Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MPC and ZMPC Preparation
2.3. Chemical Composition and Surface Morphology
2.4. Physicochemical Properties: Compressive Strength, Degradation, Mg2+ Release, pH Variation, Setting Time and Porosity
2.5. In Vitro Studies
2.5.1. Cell Proliferation and Adhesion
2.5.2. Quantitative Real-Time Polymerase Chain Reaction
2.5.3. Western Blotting
2.5.4. Immunofluorescence Microscopy
2.6. In Vivo Experiments
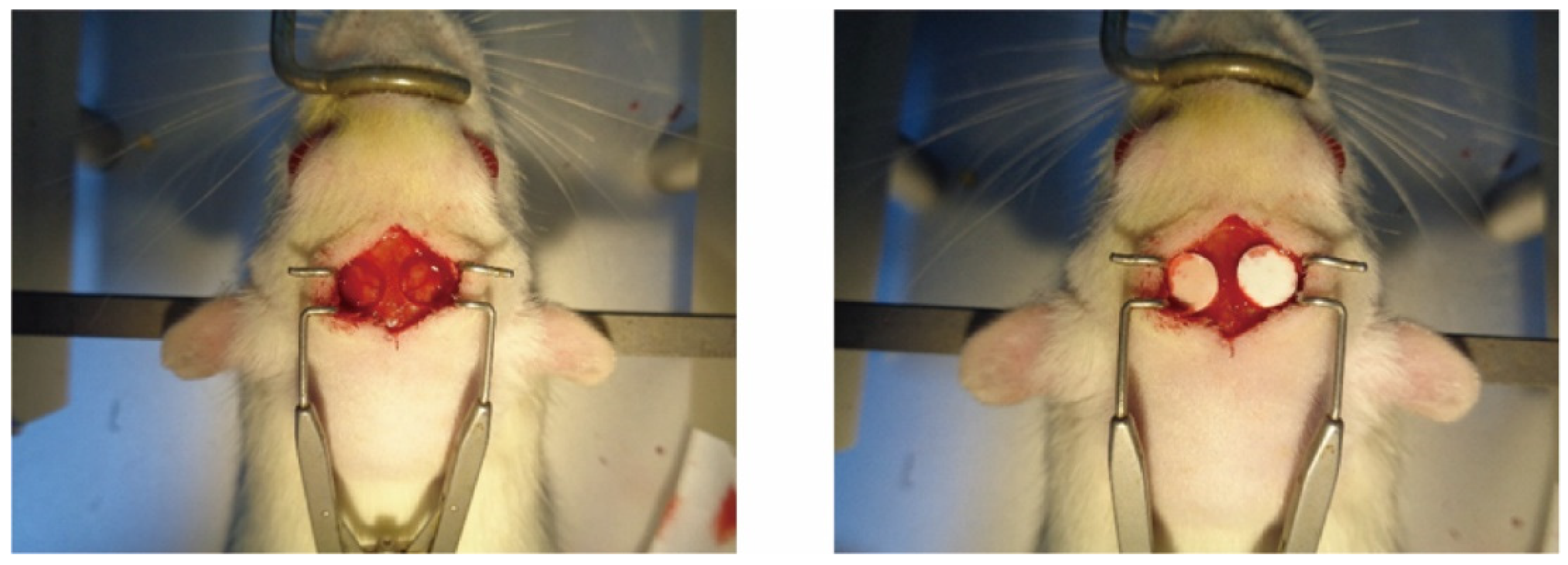
2.6.1. Construction of Animal Models
2.6.2. Micro-Computed Tomography
2.6.3. Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Morphological and Physicochemical Characterization of the MPC and ZMPCs
3.1.1. Field Emission Scanning Electron Microscopy (FE-SEM) Examination
3.1.2. XRD and Raman Examination
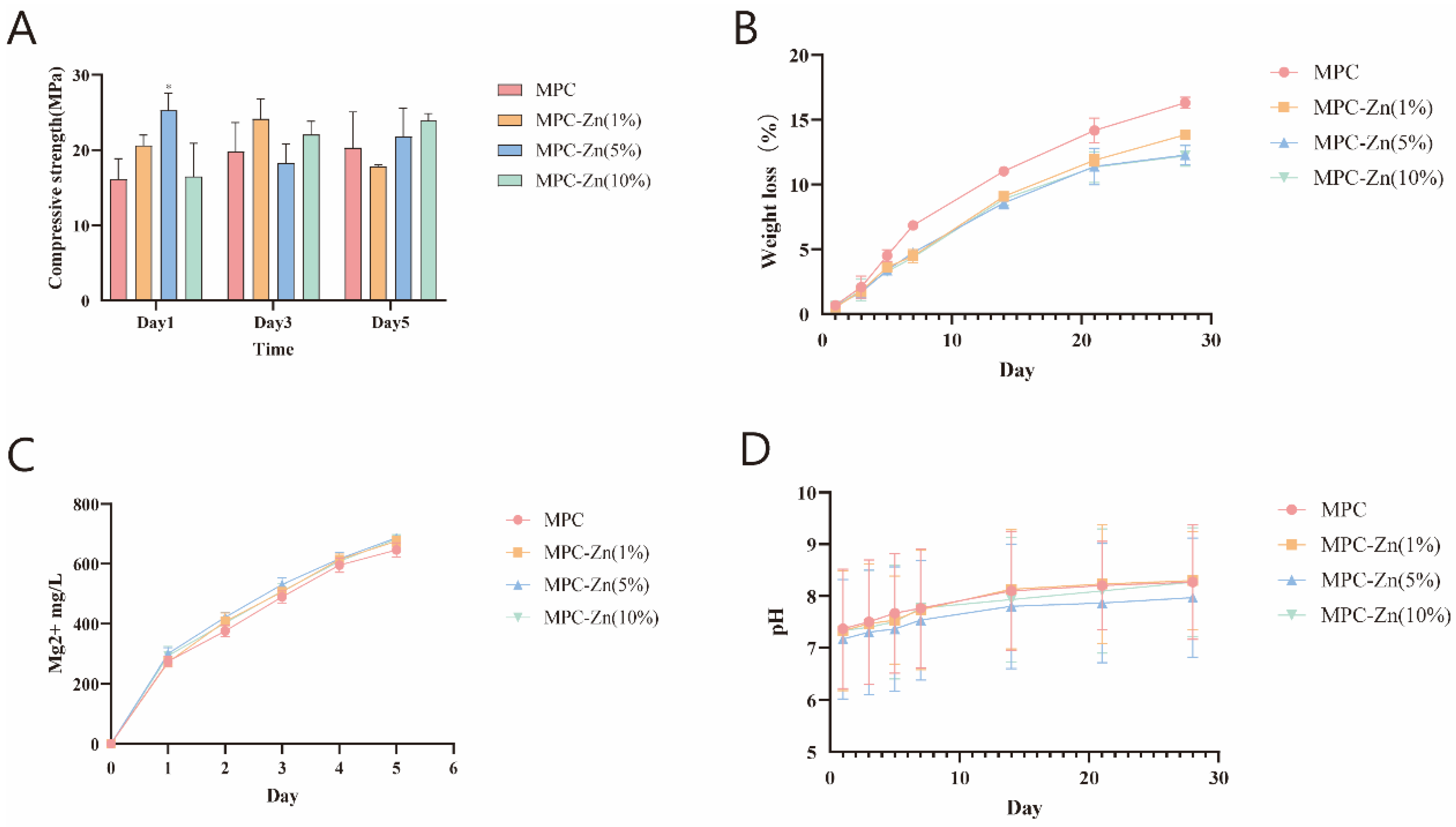
3.1.3. Mechanical and Physicochemical Properties of the Scaffolds
3.2. In Vitro Experiments on the MPC and ZMPCs
3.2.1. Cell Proliferation and Adhesion on Samples
3.2.2. Osteogenic Differentiation Behaviors
3.2.3. In Vitro Osteogenesis of Samples
3.3. In Vivo Experiments on the MPC and ZMPCs
3.3.1. Micro-CT Analysis
3.3.2. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koski, C.; Onuike, B.; Bandyopadhyay, A.; Bose, S. Starch-Hydroxyapatite Composite Bone Scaffold Fabrication Utilizing a Slurry Extrusion-Based Solid Freeform Fabricator. Addit. Manuf. 2018, 24, 47–59. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Jiang, Y.; Liu, S.; Zheng, J.; Li, N.; Wang, G.; Dong, F.; Chen, J.; Xie, Y.; et al. A 3D biomimetic optoelectronic scaffold repairs cranial defects. Sci. Adv. 2023, 9, eabq7750. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H.L. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef]
- WE, B. A new calcium phosphate water setting cement. Cem. Res. Prog. 1986, 31, 352–379. [Google Scholar]
- Ali, A.; Paladhi, A.; Hira, S.K.; Singh, B.N.; Pyare, R. Bioactive ZnO-assisted 1393 glass scaffold promotes osteogenic differentiation: Some studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1059–1073. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, J.; Pei, X.; Sun, S.; Cai, J.; Liu, Y.; Wang, Y.; Zheng, L.; Zhou, H. Ultrasound-driven electrical stimulation based on 3D hierarchical porous piezoelectric nanofiber-aerogel scaffold promotes bone defect repair. Chem. Eng. J. 2023, 470, 144305. [Google Scholar] [CrossRef]
