Association of LVV-Hemorphin-7 with Sepsis and Shock: Roles of Cathepsin D and G in Hemoglobin Metabolism in a Prospective ICU Cohort Study
Abstract
1. Introduction
2. Methods
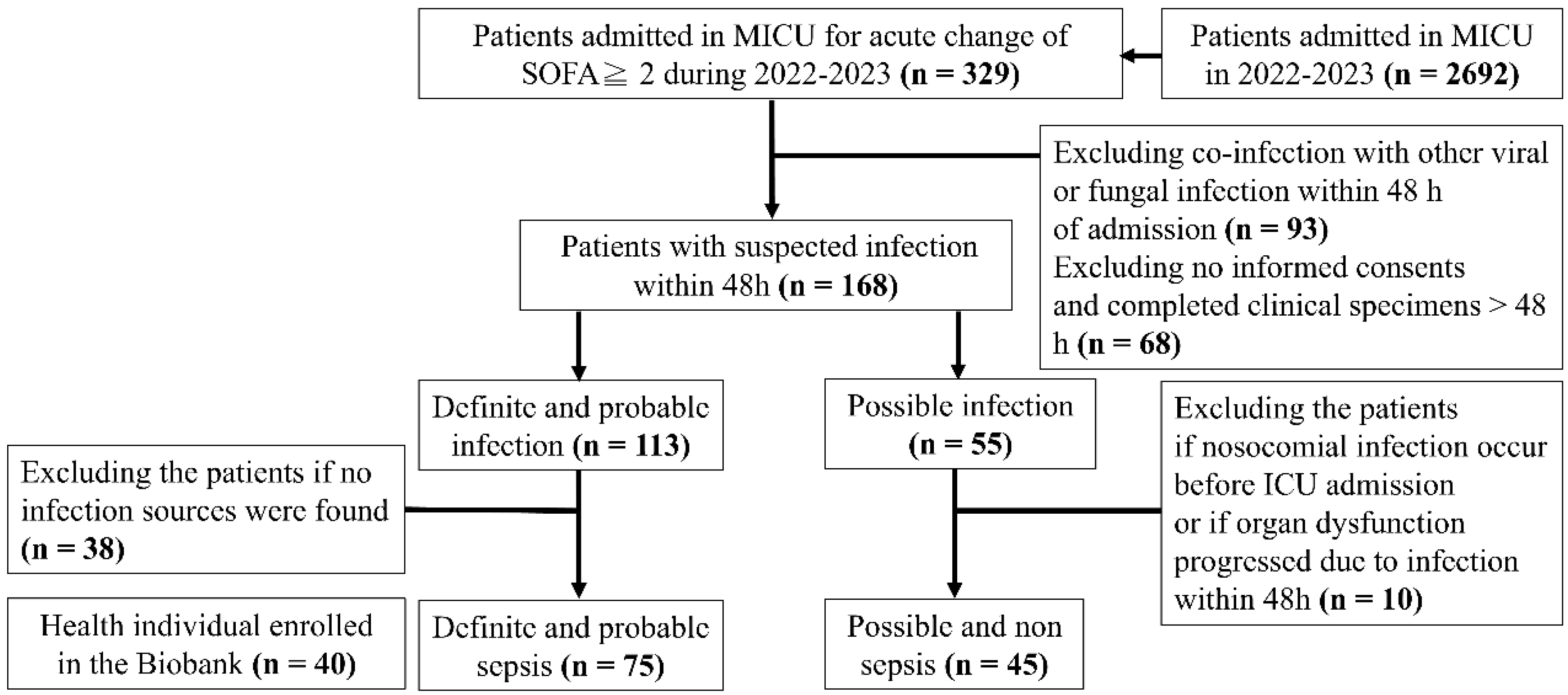
2.1. Study Design and Participant Enrolment
- A definite or probable source of infection (e.g., positive cultures or radiographic evidence with a compatible clinical syndrome).
- Organ dysfunction due to infection without any discernable cause other than infection.
- Organ dysfunction most likely attributable to infection, although other potential contributors were present.
