine Metabolism with Arterial Ischemic Stroke in Children. Clin. Appl. Thromb. Hemost. 2017, 23, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Cernera, G.; Comegna, M.; Gelzo, M.; Savoia, M.; Bruzzese, D.; Mormile, M.; Zarrilli, F.; Amato, F.; Micco, P.D.; Castaldo, G. Molecular Analysis of Prothrombotic Gene Variants in Patients with Acute Ischemic Stroke and with Transient Ischemic Attack. Medicina 2021, 57, 723. [Google Scholar] [CrossRef]
- Krleza, J.L.; Coen Herak, D.; Dakovic, I.; Vulin, K.; Roic, G.; Tripalo Batos, A.; Ceri, A.; Zadro, R.; Duranovic, V. Inherited Thrombophilia Associated with Ischemic Pediatric Stroke in Parent-Child Pairs. Pediatr. Neurol. 2023, 146, 119–128. [Google Scholar] [CrossRef]
- Kopyta, I.A.; Emich-Widera, E.; Balcerzyk, A.; Niemiec, P.; Zak, I.; Pilarska, E.; Kacinski, M.; Wendorff, J.; Nowak, T.; Iwanicki, T.; et al. Polymorphisms of genes encoding coagulation factors II, V, VII, and XIII in relation to pediatric ischemic stroke: Family-based and case-control study. Neurologist 2012, 18, 282–286. [Google Scholar] [CrossRef]
- Komitopoulou, A.; Platokouki, H.; Kapsimali, Z.; Pergantou, H.; Adamtziki, E.; Aronis, S. Mutations and polymorphisms in genes affecting hemostasis proteins and homocysteine metabolism in children with arterial ischemic stroke. Cerebrovasc. Dis. 2006, 22, 13–20. [Google Scholar] [CrossRef]
- Sarecka-Hujar, B.; Kopyta, I.; Skrzypek, M. Lack of Associations Between PAI-1 and FXIII Polymorphisms and Arterial Ischemic Stroke in Children: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619869500. [Google Scholar] [CrossRef]
- Szegedi, I.; Orban-Kalmandi, R.; Nagy, A.; Sarkady, F.; Vasas, N.; Sik, M.; Lanczi, L.I.; Berenyi, E.; Olah, L.; Crisan, A.; et al. Decreased clot burden is associated with factor XIII Val34Leu polymorphism and better functional outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. PLoS ONE 2021, 16, e0254253. [Google Scholar] [CrossRef]
- Gonzalez-Conejero, R.; Fernandez-Cadenas, I.; Iniesta, J.A.; Marti-Fabregas, J.; Obach, V.; Alvarez-Sabin, J.; Vicente, V.; Corral, J.; Montaner, J.; Proyecto Ictus Research, G. Role of fibrinogen levels and factor XIII V34L polymorphism in thrombolytic therapy in stroke patients. Stroke 2006, 37, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Luo, Y.; Tang, N. Predictive value of coagulation factor XIII on bleeding risk in ischemic stroke patients treated with intravenous thrombolysis. Ann. Palliat. Med. 2021, 10, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
- Cocho, D.; Borrell, M.; Marti-Fabregas, J.; Montaner, J.; Castellanos, M.; Bravo, Y.; Molina-Porcel, L.; Belvis, R.; Diaz-Manera, J.A.; Martinez-Domeno, A.; et al. Pretreatment hemostatic markers of symptomatic intracerebral hemorrhage in patients treated with tissue plasminogen activator. Stroke 2006, 37, 996–999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marti-Fabregas, J.; Borrell, M.; Cocho, D.; Belvis, R.; Castellanos, M.; Montaner, J.; Pagonabarraga, J.; Aleu, A.; Molina-Porcel, L.; Diaz-Manera, J.; et al. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-PA. Neurology 2005, 65, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Briggs, B.; James, K.N.; Chowdhury, S.; Thornburg, C.; Farnaes, L.; Dimmock, D.; Kingsmore, S.F.; Investigators, R. Novel Factor XIII variant identified through whole-genome sequencing in a child with intracranial hemorrhage. Cold Spring Harb. Mol. Case Stud. 2018, 4, a003525. [Google Scholar] [CrossRef] [PubMed]
- Szekely, E.G.; Czuriga-Kovacs, K.R.; Bereczky, Z.; Katona, E.; Mezei, Z.A.; Nagy, A.; Toth, N.K.; Berenyi, E.; Muszbek, L.; Csiba, L.; et al. Low factor XIII levels after intravenous thrombolysis predict short-term mortality in ischemic stroke patients. Sci. Rep. 2018, 8, 7662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, S.; Yao, Y.; Ding, J.; Wang, Y.; Zhao, Z.; Liao, L.; Li, P.; Zang, F.; Teng, G.J. In vivo near-infrared imaging of fibrin deposition in thromboembolic stroke in mice. PLoS ONE 2012, 7, e30262. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Hutanu, A.; Chiriac, L.; Dobreanu, M.; Oradan, A.; Nagy, E.E. Ischemic damage and early inflammatory infiltration are different in the core and penumbra lesions of rat brain after transient focal cerebral ischemia. J. Neuroimmunol. 2018, 324, 35–42. [Google Scholar] [CrossRef]
- Chen, J.W.; Figueiredo, J.L.; Wojtkiewicz, G.R.; Siegel, C.; Iwamoto, Y.; Kim, D.E.; Nolte, M.W.; Dickneite, G.; Weissleder, R.; Nahrendorf, M. Selective factor XIIa inhibition attenuates silent brain ischemia: Application of molecular imaging targeting coagulation pathway. JACC Cardiovasc. Imaging 2012, 5, 1127–1138. [Google Scholar] [CrossRef]
- Cucuianu, M.; Dican, L. Coagulation factor XIII and atherothrombosis. A mini-review. Rom. J. Intern. Med. 2003, 41, 339–355. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traub, J.; Weber, M.S.; Frey, A. Differential Role of Factor XIII in Acute Myocardial Infarction and Ischemic Stroke. Biomedicines 2024, 12, 497. https://doi.org/10.3390/biomedicines12030497
Traub J, Weber MS, Frey A. Differential Role of Factor XIII in Acute Myocardial Infarction and Ischemic Stroke. Biomedicines. 2024; 12(3):497. https://doi.org/10.3390/biomedicines12030497
Chicago/Turabian StyleTraub, Jan, Martin S. Weber, and Anna Frey. 2024. "Differential Role of Factor XIII in Acute Myocardial Infarction and Ischemic Stroke" Biomedicines 12, no. 3: 497. https://doi.org/10.3390/biomedicines12030497
APA StyleTraub, J., Weber, M. S., & Frey, A. (2024). Differential Role of Factor XIII in Acute Myocardial Infarction and Ischemic Stroke. Biomedicines, 12(3), 497. https://doi.org/10.3390/biomedicines12030497