- Chopra, V.; Thomas, J.; Sharma, A.; Panwar, V.; Kaushik, S.; Sharma, S.; Porwal, K.; Kulkarni, C.; Rajput, S.; Singh, H.; et al. Synthesis and Evaluation of a Zinc Eluting rGO/Hydroxyapatite Nanocomposite Optimized for Bone Augmentation. ACS Biomater. Sci. Eng. 2020, 6, 6710–6725. [Google Scholar] [CrossRef]
- Han, F.; Yan, Z.; Wu, Q.; Hu, Y.; Li, J.; Du, F.; Tan, Y. Different dimension ZnO nano materials in the rehabilitation of patients with limb fracture and injury. Ferroelectrics 2021, 578, 95–107. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Martinez-Zelaya, V.R.; Zarranz, L.; Herrera, E.Z.; Alves, A.T.; Uzeda, M.J.; Mavropoulos, E.; Rossi, A.L.; Mello, A.; Granjeiro, J.M.; Calasans-Maia, M.D. In vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: Zinc and calcium bioavailability and bone regeneration. Int. J. Nanomed. 2019, 14, 3471–3490. [Google Scholar] [CrossRef]
- Li, P.; Dai, J.; Schweizer, E.; Rupp, F.; Heiss, A.; Richter, A.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L.; Alexander, D. Response of human periosteal cells to degradation products of zinc and its alloy. Mater. Sci. Eng. C 2020, 108, 110208. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, X.; Yang, H.; Li, L.; He, C.; Cheng, C.; Haag, R. ZnO/Nanocarbons-Modified Fibrous Scaffolds for Stem Cell-Based Osteogenic Differentiation. Small 2020, 16, 2003010. [Google Scholar] [CrossRef]
- He, T.; Chen, H.; Liu, P.; Shi, H.; Xu, X.; Feng, C.; Wang, Y.; Li, X.; Lei, N.; Xiao, Y.; et al. One-step co-doping of ZnO and Zn2+in osteoinductive calcium phosphate ceramics with synergistic antibacterial activity for regenerative repair of infected bone defect. J. Mater. Sci. Technol. 2023, 163, 168–181. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, H.; Xue, J.; Luo, J.; Liu, Q.; Chen, X.; Pan, Y.; Zhou, J.; Liu, Z.; Chen, T. Near-Infrared Radiation-Assisted Drug Delivery Nanoplatform to Realize Blood-Brain Barrier Crossing and Protection for Parkinsonian Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37746–37760. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Dowaidar, M.; Langel, Ü. Carbonized chitosan encapsulated hierarchical porous zeolitic imidazolate frameworks nanoparticles for gene delivery. Microporous Mesoporous Mater. 2020, 302, 110200. [Google Scholar] [CrossRef]
