Abstract
Prenatal exposure to alcohol can cause Fetal Alcohol Spectrum Disorders (FASDs) after birth, encompassing a spectrum of physical, cognitive, and behavioral abnormalities. FASD represents a severe non-genetic disability avoidable through alcohol abstinence during pregnancy and when planning it. Clinical severity depends on alcohol impact, symptomatology, and resulting disabilities. FASD is a permanent disability with no recognized specific medical care. Conversely, secondary FASD-related disabilities can be symptomatically treated. This integrative review aims to provide information about the novel pharmacological treatments of FASD-associated comorbidities by selecting the last ten years of studies carried out on animals and humans. PRISMA guidelines were followed to search human/animal model studies of pharmacological interventions on FASD comorbidities, using different databases (PubMed, Cochrane, etc.). From 1348 articles, 44 met the criteria after full-text analysis. Firstly, all the reported studies point out that early diagnosis and tailored interventions are the principal tools to reduce FASD-related secondary disabilities, due to the fact that there is currently no approved pharmacological treatment for the tissue damage which produces FASD. Despite limitations in study designs and small sample sizes, these review results highlight how the treatment strategies of children with FASD have changed. In the past, studies focused on treating symptoms, but in the last years, researchers have turned their attention to the prevention targeting central nervous system embryogenesis. Novel treatments like choline and natural antioxidants and nutritional supplements are the most investigated treatments in humans with promising results. More follow-up studies need to be performed, to confirm and generalize reported efficacy to a wide sample size.
1. Introduction
Prenatal Exposure to Alcohol (PAE) can exert toxic effects on the fetus, causing a broad range of physical, cognitive, and behavioral abnormalities that are classified as Fetal Alcohol Spectrum Disorders (FASDs) [1,2,3]. FASD is the primary non-genetic cause of a severe permanent disability, which can be totally prevented by abstaining from alcohol consumption during pregnancy. FASD is a complex set of clinical conditions that vary depending on the damage caused by alcohol exposure, the severity of symptoms observed, and the type of disability exhibited [4] with Fetal Alcohol Syndrome (FAS) being the most severe condition.
Growth deficits, craniofacial dysmorphology, birth malformations, and Neurodevelopmental Disorders (NDs) are clinical and phenotypic manifestations of FASD that can vary in severity and expression according to dose, amount, and frequency of consumption, as well as the gestational period of exposure, maternal nutrition, and genetic background [5,6,7,8]. FAS, partial FAS (pFAS), Alcohol-Related Neurodevelopmental Disorder (ARND), and Alcohol-Related Brain Disorders (ARBDs) have specific diagnostic criteria based on the co-occurrence of one or more clinical and above-mentioned phenotypic manifestations. However, a specific neuropsychological profile has not yet been established for patients affected by FASD [9].
The cognitive and behavioral difficulties are a consequence of Central Nervous System (CNS) permanent damage due to PAE [10,11]. Some of the difficulties commonly referred to as Primary Disabilities (PDs) include intellectual disability, deficits in executive functioning, memory and information processing, language delay or dysfunction, attention deficits and hyperactivity, and general difficulty in learning [12,13,14,15]. If these are not promptly treated, they can lead to Secondary Disabilities (SDs), that develop later in life and include health problems, employment issues, failed school experiences, legal issues, inappropriate sexual behavior, drug and/or alcohol use problems.
Early diagnosis allows for the correlation of symptoms with an accurate diagnosis and treatment and helps to minimize SD. Indeed, SDs decrease significantly in adolescence and in adulthood if the intervention starts before the age of six, due to the large neuronal plasticity of a child’s brain [16,17].
Moreover, early diagnosis cannot be carried out in cases of abandoned children when maternal alcohol consumption is unknown or when they rarely admit using ethanol during pregnancy [18].
Late diagnosis in adulthood leads to greater treatment challenges, as the pathological mechanisms have already been established. However, it is still possible to activate pathways of awareness, treatment, and support by starting from the family situation and the emerged disabilities [19].
Language problems, which are characterized as difficulties in expression and understanding, require special attention in individuals with FASD [20,21]. In FASD individuals, metacognition is reduced or absent and it is associated with language impairment, preventing them from expressing their emotions verbally. Emotions are often the consequence of a painful experience resulting in physical discomfort (e.g., tachycardia, abdominal pain, etc.) [22]. Moreover, FASD individuals are not able to distinguish their own internal reality from the external one, resulting in their inability to grasp the difference between dreams, fantasies, beliefs, and hypotheses. Thinking, reflecting, and reasoning about one’s own and others’ mental states is another challenge for FASD individuals [23].
FASD is related to five comorbid conditions with the highest aggregate prevalence (between 50% and 91%) including abnormal findings on functional studies of the peripheral nervous system and special senses, conduct disorder, receptive language disorder, chronic serous otitis media, and expressive language disorder [24].
Moreover, the most common comorbidities concern the diagnoses of Cognitive Delay, Attention Deficit/Hyperactivity Disorder (ADHD), Specific Learning Disorders (SLDs), Pervasive Developmental Disorders (autism and other pervasive disorders), and Externalizing Disorders (Oppositional Defiant Disorder, Conduct Disorder, Intermittent Explosive Disorder) [25,26]. Neurobehavioral Disorders associated with Prenatal Exposure to Alcohol (NDs-PAE) have been well defined in the Diagnostic and Statistical Manual of Mental Disorders [27], and represent a specific diagnostic category highlighting the evolution of the disorder throughout the life span.
The treatment of FASD requires an integrated approach, involving different disciplines (psychiatry, neurology cardiology, nutritional rehabilitation courses, etc.) and professionals [28,29]. To date, there are no specific treatments for FASD. Hence, it is necessary to evaluate each affected subject individually to determine an appropriate targeted treatment and improve the quality of life [30,31].
Due to their neurocognitive particularities, individuals with FASD may not be consistently able to adhere to a psychotherapeutic process and complete it successfully. Cognitive-Behavioral Therapy (CBT) and Parent Training are successful therapeutic interventions supporting both FASD patients and the whole family group [32].
Sometimes, despite optimization of behavioral interventions, some individuals will need pharmacological treatments for a long period to manage their symptoms and comorbidities [33].
Drugs commonly used in the FASD clinical manifestations are different for clusters of signs and symptoms. Adrenergic agents are the primary treatment for hyperactivity including irritability, aggression, insomnia, agitation, and anxiety. Mood stabilizers (such as Divalproex and Lamotrigine) can be administered to treat conditions associated with emotional regulation (e.g., anxiety, depression, mood swings, etc.), while SSRIs (Fluoxetine, Citalopram, Sertraline) are used as a second option for both these symptom groups, but they are not recommended for preschool children. Concerning disorders linked to executive functions and/or hyperactivity (e.g., impulsivity, poor concentration, restless movement), stimulant-based amphetamines (like Lisdexamfetamine and Dexedrine) are recommended as the first-line treatment, followed by other stimulants such as Methylphenidate, Atomoxetine, or Bupropion. Finally, for symptoms pertaining to cognitive inflexibility, such as perspective disorders, frustration tolerance, social skills, reasoning, etc., atypical neuroleptics like Risperidone are recommended as the first-line treatment, while Olanzapine and Aripiprazole are suggested as second-line options.
The aim of this integrative review is the analysis of the pharmacological interventions for the treatment of FASD and its associated comorbidities such as anxiety, depression, hyperactivity, and Attention Deficit Disorder (ADHD), aggressiveness, etc. Moreover, this review analyzes novel treatments for FASD or its comorbidities in either preclinical and analytical studies or clinical trials, with special mention to treatments with natural antioxidants and choline during the perinatal period and infancy. We could not consider a meta-analysis due to the differences in the experimental design depending on preclinical studies in FASD-like animal models or trials in FASD subjects. In addition, the differences of the molecular mechanism targeted by the diverse pharmacological interventions evaluated in this review would generate an important bias in the statistical results in a meta-analysis.
2. Materials and Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was the methodology selected for the present integrative review [34,35]. According to guidelines of the 2009 as well as the update of the 2020 PRISMA statement [36], the research team evaluated the following items: definition of the research question, hypothesis and objectives; bibliographic search; data collection, evaluation, synthesis, and comparison; critical evaluation of the scientific papers selected and finally, analysis of the main findings and conclusions including the strengths and weakness of these studies (Figure 1).
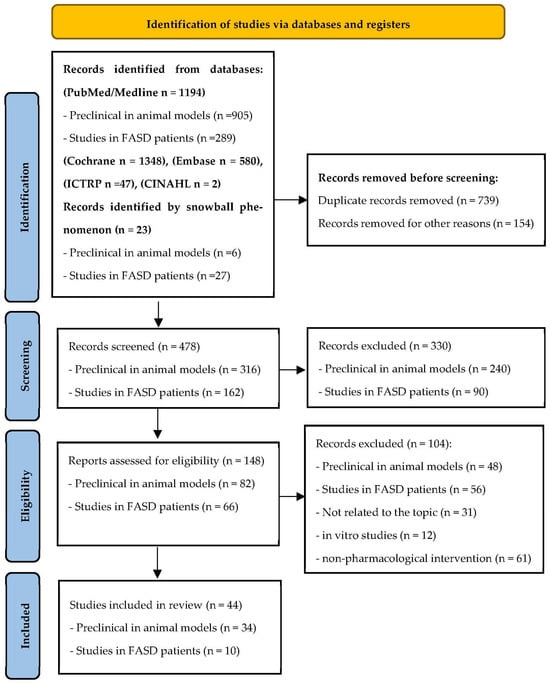
Figure 1.
Methodological flowchart based on PRISMA 2020 update following the preferred reporting items for systematic review [36].
PubMed (MeSH), Cochrane Central Register of Controlled Trials, Embase, ICTRP, and CINAHL were the electronic databases consulted to collect the data. We performed an initial search using the following descriptors (as MeSH terms or not) and similar terms with the Boolean operators (AND/OR) in multiple combinations “((fetal alcohol spectrum disorders) OR (fetal alcohol syndrome)) AND ((pharmacological treatment) or (pharmacological intervention)) or (pharmacology)” “((fetal alcohol spectrum disorders) OR (fetal alcohol syndrome)) AND (choline)” “((fetal alcohol spectrum disorders) OR (fetal alcohol syndrome)) AND (natural antioxidants)” to focus the scope of this review on those pharmacological interventions whose effects on FASD or associated comorbidities have been analyzed in both humans and animal models studies. MeSH terms also included various expressions of FASD and psychotropic or antipsychotics medications as anxiolytics. All terms were adapted for the databases consulted for this review.
Inclusion criteria comprised papers written in English (with no geographical restrictions) published from 26 June 2013 to 26 June 2023 with evidence of FASD diagnosis and pharmacological treatment. Papers before 2013 were not included because they mainly focused on the PAE effects investigation rather than research based on novel treatments for FASD comorbidities exhibited both in animal models and humans.
The last ten years were selected to include the following: current information regarding novel pharmacological treatments or nutritional supplements applied to individuals with FASD; the presence of the selected terms in the title or as keywords; original research performed in humans or animal models when focused on the use of therapeutic drugs or novel pharmacological therapies to treat FASD or a specific comorbidity associated with FASD.
