Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma
Abstract
1. Introduction
2. Insulin Resistance
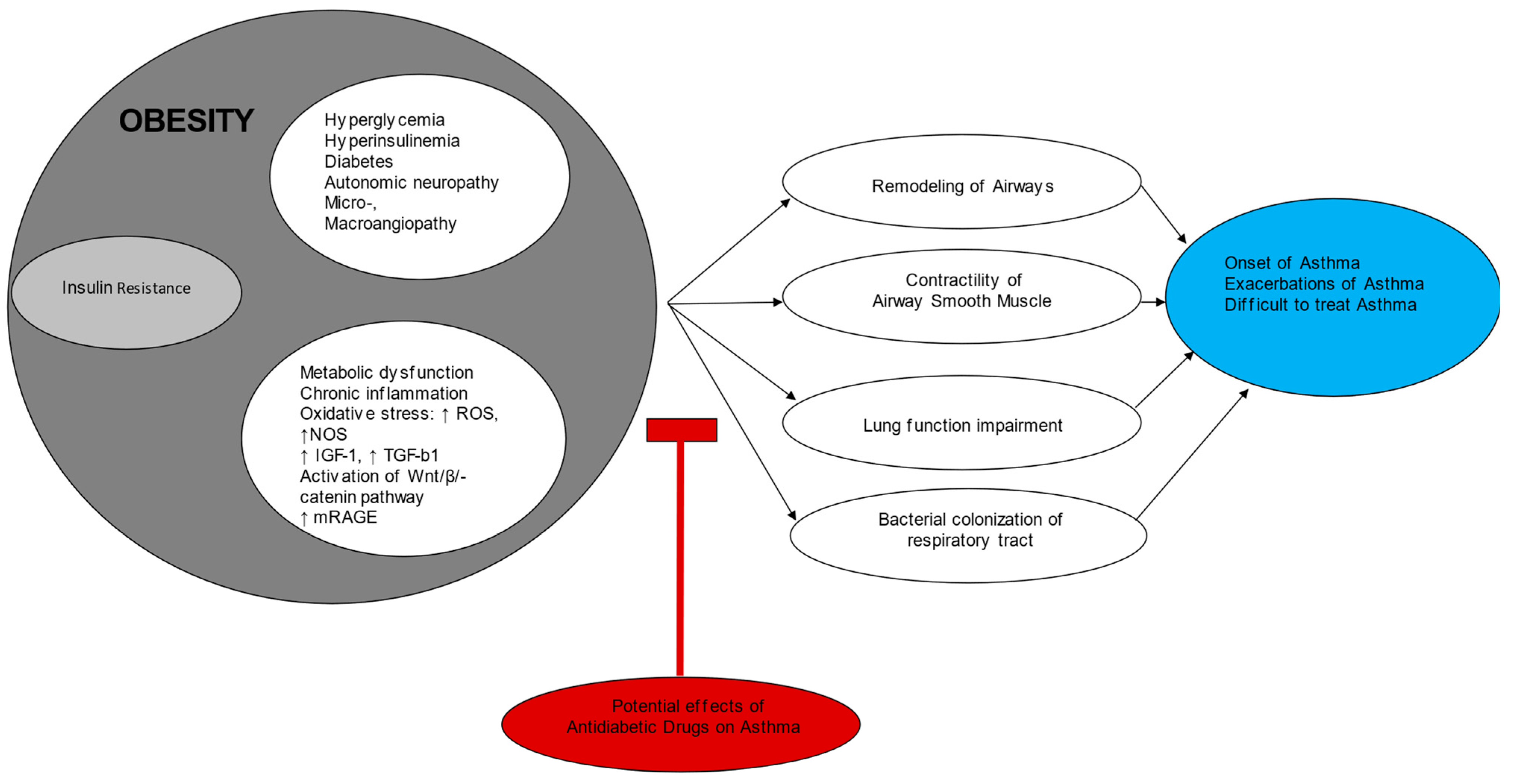
3. Insulin Resistance and Asthma Pathophysiology: A Missing Link
4. Epidemiological-Observational Studies
5. Current Antidiabetic Drugs and Asthma
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.-P. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef]
- GBD 2019 Chronic Respiratory Diseases Collaborators. Global Burden of Chronic Respiratory Diseases and Risk Factors, 1990-2019: An Update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- 2023 GINA Main Report. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 12 December 2023).