- Kim, S.-K.; Murugan, S.S.; Dalavi, P.A.; Gupta, S.; Anil, S.; Seong, G.H.; Venkatesan, J. Biomimetic chitosan with biocomposite nanomaterials for bone tissue repair and regeneration. Beilstein J. Nanotechnol. 2022, 13, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Dai, C.; Yin, Y.; Gong, L.; Pan, W.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; et al. Bioactive Three-Dimensional Graphene Oxide Foam/Polydimethylsiloxane/Zinc Silicate Scaffolds with Enhanced Osteoinductivity for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Yao, Y.; Miao, X. Application prospect of nano-zinc oxide in orthopedics surgery. Orthop. J. China 2017, 25, 1675–1678. [Google Scholar]
- Liu, W.; Zhang, K.; Nan, J.; Lei, P.; Sun, Y.; Hu, Y. Nano artificial periosteum PCL/Ta/ZnO accelerates repair of periosteum via antibacterial, promoting vascularization and osteogenesis. Biomater. Adv. 2023, 154, 213624. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.; Abdullah, T.; Russell, C.S.; Mostafavi, E.; Williams, T.J.; Salah, N.; Alshahrie, A.; Harris, S.; Basri, S.M.M.; Mishra, Y.K.; et al. In situ printing of scaffolds for reconstruction of bone defects. Acta Biomater. 2021, 127, 313–326. [Google Scholar] [CrossRef]
- Reyna-Urrutia, V.A.; Rosales-Ibanez, R.; Gonzalez-Gonzalez, A.M.; Estevez, M.; Rodriguez-Martinez, J.J.; Gonzalez-Reyna, M.A. Biological activity of a chitosan-carboxymethylcellulose-zinc oxide and calcium carbonate in 3D scaffolds stabilized by physical links for bone tissue engineering. J. Biomater. Appl. 2023, 37, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, Z.; Shi, Y.; Dong, L.; Wang, C. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Bioact. Mater. 2021, 6, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, U.; van Weeren, R.; Castilho, M.; Malda, J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef]
- Tarasenko, S.V.; Grigor’janc, L.A.; Morozova, E.A.; Gor, I.A.; Simonjan, D.V.; Kamilov, S.T. Histological evaluation of inflammatory response to zinc-oxide-eugenol filling materials in a soft and bone tissues. Stomatologiia 2019, 98, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ma, S.; Fu, M.; Wu, M.; Li, J.; Wu, K.; Zhuang, X.; Lu, Z.; Guo, J. Injective Programmable Proanthocyanidin-Coordinated Zinc-Based Composite Hydrogel for Infected Bone Repair. Adv. Healthc. Mater. 2023, 2302690. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Zalama, E.; Karrouf, G.; Rizk, A.; Salama, B.; Samy, A. Does zinc oxide nanoparticles potentiate the regenerative effect of platelet-rich fibrin in healing of critical bone defect in rabbits? BMC Vet. Res. 2022, 18, 130. [Google Scholar] [CrossRef]