The types of selected experimental designs were clinical studies, clinical trials, comparative studies, case-control studies, longitudinal cohorts, cross-sectional and case report studies with a sample size of a minimum of 10 participants, as well as preclinical studies with animal models. Selected outcomes were evidence of neuropsychological or behavior evaluation as well as the measure of specific biomarkers of CNS in human or animal models. Exclusion criteria were non-systematic reviews, lack of a control or comparative group with no pharmacological treatment, experimental designs using different drugs to treat different symptoms interfering with the effects of a specific treatment, the presence of genetic or other diseases in FASD subjects which could interfere with the alterations analyzed in this review, and papers in which output variables were not related to pharmacological treatments for FASD.
The researchers M.M., V.A.F., A.M., N.L.M. and S.P. performed the initial selection of original manuscripts by screening titles and abstracts, creating a reference list of papers for the topics evaluated in the present review using Rayyan software version 1.3.3 to perform systematic reviews [37]. Four investigators (M.M., V.A.F., A.M. and S.P.) conducted all of the stages of the studies’ selection, deleted duplicate inputs, and reviewed studies as excluded or requiring further assessment. All data were extracted by two investigators (M.M. and V.A.F.) and cross-checked by the other four investigators (N.L.M., A.M., S.P., and O.G). In the case of discrepancies in the selected studies, we opted for reconciliation through team discussion. Moreover, the reference lists of some selected papers were manually checked to include some high impact additional studies from the bibliography of the original papers or meta-analyses which were relevant to the topics addressed following the snowball strategy (Figure 1). The information evaluated from each study included the following: pharmacological intervention, first author, year of publication, objective, experimental design (treatment duration and study groups), and number of participants in treated and control group; main outcomes/findings; conclusions; strengths and limitations (including biases). The eligibility criteria followed the PICOS approach (patient, intervention, comparators, outcome, and study design); population included infants diagnosed with FASD or FASD-like animal models; intervention included any dose of pharmacological treatment selected for this review; comparators included placebo, if applicable; the primary outcome was the neurocognitive or behavioral response of the FASD individuals after a pharmacological treatment; changes in levels or expression of selected molecular biomarkers related to CNS alterations and diverse neurological functions. All authors performed a critical appraisal for the selected studies following the inclusion criteria, as well as analyzed the methodology and key results.
The outputs evaluated following PRISMA were heterogeneous by the need to include animal models as well as human studies; the different populations of analyzed infants; the limited sample size observed in many of these studies and the few randomized trials using novel molecules as a pharmacological treatment for FASD or its associated comorbidities. The studies identified through database searching, selection after meeting the inclusion criteria, and the application of the exclusion criteria are indicated in Figure 1. All citations were collated and imported into Mendeley citation manager. This systematic review protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews, on 27 June 2023, with the registration number CRD42023439958, available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=439958 (accessed on 12 January 2024).
3. Results
3.1. Characteristics of the Included Studies
A total of 1348 published articles were identified after bibliographic search in the databases indicated in the Materials and Methods Section. A total of 23 studies were included via citation searching following the snowball strategy. Once duplicate papers were deleted (893), the abstract and title of the potentially relevant 478 articles were reviewed. After applying inclusion and exclusion criteria, 330 references were eliminated. The full text of the remaining 148 references was carefully analyzed, removing 104 articles because they did not meet eligibility criteria. Since they were not related to the topic or the analyzed outcomes, they were in vitro studies or they referred to a non-pharmacological treatment. Finally, eligible and included studies in this integrative review are shown in the flowchart of Figure 1.
3.2. Results from Animal Studies
Animal studies explored the effects of various interventions on alcohol exposure and its neurological consequences, although not all studies were conducted considering the condition of prenatal alcohol exposure (Table 1).
These interventions included choline, minocycline, cannabidiol, omega-3 fatty acids, metformin, Shh signaling activation, rapamycin, DAT inhibitor CE-123, curcumin, crocin, Dihydromyricetin (DHM), Epigallocatechin Gallate (EGCG), folic acid, betaine, osmotin, adiponectin, Trichostatin A (TSA), Docosahexaenoic acid (DHA), papaverine, and HX106.
The findings revealed various promising outcomes. Choline demonstrated efficacy in preventing developmental anomalies, motor difficulties, and cognitive impairments linked with PAE [38,39,40,41,42]. Omega-3 fatty acids were found to reverse deficits associated with PAE, specifically in male animals [43]. Metformin exhibited significant effects in reducing oxidative stress and apoptosis in the hippocampus [44]. Some interventions, such as Rapamycin, Curcumin, DHM, EGCG, and TSA, showed potential in reversing ethanol-induced deficits in memory, neuronal damage, or neurobehavioral alterations [45,46,47,48,49].
Several interventions, including folic acid, betaine, osmotin, and adiponectin, demonstrated protective effects against ethanol-induced teratogenic effects and neuronal apoptosis [50,51,52]. Others, like DHA and papaverine, improved ethanol-induced deficits in behavior and neurodevelopmental markers [53,54]. Additionally, some interventions, such as HX106 and DAT inhibitor CE-123, improved enhanced cognitive abilities and regulated dopamine-related receptors in PAE animals [55,56].
These different interventions offer promising avenues for addressing the detrimental impacts of PAE on neurodevelopment and behavior, showing potential in mitigating or reversing ethanol-induced neurological deficits.

Table 1.
Preclinical treatments in animal models of fetal alcohol spectrum disorders.
Table 1.
Preclinical treatments in animal models of fetal alcohol spectrum disorders.
| Author Year Country | Pharmacological Intervention | Study Design and Objectives | Population | EtOH Exposure | Dose/Intervention Period | Variables Studied | Key Results |
|---|---|---|---|---|---|---|---|
| Patten et al. [43], 2013 Canada | Omega-3 fatty acids | RCT to determine whether feeding an omega-3 fatty acid-enriched diet from birth is able to overcome the deficits in synaptic plasticity that occur with PNE | Sprague-Dawley rats (N = 24) Offspring (N = 10 each group) | Prenatal exposure to EtOH Offspring exposure | Dams: ‣on GD1: prenatal diets EtOH or Pair-fed or Ad libitum control ‣on GD 15: BAC assessment. When pups were born, the dams were placed on either a regular chow diet or an omega-3-enriched diet Pups: ‣at P22: they were continued on The same postnatal diet as their mothers until they reached an experimental age (P55–70) ‣at P70: animals were used for in vivo electrophysiological experiments ‣Pre-stimulation: pulse 0.12 ms (duration) at 0.067 Hz LTP induced by applying TBS ‣Post-stimulation: 60 min at 0.067 Hz | EtOH, LTP, omega-3 fatty acids | omega-3 fatty acids can reduce oxidative stress and enhance antioxidant protection in PNE animals |
| Naseer et al. [52], 2014 | Osmotin | In vitro and in vivo study to examine the neuroprotective effects of adiponectin-activated pathways against FAS | Rats Sprague-Dawley rats (P7) | exposed to EtOH for 12 and 24 h no prenatal exposure to EtOH | ‣CT: no treatment ‣TG1: EtOH (100 mM) ‣TG2: Osm (0.16 mM) + ‣EtOH (100 mM) after 24 h staining with FJB and PI ‣20% EtOH in normal saline (4 mg/g body weight) | Osm, EtOH, caspase-3, AMPK activity | ‣AMPK-activating drugs such as metformin, adiponectin, and osmotin prevent the effects of FAS ‣Osmotin is an experimental alternative to adiponectin |
| Liang et al. [47], 2014 USA | Dihydromyricetin | RCT to study the effect of FAE on physiology, behavior, and GABAAR function of early adolescent rats to test the utility of DHM as a preventative treatment for FAE-induced disturbances | Sprague-Dawley young rats | Prenatal exposure to EtOH Offspring exposure | ‣Dams: pregnant dams received the following treatments: (1) EtOH (1.5 g/kg, gavage); (2) EtOH (2.5 g/kg); (3) EtOH (5 g/kg); (4) EtOH (1.5 g/kg) + DHM (1 mg/kg); (5) EtOH (2.5 g/kg) + DHM (1 mg/kg); (6) EtOH (5.0 g/kg) + DHM (1 mg/kg); (7) DHM (1 mg/kg); (8) vehicle (water, 20 mL/kg) ‣Offspring: Behavioral and electrophysiological experiments were performed on P25-35 | EtOH; DHM; anxiety-related behavior; Plasma EtOH Concentration assessment; brain analysis | The absence of adverse side effects and the ability of DHM to completely prevent FAE consequences suggest that DHM is an attractive candidate for development as a treatment for the prevention of FASD |
| Bearer et al. [38], 2015 USA | Choline | RCT to determine if choline supplementation prior to an acute exposure to ethanol on P5 would reduce the effects of EtOH on the dowel crossing test | C57B16/J mice pups | No prenatal exposure to EtOH | Prenatal treatment from E 4.5 to P21: pups received choline-deficient pellet diet Postnatal treatment (from P2): Pre-exposure treatment: S group: 10 μL C group: 10 μL of 18/8 mg/mL Exposure (on P5): EtOH group (6 g/kg) Intralipid® group Post-exposure (from P6 to P20): S group: 10 μL C group: 10 μL of 18/8 mg/mL On P30: balance and coordination assessment (6 animals per sex per treatment group) | BAC; balance and coordination; Dowel Crossing Test | Choline fortification of common foodstuffs may reduce the effects of alcohol |
| Wellmann et al. [53], 2016 USA | Docosahexaenoic acid (DHA) | RCT to evaluate if DHA reduction underlies (or contributes to) the neurobehavioral problems observed in FASD, to determine if postnatal DHA supplementation will ameliorate neurobehavioral problems in animal model of FASD | Long-Evans rats | Prenatal exposure to EtOH Offspring exposure | Dams: Timed pregnant rats were assigned to one of 3 groups: ‣ad libitum access to an ethanol-containing liquid diet; ‣pair fed an isocaloric isonutritive non-alcohol liquid diet; ‣ad libitum access to chow and water. Pups: From P11 to P20 pups were assigned to: ‣DHA (10 g/kg in artificial rat milk); ‣artificial rat milk 0.01 mL/g; ‣CT. | P14: iUSVs assessment. P28 or P42: Social behavior and play-induced USVs assessment. P33 or P42: somatosensory performance assessment. P35: anxiety assessment. | DHA administration may have therapeutic value to reverse some of ethanol’s damaging effects |