- Tiotiu, A.; Novakova, P.; Baiardini, I.; Bikov, A.; Chong-Neto, H.; de-Sousa, J.C.; Emelyanov, A.; Heffler, E.; Fogelbach, G.G.; Kowal, K.; et al. Manifesto on United Airways Diseases (UAD): An Interasma (global Asthma Association—GAA) Document. J. Asthma 2022, 59, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Fouka, E.; Domvri, K.; Gkakou, F.; Alevizaki, M.; Steiropoulos, P.; Papakosta, D.; Porpodis, K. Recent Insights in the Role of Biomarkers in Severe Asthma Management. Front. Med. 2022, 9, 992565. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Fouka, E.; Bartziokas, K.; Kallieri, M.; Vontetsianos, A.; Porpodis, K.; Rovina, N.; Loukides, S.; Bakakos, P. Defining Response to Therapy with Biologics in Severe Asthma: From Global Evaluation to Super Response and Remission. Expert Rev. Respir. Med. 2023, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Loza, M.J.; Djukanovic, R.; Chung, K.F.; Horowitz, D.; Ma, K.; Branigan, P.; Barnathan, E.S.; Susulic, V.S.; Silkoff, P.E.; Sterk, P.J.; et al. Validated and Longitudinally Stable Asthma Phenotypes Based on Cluster Analysis of the ADEPT Study. Respir. Res. 2016, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D. Diabetes, Insulin Resistance, and Asthma: A Review of Potential Links. Curr. Opin. Pulm. Med. 2021, 27, 29–36. [Google Scholar] [CrossRef]
- Park, J.-W. Asthma Phenotype with Metabolic Dysfunction. Yonsei Med. J. 2022, 63, 1–7. [Google Scholar] [CrossRef]
- Škrgat, S.; Harlander, M.; Janić, M. Obesity and Insulin Resistance in Asthma Pathogenesis and Clinical Outcomes. Biomedicines 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Verma, M.; Michalec, L.; Liu, W.; Sripada, A.; Rollins, D.; Good, J.; Ito, Y.; Chu, H.; Gorska, M.M.; et al. Steroid Resistance of Airway Type 2 Innate Lymphoid Cells from Patients with Severe Asthma: The Role of Thymic Stromal Lymphopoietin. J. Allergy Clin. Immunol. 2018, 141, 257–268.e6. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Nanzer, A.M.; Pfeffer, P.E.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Distinct Endotypes of Steroid-Resistant Asthma Characterized by IL-17A(high) and IFN-γ(high) Immunophenotypes: Potential Benefits of Calcitriol. J. Allergy Clin. Immunol. 2015, 136, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Han, Y.-Y.; Forno, E.; Yan, Q.; Rosser, F.; Chen, W.; Celedón, J.C. Glycated Hemoglobin A, Lung Function, and Hospitalizations Among Adults with Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3409–3415.e1. [Google Scholar] [CrossRef]
- Peters, M.C.; Mauger, D.; Ross, K.R.; Phillips, B.; Gaston, B.; Cardet, J.C.; Israel, E.; Levy, B.D.; Phipatanakul, W.; Jarjour, N.N.; et al. Evidence for Exacerbation-Prone Asthma and Predictive Biomarkers of Exacerbation Frequency. Am. J. Respir. Crit. Care Med. 2020, 202, 973–982. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of Type 1 and Type 2 Diabetes Mellitus: A 90-Year Perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; McGuire, L.C.; Mokdad, A.H.; Little, R.R.; Reaven, G.M. Trends in Hyperinsulinemia among Nondiabetic Adults in the U.S. Diabetes Care 2006, 29, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Statistics Report. 2022. Available online: https://repository.gheli.harvard.edu/repository/11854/ (accessed on 12 December 2023).
- Terzano, C.; Morano, S.; Ceccarelli, D.; Conti, V.; Paone, G.; Petroianni, A.; Graziani, E.; Carnovale, A.; Fallarino, M.; Gatti, A.; et al. Effect of Insulin on Airway Responsiveness in Patients with Type 2 Diabetes Mellitus: A Cohort Study. J. Asthma 2009, 46, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Husemoen, L.L.N.; Hersoug, L.-G.; Pisinger, C.; Linneberg, A. Insulin Resistance as a Predictor of Incident Asthma-like Symptoms in Adults. Clin. Exp. Allergy 2009, 39, 700–707. [Google Scholar] [CrossRef]
- Appleton, S.L.; Adams, R.J.; Wilson, D.H.; Taylor, A.W.; Ruffin, R.E. North West Adelaide Health Study Team Central Obesity Is Associated with Nonatopic but Not Atopic Asthma in a Representative Population Sample. J. Allergy Clin. Immunol. 2006, 118, 1284–1291. [Google Scholar] [CrossRef]
- Scholtens, S.; Wijga, A.H.; Brunekreef, B.; Kerkhof, M.; Postma, D.S.; Oldenwening, M.; de Jongste, J.C.; Smit, H.A. Maternal Overweight before Pregnancy and Asthma in Offspring Followed for 8 Years. Int. J. Obes. 2010, 34, 606–613. [Google Scholar] [CrossRef]
- Lee, E.J.; In, K.H.; Ha, E.S.; Lee, K.J.; Hur, G.Y.; Kang, E.H.; Jung, K.H.; Lee, S.Y.; Kim, J.H.; Lee, S.Y.; et al. Asthma-like Symptoms Are Increased in the Metabolic Syndrome. J. Asthma 2009, 46, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Mabalirajan, U.; Ahmad, T.; Ghosh, B. Emerging Interface between Metabolic Syndrome and Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 270–275. [Google Scholar] [CrossRef]