- Patients were treated for presumed sepsis but lacked definitive evidence of infection.
- Patients had alternative definite or possible explanations for organ dysfunction.
- Acute or chronic renal failure leading to acute pulmonary edema with hypoxemic respiratory failure or hyperkalemia with bradycardic heart failure.
- Cardiac dysfunction-related cardiogenic hypotension, including acute myocardial infarction, decompensated heart failure, and arrhythmias.
- Pulmonary disease causing impaired gas exchange and hypoxemia, such as chronic obstructive pulmonary disease, asthma, and interstitial lung disease.
- Gastrointestinal tract hemorrhage with hypovolemic hypotension, including esophageal varices bleeding and upper or lower gastrointestinal tract bleeding.
- Acute pancreatitis with volume depletion and distributive hypotension.
- Metabolic acidosis-related hypotension or cardiac suppression, including diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome.
- Anaphylaxis resulting in distributive hypotension.
2.2. Data Collection
2.3. Free Hemoglobin, Cathepsin D, Cathepsin G, LVV-H7, and Angiotensin II Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.4. Statistical Analysis
3. Results
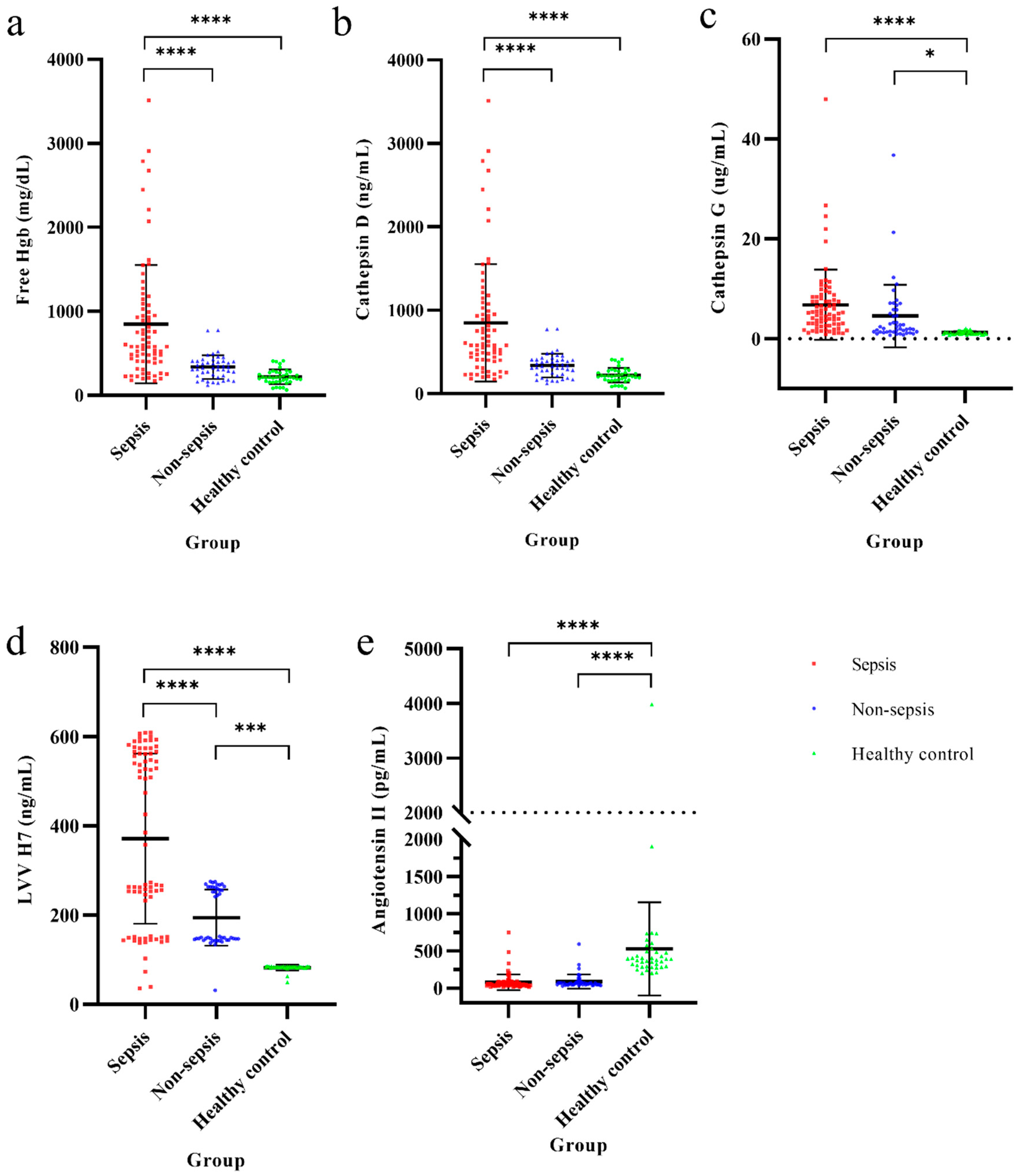
3.1. Hb Metabolic Biomarkers Among Sepsis, Non-Sepsis, and Healthy Control Groups
3.2. The Isolated Microorganisms in the Sepsis Group and the Diagnoses in the Non-Sepsis Group
3.3. Comparisons of Parameters Between Sepsis and Non-Sepsis Groups
3.4. Potential Factors for Diagnosis of Sepsis, Shock, or Septic Shock in Critical Ill Patients with Acute Change of SOFA Score ≥ 2
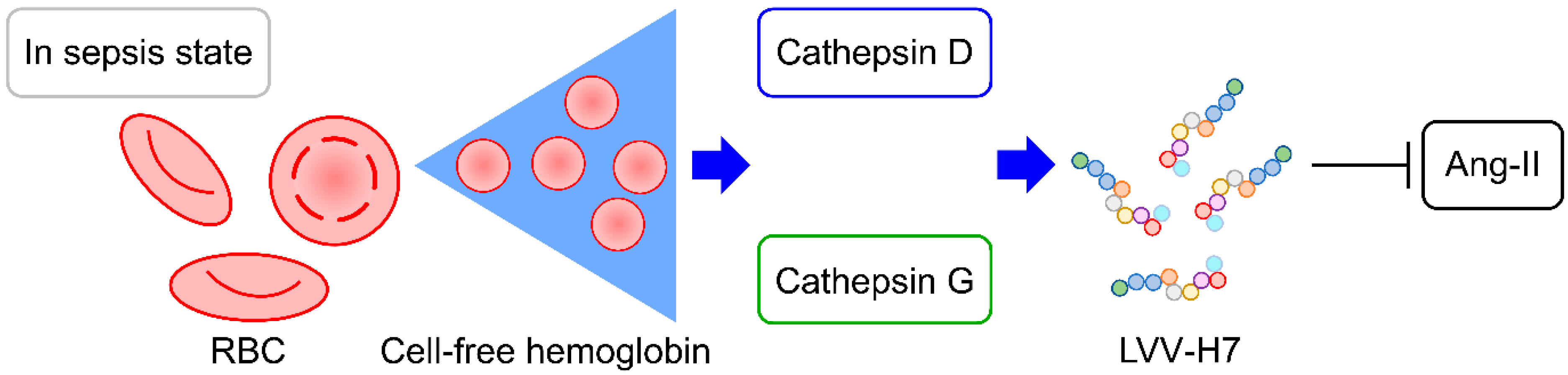
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Jansma, G.; de Lange, F.; Kingma, W.P.; Vellinga, N.A.; Koopmans, M.; Kuiper, M.A.; Boerma, E.C. ‘Sepsis-related anemia’ is absent at hospital presentation; a retrospective cohort analysis. BMC Anesthesiol. 2015, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Nissenson, A.R.; Dylan, M.L.; Griffiths, R.I.; Yu, H.T.; Dubois, R.W. Septicemia in patients with ESRD is associated with decreased hematocrit and increased use of erythropoietin. Clin. J. Am. Soc. Nephrol. 2006, 1, 505–510. [Google Scholar] [CrossRef] [PubMed]