| Balaraman et al. [39], 2017 USA | Choline | RCT to examine whether the nutrient choline would modify ethanol’s effects on miRNAs | Sprague-Dawley rats (N = 48; 6 for each experimental group) | No prenatal alcohol exposure | From P4 to P21: ‣EtOH (2.625 g/kg) + C (100 mg/kg/day) +  (or (or  ) )‣EtOH (2.625 g/kg) + S +  (or (or  ) )‣Sham + C (100 mg/kg/day) +  (or (or  ) )‣Sham + S +  (or (or  ) ) | EtOH, choline, P22: brain examination, with dissection of the hippocampus and isolation of RNA | Choline modification of EtOH effects on miRNAs provide another avenue through which choline may protect against ethanol’s teratogenic effects |
| Waddell et al. [40], 2017 USA | Choline | RCT to test if working memory training during adolescence combined with choline supplementation would improve cognitive flexibility in rat model of FASD more effectively than either intervention alone | Rats (GD3) | Prenatal exposure to EtOH Offspring exposure | Dams: ‣CT group (N = 9) ‣ET group (N = 10): diet containing 3% ethanol for the duration of the exposure From P16 to P30: ‣SC saline (0.9% Sal; pH 7.4) group ‣SC choline (Cho; 100 mg/kg) group From P30 to P38: ‣Tr group ‣Utr group Offspring assigned to one of 4 postnatal groups: Sal-Utr; Sal-Tr; Cho-Utr; Cho-Tr | EtOH, Choline, working memory | Possible beneficial effect of choline in FASD; it may be more efficacious when coupled with cognitive training |
| Sogut et al. [50], 2017 Turkey | Folic acid and betaine | RCT to investigate prenatal EtOH exposure-related neuroapoptosis on the cerebral cortex tissues of newborn rats and possible neuroprotective effects of betaine, folic acid, and combined therapy | Sprague-Dawley rats Pregnant rats N = n.d. Offspring N = 65 | Pregnant rats were divided into 5 experimental groups: control, EtOH, EtOH + betaine, EtOH + folic acid, and EtOH + betaine + folic acid combined therapy groups | Betaine, Calpain, cathepsin B and L, enzyme activities Caspase-3, Cytochrome c, Folic acid | Folic acid, betaine, and combined therapy of these supplements may reduce neuroapoptosis related to prenatal alcohol consumption and might be effective in preventing FAS | |
| Wang et al. [57], 2018 USA | Minocycline | In vitro and in vivo study to demonstrate the neuroprotective role of minocycline | C57BL/6 Mice | No prenatal EtOH exposure | On PD5, pups were randomly assigned to: CG, minocycline group, EtOH group, and EtOH + minocycline | Minocycline, EtOH, microglial cells and neurons | minocycline may ameliorate EtOH neurotoxicity in the development by alleviating GSK3β-mediated neuroinflammation |
| Skorput et al. [58], 2019 USA | NKCC1 inhibitor Bumetanide | In vivo and ex vivo study to assess whether the NKCC1 cotransporter is a tractable pharmacological target for normalizing in utero ethanol exposure-induced escalation of tangential migration to the prefrontal cortex | Nkx2.1Cre/Ai14 mice harboring Tomato-fluorescent (Nkx2.1+) | Dams EtOH exposure Dams CT | ‣5% w/w EtOH alone, from E13.5 to E16.5 ‣5% w/w EtOH + Bumetanide DMSO solution: 0.15 mg/kg/day, 3 days ‣Isocaloric control diet containing maltose | ‣Density of Nkx2.1+ profiles in the embryonic prefrontal cortex at E16.5 ‣PV+ interneurons in the PFC of young adult mice exposed to EtOH and EtOH + Bumetanide | ‣EtOH alone ↑ Density of Nkx2.1+ neurons ‣Bumetanide ↓ ethanol-induced enhancement of tangential migration of GABAergic cortical interneurons vs. CT |
| Ren et al. [59], 2019 USA | Minocycline | RCT to determine whether minocycline’s neuroprotective and anti-inflammatory properties can reduce EtOH-induced damage to spinal cord neuron development | C57BL/6 mice | No prenatal EtOH exposure | On PD5: ‣CT (SC injections of sterile saline) ‣Min group (2 SC injections/30 mg/kg) ‣EtOH group (5 g/kg) ‣EtOH (5 g/kg) + Min (2 SC injections/30 mg/kg) BAC = 338 mg/dL 8 h after the first injection | EtOH, minocycline, spinal cord | Minocycline may have neuroprotective property against ethanol-induced spinal cord damage. More investigation of the dosage and timeline of minocycline’s action is necessary. |
| Montagud-Romero et al. [49], 2019 | Trichostatin A (TSA) | RCT to assess the effects of TSA on emotional and cognitive impairments caused by PLAE | C57BL/6 mice | Prenatal exposure to EtOH (N = 30) Offspring (N = 80 males) | Dams: binge alcohol drinking model during gestation and lactation (20% v/v alcohol solution) Pups: From P28 to P35: treatment with TSA from P36 to P55 behavior assessment | EtOH, TSA, anxiogenic-like responses, and memory deterioration | potential benefits of HDAC inhibitors for some aspects FASD |
| Cantacorps et al. [46], 2020 Spain | Curcumin | RCT to assess the effects of curcumin in counteracting behavioral and molecular alterations induced by EtOH exposure during development | C57BL/6 mice (N = 24 for PAE) | Prenatal exposure to EtOH Offspring | Dams: ‣DID test ‣CG: water EG: 20% EtOH (oral) Pups: ‣P28: daily treatment with curcumin and vehicle solution ‣P60: EPM ‣P61: Y-maze ‣P62: NOR ‣P66: T-maze ‣P70: brain extraction | Curcumin; anxiety-like behavior; working memory; recognition memory; spatial ability | Curcumin could be a useful treatment for attenuating the alcohol-induced deficits observed in FASD |
| Cadena et al. [51], 2020 USA | Folic Acid | RCT to test FA ability to protect against behavioral defects induced by EtOH exposure | zebrafish | No prenatal exposure to EtOH | Healthy embryos were maintained in embryo medium until 2 hpf ‣CT group: animals transferred to embryo medium, no treatment ‣EtOH group: embryo medium containing 100 mM EtOH ‣EtOH + FA group: embryo medium containing 100 mM EtOH and 75 μM FA ‣FA group: embryo medium containing 75 μM FA | FA, EtOH, Teratogenic effects on morphological development (COA, ED, EY, PE, SB, SD, TD, YSE, YSR, HR, LP) Behavior (locomotor activity, sleep, anxiety like-behavior, visual ability) | FA alone produced different behavioral responses, indicating that FA may independently modify fish development. This zebrafish behavior on FASD model also provides an opportunity to test whether other compounds reduce the EtOH toxic effects. |
| Bottom et al. [41], 2020 USA | Choline | RCT to assess the ability of concurrent choline supplementation to ameliorate atypical neocortical and behavioral development following fully or partially PAE | CD-1 Mice | Prenatal exposure to EtOH Offspring | Dams (N = 10 for each group): ‣group 1: Water (Control) ‣group 2: 25% EtOH in water ‣group 3: 25% EtOH in water with 642 mg/L choline chloride ‣group 4: 642 mg/L choline chloride in water (CW). Separate subsets of dams were sacrificed at either GD9 and 19 for BEC and POSM measurements during gestation (n = 10, all groups). Pups: ‣Experimental analyses in brain: 1 ± 1 pups (P0) were selected pseudo-randomly from each litter. ‣Behavioral analysis: subsets of each litter in all groups were cross-fostered to alcohol-naïve mothers until P20 when behavioral testing of 3 ± 1 pups per litter took place. | EtOH, Choline, BEC, POSM, anxiety-like behaviors, motor function | Choline supplementation may represent a potent preventative measure for the adverse outcomes associated with PAE |
| Mohammad et al. [60], 2020 USA | Kcnn2 blockers | In vivo study to assess if PAE leads to deficits in gross and fine motor skill learning | Mice | Prenatal exposure to EtOH | Dams exposed to EtOH (1.0 g/kg weight), at E 16 and 17, during which upper cortical layer neurons are predominantly generated | Kcnn2, motor cortex, motor learning deficits | Kcnn2 blockers may be a novel intervention for learning disabilities in FASD |
| Ju et al. [1], 2020 Republic of Korea | HX106 | RCT to assess the effects of HX106 on PAE-induced ADHD-like phenotype and metabolic changes in the offspring mice | ICR mice (28–30 g weight) | Prenatal exposure to EtOH Offspring | Female at GD 3. Stabilized animals divided into 3 groups (from GD 6 to GD 15): ‣CT: saline (0.9% NaCl) ‣EtOH 2 groups: 6 g/kg/day; 50 v/v%) Pups (from P21; n = 10 per each group) ‣CT group: saline ‣OPAE groups: saline + HX106 at 200 mg/kg/day | ADHD-Like Symptoms, HX106, EtOH | ADHD pathology may involve metabolic disruptions and HX106 can be a promising supplement to attenuate the hyperactivity appearance in PAE-induced ADHD-like neuropathology |
| Almeida-Toledano et al. [61], 2021 Spain | Epigallocatechin Gallate | RCT to describe the effect of EGCG administration on oxidative stress, fetal growth, placental development, and neurogenesis processes in 2 human-like patterns of alcohol use FAS mouse models | 283 mouse fetuses | ‣Group 1: 42 Med control ‣group 2: 47 Med EtOH ‣group 3: 45 EtOH Med + EGCG ‣group 4: 54 Bin control ‣group 5: 44 Bin EtOH ‣group 6: 47 Bin + EGCG EtOH | Blood EtOH levels; EGCG; fetal growth; EtOH effect on oxidative stress; neuronal maturation and plasticity; astrocyte differentiation | EGCG is a promising antioxidant therapy to attenuate the consequences of PAE | |
| Gibula-Tarlowska et al. [56], 2021 Poland | CE-123 DAT inhibitor | RCT to demonstrate that dopamine signaling is one of the main factors underlying hyperactive, inattentive, and impulsive behaviors | Male rat (N = 163) | No prenatal exposure to EtOH | EtOH on P4-9. ‣Weaning: P20 group 1 was tested in early adolescence (P21) ‣group 2 was tested as adults (P45-50) group 3 was tested at P50 | BEC; Locomotor activity test; EPM test; Barnes maze task | CE-123 may be helpful in overcoming behavioral disorders in subjects perinatally exposed to ethanol |
| Lopatynska-Mazurek et al. [45], 2021 Poland | Rapamycin | Prospective cohort study to evaluate the hypothesis that development of emotional learning deficits and depressive-like behaviors in adult rats exposed to EtOH during the neonatal period are a function of oxidative stress that, in turn, depends on the mTOR signaling pathway | Male rats (N = 64) | No prenatal exposure to EtOH | from P4 to P9: ‣S (0.9%) + SI ‣Rapamycin + SI ‣Torin 2 + SI ‣FK-506 + SI ‣S (0.9%) + EtOH (5 g/Kg/day) ‣Rapamycin + EtOH (5 g/Kg/day) ‣Torin 2 + EtOH (5 g/Kg/day) ‣FK-506 + EtOH (5 g/Kg/day) ‣P60 (adults): aversive learning and memory processes assessment; depressive-like behavior assessment | EtOH, Rapamycin, Torin, FK-506 | Rapamycin but not Torin-2 or FK-506 could have protective effects against the ethanol-induced LPO and AP site levels in the hippocampus and prefrontal cortex. Rapamycin could be useful as a preventive therapy in disorders related to PAE. |