- Scichilone, N.; Rizzo, M.; Benfante, A.; Catania, R.; Giglio, R.V.; Nikolic, D.; Montalto, G.; Bellia, V. Serum Low Density Lipoprotein Subclasses in Asthma. Respir. Med. 2013, 107, 1866–1872. [Google Scholar] [CrossRef]
- Rasmussen, F.; Hancox, R.J.; Nair, P.; Hansen, H.S.; Siersted, H.C.; Nybo, M. Associations between Airway Hyperresponsiveness, Obesity and Lipoproteins in a Longitudinal Cohort. Clin. Respir. J. 2013, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Macian, F. Inflammation, Metabolic Dysregulation, and Pulmonary Function among Obese Urban Adolescents with Asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and Asthma: An Inflammatory Disease of Adipose Tissue Not the Airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin Resistance Causes Inflammation in Adipose Tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. Plasma Interleukin-6 Concentrations, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis of Two Cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Neveu, W.A.; Allard, J.L.; Raymond, D.M.; Bourassa, L.M.; Burns, S.M.; Bunn, J.Y.; Irvin, C.G.; Kaminsky, D.A.; Rincon, M. Elevation of IL-6 in the Allergic Asthmatic Airway Is Independent of Inflammation but Associates with Loss of Central Airway Function. Respir. Res. 2010, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Baffi, C.W.; Wood, L.; Winnica, D.; Strollo, P.J., Jr.; Gladwin, M.T.; Que, L.G.; Holguin, F. Metabolic Syndrome and the Lung. Chest 2016, 149, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Tamborlane, W.V.; Heptulla, R.; Maggs, D.; Grozman, A.; Sherwin, R.S.; Caprio, S. The Metabolic Syndrome and Insulin-like Growth Factor I Regulation in Adolescent Obesity. J. Clin. Endocrinol. Metab. 1998, 83, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prakash, Y.S.; Linneberg, A.; Agrawal, A. Insulin and the Lung: Connecting Asthma and Metabolic Syndrome. J. Allergy 2013, 2013, 627384. [Google Scholar] [CrossRef]
- Dekkers, B.G.J.; Schaafsma, D.; Tran, T.; Zaagsma, J.; Meurs, H. Insulin-Induced Laminin Expression Promotes a Hypercontractile Airway Smooth Muscle Phenotype. Am. J. Respir. Cell Mol. Biol. 2009, 41, 494–504. [Google Scholar] [CrossRef]
- Singh, S.; Bodas, M.; Bhatraju, N.K.; Pattnaik, B.; Gheware, A.; Parameswaran, P.K.; Thompson, M.; Freeman, M.; Mabalirajan, U.; Gosens, R.; et al. Hyperinsulinemia Adversely Affects Lung Structure and Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L837–L845. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- George, S.J. Wnt Pathway: A New Role in Regulation of Inflammation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 400–402. [Google Scholar] [CrossRef]
- Henderson, W.R., Jr.; Chi, E.Y.; Ye, X.; Nguyen, C.; Tien, Y.-T.; Zhou, B.; Borok, Z.; Knight, D.A.; Kahn, M. Inhibition of Wnt/beta-catenin/CREB Binding Protein (CBP) Signaling Reverses Pulmonary Fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14309–14314. [Google Scholar] [CrossRef]
- de Jesus Perez, V.; Yuan, K.; Alastalo, T.-P.; Spiekerkoetter, E.; Rabinovitch, M. Targeting the Wnt Signaling Pathways in Pulmonary Arterial Hypertension. Drug Discov. Today 2014, 19, 1270–1276. [Google Scholar] [CrossRef]
- Kwak, H.J.; Park, D.W.; Seo, J.-Y.; Moon, J.-Y.; Kim, T.H.; Sohn, J.W.; Shin, D.H.; Yoon, H.J.; Park, S.S.; Kim, S.-H. The Wnt/β-Catenin Signaling Pathway Regulates the Development of Airway Remodeling in Patients with Asthma. Exp. Mol. Med. 2015, 47, e198. [Google Scholar] [CrossRef]
- Kumawat, K.; Koopmans, T.; Gosens, R. β-Catenin as a Regulator and Therapeutic Target for Asthmatic Airway Remodeling. Expert Opin. Ther. Targets 2014, 18, 1023–1034. [Google Scholar] [CrossRef]
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. Asthma. From Bronchoconstriction to Airways Inflammation and Remodeling. Am. J. Respir. Crit. Care Med. 2000, 161, 1720–1745. [Google Scholar] [CrossRef]
- Fernandes, D.J.; Bonacci, J.V.; Stewart, A.G. Extracellular Matrix, Integrins, and Mesenchymal Cell Function in the Airways. Curr. Drug Targets 2006, 7, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Ebina, M.; Takahashi, T.; Chiba, T.; Motomiya, M. Cellular Hypertrophy and Hyperplasia of Airway Smooth Muscles Underlying Bronchial Asthma. A 3-D Morphometric Study. Am. Rev. Respir. Dis. 1993, 148, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Altraja, A.; Laitinen, A.; Virtanen, I.; Kämpe, M.; Simonsson, B.G.; Karlsson, S.E.; Håkansson, L.; Venge, P.; Sillastu, H.; Laitinen, L.A. Expression of Laminins in the Airways in Various Types of Asthmatic Patients: A Morphometric Study. Am. J. Respir. Cell Mol. Biol. 1996, 15, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, D.; McNeill, K.D.; Stelmack, G.L.; Gosens, R.; Baarsma, H.A.; Dekkers, B.G.J.; Frohwerk, E.; Penninks, J.-M.; Sharma, P.; Ens, K.M.; et al. Insulin Increases the Expression of Contractile Phenotypic Markers in Airway Smooth Muscle. Am. J. Physiol. Cell Physiol. 2007, 293, C429–C439. [Google Scholar] [CrossRef]
