Association of BRAF V600E Mutant Allele Proportion with the Dissemination Stage of Papillary Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Histological Analysis of the Primary Tumor
2.3. DNA Extraction and BRAF V600E Mutation Detection
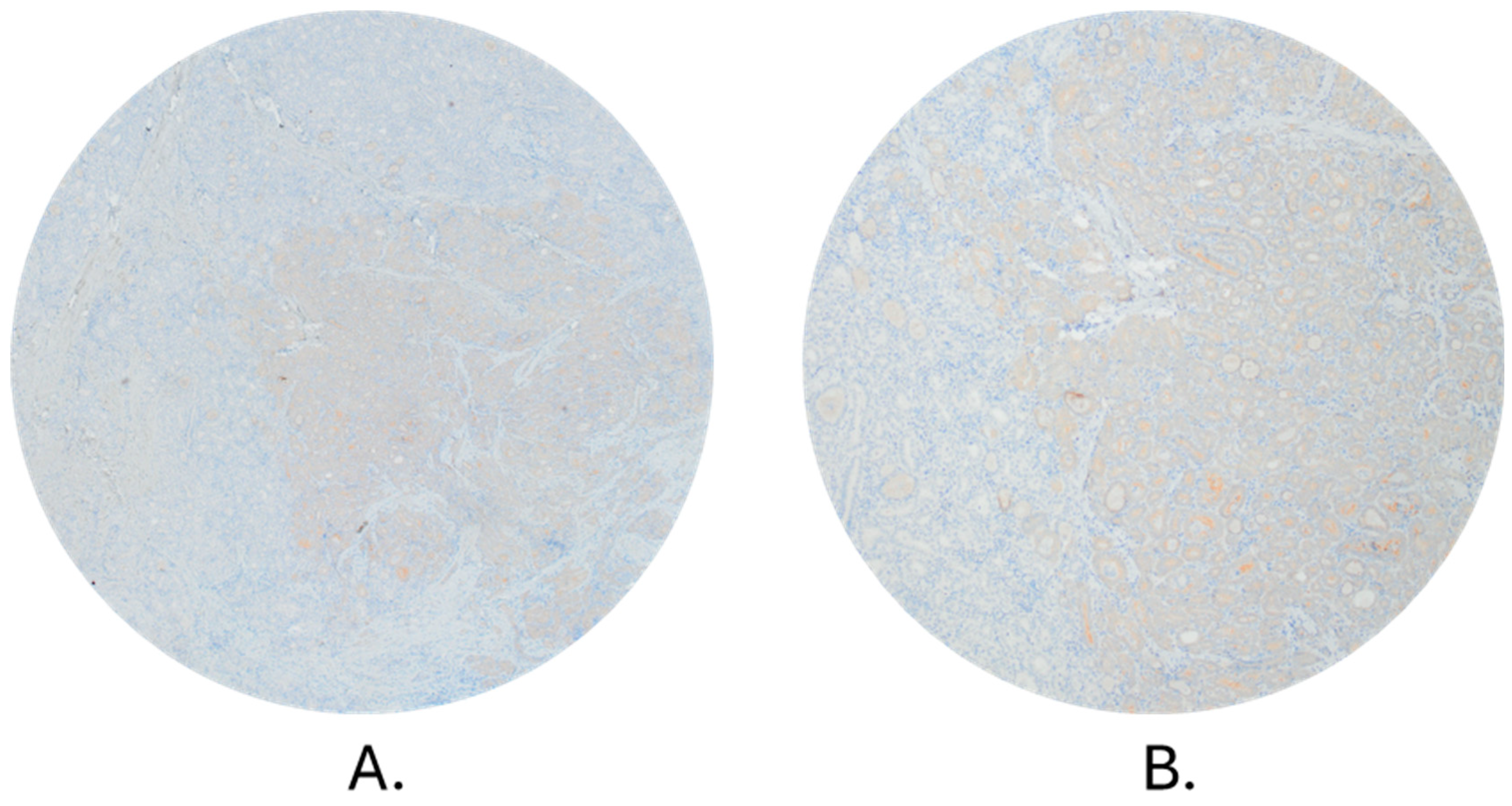
2.4. Immunohistochemical (IHC) Analysis
2.5. Statistical Methods in Data Processing
3. Results