| García-Baos et al. (2021) [62] Spain | Cannabidiol | RDBPC to explore CBD therapeutic effects demonstrating that it might reduce through an anti-inflammatory mechanism. Cognitive deficits induced by EtOH exposure. | C57BL/6 mice | Prenatal exposure to EtOH Offspring | Pregnant females: DID test during PLAE. Day 4: six binge-like drinking sessions. Dose ethyl alcohol n.d. Offspring: CBD 20 mg/kg during 10 consecutive days from P25 until P34 Behavioral assessment: P60–P66 P70: PFC and HPC extraction | CBD, EtOH, spatial, working and recognition memory | CBD appears to be a promising therapeutic drug since it could hamper cognitive impairments caused by several pathological conditions, potentially through neuroimmune modulation |
| Grafe et al. [42], 2021 Canada | Choline | RCT to examine the impact of acute choline administration on synaptic transmission in the in vitro DG and how these mechanisms may be altered by EtOH exposure of male and female offspring | Sprague-Dawley rats | GD1 2 experimental groups: ‣PNE diet was a protein and liquid diet containing 35.5% ethanol-derived calories. Dams were gradually introduced to the ethanol diet and subsequently consumed only the ethanol diet from GD 22 until the day before birth. ‣On GD 21: dams were returned to a solid control chow diet and remained on this diet until parturition. CT diet. | EtOH, choline, NMDA, M1 receptors | choline can modulate hippocampal transmission at the level of the synapse and it can have unique effects following EtOH exposure | |
| Sharma et al. [2], 2021 India | Papaverine | RCT to assess the outcome of papaverine administration on PAE affected behavioral (hyperactivity, repetitive behavior, and anxiety) and biochemical markers in several brain areas associated with ADHD | N = 40 Adults albino wistar rats | Prenatal exposure to EtOH Offspring | Dams (from GD8 to GD20): ‣EtOH (20% w/v; 6 g kg−1 day−1-weekdays; 4 g kg−1 day−1-weekends; N = 8); CT group: received calorifically equivalent sucrose solution (30% w/v; N = 5) Pups (from P21 to P48), 4 Exp groups (each with 8 males): ‣Group I and II (VEH/Drug per-se); sucrose solution-treated females were administered with normal saline—2 mL kg−1 i.p., or Papaverine—30 mg kg−1 day−1, i.p., as per the treatment assigned. ‣Group III (PAE); females treated with EtOH during gestation were treated with NS—2 mL kg−1, i.p. ‣Groups IV and V (Papaverine treatment); PAE pups were divided further into PAE + P (15/30) groups and received papaverine (15/30 mg/kg day−1 i.p.) in a volume of 2 mL kg−1. ‣Behavioral assessment: P44 to P48. | ADHD-Like Symptoms; papaverine; EtOH | Papaverine, a selective PDE10A inhibitor rectified behavioral phenotypes associated with ADHD, possibly by altering the protein markers associated with neuronal survival, neuronal transcription factor, brain inflammation, and brain oxidative stress. Implicating PDE10A as a possible target for furthering our understanding of ADHD phenotypes. |
| Chen et al. [63], 2022 Taiwan | AST | RCT to explore how AST treatment can ameliorate morphological changes in the hippocampus and cognitive impairment in FASD rats by reducing oxidative stress and neuroinflammation in the brain | 40 male rat pups | No prenatal exposure to EtOH | 4 groups: CT; normal with AST; FASD group; ethanol-inhaled with AST treatment. FASD induction started on P2 and continued until P10. To investigate AST effects in a FASD rat model, it was administered on P53 and kept until sacrifice (P60). | EtOH, AST, MWM task, BWT | AST could be considered to treat FASD |
| Sabzali et al. [44], 2022 Iran | Metformin | In vivo and ex vivo study to evaluate the protective effects of metformin on EtOH-related neuroinflammation, as well as neuron apoptosis in the hippocampus of adult male rat model of FASD | 60 Wistar male rat pups (8–10 g) | No prenatal exposure to EtOH | EtOH in milk solution (5.25 and 27.8 g/kg, respectively) by intragastric intubation at 2–10 days after birth | tumor necrosis factor-α (TNF-α) and antioxidant enzyme concentrations | Metformin reduces cell apoptosis in the hippocampus. It can be considered a promising therapeutic option for FASD; however, more research is required. |
| Burton et al. [64], 2022 USA | Smoothened Agonist (SAG); purmorphamine (PUR) | In vivo study to demonstrate that a Shh pathway agonist given at an appropriate dose and timing relative to ETOH exposure during embryogenesis may alleviate the effects of PAE | Zebrafish embryos (AB strain, ZFIN ID: ZDB-GENO-960809-7) | Zebrafish embryos exposed to EtOH from 8–10 hpf | Embryos exposed to varying concentrations of SAG or PUR at 6–8 hpf or 10–12 hpf by diluting with egg water | EtOH, Eye size data, gene expression, novel tank diving data, midbrain hindbrain data, and pax6a in situ expression data | Pharmacological activation of the Shh pathway at specific developmental timing markedly diminishes the severity of alcohol-induced birth defects as altered eye size and midline brain development |
| Farhadi et al. [65], 2022 Iran | Crocin | RCT to evaluate the protective impact of crocin on ethanol-related neuroinflammation | 72 wistar rat pups (8 to 10 g) | No prenatal exposure to EoTH | Pups treated from P2 to P10. Group 1: CT Group 2: milk + saline solution (dose n.a.) Group 3: milk solution + ethanol (total daily dose of ethanol (5.25 g/kg) Groups 4, 5, 6: milk solution + ethanol + crocin (15, 30, and 45 mg/kg) | EtOH; Crocin; astrogliosis evaluation; inflammation measurement; enzyme evaluation; spatial memory | Crocin is applicable for the treatment of FASD |
| Gasparyan et al. [66] 2023 Spain | Cannabidiol | RCT to evaluate the effects of early and chronic CBD administration on offspring exposed to an animal model of FASD | N = 190 c57bl/6j mice 50 female and 20 male 5-week-old mice Offspring: 120 (60 males and 60 females) | Prenatal exposure to EtOH Offspring | PAE: N = 15 females exposed to 2 bottles with tap water. N = 35 females exposed to 2 bottles, one always with tap water and the other increasing EtOH concentrations. PAE: at GD7, EtOH gavage was started until the pup’s weaning at P21. Pups: treated with CBD 30 mg/kg/day or VEH from the day of weaning for 10 weeks. | CBD, ethanol, anxiety-like behaviors, depressive-like behaviors; ASR; recognition memory; long-term aversive memory; gene expression analyses; brain analysis | Potential suitability of CBD in children and young adults with FASD |
Abbreviations:  , male gender;
, male gender;  , female gender; ADHD, attention deficit hyperactivity disorder; AP, apurinic/apyrimidinic; AST, astaxanthin; EC or BAC, blood ethanol concentration; Bin, binge; BWT, beam walking test; C or Cho, choline; CBD, cannabidiol; Cho-Tr, choline-trained; Cho-Utr, choline-untrained; COA, coagulation; CT, control group; DAT, dopamine transporter; DHA, docosahexaenoic acid; DHM, dihydromyricetin; DG, dentate gyrus; DID, drinking in the dark; E, embryonic day; ED, large inter-eye distance; EGCG, epigallocatechin-3-gallate; EPM, elevated pus maze test; ET, ethanol-exposed group; EtOH, ethanol or alcohol; EY, small eyes; FAE, Fetal alcohol exposure; FAS, fetal alcohol syndrome; FJB, fluoro-jade B; FST, forced swimming test; GABAAR, γ-aminobutyric acid type A receptor; GD, gestational day; GFAP, glial fibrillary acidic protein; GSH-Px, glutathione peroxidase; GSK3β, glycogen synthase kinase 3 beta; HAT, histone acetyltransferase; HDAC, histone deacetylases enzymes; HPC, hippocampus; hpf, hour post-fertilization; HR, hatching rate; larvae paralysis; iUSVs, isolation-induced ultrasonic vocalizations; LPO, lipid peroxidation; LTD, long-term depression; LTP, long-term potentiation; Med, mediterranean; Min, minocycline; mIPSCs, inhibitory postsynaptic currents; mTOR, mammalian target of the rapamycin; MWM, morris water maze; n.d., not declared; NMDA, N-methyl-d-aspartate; NOR, novel object recognition test; OPAE, Offspring prenatal alcohol exposure; Osm, osmotin; P or PD, postnatal day; PAE, prenatal alcohol exposure; PE, pericardial edema; PFC, prefrontal cortex; PI, propidium iodide; PLAE, prenatal and lactational alcohol exposure; PNTG, postnatal treatment group; PNUn, postnatal untreated group; POSM, plasma osmolality; PUFAs, polyunsaturated fatty acids; RCT, randomized controlled trial; RDBPC, randomized double blind placebo control study; S, saline; Sal-Tr, saline-trained; Sal-Utr, saline-untrained; SB, delayed swim bladder inflation; SC, subcutaneous; SD, spine deformation; Shh, sonic hedgehog; SI, sham intubation; SOD, superoxide dismutase; TBS, theta burst stimulation; TD, tail deformation; TG, treatment group; THIP, gaboxadol hydrochloride; TNF-α, tumor necrosis factor-α; Tr, working memory training; TSA, trichostatin A; Utr, untrained; VEH, vehicle; YSE, yolk sac edema/unusual large yolk sac; YSR, delayed yolk sac resorption.
, female gender; ADHD, attention deficit hyperactivity disorder; AP, apurinic/apyrimidinic; AST, astaxanthin; EC or BAC, blood ethanol concentration; Bin, binge; BWT, beam walking test; C or Cho, choline; CBD, cannabidiol; Cho-Tr, choline-trained; Cho-Utr, choline-untrained; COA, coagulation; CT, control group; DAT, dopamine transporter; DHA, docosahexaenoic acid; DHM, dihydromyricetin; DG, dentate gyrus; DID, drinking in the dark; E, embryonic day; ED, large inter-eye distance; EGCG, epigallocatechin-3-gallate; EPM, elevated pus maze test; ET, ethanol-exposed group; EtOH, ethanol or alcohol; EY, small eyes; FAE, Fetal alcohol exposure; FAS, fetal alcohol syndrome; FJB, fluoro-jade B; FST, forced swimming test; GABAAR, γ-aminobutyric acid type A receptor; GD, gestational day; GFAP, glial fibrillary acidic protein; GSH-Px, glutathione peroxidase; GSK3β, glycogen synthase kinase 3 beta; HAT, histone acetyltransferase; HDAC, histone deacetylases enzymes; HPC, hippocampus; hpf, hour post-fertilization; HR, hatching rate; larvae paralysis; iUSVs, isolation-induced ultrasonic vocalizations; LPO, lipid peroxidation; LTD, long-term depression; LTP, long-term potentiation; Med, mediterranean; Min, minocycline; mIPSCs, inhibitory postsynaptic currents; mTOR, mammalian target of the rapamycin; MWM, morris water maze; n.d., not declared; NMDA, N-methyl-d-aspartate; NOR, novel object recognition test; OPAE, Offspring prenatal alcohol exposure; Osm, osmotin; P or PD, postnatal day; PAE, prenatal alcohol exposure; PE, pericardial edema; PFC, prefrontal cortex; PI, propidium iodide; PLAE, prenatal and lactational alcohol exposure; PNTG, postnatal treatment group; PNUn, postnatal untreated group; POSM, plasma osmolality; PUFAs, polyunsaturated fatty acids; RCT, randomized controlled trial; RDBPC, randomized double blind placebo control study; S, saline; Sal-Tr, saline-trained; Sal-Utr, saline-untrained; SB, delayed swim bladder inflation; SC, subcutaneous; SD, spine deformation; Shh, sonic hedgehog; SI, sham intubation; SOD, superoxide dismutase; TBS, theta burst stimulation; TD, tail deformation; TG, treatment group; THIP, gaboxadol hydrochloride; TNF-α, tumor necrosis factor-α; Tr, working memory training; TSA, trichostatin A; Utr, untrained; VEH, vehicle; YSE, yolk sac edema/unusual large yolk sac; YSR, delayed yolk sac resorption.