- Gosens, R.; Nelemans, S.A.; Hiemstra, M.; Grootte Bromhaar, M.M.; Meurs, H.; Zaagsma, J. Insulin Induces a Hypercontractile Airway Smooth Muscle Phenotype. Eur. J. Pharmacol. 2003, 481, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Oh, E.Y.; Han, H.; Yang, M.; Park, H.J.; Park, K.H.; Lee, J.-H.; Park, J.-W. Insulin Resistance Mediates High-Fat Diet-Induced Pulmonary Fibrosis and Airway Hyperresponsiveness through the TGF-β1 Pathway. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Jacoby, D.B.; Fryer, A.D. Hyperinsulinemia Potentiates Airway Responsiveness to Parasympathetic Nerve Stimulation in Obese Rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Calco, G.N.; Maung, J.N.; Jacoby, D.B.; Fryer, A.D.; Nie, Z. Insulin Increases Sensory Nerve Density and Reflex Bronchoconstriction in Obese Mice. JCI Insight 2022, 7, e161898. [Google Scholar] [CrossRef] [PubMed]
- Calco, G.N.; Alharithi, Y.J.; Williams, K.R.; Jacoby, D.B.; Fryer, A.D.; Maloyan, A.; Nie, Z. Maternal High-Fat Diet Increases Airway Sensory Innervation and Reflex Bronchoconstriction in Adult Offspring. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L66–L73. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, D.; Gosens, R.; Ris, J.M.; Zaagsma, J.; Meurs, H.; Nelemans, S.A. Insulin Induces Airway Smooth Muscle Contraction. Br. J. Pharmacol. 2007, 150, 136–142. [Google Scholar] [CrossRef]
- Xu, R.; Gopireddy, R.R.; Wu, Y.; Wu, L.; Tao, X.; Shao, J.; Wang, W.; Li, L.; Jovanovic, A.; Xu, B.; et al. Hyperinsulinemia Promotes Heterologous Desensitization of β Adrenergic Receptor in Airway Smooth Muscle in Obesity. FASEB J. 2020, 34, 3996–4008. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Oliveira, M.A.; Tsujita, M.; Nunes, F.P.B.; Casagrande, F.B.; Gomes, E.; Russo, M.; Tavares de Lima, W.; Martins, J.O. Insulin Modulates the Immune Cell Phenotype in Pulmonary Allergic Inflammation and Increases Pulmonary Resistance in Diabetic Mice. Front. Immunol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Lessmann, E.; Grochowy, G.; Weingarten, L.; Giesemann, T.; Aktories, K.; Leitges, M.; Krystal, G.; Huber, M. Insulin and Insulin-like Growth Factor-1 Promote Mast Cell Survival via Activation of the Phosphatidylinositol-3-Kinase Pathway. Exp. Hematol. 2006, 34, 1532–1541. [Google Scholar] [CrossRef]
- Numata, T.; Araya, J.; Fujii, S.; Hara, H.; Takasaka, N.; Kojima, J.; Minagawa, S.; Yumino, Y.; Kawaishi, M.; Hirano, J.; et al. Insulin-Dependent Phosphatidylinositol 3-kinase/Akt and ERK Signaling Pathways Inhibit TLR3-Mediated Human Bronchial Epithelial Cell Apoptosis. J. Immunol. 2011, 187, 510–519. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Mallia, P. Modulating Airway Glucose to Reduce Respiratory Infections. Expert Rev. Respir. Med. 2019, 13, 121–124. [Google Scholar] [CrossRef]
- Baker, E.H.; Baines, D.L. Airway Glucose Homeostasis: A New Target in the Prevention and Treatment of Pulmonary Infection. Chest 2018, 153, 507–514. [Google Scholar] [CrossRef]
- Gill, S.K.; Hui, K.; Farne, H.; Garnett, J.P.; Baines, D.L.; Moore, L.S.P.; Holmes, A.H.; Filloux, A.; Tregoning, J.S. Increased Airway Glucose Increases Airway Bacterial Load in Hyperglycaemia. Sci. Rep. 2016, 6, 27636. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. RAGE: Therapeutic Target and Biomarker of the Inflammatory Response--the Evidence Mounts. J. Leukoc. Biol. 2009, 86, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, P.S.; Alcorn, J.F.; Englert, J.M.; Crum, L.T.; Oury, T.D. The Receptor for Advanced Glycation End Products Is a Central Mediator of Asthma Pathogenesis. Am. J. Pathol. 2012, 181, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Fuso, L.; Conte, E.G.; Zaccardi, F.; Condoluci, C.; Scavone, G.; Incalzi, R.A.; Ghirlanda, G. The Diabetic Lung--a New Target Organ? Rev. Diabet. Stud. 2012, 9, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ofulue, A.F.; Thurlbeck, W.M. Experimental Diabetes and the Lung. II. In Vivo Connective Tissue Metabolism. Am. Rev. Respir. Dis. 1988, 138, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Utsuyama, M.; Takizawa, T.; Thurlbeck, W.M. Changes in Lung Morphologic Features and Elasticity Caused by Streptozotocin-Induced Diabetes Mellitus in Growing Rats. Am. Rev. Respir. Dis. 1983, 128, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M.W.J. Pulmonary Function in Diabetes: A Metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Rayner, L.; McGovern, A.; Creagh-Brown, B.; Woodmansey, C.; de Lusignan, S. Type 2 Diabetes and Asthma: Systematic Review of the Bidirectional Relationship. Curr. Diabetes Rev. 2019, 15, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Koh, W.-P.; Odegaard, A.O.; Gross, M.D.; Yuan, J.-M.; Pereira, M.A. Asthma and the Risk of Type 2 Diabetes in the Singapore Chinese Health Study. Diabetes Res. Clin. Pract. 2013, 99, 192–199. [Google Scholar] [CrossRef]