3.1. Association between BRAF Mutation with Disease Dissemination and Characteristics of the Disease
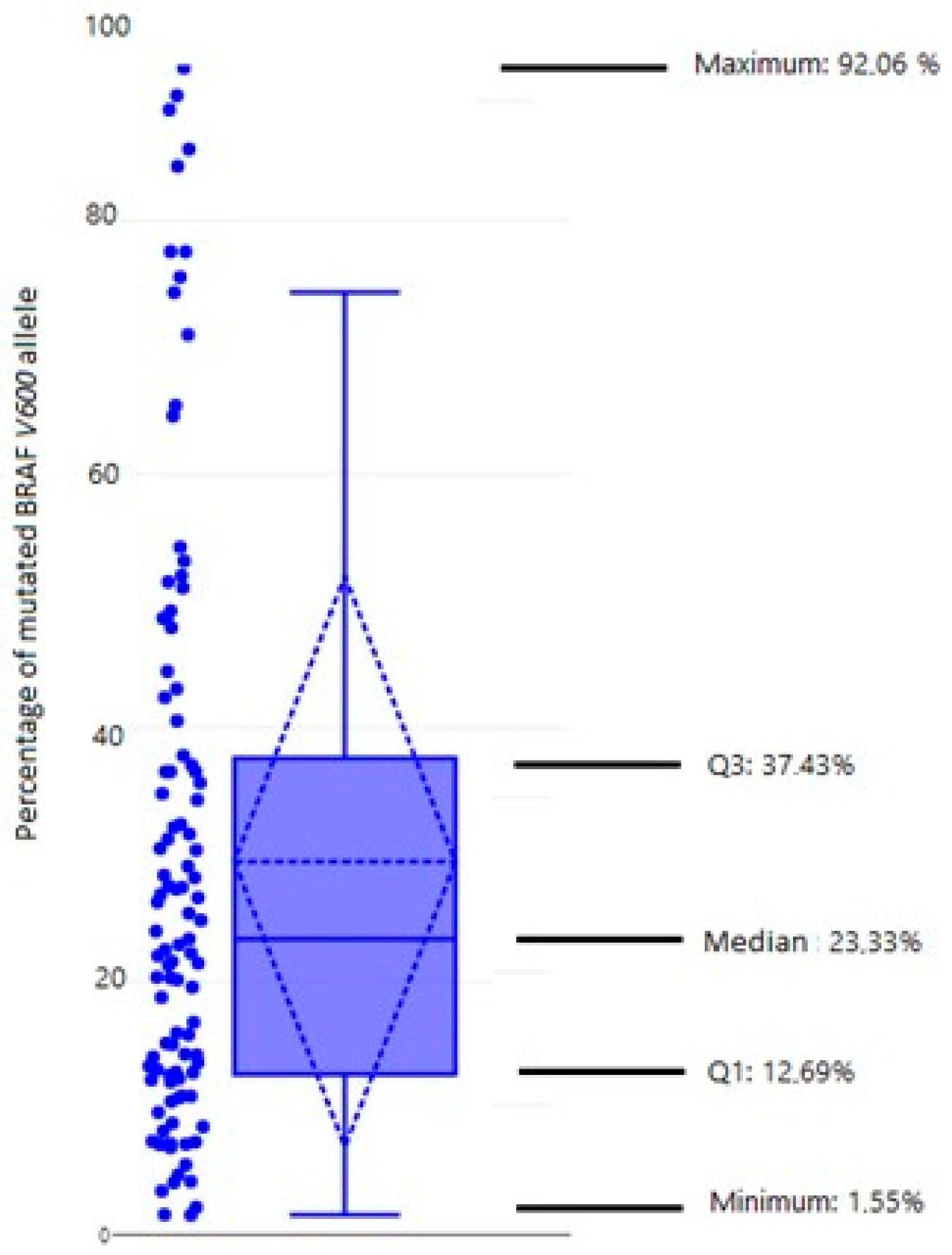
3.2. Heterogeneity of Mutated BRAF V600E Allele (qPCR)
3.3. Comparison of qPCR and Immunohistochemistry Method
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GLOBOCAN. n.d. Available online: https://gco.iarc.fr/today/ (accessed on 15 May 2022).
