Nano-Delivery Revolution: Harnessing Mesenchymal Stem Cell-Derived Exosomes’ Potential for Wound Healing
Abstract
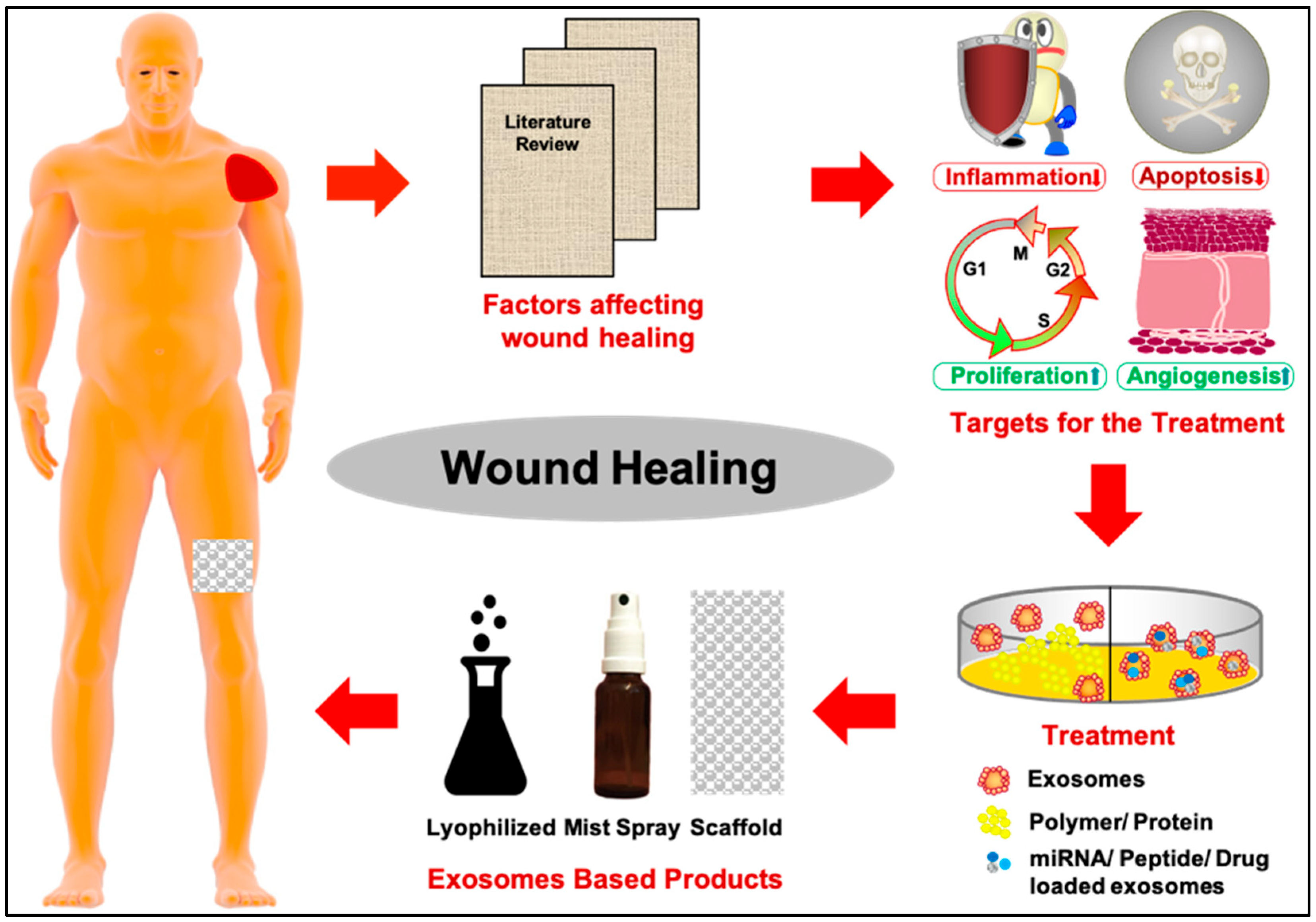
1. Introduction
2. Exosomes and Their Cargo Content
- Biocompatibility: MSC-derived exosomes are well-tolerated by the body and exhibit low immunogenicity by exhibiting anti-proliferative effects on T-, B-, NK-cells and macrophages. This is regulated primarily via ligand–receptor binding through exosome proteins tetraspanin and integrins (CD81, CD82, CD63, etc.), followed by membrane fusion via Rab GTPases, annexins, and heat shock protein (HSP70 and HSP90) and signal transduction.