- Brumpton, B.M.; Camargo, C.A., Jr.; Romundstad, P.R.; Langhammer, A.; Chen, Y.; Mai, X.-M. Metabolic Syndrome and Incidence of Asthma in Adults: The HUNT Study. Eur. Respir. J. 2013, 42, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Han, X.; Chen, Y.; Gao, Y.; Yang, W.; Huang, L. Asthma Prevalence Is Increased in Patients with High Metabolism Scores for Visceral Fat: Study Reports from the US. Front. Endocrinol. 2023, 14, 1162158. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Kim, M.-H.; Cho, Y.-J. Risk of Asthma And/or Wheezing in Obese Individuals with or without Metabolic Syndrome: From the Korea National Health and Nutrition Examination Survey Data. Allergy Asthma Proc. 2024, 45, e1–e8. [Google Scholar] [CrossRef]
- Arshi, M.; Cardinal, J.; Hill, R.J.; Davies, P.S.W.; Wainwright, C. Asthma and Insulin Resistance in Children. Respirology 2010, 15, 779–784. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Duffy, D.L.; Kyvik, K.O.; Skytthe, A.; Backer, V. Risk of Asthma in Adult Twins with Type 2 Diabetes and Increased Body Mass Index. Allergy 2011, 66, 562–568. [Google Scholar] [CrossRef]
- Ehrlich, S.F.; Quesenberry, C.P., Jr.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients Diagnosed with Diabetes Are at Increased Risk for Asthma, Chronic Obstructive Pulmonary Disease, Pulmonary Fibrosis, and Pneumonia but Not Lung Cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cardet, J.C.; Ash, S.; Kusa, T.; Camargo, C.A., Jr.; Israel, E. Insulin Resistance Modifies the Association between Obesity and Current Asthma in Adults. Eur. Respir. J. 2016, 48, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sadeghimakki, R.; McCarthy, H.D. Interactive Effects of Adiposity and Insulin Resistance on the Impaired Lung Function in Asthmatic Adults: Cross-Sectional Analysis of NHANES Data. Ann. Hum. Biol. 2019, 46, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Schiebler, M.L.; Cardet, J.C.; Johansson, M.W.; Sorkness, R.; DeBoer, M.D.; Bleecker, E.R.; Meyers, D.A.; Castro, M.; Sumino, K.; et al. The Impact of Insulin Resistance on Loss of Lung Function and Response to Treatment in Asthma. Am. J. Respir. Crit. Care Med. 2022, 206, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Fryer, A.D.; Jacoby, D.B.; Drake, M.G. Mechanisms of Obesity-Related Asthma: Is Insulin Getting on Your Nerves? Am. J. Respir. Crit. Care Med. 2023, 207, 109–110. [Google Scholar] [CrossRef]
- Kuschnir, F.C.; Felix, M.M.R.; Caetano Kuschnir, M.C.; Bloch, K.V.; Azevedo de Oliveira Costa Jordão, E.; Solé, D.; Ledo Alves da Cunha, A.J.; Szklo, M. Severe Asthma Is Associated with Metabolic Syndrome in Brazilian Adolescents. J. Allergy Clin. Immunol. 2018, 141, 1947–1949.e4. [Google Scholar] [CrossRef]
- Wu, T.D.; Brigham, E.P.; Keet, C.A.; Brown, T.T.; Hansel, N.N.; McCormack, M.C. Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J. Allergy Clin. Immunol. Pract. 2019, 7, 1868–1873.e5. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Wilson, A.M.; Chisholm, A.; Rigazio, A.; Burden, A.; Thomas, M.; King, C. Predicting Frequent Asthma Exacerbations Using Blood Eosinophil Count and Other Patient Data Routinely Available in Clinical Practice. J. Asthma Allergy 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Staggers, K.A.; Minard, C.; Byers, M.; Helmer, D.A.; Wu, T.D. Metabolic Dysfunction, Triglyceride-Glucose Index, and Risk of Severe Asthma Exacerbation. J. Allergy Clin. Immunol. Pract. 2023, 11, 3700–3705.e2. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Punjabi, N.M.; Wang, N.-Y.; Pankow, J.S.; Duncan, B.B.; Cox, C.E.; Selvin, E.; Brancati, F.L. Cross-Sectional and Prospective Study of Lung Function in Adults with Type 2 Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2008, 31, 741–746. [Google Scholar] [CrossRef]