- HZJZ. Hrvatsku Zavod za Javno Zdravstvo. Available online: http://www.hzjz.hr/wp-content/uploads/2021/12/Bilten44_2019.pdf (accessed on 16 May 2022).
- Dal Maso, L.; Tavilla, A.; Pacini, F.; Serraino, D.; Dijk, B.A.C.; Chirlaque, M.D.; Capocaccia, R.; Larrañaga, N.; Colonna, M.; Agius, D.; et al. Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur. J. Cancer 2017, 77, 140–152. [Google Scholar] [CrossRef]
- Li, M.; Brito, J.P.; Vaccarella, S. Long-Term Declines of Thyroid Cancer Mortality: An International Age–Period–Cohort Analysis. Thyroid 2020, 30, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338. [Google Scholar] [CrossRef]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef]
- Liu, Y.; Su, L.; Xiao, H. Review of Factors Related to the Thyroid Cancer Epidemic. Int. J. Endocrinol. 2017, 2017, 5308635. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Shaha, A.R.; Ferlito, A.; Rinaldo, A. Distant Metastases from Thyroid and Parathyroid Cancer. ORL 2001, 63, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, T.; Siraj, A.K.; Siraj, S.; Azam, S.; Qadri, Z.; Albalawy, W.N.; Parvathareddy, S.K.; Al-Sobhi, S.S.; Al-Dayel, F.; Alkuraya, F.S.; et al. Whole-Exome Sequencing of Matched Primary and Metastatic Papillary Thyroid Cancer. Thyroid 2020, 30, 42–56. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Nowell, P.C. The Clonal Evolution of Tumor Cell Populations: Acquired genetic lability permits stepwise selection of variant sublines and underlies tumor progression. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- Abuali, I.; Lee, C.-S.; Seetharamu, N. A narrative review of the management of BRAF non-V600E mutated metastatic non-small cell lung cancer. Precis. Cancer Med. 2022, 5, 13. [Google Scholar] [CrossRef]
- McKelvey, B.A.; Umbricht, C.B.; Zeiger, M.A. Telomerase Reverse Transcriptase (TERT) Regulation in Thyroid Cancer: A Review. Front. Endocrinol. 2020, 11, 485. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21, S37–S43. [Google Scholar] [CrossRef]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. JCO 2014, 32, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Lim, J.A.; Choi, H.; Won, J.; Moon, J.H.; Cho, S.W.; Lee, K.E.; Park, Y.J.; Yi, K.H.; Park, D.J.; et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016, 122, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, M.; Veskoukis, A.S.; Karanatsiou, P.-M.; Manolakelli, A.; Kostoglou-Athanassiou, I.; Vilaras, G.; Karameris, A.; Liadaki, K. Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population. Pathol. Oncol. Res. 2020, 26, 347–354. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef]
- Wan, P.T.C.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Project, C.G.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; et al. Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Mayank, M.; Kaur, N.; Singh, N. Structural insights and influence of V599 mutations on the overall dynamics of BRAF protein against its kinase domains. Integr. Biol. 2018, 10, 646–657. [Google Scholar] [CrossRef]