- Jang, W.G.; Kim, E.J.; Kim, D.K.; Ryoo, H.M.; Lee, K.B.; Kim, S.H.; Choi, H.S.; Koh, J.T. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 2012, 287, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, F.; Araya, H.; Briceño, P.; Varela, N.; Stevenson, A.; Jerez, S.; Tempio, F.; Chnaiderman, J.; Perez, C.; Villarroel, M.; et al. The cancer-related transcription factor RUNX2 modulates expression and secretion of the matricellular protein osteopontin in osteosarcoma cells to promote adhesion to endothelial pulmonary cells and lung metastasis. J. Cell. Physiol. 2019, 234, 13659–13679. [Google Scholar] [CrossRef] [PubMed]
- Samelson, E.J.; Broe, K.E.; Xu, H.; Yang, L.; Boyd, S.; Biver, E.; Szulc, P.; Adachi, J.; Amin, S.; Atkinson, E.; et al. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): A prospective study. Lancet Diabetes Endocrinol. 2019, 7, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Guo, B.; Wang, Q.; Chen, L.; Zhu, L.; Zhang, T.; Wang, R.; Li, W.; Luo, D.; et al. A Zinc Oxide Nanowire-Modified Mineralized Collagen Scaffold Promotes Infectious Bone Regeneration. Small 2023, 2309230. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Solano, M.; Pompolo, S.; Fernandes, T.J.; Constable, M.J.; Nicholson, G.C.; Zhang, J.G.; Nicola, N.A.; et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J. Clin. Investig. 2010, 120, 582–592. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Standardization Administration (SAC): Beijing, China, 2011.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Vrahnas, C.; Blank, M.; Dite, T.A.; Tatarczuch, L.; Ansari, N.; Crimeen-Irwin, B.; Nguyen, H.; Forwood, M.R.; Hu, Y.; Ikegame, M. Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone. Nat. Commun. 2019, 10, 3436. [Google Scholar] [CrossRef]