- van Beest, P.A.; Hofstra, J.J.; Schultz, M.J.; Boerma, E.C.; Spronk, P.E.; Kuiper, M.A. The incidence of low venous oxygen saturation on admission to the intensive care unit: A multi-center observational study in The Netherlands. Crit. Care 2008, 12, R33. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Hartmann, M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018, 41, 1569–1581. [Google Scholar] [CrossRef]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassu, A.M.; Bonaparte, D.; Cavalcante, M.M.; Chora, A.; Ferreira, A.; et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010, 2, 51ra71. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef]
- Adamzik, M.; Hamburger, T.; Petrat, F.; Peters, J.; de Groot, H.; Hartmann, M. Free hemoglobin concentration in severe sepsis: Methods of measurement and prediction of outcome. Crit. Care 2012, 16, R125. [Google Scholar] [CrossRef]
- Janz, D.R.; Bastarache, J.A.; Peterson, J.F.; Sills, G.; Wickersham, N.; May, A.K.; Roberts, L.J., 2nd; Ware, L.B. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: An observational study. Crit. Care Med. 2013, 41, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Starkey, P.M.; Barrett, A.J. Human cathepsin G. Catalytic and immunological properties. Biochem. J. 1976, 155, 273–278. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Diment, S.; Leech, M.S.; Stahl, P.D. Cathepsin D is membrane-associated in macrophage endosomes. J. Biol. Chem. 1988, 263, 6901–6907. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, P.; Hartman, K.; Drabik, A.; Hung, H.Y.; Huang, E.Y.; Gibula-Tarlowska, E.; Kotlinska, J.H.; Silberring, J. Hemorphins-From Discovery to Functions and Pharmacology. Molecules 2021, 26, 3879. [Google Scholar] [CrossRef] [PubMed]
- Dejouvencel, T.; Feron, D.; Rossignol, P.; Sapoval, M.; Kauffmann, C.; Piot, J.M.; Michel, J.B.; Fruitier-Arnaudin, I.; Meilhac, O. Hemorphin 7 reflects hemoglobin proteolysis in abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 269–275. [Google Scholar] [CrossRef]
- Ali, A.; Palakkott, A.; Ashraf, A.; Al Zamel, I.; Baby, B.; Vijayan, R.; Ayoub, M.A. Positive Modulation of Angiotensin II Type 1 Receptor-Mediated Signaling by LVV-Hemorphin-7. Front. Pharmacol. 2019, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Cohen, J.; FRCP for the International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 2005, 33, 1538–1548. [Google Scholar] [CrossRef]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef]