- Gouveia, C.; Can, N.T.; Bostrom, A.; Grenert, J.P.; Zante, A.; Orloff, L.A. Lack of Association of BRAF Mutation With Negative Prognostic Indicators in Papillary Thyroid Carcinoma: The University of California, San Francisco, Experience. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1164. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Muzza, M.; Proverbio, M.C.; Tosi, D.; Soranna, D.; Pesenti, C.; Rossi, S.; Cirello, V.; De Leo, S.; Fusco, N.; et al. Impact of Mutation Density and Heterogeneity on Papillary Thyroid Cancer Clinical Features and Remission Probability. Thyroid 2019, 29, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, A.; Kowalska, A.; Kowalik, A.; Sygut, J.; Wypiórkiewicz, E.; Chodurska, R.; Pięciak, L.; Góźdź, S. The BRAFV600E mutation in papillary thyroid microcarcinoma: Does the mutation have an impact on clinical outcome? Clin. Endocrinol. 2014, 80, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Sciammarella, C.; Capasso, M.; Testori, A.; Pivonello, C.; Chiofalo, M.G.; Gambardella, C.; Grasso, M.; Antonino, A.; Annunziata, A.; et al. Germline Polymorphisms of the VEGF Pathway Predict Recurrence in Nonadvanced Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wei, S.; Han, Y.; Li, Y.; Yu, Y.; Yun, X.; Ren, X.; Gao, M. Papillary Microcarcinoma of the Thyroid: Clinical Characteristics and BRAFV600E Mutational Status of 977 Cases. Ann. Surg. Oncol. 2013, 20, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Martini, M.; Capodimonti, S.; Lombardi, C.P.; Pontecorvi, A.; Vellone, V.G.; Zannoni, G.F.; Larocca, L.M.; Fadda, G. BRAF (V600E) mutation analysis on liquid-based cytology-processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid microcarcinoma: BRAF Mutation in Micro-PTC on LBC. Cancer Cytopathol. 2013, 121, 291–297. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients With Papillary Thyroid Cancer. JAMA 2013, 309, 1493. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. JCO 2015, 33, 42–50. [Google Scholar] [CrossRef]
- Guerra, A.; Di Stasi, V.; Zeppa, P.; Faggiano, A.; Marotta, V.; Vitale, M. BRAF V600E assessment by pyrosequencing in fine needle aspirates of thyroid nodules with concurrent Hashimoto’s thyroiditis is a reliable assay. Endocrine 2014, 45, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Fugazzola, L.; Marotta, V.; Cirillo, M.; Rossi, S.; Cirello, V.; Forno, I.; Moccia, T.; Budillon, A.; Vitale, M. A High Percentage of BRAFV600E Alleles in Papillary Thyroid Carcinoma Predicts a Poorer Outcome. J. Clin. Endocrinol. Metab. 2012, 97, 2333–2340. [Google Scholar] [CrossRef]
- Guerra, A.; Sapio, M.R.; Marotta, V.; Campanile, E.; Rossi, S.; Forno, I.; Fugazzola, L.; Budillon, A.; Moccia, T.; Fenzi, G.; et al. The Primary Occurrence of BRAF V600E Is a Rare Clonal Event in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Biase, D.; Cesari, V.; Visani, M.; Casadei, G.P.; Cremonini, N.; Gandolfi, G.; Sancisi, V.; Ragazzi, M.; Pession, A.; Ciarrocchi, A.; et al. High-Sensitivity BRAF Mutation Analysis: BRAF V600E Is Acquired Early During Tumor Development but Is Heterogeneously Distributed in a Subset of Papillary Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E1530–E1538. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Sancisi, V.; Torricelli, F.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A. Allele Percentage of the BRAF V600E Mutation in Papillary Thyroid Carcinomas and Corresponding Lymph Node Metastases: No Evidence for a Role in Tumor Progression. J. Clin. Endocrinol. Metab. 2013, 98, E934–E942. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-P.; Hsu, Y.-C.; Liu, C.-L.; Liu, T.-P.; Chien, M.-N.; Wang, T.-Y.; Lee, J.