 , male gender;
, male gender;  , female gender; ADHD, attention deficit hyperactivity disorder; AP, apurinic/apyrimidinic; AST, astaxanthin; EC or BAC, blood ethanol concentration; Bin, binge; BWT, beam walking test; C or Cho, choline; CBD, cannabidiol; Cho-Tr, choline-trained; Cho-Utr, choline-untrained; COA, coagulation; CT, control group; DAT, dopamine transporter; DHA, docosahexaenoic acid; DHM, dihydromyricetin; DG, dentate gyrus; DID, drinking in the dark; E, embryonic day; ED, large inter-eye distance; EGCG, epigallocatechin-3-gallate; EPM, elevated pus maze test; ET, ethanol-exposed group; EtOH, ethanol or alcohol; EY, small eyes; FAE, Fetal alcohol exposure; FAS, fetal alcohol syndrome; FJB, fluoro-jade B; FST, forced swimming test; GABAAR, γ-aminobutyric acid type A receptor; GD, gestational day; GFAP, glial fibrillary acidic protein; GSH-Px, glutathione peroxidase; GSK3β, glycogen synthase kinase 3 beta; HAT, histone acetyltransferase; HDAC, histone deacetylases enzymes; HPC, hippocampus; hpf, hour post-fertilization; HR, hatching rate; larvae paralysis; iUSVs, isolation-induced ultrasonic vocalizations; LPO, lipid peroxidation; LTD, long-term depression; LTP, long-term potentiation; Med, mediterranean; Min, minocycline; mIPSCs, inhibitory postsynaptic currents; mTOR, mammalian target of the rapamycin; MWM, morris water maze; n.d., not declared; NMDA, N-methyl-d-aspartate; NOR, novel object recognition test; OPAE, Offspring prenatal alcohol exposure; Osm, osmotin; P or PD, postnatal day; PAE, prenatal alcohol exposure; PE, pericardial edema; PFC, prefrontal cortex; PI, propidium iodide; PLAE, prenatal and lactational alcohol exposure; PNTG, postnatal treatment group; PNUn, postnatal untreated group; POSM, plasma osmolality; PUFAs, polyunsaturated fatty acids; RCT, randomized controlled trial; RDBPC, randomized double blind placebo control study; S, saline; Sal-Tr, saline-trained; Sal-Utr, saline-untrained; SB, delayed swim bladder inflation; SC, subcutaneous; SD, spine deformation; Shh, sonic hedgehog; SI, sham intubation; SOD, superoxide dismutase; TBS, theta burst stimulation; TD, tail deformation; TG, treatment group; THIP, gaboxadol hydrochloride; TNF-α, tumor necrosis factor-α; Tr, working memory training; TSA, trichostatin A; Utr, untrained; VEH, vehicle; YSE, yolk sac edema/unusual large yolk sac; YSR, delayed yolk sac resorption.
, female gender; ADHD, attention deficit hyperactivity disorder; AP, apurinic/apyrimidinic; AST, astaxanthin; EC or BAC, blood ethanol concentration; Bin, binge; BWT, beam walking test; C or Cho, choline; CBD, cannabidiol; Cho-Tr, choline-trained; Cho-Utr, choline-untrained; COA, coagulation; CT, control group; DAT, dopamine transporter; DHA, docosahexaenoic acid; DHM, dihydromyricetin; DG, dentate gyrus; DID, drinking in the dark; E, embryonic day; ED, large inter-eye distance; EGCG, epigallocatechin-3-gallate; EPM, elevated pus maze test; ET, ethanol-exposed group; EtOH, ethanol or alcohol; EY, small eyes; FAE, Fetal alcohol exposure; FAS, fetal alcohol syndrome; FJB, fluoro-jade B; FST, forced swimming test; GABAAR, γ-aminobutyric acid type A receptor; GD, gestational day; GFAP, glial fibrillary acidic protein; GSH-Px, glutathione peroxidase; GSK3β, glycogen synthase kinase 3 beta; HAT, histone acetyltransferase; HDAC, histone deacetylases enzymes; HPC, hippocampus; hpf, hour post-fertilization; HR, hatching rate; larvae paralysis; iUSVs, isolation-induced ultrasonic vocalizations; LPO, lipid peroxidation; LTD, long-term depression; LTP, long-term potentiation; Med, mediterranean; Min, minocycline; mIPSCs, inhibitory postsynaptic currents; mTOR, mammalian target of the rapamycin; MWM, morris water maze; n.d., not declared; NMDA, N-methyl-d-aspartate; NOR, novel object recognition test; OPAE, Offspring prenatal alcohol exposure; Osm, osmotin; P or PD, postnatal day; PAE, prenatal alcohol exposure; PE, pericardial edema; PFC, prefrontal cortex; PI, propidium iodide; PLAE, prenatal and lactational alcohol exposure; PNTG, postnatal treatment group; PNUn, postnatal untreated group; POSM, plasma osmolality; PUFAs, polyunsaturated fatty acids; RCT, randomized controlled trial; RDBPC, randomized double blind placebo control study; S, saline; Sal-Tr, saline-trained; Sal-Utr, saline-untrained; SB, delayed swim bladder inflation; SC, subcutaneous; SD, spine deformation; Shh, sonic hedgehog; SI, sham intubation; SOD, superoxide dismutase; TBS, theta burst stimulation; TD, tail deformation; TG, treatment group; THIP, gaboxadol hydrochloride; TNF-α, tumor necrosis factor-α; Tr, working memory training; TSA, trichostatin A; Utr, untrained; VEH, vehicle; YSE, yolk sac edema/unusual large yolk sac; YSR, delayed yolk sac resorption.3.3. Results from Human Studies
As reported in Table 2, most of the clinical studies carried out in the last ten years concerned both perinatally [67,68,69,70] and postnatally [71,72,73,74,75,76] the use of choline and/or vitamins as nutritional interventions for ND due to alcohol exposure. After the promising results obtained in animal studies, tolerability, adherence and side effects of choline were evaluated in humans, when perinatally and postnatally administered for approximately 6 and 9 months to pregnant alcohol users and 2–5 year-old children, respectively. These studies [68,71] demonstrated a good feasibility and acceptability of choline both in heavy drinking pregnant women and children with minimal or no side effects (details in Table 2).
When exploring choline efficacy on cognitive development of PAE children, two studies from the same research group [72,75] observed potentially positive effects on memory functions in children aged 2–4 years old, while a little decrease in behavioral problems was assessed at 8 years old. These results highlighted the need to perform long-term follow-up for the monitoring of choline effects on neurodevelopmental functions in PAE children. Another study with choline [73] did not obtain significant cognitive improvements in school-aged children. Indeed, according to preclinical studies, choline supplementation has greater efficacy in early ages and a choline therapeutic window for FASDs children has to be considered in clinical studies. Nevertheless, even when prenatally administrated, choline did not minimize PAE effects, when measured at 6 months of age and only the use of multivitamins had small positive effects [67].
Warton et al. reported that high doses of choline supplementation (2 g/day) can mitigate PAE-related brain structural deficits in humans. Gimbel et al. [76] obtained similar results in a follow-up study, but with a lower dose. The authors showed that early choline supplementation may affect white matter development in terms of the structure and organization of axons in the corpus callosum.

Table 2.
Pharmacological interventions for fetal alcohol spectrum disorders.
Table 2.
Pharmacological interventions for fetal alcohol spectrum disorders.
| Author Year Country | Study Design and Objectives | Pharmacological Intervention | Dose Intervention Period | Population (N) | Patient Age, Mean (SD) | Variables Studied | Key Results/Assessment |
|---|---|---|---|---|---|---|---|
| Wozniak J.R. et al. [71], 2013 USA | RDBPC to evaluate tolerability and bioavailability of choline as supplement for FASD children | Postnatal choline bitartrate (1.25 g) | 514 mg/day, 9 m | Children: ‣10 active ‣9 placebo | 2–5 y (range) | ‣Feasibility ‣Tolerability ‣Adverse effects ‣Serum choline levels | ‣Minimal and equivalent adverse effects were observed in choline and placebo groups. Fishy body odor was reported for choline group ‣Acceptable feasibility and tolerability |
| Wozniak J.R. et al. [72] 2015 USA | RDBPC to assess hippocampal-dependent memory improvement by postnatal choline supplementation in FASD children | Postnatal choline bitartrate (1.25 g) | 514 mg/day, 9 m | Children: ‣31 active ‣29 placebo | 3.8 y (0.80) | ‣Mullen Scales of Early Learning (global cognitive functioning) ‣Elicited imitation memory tasks | ‣No choline effects were observed in global cognitive functioning ‣Choline ↑ hippocampal-dependent memory tasks especially in younger children (≤4 y) and best score were obtained in long-delayed memory |
| Coles C.D. et al. [67], 2015 USA Ukraine | PCS to evaluate mother nutritional supplements use impact on children prenatally exposed to alcohol | ‣prenatal MVM ‣prenatal MVM + Choline | ‣nd ‣nd + 750 mg Choline from first prenatal clinic visit until delivery | 367 Children | ‣Children development assessment through BSID-II at 6 m of age | ‣MVM ↑ cognitive development ‣Choline has no effect on cognitive development and negative effects on motor outcomes ‣No differences between prenatally alcohol-exposed and non-exposed children | |
| Nguyen T.T. et al. [73], 2016 USA | RDBPC to investigate if choline supplements have positive effects on the memory impairments, executive function, and attention deficits in school-aged (5–10 y) FASDs children | Postnatal Glicerophospho-choline | 625 mg/day, 6 wk | 55 Children: ‣29 active ‣26 placebo | 8.3 y (1.75) | ‣Cognitive abilities in the domains of learning and memory, executive function, (attention, fine motor functioning) | ‣Choline supplements did not improve memory, executive and attention functioning in school-aged (5–10 y) children with FASDs |
| Jacobson S.W. et al. [68], 2018 South Africa | RDBPC to assess adherence and side effects to choline supplementation in heavy drinking pregnant women | Prenatal choline bitartrate | 2 g/day, from mid-pregnancy until delivery | Mothers: ‣34 active ‣35 placebo | 26.4 y (5.7) | ‣Adherence ‣Side effects ‣Choline plasma concentration | ‣Choline adherence was excellent (≥84%) and good to excellent (≥68%) for 42% and 58% of participants, respectively. It was not related to maternal education, depression, intellectual function, stressful life events or socioeconomic status. ‣Increase in nausea/dyspepsia symptoms, especially when choline is consumed on an empty stomach. ‣Choline levels in plasma were significantly higher than those of the placebo group. |
| Jacobson S.W. et al. [69], 2018 South Africa | RDBPC to assess prenatal choline supplementation effects on EBC, on growth, recognition memory and information processing speed deficit | Prenatal choline bitartrate | 2 g/day, 9–27 wk (range) | 62 Infants: ‣31 active ‣31 placebo | ‣6.5 m ‣1 y | ‣EBC assessed at 6.5 m ‣Infant growth assessed at 6.5 m and 1 y ‣Visual recognition memory assessed at 6.5 m and 1 y through FTII ‣FASD diagnosis | ‣Prenatal choline supplementation ↑ conditioned responses more than placebo, especially in mother with high adherence to the treatment. ‣Newborns in the placebo and choline group were both small at birth. Infants in choline group; nevertheless, a weight and head circumference growth increase between 6.5 m and 1 y ‣FAS and pFAS diagnosis incidence for the choline and placebo group was almost the same |