- Mellhammar, L.; Elen, S.; Ehrhard, S.; Bouma, H.; Ninck, L.; Muntjewerff, E.; Wunsch, D.; Bloos, F.; Malmstrom, E.; Linder, A. New, Useful Criteria for Assessing the Evidence of Infection in Sepsis Research. Crit. Care Explor. 2022, 4, e0697. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Janz, D.R.; Ware, L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J. Intensive Care 2015, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Zamolodchikova, T.S.; Tolpygo, S.M.; Svirshchevskaya, E.V. Cathepsin G-Not Only Inflammation: The Immune Protease Can Regulate Normal Physiological Processes. Front. Immunol. 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef]
- Fruitier, I.; Garreau, I.; Piot, J.M. Cathepsin D is a good candidate for the specific release of a stable hemorphin from hemoglobin in vivo: VV-hemorphin-7. Biochem. Biophys. Res. Commun. 1998, 246, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Fruitier-Arnaudin, I.; Sauvan, R.; Birnbaum, D.; Piot, J.M. Serum levels of Hemorphin-7 peptides in patients with breast cancer. Clin. Chim. Acta 2003, 337, 59–67. [Google Scholar] [CrossRef]
- Maraninchi, M.; Feron, D.; Fruitier-Arnaudin, I.; Begu-Le Corroller, A.; Nogueira, J.P.; Mancini, J.; Valero, R.; Piot, J.M.; Vialettes, B. Serum hemorphin-7 levels are decreased in obesity. Obesity 2013, 21, 378–381. [Google Scholar] [CrossRef]
- Fruiter, A., II; Cohen, M.M.; Nervi, S.S.; Bordenave, S.S.; Sannier, F.F.; Piot, J.M. Reduced level of opioid peptides, hemorphin-7 peptides, in serum of diabetic patients. Diabetes Care 2003, 26, 2480. [Google Scholar] [CrossRef]
- Kearon, C.; de Wit, K.; Parpia, S.; Schulman, S.; Afilalo, M.; Hirsch, A.; Spencer, F.A.; Sharma, S.; D’Aragon, F.; Deshaies, J.F.; et al. Diagnosis of Pulmonary Embolism with d-Dimer Adjusted to Clinical Probability. N. Engl. J. Med. 2019, 381, 2125–2134. [Google Scholar] [CrossRef]