-J. Significance of Allelic Percentage of BRAF c.1799T > A (V600E) Mutation in Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 2014, 21, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; O’Neill, C.; Chou, A.; Clarkson, A.; Dodds, T.; Toon, C.; Sywak, M.; Sidhu, S.B.; Delbridge, L.W.; Robinson, B.G.; et al. Utilization of a MAB for BRAFV600E detection in papillary thyroid carcinoma. Endocr.-Relat. Cancer 2012, 19, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Namba, H.; Nakashima, M.; Hayashi, T.; Hayashida, N.; Maeda, S.; Rogounovitch, T.I.; Ohtsuru, A.; Saenko, V.A.; Kanematsu, T.; Yamashita, S. Clinical Implication of Hot Spot BRAF Mutation, V599E, in Papillary Thyroid Cancers. J. Clin. Endocrinol. Metab. 2003, 88, 4393–4397. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.-Y.; Shibru, D.; Bastian, B.; Griffin, A. The Prevalence and Prognostic Value of BRAF Mutation in Thyroid Cancer. Ann. Surg. 2007, 246, 466–471. [Google Scholar] [CrossRef]
- Lee, X.; Gao, M.; Ji, Y.; Yu, Y.; Feng, Y.; Li, Y.; Zhang, Y.; Cheng, W.; Zhao, W. Analysis of Differential BRAFV600E Mutational Status in High Aggressive Papillary Thyroid Microcarcinoma. Ann. Surg. Oncol. 2009, 16, 240–245. [Google Scholar] [CrossRef]
- Kim, J.; Giuliano, A.E.; Turner, R.R.; Gaffney, R.E.; Umetani, N.; Kitago, M.; Elashoff, D.; Hoon, D.S.B. Lymphatic Mapping Establishes the Role of BRAF Gene Mutation in Papillary Thyroid Carcinoma: Ann. Surg. 2006, 244, 799–804. [Google Scholar] [CrossRef]
- Frasca, F.; Nucera, C.; Pellegriti, G.; Gangemi, P.; Attard, M.; Stella, M.; Loda, M.; Vella, V.; Giordano, C.; Trimarchi, F.; et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr. Relat. Cancer 2008, 15, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sapio, M.R.; Posca, D.; Troncone, G.; Pettinato, G.; Palombini, L.; Rossi, G.; Fenzi, G.; Vitale, M. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA). Eur. J. Endocrinol. 2006, 154, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Finkel, A.; Liba, L.; Simon, E.; Bick, T.; Prinz, E.; Sabo, E.; Ben-Izhak, O.; Hershkovitz, D. Subclonality for BRAF Mutation in Papillary Thyroid Carcinoma Is Associated With Earlier Disease Stage. J. Clin. Endocrinol. Metab. 2016, 101, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Bae, J.S.; Lim, D.-J.; Lee, H.; Jeon, S.R.; Park, G.S.; Jung, C.K. Quantification of BRAF V600E alleles predicts papillary thyroid cancer progression. Endocr.-Relat. Cancer 2014, 21, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, R.A.; Katabi, N.; Fagin, J.A. Immunohistochemical Detection of Mutated BRAF V600E Supports the Clonal Origin of BRAF-Induced Thyroid Cancers Along the Spectrum of Disease Progression. J. Clin. Endocrinol. Metab. 2013, 98, E1414–E1421. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.Y.; An, J.; Cho, H.Y.; Kim, Y.; Ha, S.Y. Nuclear features of papillary thyroid carcinoma: Comparison of Core needle biopsy and thyroidectomy specimens. Ann. Diagn. Pathol. 2018, 32, 35–40. [Google Scholar] [CrossRef]
- Lee, T.-K.; Myers, R.T.; Bond, M.G.; Marshall, R.B.; Kardon, B. The significance of nuclear diameter in the biologic behavior of thyroid carcinomas: A retrospective study of 127 cases. Hum. Pathol. 1987, 18, 1252–1256. [Google Scholar] [CrossRef]

| Basic Characteristics of the Patient | All Patients | BRAF + | BRAF − | p Value | |
|---|---|---|---|---|---|
| Gender (n = 173); n (%) | |||||
| Women | 109 (63.0) | 62 | 47 | p = 0.257 a | |
| Men | 64 (37.0) | 42 | 22 | ||
| Age, mean ± SD, years | 46.56 ± 16.62 | 49.58 ± 15.5 | 42.01 ± 17.3 | p = 0.003 *,b | |