| Sarkar et al. [74], 2019 USA | RDBPC to assess if choline can reduce DNA methylation and improve POMC and PER2 gene expression by analyzing blood sample of FASD children treated with the supplement | Postnatal choline bitartrate | 514 mg/day, 9 m | 32 Children: ‣16 active ‣16 placebo | 2.5–5 y (range) | ‣POMC and PER2 mRNA levels measured by quantitative real-time PCR ‣DNA methylation assessed by methylation-specific real time PCR assay | ‣Choline increased POMC and PER2 gene expression following 9 m of treatment ‣Choline reduced PER2 and POMC DNA methylation following 9 m of treatment |
| Wozniak J.R. et al. [75], 2020 USA | RDBPC follow-up study evaluating postnatal choline supplementation potential on long-term cognitive and behavioral functions of PAE children | Postnatal choline | 514 mg/day, 9 m | 31 Children: ‣15 active ‣16 placebo | 8.6 y (1.0) | ‣Cognitive, memory, executive, behavioral and emotional functioning | ‣Significant non-verbal Visual-Spatial Reasoning and non-verbal Working Memory components in the choline group vs. placebo ‣choline had non-significant effects on quantitative reasoning, fluid reasoning, and a range of verbally-mediated skills ‣Choline group had fewer ADHD-related behavioral problems than the placebo group |
| Warton F.L. et al. [70], 2021 South Africa | Follow-up study observing if prenatal choline supplementation protected PAE newborn brain from volume reduction and improved infant recognition memory | Prenatal choline | 2 g/day | 50 Children: ‣27 active ‣23 placebo | 2.8 wk median | ‣MRI data of newborn brains ‣Recognition memory through FTII at 12 months | ‣Larger total intracranial volume in choline group vs. placebo ‣Larger right putamen and corpus callosum were associated with improved recognition memory |
| Gimbel B.A. et al. [76], 2022 USA | Follow-up study evaluating postnatal choline effects on executive functioning and white matter microstructure | Postnatal choline bitartrate | 514 mg/day, 9 m | 18 Children: ‣9 active ‣9 placebo | 11.0 y | ‣Executive functioning ‣Differences in corpus callosum and white matter microstructure through diffusion-weighted MRI and the NODDI biophysical model | ‣Improved executive functioning in choline group vs. placebo ‣Lower corpus callosum orientation dispersion index (more coherent fibers) in choline group vs. placebo |
Abbreviations: ↑, improve; ADHD, attention deficit/hyperactivity disorder; BSID-II, Bayley Scales of Infant Development 2nd Ed; EBC, eyeblink conditioning; FASDs, Fetal Alcohol Spectrum Disorders; FAS, Fetal Alcohol Spectrum; FTII, Fagan Test of Infant Intelligence; m, months; MRI, magnetic resonance imaging; MVM, multivitamin/mineral supplementation; nd, not declared; NODDI, neurite orientation dispersion and density imaging; PAE, prenatal alcohol exposure; PCS, prospective cohort study; pFAS, partial fetal alcohol spectrum; RDBPC, randomized double blind placebo control study; wk, weeks; y, years.
4. Discussion
PAE has long been recognized as a significant risk factor contributing to FASD. Early diagnosis plays a crucial role in addressing FASD symptoms and minimizing subsequent SD that may arise later in life.
FASD is associated with various comorbidities [24], significantly impacting the individual’s lifelong health and functionality. ND, such as Cognitive Delay, ADHD, SLD, and Externalizing Disorders, are commonly observed in FASD subjects.
The therapeutic approach for FASD individuals requires a multidisciplinary strategy, including medical, psychosocial, and educational interventions [19].
Several drugs have shown potential in managing FASD-related symptoms. Mood stabilizers, CNS stimulants, atypical antipsychotics, SSRIs, benzodiazepines, and other medications have been used to address aggression, ADHD, intellectual disabilities, and depressive or anxiety disorders. However, it is crucial to consider potential side effects and long-term impact, especially given the teratogenic effects of certain drugs.
Stimulants are primarily used to counteract ADHD symptoms including hyperactivity, inattention, and impulsivity. For example, the use of methylphenidate in a pilot study showed improvements in the symptoms of ADHD although there are opinions against its use due to side effects (decreased appetite, stomach aches and headaches, growth retarding effects, diversion) [77]. Doing et al. [78] reported contradictory findings about the use of methylphenidate in cases of comorbidities associated with FASD (symptoms of hyperactivity and inattention). Even considering study limitations, inattention was found to respond better to dextroamphetamine.
If stimulants are not effective, selective norepinephrine reuptake inhibitors (atomoxetine) may be used to treat ADHD symptoms. Anxiolytics can treat anxiety symptoms commonly seen in children with FASD. Alpha-2-adrenergic agonists are used to counteract the symptoms of anxiety and have also been shown to address problems with attention, hyperactivity, and impulsivity, as well as helping to reduce tics. Because alpha-2-adrenergic agonists can cause sedation, their daytime use may be limited, but they may be helpful for children who have difficulty sleeping. Antidepressant medications can treat depressive symptoms such as sad mood, loss of interest, sleep problems, and anxiety. Neuroleptics (for example, risperidone or aripiprazole) may be used to treat serious symptoms such as aggression, anxiety, disruptive behavior, tics, and impulsivity. The most common side effect of neuroleptic use is significant weight gain, and rarely the drug can cause extrapyramidal seizures. Melatonin supplementation can help with sleep disorders. Some subjects with FASD who exhibit sleep difficulties may respond positively to melatonin supplementation to aid sleep onset [79].
Moreover, when evaluating individuals by a Social Skills Rating System, in a Children’s Friendship Training treated with psychotropic drugs, different outcomes have been recorded. For example, neuroleptics improved Social Skills Rating System scores (parent- and teacher reported self-control, parent assertation and problem behaviors) more than stimulants, while antidepressants and nonstimulants did not produce statistically significant main outcomes. In some cases, no outcome differences between children prescribed stimulants or no medication were described [80].
The choice of a pharmacological intervention for treating FASD depends on many factors such as the severity of the disease spectrum and on gender and age. Although some clinical evidence has confirmed the validity of different therapy approaches, there are still many studies needed to determine the effectiveness of appropriate treatment [81].
In the past 10 years, new experiments have been conducted with new substances, especially non-psychotropic molecules.
This integrative review shows that in the last 10 years, the focus has changed from treating symptoms to prevention. The new animal and human studies have emphasized the importance of testing substances that can affect the embryogenesis of the various brain regions altered by alcohol exposure at the subcellular level. Acting during embryogenesis on the negative effects of alcohol like neuronal apoptosis, synaptic connections, cascade of protein signaling, and morphologic modifications, could reduce PD and ameliorate SD. Alternative interventions in animal studies such as natural antioxidants like EGCG and cannabidiol have shown promise in ameliorating FASD-related cognitive impairments or addressing associated symptoms.
For example, Epigallocatechin Gallate (EGCG) has been suggested to be a protective agent against FASD, ameliorating fetal growth restriction and preventing FASD-related cognitive impairment [48].
The majority of the trials are still in preclinical stages, and choline has also been studied in clinical trials. Despite the results of the studies agreeing on choline’s beneficial effect such as nonverbal intelligence, visuospatial abilities, verbal memory, and working memory, there are disparate conclusions regarding its use. There are still some issues to be addressed like establishing the right timing and doses of administration (both prenatally and postnatally). Moreover, further follow-up studies need to be performed to evaluate its impact on older age. When thinking about this treatment approach (both during the prenatal and postnatal period), the early diagnosis is of fundamental importance not only in managing SD, but also in PD, even though the biggest limit in perinatal cases is the detection of pregnant women who declare that they drink.
The selected studies encompassed human and animal models, evaluating pharmacological treatments’ efficacy on neurocognitive and behavioral responses in FASD patients.
However, limitations include the varied study designs and small population sample size with limited randomized trials using novel molecules, hindering the generalization of results.
The review strengths lie in its comprehensive assessment of the new insights in pharmacological therapies tested for children with FASD with diverse studies, including the treatment with promising nutritional supplements as choline and EGCG. Moreover, another strength of this study is that it highlights key results from previous papers using a snowball strategy to cover all the known treatments for FASD comorbidities.
As a limitation, this review did not evaluate the quality of evidence following the GRADE statement, which may lead to bias in relevance or specific value among the different studies. Furthermore, FASD is associated with several comorbidities, which complicates the search strategy. Hence, we decided to focus on the most representative comorbidities associated with FASD.
5. Conclusions
In conclusion, up to date, there are specific treatments for FASD-associated symptoms caused by the cellular and tissue injury that originated from prenatal exposure to alcohol, but there are no medicaments addressed to an irreversible damage. However, recent studies included in this review have performed multidisciplinary approaches involving psycho-social interventions and pharmacological treatments that aim to improve FASD individuals’ quality of life and manage its associated symptoms. Moreover, natural molecules such as choline or EGCG have shown promising results in recent clinical studies; therefore, they could be used as a complementary treatment for some of the clinical manifestations in subjects with FASD. However, further research and longer-term follow up need to be performed to better understand the efficacy, safety, and long-lasting impact of these interventions in FASD individuals. Furthermore, the restorative therapies that have been used in both animal and human models have to be aimed to improve damaged neural functioning. An example is antioxidants that can stabilize neural membranes, in a similar way as the treatment of fever associated with meningitis or pneumonia. The authors believe that this should be the main investigation line for studies in the next future.
This integrative review serves as a crucial reference for clinicians and researchers seeking insights into the novel approach of pharmacological interventions for FASD and associated comorbidities, driving the need to investigate in more targeted and effective treatments for affected individuals.