- McKeever, T.M.; Weston, P.J.; Hubbard, R.; Fogarty, A. Lung Function and Glucose Metabolism: An Analysis of Data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2005, 161, 546–556. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.-Y.; Muzumdar, R.H.; Celedón, J.C. Insulin Resistance, Metabolic Syndrome, and Lung Function in US Adolescents with and without Asthma. J. Allergy Clin. Immunol. 2015, 136, 304–311.e8. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Kato, K.; Tomita, M.; Katsuki, T.; Oikawa, Y.; Shimada, A. Association of Glycemic Status with Impaired Lung Function among Recipients of a Health Screening Program: A Cross-Sectional Study in Japanese Adults. J. Epidemiol. 2014, 24, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Hickson, D.A.; Burchfiel, C.M.; Liu, J.; Petrini, M.F.; Harrison, K.; White, W.B.; Sarpong, D.F. Diabetes, Impaired Glucose Tolerance, and Metabolic Biomarkers in Individuals with Normal Glucose Tolerance Are Inversely Associated with Lung Function: The Jackson Heart Study. Lung 2011, 189, 311–321. [Google Scholar] [CrossRef][Green Version]
- Dennis, R.J.; Maldonado, D.; Rojas, M.X.; Aschner, P.; Rondón, M.; Charry, L.; Casas, A. Inadequate Glucose Control in Type 2 Diabetes Is Associated with Impaired Lung Function and Systemic Inflammation: A Cross-Sectional Study. BMC Pulm. Med. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Foer, D.; Cahill, K.N. Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity. Pulm. Ther. 2023, 9, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Calixto, M.C.; Lintomen, L.; André, D.M.; Leiria, L.O.; Ferreira, D.; Lellis-Santos, C.; Anhê, G.F.; Bordin, S.; Landgraf, R.G.; Antunes, E. Metformin Attenuates the Exacerbation of the Allergic Eosinophilic Inflammation in High Fat-Diet-Induced Obesity in Mice. PLoS ONE 2013, 8, e76786. [Google Scholar] [CrossRef]
- Park, C.S.; Bang, B.-R.; Kwon, H.-S.; Moon, K.-A.; Kim, T.-B.; Lee, K.-Y.; Moon, H.-B.; Cho, Y.S. Metformin Reduces Airway Inflammation and Remodeling via Activation of AMP-Activated Protein Kinase. Biochem. Pharmacol. 2012, 84, 1660–1670. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin Inhibits Cytokine-Induced Nuclear Factor kappaB Activation via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef]
- Executive Summary: Standards of Medical Care in Diabetes—2013. Diabetes Care 2012, 36, S4–S10. [CrossRef]
- Wu, T.D.; Keet, C.A.; Fawzy, A.; Segal, J.B.; Brigham, E.P.; McCormack, M.C. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-Based Cohort Study. Ann. Am. Thorac. Soc. 2019, 16, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Erickson, S.R.; Wu, C.-H. Metformin Use and Asthma Outcomes among Patients with Concurrent Asthma and Diabetes. Respirology 2016, 21, 1210–1218. [Google Scholar] [CrossRef]
- Wu, T.D.; Fawzy, A.; Akenroye, A.; Keet, C.; Hansel, N.N.; McCormack, M.C. Metformin Use and Risk of Asthma Exacerbation Among Asthma Patients with Glycemic Dysfunction. J. Allergy Clin. Immunol. Pract. 2021, 9, 4014–4020.e4. [Google Scholar] [CrossRef]
- Pabreja, K.; Mohd, M.A.; Koole, C.; Wootten, D.; Furness, S.G.B. Molecular Mechanisms Underlying Physiological and Receptor Pleiotropic Effects Mediated by GLP-1R Activation. Br. J. Pharmacol. 2014, 171, 1114–1128. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The Anti-Inflammatory and Immunological Properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Wang, N.; Xue, P.; Li, Y. Liraglutide, a Glucagon-like Peptide-1 Receptor Agonist, Suppresses Osteoclastogenesis through the Inhibition of NF-κB and MAPK Pathways via GLP-1R. Biomed. Pharmacother. 2020, 130, 110523. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Nguyen, D.-V.; Linderholm, A.; Haczku, A.; Kenyon, N. Glucagon-like Peptide 1: A Potential Anti-Inflammatory Pathway in Obesity-Related Asthma. Pharmacol. Ther. 2017, 180, 139–143. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, X.-L.; Zhang, W.; Xiao, M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int. J. Mol. Sci. 2015, 16, 20195–20211. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Reiss, S.; Zhang, J.; Bloodworth, M.H.; Stier, M.T.; Zhou, W.; Newcomb, D.C.; Ware, L.B.; Stanwood, G.D.; et al. Glucagon-like Peptide 1 Signaling Inhibits Allergen-Induced Lung IL-33 Release and Reduces Group 2 Innate Lymphoid Cell Cytokine Production in Vivo. J. Allergy Clin. Immunol. 2018, 142, 1515–1528.e8. [Google Scholar] [CrossRef]
- Bloodworth, M.H.; Rusznak, M.; Pfister, C.C.; Zhang, J.; Bastarache, L.; Calvillo, S.A.; Chappell, J.D.; Boyd, K.L.; Toki, S.; Newcomb, D.C.; et al. Glucagon-like Peptide 1 Receptor Signaling Attenuates Respiratory Syncytial Virus-Induced Type 2 Responses and Immunopathology. J. Allergy Clin. Immunol. 2018, 142, 683–687.e12. [Google Scholar] [CrossRef]
- Toki, S.; Newcomb, D.C.; Printz, R.L.; Cahill, K.N.; Boyd, K.L.; Niswender, K.D.; Peebles, R.S., Jr. Glucagon-like Peptide-1 Receptor Agonist Inhibits Aeroallergen-Induced Activation of ILC2 and Neutrophilic Airway Inflammation in Obese Mice. Allergy 2021, 76, 3433–3445. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Capuani, B.; Facciolo, F.; Cazzola, M.; Lauro, D.; Matera, M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2016, 55, 804–814. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Salter, B.M.; Oliveria, J.P.; El-Gammal, A.; Tworek, D.; Smith, S.G.; Sehmi, R.; Gauvreau, G.M.; Butler, M.; O’Byrne, P.M. Glucagon-like Peptide-1 Receptor Expression on Human Eosinophils and Its Regulation of Eosinophil Activation. Clin. Exp. Allergy 2017, 47, 331–338. [Google Scholar] [CrossRef]