- Koozi, H.; Lengquist, M.; Frigyesi, A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J. Crit. Care 2020, 56, 73–79. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Klempner, M.S.; Austen, K.F.; Wintroub, B.U. Identification of a human neutrophil angiotension II-generating protease as cathepsin G. J. Clin. Investig. 1982, 69, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rykl, J.; Thiemann, J.; Kurzawski, S.; Pohl, T.; Gobom, J.; Zidek, W.; Schlüter, H. Renal cathepsin G and angiotensin II generation. J. Hypertens. 2006, 24, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; G, M. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]



| Variables | Definite and Probable Sepsis (n = 75) | Possible and Non-Sepsis (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 68.81 ± 14.17 | 71.09 ± 13.56 | 0.388 c |
| Sex | 0.530 a | ||
| Female | 31 (41.3%) | 16 (35.6%) | |
| Male | 44 (58.7%) | 29 (64.4%) | |
| Body mass index (kg/m2) | 22.96 ± 4.73 | 22.85 ± 4.41 | 0.900 c |
| Vital signs | |||
| Body temperature (°C) | 36.99 ± 1.45 | 36.38 ± 0.81 | 0.004 c* |
| Respiratory rate (/min) | 21.17 ± 5.38 | 20.76 ± 4.69 | 0.667 c |
| Heart rate (/min) | 106.33 ± 25.76 | 100.16 ± 26.57 | 0.211 c |
| MAP (mmHg) | 82.53 ± 22.00 | 97.58 ± 21.33 | <0.001 c** |
| SpO2 (%) | 94.85 ± 4.94 | 94.36 ± 5.03 | 0.597 c |
| GCS | 12.13 ± 4.06 | 12.27 ± 4.08 | 0.862 c |
| CCI | 5.55 ± 2.80 | 5.38 ± 2.31 | 0.734 c |
| Comorbidities | |||
| Diabetes | 33 (44.0%) | 18 (40.0%) | 0.668 a |
| Cardiovascular disease | 26 (34.7%) | 22 (48.9%) | 0.124a |
| Chronic kidney disease | 24 (32.0%) | 12 (26.7%) | 0.537 a |
| Neurologic diseases | 19 (25.3%) | 11 (24.4%) | 0.913 a |
| Pulmonary disease | 15 (20.0%) | 12 (26.7%) | 0.397 a |
| Malignancy | 15 (20.0%) | 6 (13.3%) | 0.352 a |
| Chronic liver disease | 8 (10.7%) | 4 (8.9%) | 1.000 b |
| Autoimmune disease | 2 (2.7%) | 2 (4.4%) | 0.630 b |
| Disease severity | |||
| qSOFA | 1.09 ± 0.83 | 0.84 ± 0.74 | 0.099 c |
| SOFA score | 6.61 ± 2.91 | 5.11 ± 2.80 | 0.006 c |
| APACHE II score | 23.07 ± 9.92 | 21.36 ± 8.40 | 0.336 c |
| Oxygenation device status | 0.512 a | ||
| IMV | 35 (46.7%) | 26 (57.8%) | |
| NIV | 5 (6.7%) | 4 (8.9%) | |
| Oxygen supplement | 31 (41.3%) | 14 (31.1%) | |
| Oxygen not needed | 4 (5.3%) | 1 (2.2%) | |
| Clinical outcomes | |||
| Length of stay in ICU | 12.44 ± 12.84 | 15.31 ± 14.65 | 0.263 c |
| Hospital days | 23.20 ± 19.71 | 27.36 ± 23.91 | 0.307 c |