Author Contributions
Conceptualization, M.M., V.A.-F., A.M. (Adele Minutillo) and N.L.M.; methodology, M.M. and V.A.-F.; validation, A.M. (Adele Minutillo) and N.L.M.; formal analysis, A.M. (Adele Minutillo) and N.L.M.; investigation, A.M. (Adele Minutillo), N.L.M., M.M., V.A.-F. and A.M. (Afrouz Mirahi); resources, M.M. and V.A.-F.; data curation, S.P. and O.G.-A.; writing—original draft preparation V.A.-F., A.M. (Adele Minutillo) and N.L.M.; writing—review and editing, M.M., V.A.-F., A.M. (Adele Minutillo), N.L.M., A.M. (Afrouz Mirahi), S.P. and O.G.-A.; visualization, M.M., V.A.-F., A.M. (Adele Minutillo), N.L.M. and A.M. (Afrouz Mirahi); supervision, S.P. and O.G.-A.; project administration, S.P. and O.G.-A.; funding acquisition, S.P. and O.G.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Red de Salud Materno-Infantil y del Desarrollo (SAMID) (RD12/0026/0003 and RD16/0022/0002) and Instituto de Salud Carlos III (ISCIII) (PI19/01853 and PI21/01415), and co-funded by Redes de Investigación Cooperativa Orientadas a Resultados en Salud (RICORS) (RD21/0012/0017), teams RG6 and RG19 (supported V.A., M.V.) funded by Instituto de Salud Carlos III (ISCIII) and European Union NextGenerationEU/Mecanismo para la Recuperación y la Resiliencia (MRR)/Plan de Recuperación, Transformación y Resiliencia (PRTR). This study has also been carried out thanks to the support of the Departament de Recerca i Universitats de la Generalitat de Catalunya al Grup de Recerca Infància i Entorn (GRIE) (2021 SGR 01290) and of Fundación Mutua Madtileña (2023 AP183662023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jones, K.L.; Smith, D.W.; Ulleland, C.N. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973, 301, 1267–1271. [Google Scholar] [CrossRef]
- Riley, E.P.; Infante, M.A.; Warren, K.R. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychol. Rev. 2011, 21, 73–80. [Google Scholar] [CrossRef]
- Brown, J.M.; Bland, R.; Jonsson, E.; Greenshaw, A.J.; Riley, E.P.; Infante, M.A.; Warren, K.R.; Jones, K.L.; Smith, D.W.; Ulleland, C.N. The Standardization of Diagnostic Criteria for Fetal Alcohol Spectrum Disorder (FASD): Implications for Research, Clinical Practice and Population Health. Can. J. Psychiatry 2011, 64, 73–80. [Google Scholar] [CrossRef]
- Elliott, L.; Coleman, K.; Suebwongpat, A.; Norris, S. Fetal Alcohol Spectrum Disorders (FASD): Systematic Reviews of Prevention, Diagnosis and Management; Health Services Assessment Collaboration, University of Canterbury: Christchurch, New Zealand, 2008. [Google Scholar]
- May, P.A.; Gossage, J.P. Maternal Risk Factors for Fetal Alcohol Spectrum Disorders: Not as Simple as It Might Seem. Alcohol Res. Health J. Natl. Inst. Alcohol Abuse Alcohol. 2011, 34, 15–26. [Google Scholar]
- Bell, J.C.; Raynes-Greenow, C.; Turner, R.M.; Bower, C.; Nassar, N.; O’Leary, C.M. Maternal Alcohol Consumption during Pregnancy and the Risk of Orofacial Clefts in Infants: A Systematic Review and Meta-Analysis. Paediatr. Perinat. Epidemiol. 2014, 28, 322–332. [Google Scholar] [CrossRef]
- Caputo, C.; Wood, E.; Jabbour, L. Impact of Fetal Alcohol Exposure on Body Systems: A Systematic Review. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 174–180. [Google Scholar] [CrossRef]
- Abel, E.L. An Update on Incidence of FAS: FAS Is Not an Equal Opportunity Birth Defect. Neurotoxicol. Teratol. 1995, 17, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Maya-Enero, S.; Ramis-Fernández, S.M.; Astals-Vizcaino, M.; García-Algar, Ó. Neurocognitive and Behavioral Profile of Fetal Alcohol Spectrum Disorder. An. Pediatría Engl. Ed. 2021, 95, 208.e1–208.e9. [Google Scholar] [CrossRef]
- Pei, J.; Flannigan, K. Interventions for Fetal Alcohol Spectrum Disorder: Meeting Needs Across the Lifespan. Int. J. Neurorehabilit. 2016, 3, 192. [Google Scholar] [CrossRef]
- Flannigan, K.; Kapasi, A.; Pei, J.; Murdoch, I.; Andrew, G.; Rasmussen, C. Characterizing Adverse Childhood Experiences among Children and Adolescents with Prenatal Alcohol Exposure and Fetal Alcohol Spectrum Disorder. Child Abus. Negl. 2021, 112, 104888. [Google Scholar] [CrossRef]
- Passmore, H.M.; Mutch, R.C.; Burns, S.; Watkins, R.; Carapetis, J.; Hall, G.; Bower, C. Fetal Alcohol Spectrum Disorder (FASD): Knowledge, Attitudes, Experiences and Practices of the Western Australian Youth Custodial Workforce. Int. J. Law Psychiatry 2018, 59, 44–52. [Google Scholar] [CrossRef]
- Roszel, E.L. Central Nervous System Deficits in Fetal Alcohol Spectrum Disorder. Nurse Pract. 2015, 40, 24–33. [Google Scholar] [CrossRef]
- Caley, L.M.; Kramer, C.; Robinson, L.K. Fetal Alcohol Spectrum Disorder. J. Sch. Nurs. 2005, 21, 139–146. [Google Scholar] [CrossRef]
- Kalberg, W.O.; May, P.A.; Buckley, D.; Hasken, J.M.; Marais, A.-S.; Vries, M.M.D.; Bezuidenhout, H.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; et al. Early-Life Predictors of Fetal Alcohol Spectrum Disorders. Pediatrics 2019, 144, e20182141. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Bookstein, F.L.; Barr, H.M.; Sampson, P.D.; O’Malley, K.; Young, J.K. Risk Factors for Adverse Life Outcomes in Fetal Alcohol Syndrome and Fetal Alcohol Effects. J. Dev. Behav. Pediatr. JDBP 2004, 25, 228–238. [Google Scholar] [CrossRef]
- Premji, S.; Benzies, K.; Serrett, K.; Hayden, K.A. Research-Based Interventions for Children and Youth with a Fetal Alcohol Spectrum Disorder: Revealing the Gap. Child Care Health Dev. 2007, 33, 389–397. [Google Scholar] [CrossRef]
- Bertrand, J.; Floyd, R.; Weber, M.; O’Connor, M.; Riley, E.; Johnson, K.; Cohen, D. National Task Force on FAS/FAE. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2004. [CrossRef]
- Petrenko, C.L.M.; Tahir, N.; Mahoney, E.C.; Chin, N.P. Prevention of Secondary Conditions in Fetal Alcohol Spectrum Disorders: Identification of Systems-Level Barriers. Matern. Child Health J. 2014, 18, 1496–1505. [Google Scholar] [CrossRef]
- Marrus, N.; Hall, L. Intellectual Disability and Language Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2017, 26, 539–554. [Google Scholar] [CrossRef]
- Terband, H.; Spruit, M.; Maassen, B. Speech Impairment in Boys with Fetal Alcohol Spectrum Disorders. Am. J. Speech Lang. Pathol. 2018, 27, 1405–1425. [Google Scholar] [CrossRef] [PubMed]
- Temple, V.K.; Cook, J.L.; Unsworth, K.; Rajani, H.; Mela, M. Mental Health and Affect Regulation Impairment in Fetal Alcohol Spectrum Disorder (FASD): Results from the Canadian National FASD Database. Alcohol Alcohol. 2019, 54, 545–550. [Google Scholar] [CrossRef]
- Mattson, S.N.; Crocker, N.; Nguyen, T.T. Fetal Alcohol Spectrum Disorders: Neuropsychological and Behavioral Features. Neuropsychol. Rev. 2011, 21, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Shield, K.; Mihic, A.; Chudley, A.E.; Mukherjee, R.A.S.; Bekmuradov, D.; Rehm, J. Comorbidity of Fetal Alcohol Spectrum Disorder: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Weyrauch, D.; Schwartz, M.; Hart, B.; Klug, M.G.; Burd, L. Comorbid Mental Disorders in Fetal Alcohol Spectrum Disorders: A Systematic Review. J. Dev. Behav. Pediatr. 2017, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Rehm, J.; Anagnostou, E.; Popova, S. Prevalence of Externalizing Disorders and Autism Spectrum Disorders among Children with Fetal Alcohol Spectrum Disorder: Systematic Review and Meta-Analysis. Biochem. Cell Biol. 2018, 96, 241–251. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Bastons-Compta, A.; Astals, M.; Andreu-Fernandez, V.; Navarro-Tapia, E.; Garcia-Algar, O. Postnatal Nutritional Treatment of Neurocognitive Deficits in Fetal Alcohol Spectrum Disorder. Biochem. Cell Biol. 2017, 96, 213–221. [Google Scholar] [CrossRef] [PubMed]
- CDC. FASDs: Treatments. Available online: https://www.cdc.gov/ncbddd/fasd/treatments.html (accessed on 6 December 2023).