- Khan, F.; Mat, A.; Hogan, A.E.; Kent, B.D.; Eigenheer, S.; Corrigan, M.A.; O’Shea, D.; Butler, M.W. Preliminary Asthma-Related Outcomes Following Glucagon-like Peptide 1 Agonist Therapy. QJM Int. J. Med. 2017, 110, 853–854. [Google Scholar] [CrossRef]
- Foer, D.; Beeler, P.E.; Cui, J.; Karlson, E.W.; Bates, D.W.; Cahill, K.N. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am. J. Respir. Crit. Care Med. 2021, 203, 831–840. [Google Scholar] [CrossRef]
- Albogami, Y.; Cusi, K.; Daniels, M.J.; Wei, Y.-J.J.; Winterstein, A.G. Glucagon-Like Peptide 1 Receptor Agonists and Chronic Lower Respiratory Disease Exacerbations Among Patients With Type 2 Diabetes. Diabetes Care 2021, 44, 1344–1352. [Google Scholar] [CrossRef]
- López-Cano, C.; Ciudin, A.; Sánchez, E.; Tinahones, F.J.; Barbé, F.; Dalmases, M.; García-Ramírez, M.; Soto, A.; Gaeta, A.M.; Pellitero, S.; et al. Liraglutide Improves Forced Vital Capacity in Individuals with Type 2 Diabetes: Data From the Randomized Crossover LIRALUNG Study. Diabetes 2022, 71, 315–320. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Calzetta, L.; Hanania, N.A.; Page, C.; Rossi, I.; Andreadi, A.; Galli, A.; Coppola, A.; Cazzola, M.; et al. Long-Term Observational Study on the Impact of GLP-1R Agonists on Lung Function in Diabetic Patients. Respir. Med. 2019, 154, 86–92. [Google Scholar] [CrossRef]
- Kudaravalli, J.; Vijayalakshmi, G.; Kiran Kishore, K. Safety and Efficacy of Sulfonylurea Drugs in Type 2 Diabetes Mellitus. Apollo Med. 2013, 10, 165–168. [Google Scholar] [CrossRef]
- Hougen, I.; Whitlock, R.H.; Komenda, P.; Rigatto, C.; Clemens, K.K.; Tangri, N. Safety of Add-on Sulfonylurea Therapy in Patients with Type 2 Diabetes Using Metformin: A Population-Based Real-World Study. BMJ Open Diabetes Res. Care 2021, 9, e002352. [Google Scholar] [CrossRef]
- Douros, A.; Dell’Aniello, S.; Yu, O.H.Y.; Filion, K.B.; Azoulay, L.; Suissa, S. Sulfonylureas as Second Line Drugs in Type 2 Diabetes and the Risk of Cardiovascular and Hypoglycaemic Events: Population Based Cohort Study. BMJ 2018, 362, k2693. [Google Scholar] [CrossRef]
- Rayner, L.H.; Mcgovern, A.; Sherlock, J.; Gatenby, P.; Correa, A.; Creagh-Brown, B.; deLusignan, S. The Impact of Therapy on the Risk of Asthma in Type 2 Diabetes. Clin. Respir. J. 2019, 13, 299–305. [Google Scholar] [CrossRef]
- GRADE Study Research Group; Nathan, D.M.; Lachin, J.M.; Balasubramanyam, A.; Burch, H.B.; Buse, J.B.; Butera, N.M.; Cohen, R.M.; Crandall, J.P.; Kahn, S.E.; et al. Glycemia Reduction in Type 2 Diabetes—Glycemic Outcomes. N. Engl. J. Med. 2022, 387, 1063–1074. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Diabetic Actions of Glucagon-like Peptide-1 on Pancreatic Beta-Cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef]
- Lyu, X.; Zhu, X.; Zhao, B.; Du, L.; Chen, D.; Wang, C.; Liu, G.; Ran, X. Effects of Dipeptidyl Peptidase-4 Inhibitors on Beta-Cell Function and Insulin Resistance in Type 2 Diabetes: Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2017, 7, 44865. [Google Scholar] [CrossRef]
- Sun, J.; Chu, S.; Lu, M.; Pan, Q.; Li, D.; Zheng, S.; Ma, L. The Roles of Dipeptidyl Peptidase-4 and Its Inhibitors in the Regulation of Airway Epithelial-Mesenchymal Transition. Exp. Lung Res. 2020, 46, 163–173. [Google Scholar] [CrossRef]
- Ma, L.; Chang, E.; Ruan, X.; Zhang, B.; Tang, F.; Zhang, J. The Protective Effects of Omarigliptin against Lipopolysaccharide (LPS)- Induced Inflammatory Response and Expression of Mucin 5AC (MUC5AC) in Human Bronchial Epithelial Cells. Mol. Immunol. 2022, 141, 108–115. [Google Scholar] [CrossRef]
- Colice, G.; Price, D.; Gerhardsson de Verdier, M.; Rabon-Stith, K.; Ambrose, C.; Cappell, K.; Irwin, D.E.; Juneau, P.; Vlahiotis, A. The Effect of DPP-4 Inhibitors on Asthma Control: An Administrative Database Study to Evaluate a Potential Pathophysiological Relationship. Pragmat. Obs. Res. 2017, 8, 231–240. [Google Scholar] [CrossRef]
- Wang, A.; Tang, H.; Zhang, N.; Feng, X. Association between Novel Glucose-Lowering Drugs and Risk of Asthma: A Network Meta-Analysis of Cardiorenal Outcome Trials. Diabetes Res. Clin. Pract. 2022, 183, 109080. [Google Scholar] [CrossRef]
- Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes. Available online: https://www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes (accessed on 20 December 2023).