| Shock episodes | 61 (81.3%) | 20 (44.4%) | <0.001 a** |
| Survival | 54 (72.0%) | 35 (79.5%) | 0.360 a |
| Variables | Definite and Probable Sepsis (n = 75) | Possible and Non-Sepsis (n = 45) | p-Value |
|---|---|---|---|
| WBC (103/µL) | 13.16 ± 7.71 | 9.36 ± 4.27 | 0.001 * |
| RBC (106/µL) | 3.67 ± 0.84 | 3.60 ± 0.92 | 0.650 |
| Hemoglobin (g/dL) | 11.00 ± 2.58 | 10.97 ± 2.58 | 0.948 |
| Platelets (103/µL) | 186.84 ± 129.37 | 202.96 ± 93.94 | 0.433 |
| Neutrophil (103/µL) | 10.12 ± 6.70 | 6.91 ± 3.64 | 0.001 * |
| Lymphocyte (103/µL) | 0.91 ± 0.80 | 1.71 ± 1.96 | 0.011 * |
| Monocyte (103/µL) | 0.50 ± 0.41 | 0.47 ± 0.31 | 0.602 |
| Na (mEq/L) | 135.13 ± 6.93 | 136.76 ± 5.22 | 0.149 |
| K (mEq/L) | 4.23 ± 1.04 | 4.17 ± 0.84 | 0.748 |
| AST (U/L) | 92.60 ± 167.37 | 71.24 ± 147.65 | 0.481 |
| ALT (U/L) | 41.43 ± 59.41 | 39.49 ± 42.64 | 0.849 |
| Albumin (g/dL) | 3.00 ± 0.58 | 3.19 ± 0.49 | 0.075 |
| BUN (mg/dL) | 52.77 ± 41.87 | 43.53 ± 32.13 | 0.206 |
| Creatinine (mg/dL) | 2.93 ± 2.57 | 2.68 ± 3.19 | 0.642 |
| Random Glucose (mg/dL) | 179.30 ± 80.1 | 176.15 ± 82.68 | 0.837 |
| Lactate (mmol/L) | 3.62 ± 3.49 | 3.75 ± 4.57 | 0.863 |
| CRP (mg/dL) | 13.50 ± 11.38 | 5.11 ± 7.53 | <0.001 ** |
| PCT (ng/mL) | 19.03 ± 37.18 | 14.57 ± 43.85 | 0.553 |
| Total bilirubin (mg/dL) | 1.71 ± 2.56 | 0.95 ± 0.77 | 0.019 * |
| PT (second) | 12.30 ± 2.49 | 11.55 ± 1.84 | 0.084 |
| INR (ratio) | 1.23 ± 0.32 | 1.18 ± 0.32 | 0.404 |
| aPTT (second) | 32.65 ± 8.04 | 28.61 ± 6.12 | 0.002 * |
| ABG pH | 7.36 ± 0.12 | 7.39 ± 0.10 | 0.093 |
| ABG SaO2 | 95.89 ± 3.43 | 96.82 ± 3.43 | 0.154 |
| P/F ratio | 243.97 ± 137.03 | 289.95 ± 148.78 | 0.088 |
| Hemoglobin catabolism | |||
| Free Hemoglobin (mg/dL) | 15.27 ± 8.81 | 9.75 ± 3.51 | <0.001 ** |
| Cathepsin D (ng/mL) | 849.19 ± 703.08 | 337.44 ± 141.09 | <0.001 ** |
| Cathepsin G (μg/mL) | 6.79 ± 7.04 | 4.54 ± 6.28 | 0.080 |
| LVV-H7 (ng/mL) | 371.68 ± 190.52 | 194.48 ± 63.10 | <0.001 ** |
| Angiotensin II (pg/mL) | 78.50 ± 105.78 | 87.81 ± 95.54 | 0.630 |
| Variables | SE | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| LVV-H7 | 0.009 | 0.002 | 1.009 | 1.005 | 1.014 | <0.001 ** |
| CRP | 0.083 | 0.030 | 1.086 | 1.024 | 1.153 | 0.006 * |
| aPTT | 0.075 | 0.039 | 1.078 | 0.999 | 1.163 | 0.052 |