- Kalberg, W.O.; Buckley, D. FASD: What Types of Intervention and Rehabilitation Are Useful? Neurosci. Biobehav. Rev. 2007, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, C.L.M. Positive Behavioral Interventions and Family Support for Fetal Alcohol Spectrum Disorders. Curr. Dev. Disord. Rep. 2015, 2, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, C.L.M.; Alto, M.E. Interventions in Fetal Alcohol Spectrum Disorders: An International Perspective. Eur. J. Med. Genet. 2017, 60, 79–91. [Google Scholar] [CrossRef]
- Ozsarfati, J.; Koren, G. Medications Used in the Treatment of Disruptive Behavior in Children with FASD—A Guide. J. Popul. Ther. Clin. Pharmacol. J. Ther. Popul. Pharmacol. Clin. 2015, 22, e59–e67. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Bearer, C.F.; Wellmann, K.A.; Tang, N.; He, M.; Mooney, S.M. Choline Ameliorates Deficits in Balance Caused by Acute Neonatal Ethanol Exposure. Cerebellum 2015, 14, 413–420. [Google Scholar] [CrossRef]
- Balaraman, S.; Idrus, N.M.; Miranda, R.C.; Thomas, J.D. Postnatal Choline Supplementation Selectively Attenuates Hippocampal microRNA Alterations Associated with Developmental Alcohol Exposure. Alcohol 2017, 60, 159–167. [Google Scholar] [CrossRef]
- Waddell, J.; Mooney, S. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients 2017, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Bottom, R.T.; Abbott, C.W.; Huffman, K.J. Rescue of Ethanol-Induced FASD-like Phenotypes via Prenatal Co-Administration of Choline. Neuropharmacology 2020, 168, 107990. [Google Scholar] [CrossRef] [PubMed]
- Grafe, E.L.; Fontaine, C.J.; Thomas, J.D.; Christie, B.R. Effects of Prenatal Ethanol Exposure on Choline-Induced Long-Term Depression in the Hippocampus. J. Neurophysiol. 2021, 126, 1622–1634. [Google Scholar] [CrossRef]
- Patten, A.R.; Sickmann, H.M.; Dyer, R.A.; Innis, S.M.; Christie, B.R. Omega-3 Fatty Acids Can Reverse the Long-Term Deficits in Hippocampal Synaptic Plasticity Caused by Prenatal Ethanol Exposure. Neurosci. Lett. 2013, 551, 7–11. [Google Scholar] [CrossRef]
- Sabzali, M.; Eidi, A.; Khaksari, M.; Khastar, H. Anti-Inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox. Res. 2022, 40, 605–613. [Google Scholar] [CrossRef]
- Lopatynska-Mazurek, M.; Komsta, L.; Gibula-Tarlowska, E.; Kotlinska, J.H. Aversive Learning Deficits and Depressive-Like Behaviors Are Accompanied by an Increase in Oxidative Stress in a Rat Model of Fetal Alcohol Spectrum Disorders: The Protective Effect of Rapamycin. Int. J. Mol. Sci. 2021, 22, 7083. [Google Scholar] [CrossRef]
- Cantacorps, L.; Montagud-Romero, S.; Valverde, O. Curcumin Treatment Attenuates Alcohol-Induced Alterations in a Mouse Model of Foetal Alcohol Spectrum Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109899. [Google Scholar] [CrossRef]
- Liang, J.; Shen, Y.; Shao, X.M.; Scott, M.B.; Ly, E.; Wong, S.; Nguyen, A.; Tan, K.; Kwon, B.; Olsen, R.W.; et al. Dihydromyricetin Prevents Fetal Alcohol Exposure-Induced Behavioral and Physiological Deficits: The Roles of GABAA Receptors in Adolescence. Neurochem. Res. 2014, 39, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Fernández, V.; Serra-Delgado, M.; Almeida-Toledano, L.; García-Meseguer, À.; Vieiros, M.; Ramos-Triguero, A.; Muñoz-Lozano, C.; Navarro-Tapia, E.; Martínez, L.; García-Algar, Ó.; et al. Effect of Postnatal Epigallocatechin-Gallate Treatment on Cardiac Function in Mice Prenatally Exposed to Alcohol. Antioxidants 2023, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Romero, S.; Cantacorps, L.; Valverde, O. Histone Deacetylases Inhibitor Trichostatin A Reverses Anxiety-like Symptoms and Memory Impairments Induced by Maternal Binge Alcohol Drinking in Mice. J. Psychopharmacol. 2019, 33, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Sogut, I.; Uysal, O.; Oglakci, A.; Yucel, F.; Kartkaya, K.; Kanbak, G. Prenatal Alcohol–Induced Neuroapoptosis in Rat Brain Cerebral Cortex: Protective Effect of Folic Acid and Betaine. Childs Nerv. Syst. 2017, 33, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Cadena, P.G.; Cadena, M.R.S.; Sarmah, S.; Marrs, J.A. Folic Acid Reduces the Ethanol-Induced Morphological and Behavioral Defects in Embryonic and Larval Zebrafish (Danio rerio) as a Model for Fetal Alcohol Spectrum Disorder (FASD). Reprod. Toxicol. 2020, 96, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.I.; Ullah, I.; Narasimhan, M.L.; Lee, H.Y.; Bressan, R.A.; Yoon, G.H.; Yun, D.J.; Kim, M.O. Neuroprotective Effect of Osmotin against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Rat Brain. Cell Death Dis. 2014, 5, e1150. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, K.A.; George, F.; Brnouti, F.; Mooney, S.M. Docosahexaenoic Acid Partially Ameliorates Deficits in Social Behavior and Ultrasonic Vocalizations Caused by Prenatal Ethanol Exposure. Behav. Brain Res. 2015, 286, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Dhiman, N.; Golani, L.K.; Sharma, B. Papaverine Ameliorates Prenatal Alcohol-induced Experimental Attention Deficit Hyperactivity Disorder by Regulating Neuronal Function, Inflammation, and Oxidative Stress. Int. J. Dev. Neurosci. 2021, 81, 71–81. [Google Scholar] [CrossRef]
- Ju, I.G.; Lee, M.Y.; Jeon, S.H.; Huh, E.; Kim, J.H.; Lee, J.K.; Lee, C.H.; Oh, M.S. GC-TOF-MS-Based Metabolomic Analysis and Evaluation of the Effects of HX106, a Nutraceutical, on ADHD-Like Symptoms in Prenatal Alcohol Exposed Mice. Nutrients 2020, 12, 3027. [Google Scholar] [CrossRef]
- Gibula-Tarlowska, E.; Korz, V.; Lopatynska-Mazurek, M.; Chlopas-Konowalek, A.; Grochecki, P.; Kalaba, P.; Dragacevic, V.; Kotlinski, R.; Kujawski, R.; Szulc, M.; et al. CE-123, a Novel Dopamine Transporter Inhibitor, Attenuates Locomotor Hyperactivity and Improves Cognitive Functions in Rat Model of Fetal Alcohol Spectrum Disorders. Behav. Brain Res. 2021, 410, 113326. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Yang, F.; Ren, Z.; Xu, M.; Frank, J.A.; Ke, Z.; Luo, J. Minocycline Protects Developing Brain against Ethanol-Induced Damage. Neuropharmacology 2018, 129, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Skorput, A.G.; Lee, S.M.; Yeh, P.W.; Yeh, H.H. The NKCC1 Antagonist Bumetanide Mitigates Interneuronopathy Associated with Ethanol Exposure in Utero. eLife 2019, 8, 48648. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, X.; Xu, M.; Frank, J.A.; Luo, J. Minocycline Attenuates Ethanol-Induced Cell Death and Microglial Activation in the Developing Spinal Cord. Alcohol 2019, 79, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Page, S.J.; Wang, L.; Ishii, S.; Li, P.; Sasaki, T.; Basha, A.; Salzberg, A.; Quezado, Z.; Imamura, F.; et al. Kcnn2 Blockade Reverses Learning Deficits in a Mouse Model of Fetal Alcohol Spectrum Disorders. Nat. Neurosci. 2020, 23, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- García-Baos, A.; Puig-Reyne, X.; García-Algar, Ó.; Valverde, O. Cannabidiol Attenuates Cognitive Deficits and Neuroinflammation Induced by Early Alcohol Exposure in a Mice Model. Biomed. Pharmacother. 2021, 141, 111813. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Hong, C.-L.; Wang, Y.-T.; Wang, T.-J.; Chen, J.-R. The Effect of Astaxanthin Treatment on the Rat Model of Fetal Alcohol Spectrum Disorders (FASD). Brain Res. Bull. 2022, 183, 57–72. [Google Scholar] [CrossRef]
- Burton, D.F.; Boa-Amponsem, O.M.; Dixon, M.S.; Hopkins, M.J.; Herbin, T.; Toney, S.; Tarpley, M.; Rodriguez, B.V.; Fish, E.W.; Parnell, S.E.; et al. Pharmacological Activation of the Sonic Hedgehog Pathway with a Smoothened Small Molecule Agonist Ameliorates the Severity of Alcohol-induced Morphological and Behavioral Birth Defects in a Zebrafish Model of Fetal Alcohol Spectrum Disorder. J. Neurosci. Res. 2022, 100, 1585–1601. [Google Scholar] [CrossRef]
- Farhadi, L.; Hojati, V.; Khaksari, M.; Vaezi, G. Neuroprotective Effects of Crocin Against Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurochem. Res. 2022, 47, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.; Navarro, D.; Navarrete, F.; Austrich-Olivares, A.; Scoma, E.R.; Hambardikar, V.D.; Acosta, G.B.; Solesio, M.E.; Manzanares, J. Cannabidiol Repairs Behavioral and Brain Disturbances in a Model of Fetal Alcohol Spectrum Disorder. Pharmacol. Res. 2023, 188, 106655. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D.; Kable, J.A.; Keen, C.L.; Lyons Jones, K.; Wertelecki, W.; Granovska, I.V.; Pashtepa, A.O.; Chambers, C.D.; The CIFASD. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern. Child Health 2015, 19, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Meintjes, E.M.; Senekal, M.S.; Lindinger, N.M.; Dodge, N.C.; Zeisel, S.H.; Duggan, C.P.; Jacobson, J.L. Feasibility and Acceptability of Maternal Choline Supplementation in Heavy Drinking Pregnant Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Warton, F.L.; Molteno, C.D.; Warton, C.M.R.; Wintermark, P.; Lindinger, N.M.; Dodge, N.C.; Zöllei, L.; van der Kouwe, A.J.W.; Carter, R.C.; Jacobson, J.L.; et al. Maternal Choline Supplementation Mitigates Alcohol Exposure Effects on Neonatal Brain Volumes. Alcohol. Clin. Exp. Res. 2021, 45, 1762–1774. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Kroupina, M.G.; Miller, N.C.; Boys, C.J.; Brearley, A.M.; Fink, B.A.; Hoecker, H.L.; Zeisel, S.H.; et al. Choline Supplementation in Children with Fetal Alcohol Spectrum Disorders Has High Feasibility and Tolerability. Nutr. Res. 2013, 33, 897–904. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Fink, B.A.; Hoecker, H.L.; Boys, C.J.; Radke, J.P.; Kroupina, M.G.; Miller, N.C.; Brearley, A.M.; et al. Choline Supplementation in Children with Fetal Alcohol Spectrum Disorders: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2015, 102, 1113–1125. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Risbud, R.D.; Mattson, S.N.; Chambers, C.D.; Thomas, J.D. Choline Supplementation in School-Aged Children With Fetal Alcohol Spectrum Disorders. Am. J. Clin. Nutr. 2016, 104, 1683–1692. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Gangisetty, O.; Wozniak, J.R.; Eckerle, J.K.; Georgieff, M.K.; Foroud, T.M.; Wetherill, L.; Wertelecki, W.; Chambers, C.D.; Riley, E.; et al. Persistent Changes in Stress-Regulatory Genes in Pregnant Women or Children Exposed Prenatally to Alcohol. Alcohol. Clin. Exp. Res. 2019, 43, 1887–1897. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-Year Follow-up of a Randomized Controlled Trial of Choline for Neurodevelopment in Fetal Alcohol Spectrum Disorder. J. Neurodev. Disord. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, B.A.; Anthony, M.E.; Ernst, A.M.; Roediger, D.J.; de Water, E.; Eckerle, J.K.; Boys, C.J.; Radke, J.P.; Mueller, B.A.; Fuglestad, A.J.; et al. Long-Term Follow-up of a Randomized Controlled Trial of Choline for Neurodevelopment in Fetal Alcohol Spectrum Disorder: Corpus Callosum White Matter Microstructure and Neurocognitive Outcomes. J. Neurodev. Disord. 2022, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oesterheld, J.R.; Kofoed, L.; Tervo, R.; Fogas, B.; Wilson, A.; Fiechtner, H. Effectiveness of Methylphenidate in Native American Children with Fetal Alcohol Syndrome and Attention Deficit/Hyperactivity Disorder: A Controlled Pilot Study. J. Child Adolesc. Psychopharmacol. 1998, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Doig, J.; McLennan, J.D.; Gibbard, W.B. Medication Effects on Symptoms of Attention-Deficit/Hyperactivity Disorder in Children with Fetal Alcohol Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 2008, 18, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Peadon, E.; Thomas, D.E.; Elliott, E.J. Pharmacological Interventions for ADHD Symptoms in Children with Fetal Alcohol Spectrum Disorders (FASD). Cochrane Database Syst. Rev. 2017, CD009724. [Google Scholar] [CrossRef]
- Frankel, F.; Paley, B.; Marquardt, R.; O’Connor, M. Stimulants, Neuroleptics, and Children’s Friendship Training for Children with Fetal Alcohol Spectrum Disorders. J. Child Adolesc. Psychopharmacol. 2006, 16, 777–789. [Google Scholar] [CrossRef]
- Mela, M.; Okpalauwaekwe, U.; Anderson, T.; Eng, J.; Nomani, S.; Ahmed, A.; Barr, A.M. The Utility of Psychotropic Drugs on Patients with Fetal Alcohol Spectrum Disorder (FASD): A Systematic Review. Psychiatry Clin. Psychopharmacol. 2018, 28, 436–445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).