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin Reduces Inflammation and Fibrosis Biomarkers: A Potential Mechanism of Action for Beneficial Effects of SGLT2 Inhibitors in Diabetic Kidney Disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ding, L.-L.; Zhan, Z.-L.; Liu, S.-Y. Use of SGLT2 Inhibitors and Occurrence of Noninfectious Respiratory Disorders: A Meta-Analysis of Large Randomized Trials of SGLT2 Inhibitors. Endocrine 2021, 73, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Cheung, P.; Mozzoli, M.; Fried, S.K. Effect of Thiazolidinediones on Glucose and Fatty Acid Metabolism in Patients with Type 2 Diabetes. Metabolism 2003, 52, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Soccio, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the Promise of Insulin Sensitization in Type 2 Diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Flynt, L.; Ghosh, S.; Mellema, M.; Banerjee, A.; Williams, E.; Panettieri, R.A., Jr.; Shore, S.A. Anti-Inflammatory Effects of Thiazolidinediones in Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yan, J.; Liu, P.; Wang, Z. Effects of Thiazolidinedione Therapy on Inflammatory Markers of Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0123703. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.T.; Feemster, L.C.; Collins, B.F.; Au, D.H.; Perkins, M.; Bryson, C.L.; O’Riordan, T.G.; Liu, C.-F. Thiazolidinediones and the Risk of Asthma Exacerbation among Patients with Diabetes: A Cohort Study. Allergy Asthma Clin. Immunol. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Qualls, C.; Murata, A.; Kroth, P.J.; Mao, J.; Schade, D.S.; Murata, G. Potential for Repurposing Oral Hypertension/diabetes Drugs to Decrease Asthma Risk in Obesity. J. Asthma 2023, 60, 802–810. [Google Scholar] [CrossRef]
- Dixon, A.E.; Subramanian, M.; DeSarno, M.; Black, K.; Lane, L.; Holguin, F. A Pilot Randomized Controlled Trial of Pioglitazone for the Treatment of Poorly Controlled Asthma in Obesity. Respir. Res. 2015, 16, 143. [Google Scholar] [CrossRef]
- Kaler, M.; Barochia, A.V.; Weir, N.A.; Cuento, R.A.; Stylianou, M.; Roth, M.J.; Filie, A.C.; Vaughey, E.C.; Nathan, S.D.; Levine, S.J. A Randomized, Placebo-Controlled, Double-Blinded, Crossover Trial of Pioglitazone for Severe Asthma. J. Allergy Clin. Immunol. 2017, 140, 1716–1718. [Google Scholar] [CrossRef]
| Enhanced Airway Remodeling |
| Increased collagen deposition in the lungs |
| Increased epithelium to mucus transition |
| Increased fibrosis |
| Increased airway smooth muscle mass |
| Increased expression of TGF-β1 |
| Increased Airway Smooth Muscle (ASM) contractility |
| Increased airway hyper-responsiveness |
| Loss of inhibitory M2 muscarinic receptor function |
| Enhanced vagally mediated bronchoconstriction |
| Lung function impairment |
| Reduced Forced Expiratory Volume in the 1st sec (FEV1) |
| Reduced Forced vital capacity (FVC) |
| Reduced Forced expiratory flow over the middle half of the FVC (FEF25–75%) |
| Increase the risk of respiratory tract bacterial colonization |
| Release of pro-inflammatory mediators from adipose tissue |
| Increased IL-6 |
| Increased TNF-α |
| Increased Th2-inflammation |
| Antidiabetic Agent | Potential Beneficial Effects on Asthma | Putative Mechanisms |
|---|---|---|
| Metformin | Yes | Reverse lung tissue eosinophilic infiltrationProinflammatory cytokines
|
| GLP-1 | Yes |
|
| Dipeptidyl peptidase-4 inhibitors (DPP-4is) | Uncertain |
|
| Sodium-glucose cotransporter-2 inhibitors (SGLT-2) | Yes |
|
| Thiazolidinediones (TZD) | No |
|
| Sulfonylureas | Uncertain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartziokas, K.; Papaioannou, A.I.; Drakopanagiotakis, F.; Gouveri, E.; Papanas, N.; Steiropoulos, P. Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma. Biomedicines 2024, 12, 437. https://doi.org/10.3390/biomedicines12020437
Bartziokas K, Papaioannou AI, Drakopanagiotakis F, Gouveri E, Papanas N, Steiropoulos P. Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma. Biomedicines. 2024; 12(2):437. https://doi.org/10.3390/biomedicines12020437
Chicago/Turabian StyleBartziokas, Konstantinos, Andriana I. Papaioannou, Fotios Drakopanagiotakis, Evanthia Gouveri, Nikolaos Papanas, and Paschalis Steiropoulos. 2024. "Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma" Biomedicines 12, no. 2: 437. https://doi.org/10.3390/biomedicines12020437
APA StyleBartziokas, K., Papaioannou, A. I., Drakopanagiotakis, F., Gouveri, E., Papanas, N., & Steiropoulos, P. (2024). Unraveling the Link between Ιnsulin Resistance and Bronchial Asthma. Biomedicines, 12(2), 437. https://doi.org/10.3390/biomedicines12020437