| WBC | 0.121 | 0.053 | 1.128 | 1.017 | 1.251 | 0.022 * |
| Biomarkers | Area | 95% CI | p-Value | Cuff Point | Sensitivity | Specificity | Youden’s J | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| LVV-H7 | 0.741 | 0.654 | 0.827 | <0.001 ** | 316.61 (ng/mL) | 0.507 | 1.000 | 0.507 |
| CRP | 0.738 | 0.647 | 0.830 | <0.001 ** | 5.85 (mg/dL) | 0.720 | 0.778 | 0.498 |
| WBC | 0.639 | 0.540 | 0.738 | 0.011 * | 12.03 (103/µL) | 0.533 | 0.822 | 0.356 |
| Variables | SE | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| For shock factors | ||||||
| Cathepsin G | −0.087 | 0.040 | 0.917 | 0.848 | 0.991 | 0.030 * |
| LVV-H7 | 0.005 | 0.002 | 1.005 | 1.002 | 1.008 | 0.004 * |
| SOFA score | 0.293 | 0.100 | 1.341 | 1.103 | 1.630 | 0.003 * |
| aPTT | 0.092 | 0.039 | 1.096 | 1.015 | 1.184 | 0.019 * |
| For septic shock factors | ||||||
| Cathepsin D | 0.001 | 0.000 | 1.001 | 1.000 | 1.002 | 0.026 * |
| Lactate | 0.167 | 0.058 | 1.182 | 1.054 | 1.324 | 0.004 * |
| CRP | 0.090 | 0.024 | 1.094 | 1.044 | 1.147 | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-K.; Chung, H.-W.; Chen, Y.-T.; Chen, H.-C.; Chen, I.-H.; Su, W.-L. Association of LVV-Hemorphin-7 with Sepsis and Shock: Roles of Cathepsin D and G in Hemoglobin Metabolism in a Prospective ICU Cohort Study. Biomedicines 2024, 12, 2789. https://doi.org/10.3390/biomedicines12122789
Wu Y-K, Chung H-W, Chen Y-T, Chen H-C, Chen I-H, Su W-L. Association of LVV-Hemorphin-7 with Sepsis and Shock: Roles of Cathepsin D and G in Hemoglobin Metabolism in a Prospective ICU Cohort Study. Biomedicines. 2024; 12(12):2789. https://doi.org/10.3390/biomedicines12122789
Chicago/Turabian StyleWu, Yao-Kuang, Hsueh-Wen Chung, Yi-Ting Chen, Hsing-Chun Chen, I-Hung Chen, and Wen-Lin Su. 2024. "Association of LVV-Hemorphin-7 with Sepsis and Shock: Roles of Cathepsin D and G in Hemoglobin Metabolism in a Prospective ICU Cohort Study" Biomedicines 12, no. 12: 2789. https://doi.org/10.3390/biomedicines12122789
APA StyleWu, Y.-K., Chung, H.-W., Chen, Y.-T., Chen, H.-C., Chen, I.-H., & Su, W.-L. (2024). Association of LVV-Hemorphin-7 with Sepsis and Shock: Roles of Cathepsin D and G in Hemoglobin Metabolism in a Prospective ICU Cohort Study. Biomedicines, 12(12), 2789. https://doi.org/10.3390/biomedicines12122789