- Yu, L.; Gao, T.; Li, W.; Yang, J.; Liu, Y.; Zhao, Y.; He, P.; Li, X.; Guo, W.; Fan, Z.; et al. Carboxymethyl chitosan-alginate enhances bone repair effects of magnesium phosphate bone cement by activating the FAK-Wnt pathway. Bioact. Mater. 2023, 20, 598–609. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Liu, L.; Wang, Y.; Ju, Y.; Zeng, C.; Lu, Z.; Xie, D.; Guo, J. Zinc-Based Tannin-Modified Composite Microparticulate Scaffolds with Balanced Antimicrobial Activity and Osteogenesis for Infected Bone Defect Repair. Adv. Healthc. Mater. 2023, 12, 2300303. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef] [PubMed]
- Prolo, D.J.; Gutierrez, R.V.; DeVine, J.S.; Oklund, S.A. Clinical utility of allogeneic skull discs in human craniotomy. Neurosurgery 1984, 14, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.A.; Júnior, S.A.; Ferraz, A.V. A study of the bioactivity, hemocompatibility and antimicrobial properties of a zinc oxide and calcium phosphate composite for bone regeneration. Cerâmica 2023, 69, 93–98. [Google Scholar] [CrossRef]
- Lakshmi, K.; Varadharajan, V.; Kanagasubbulakshmi, S.; Kadirvelu, K. Advanced bio-nanoscaffold for bone tissue regeneration in animal model. J. Drug Deliv. Sci. Technol. 2022, 74, 103593. [Google Scholar] [CrossRef]







| Samples | ZnO (wt%) |
|---|---|
| MPC | 0 |
| MPC/Zn(1%) | 1.25 |
| MPC/Zn(5%) | 6.23 |
| MPC/Zn(10%) | 12.46 |
| Primer | Sequence (5′ to 3′) |
|---|---|
| R-GAPDH-F | AGACAGCCGCATCTTCTTGT |
| R-GAPDH-R | CTTGCCGTGGGTAGAGTCAT |
| R-ALP-F | GAGGCTGAACCGCAGGATGT |
| R-ALP-R | GTCAATACCGGAAGGAGTGCT |
| R-OCN-F | GCAGACCTAGCAGACACCAT |
| R-OCN-R | TTGGACATGAAGGCTTTGTCA |
| R-RUNX2-F | GAGCACAAACATGGCTGAGA |
| R-RUNX2-R | TGGAGATGTTGCTCTGTTCG |
| R-BMP2-F | ATATGCTCGACCTGTACCGC |
| R-BMP2-R | TCCTCGATGGCTTCTTCGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yu, L.; Chen, J.; Li, S.; Wei, Z.; Guo, W. Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair. Biomedicines 2024, 12, 344. https://doi.org/10.3390/biomedicines12020344
Liu Y, Yu L, Chen J, Li S, Wei Z, Guo W. Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair. Biomedicines. 2024; 12(2):344. https://doi.org/10.3390/biomedicines12020344
Chicago/Turabian StyleLiu, Yinchu, Ling Yu, Jingteng Chen, Shiyu Li, Zhun Wei, and Weichun Guo. 2024. "Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair" Biomedicines 12, no. 2: 344. https://doi.org/10.3390/biomedicines12020344
APA StyleLiu, Y., Yu, L., Chen, J., Li, S., Wei, Z., & Guo, W. (2024). Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair. Biomedicines, 12(2), 344. https://doi.org/10.3390/biomedicines12020344