| Dissemination (n = 173); n (%) | |||||
| Confined to the thyroid | 81 (46.8) | 50 (61.7) | 31 (38.3) | p = 0.154 a | |
| Loco-regional involvement | 61 (35.3) | 40 (65.6) | 21 (34.4) | ||
| Distant metastases | 31 (17.9) | 14 (45.2) | 17 (54.8) | ||
| Tumor size, mean ± SD, mm | 2.03 ± 1.33 | 1.84 ± 1.05 | 2.35 ± 1.60 | p = 0.09 b | |
| Perforation of the thyroid capsule (n = 167); n (%) | |||||
| YES | 37 (22.2) | 21 (56.8) | 16 (43.2) | p = 0.541 a | |
| NO | 130(77.8) | 81 (62.3) | 49 (37.7) | ||
| Dissemination within the thyroid gland (n = 169); n (%) | |||||
| YES | 67(39.6) | 38 (56.7) | 29 (43.3) | p = 0.433 a | |
| NO | 102(60.4) | 64 (62.7) | 38 (37.3) | ||
| Extrathyroidal spreading (n = 164); n (%) | |||||
| YES | 37 | 22.6 | 23(62.2) | 14(37.8) | p = 0.866 a |
| NO | 127 | 77.4 | 77 (60.6) | 50 (39.4) | |
| Angioinvasion (n = 132); n (%) | |||||
| YES | 12 (9,1) | 4(33.3) | 8 (66.7) | p = 0.043 *,a | |
| NO | 120 (90.9) | 76 (63.3) | 44 (36.7) | ||
| Breakthrough of the lymph node capsule (n = 47); n (%) | |||||
| YES | 6 | 12.8 | 3 | 3 | p = 0.309 a |
| NO | 41 | 87.2 | 29 | 12 | |
| Normalized Percentage of BRAF V600E Allele | ||||
|---|---|---|---|---|
| Disease dissemination | Arithmetic mean (%) | CI− 95.00% | CI+ 95.00% | N |
| Confined to the thyroid | 29.63 | 22.99 | 36.27 | 47 |
| Loco-regional involvement | 28.23 | 20.94 | 35.52 | 39 |
| Distant metastases | 33.61 | 21.44 | 45.78 | 14 |
| ANOVA test, F(2, 97) = 0.28287, p = 0.754 | ||||
| Number of Patients | |||||
|---|---|---|---|---|---|
| Groups according to the normalized percentage of BRAF V600E allele | Total number | ||||
| Up to 40% | 40% to 60% | More than 60% | |||
| Disease dissemination | Confined to the thyroid | 36 (74.5%) | 7 (14.9%) | 5 (10.6%) | 47 |
| Loco-regional involvement | 30 (76.9%) | 5 (12.8%) | 4 (10.3%) | 39 | |
| Distant metastases | 11 (78.6%) | 0 (0%) | 3 (21.4%) | 14 | |
| Total | 76 | 12 | 12 | 100 | |
| (χ2 (4, N = 100) = 3.257; p = 0.515) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazekovic, I.; Samija, I.; Perisa, J.; Gall Troselj, K.; Regovic Dzombeta, T.; Radulovic, P.; Romic, M.; Granic, R.; Sisko Markos, I.; Frobe, A.; et al. Association of BRAF V600E Mutant Allele Proportion with the Dissemination Stage of Papillary Thyroid Cancer. Biomedicines 2024, 12, 477. https://doi.org/10.3390/biomedicines12030477
Blazekovic I, Samija I, Perisa J, Gall Troselj K, Regovic Dzombeta T, Radulovic P, Romic M, Granic R, Sisko Markos I, Frobe A, et al. Association of BRAF V600E Mutant Allele Proportion with the Dissemination Stage of Papillary Thyroid Cancer. Biomedicines. 2024; 12(3):477. https://doi.org/10.3390/biomedicines12030477
Chicago/Turabian StyleBlazekovic, Ivan, Ivan Samija, Josipa Perisa, Koraljka Gall Troselj, Tihana Regovic Dzombeta, Petra Radulovic, Matija Romic, Roko Granic, Ines Sisko Markos, Ana Frobe, and et al. 2024. "Association of BRAF V600E Mutant Allele Proportion with the Dissemination Stage of Papillary Thyroid Cancer" Biomedicines 12, no. 3: 477. https://doi.org/10.3390/biomedicines12030477
APA StyleBlazekovic, I., Samija, I., Perisa, J., Gall Troselj, K., Regovic Dzombeta, T., Radulovic, P., Romic, M., Granic, R., Sisko Markos, I., Frobe, A., Kusic, Z., & Jukic, T. (2024). Association of BRAF V600E Mutant Allele Proportion with the Dissemination Stage of Papillary Thyroid Cancer. Biomedicines, 12(3), 477. https://doi.org/10.3390/biomedicines12030477