- Low cytotoxicity: They have minimal harmful effects on cells, particularly reduced cytotoxicity over NK cells.
- Stability and ideal for storage: Exosomes are stable under various types of buffers, such as PBS supplemented with human albumin or trehalose. These buffers support both short-term and long-term storage at −80 °C throughout the freeze–thaw cycles.
- Ease of production: Numerous commercial companies are dedicated to the mass production of exosomes. MSCs can also be primed with reagents like disease-condition serum or cytokines to enhance exosome production or such that exosomes carry specific biomolecules’ load for targeted activities like drug delivery, wound healing or against tumors [1,18].
- Cargo loading ability: Exosomes can carry a diverse payload of biomolecules, with the potential to regulate host inflammatory response, epithelial regeneration and stimulating angiogenesis for wound healing. For instance, SGM-miR146a-Exo@SFP is an engineered exosome targeting diabetic wound healing [19]. Additionally, human umbilical cord-derived MSCs accelerate cutaneous wound healing via Angiopoietin-2 delivery, while lncRNA H19 exosome does so via miRNA-152-3 [20,21].
- Enhanced internalization: They can efficiently enter target cells and regulate cellular signalling primarily via two mechanisms: first, exosomes recognize and bind to target cell receptors, stimulating certain signalling pathways; or second, they fuse with the target cell membrane to release their cargo either directly or through endosomes.
3. Perspective: Exosomes in Regenerative Medicine for Wound Healing
4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jorgensen, L.N.; Sorensen, L.T.; Kallehave, F.; Schulze, S.; Gottrup, F. Increased collagen deposition in an uncomplicated surgical wound compared to a minimal subcutaneous test wound. Wound Repair Regen. 2001, 9, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing an overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.D. Overview: Acute and chronic wounds. Nurs. Clin. 2005, 40, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Hirvonen, H.; Hukkanen, V.; Salmi, T.T.; Pelliniemi, T.T.; Alitalo, R. L-myc and N-myc in hematopoietic malignancies. Leuk. Lymphoma 1993, 11, 197–205. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringden, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Lee, R.H.; Oh, J.Y.; Choi, H.; Bazhanov, N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J. Cell. Biochem. 2011, 112, 3073–3078. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Efstathiou, J.A. Immunotherapy and radiation–a new combined treatment approach for bladder cancer? Bladder Cancer 2015, 1, 15–27. [Google Scholar] [CrossRef]
- Yuan, A.; Farber, E.L.; Rapoport, A.L.; Tejada, D.; Deniskin, R.; Akhmedov, N.B.; Farber, D.B. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE 2009, 4, e4722. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Gao, D.; Jiang, L. Exosomes in cancer therapy: A novel experimental strategy. Am. J. Cancer Res. 2018, 8, 2165. [Google Scholar]
- Santangelo, L.; Battistelli, C.; Montaldo, C.; Citarella, F.; Strippoli, R.; Cicchini, C. Functional roles and therapeutic applications of exosomes in hepatocellular carcinoma. BioMed Res. Int. 2017, 2017, 2931813. [Google Scholar] [CrossRef]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front. Bioeng. Biotechnol. 2022, 9, 797359. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Huang, Q.; Yang, J.; Li, B.; Ma, K.; Wei, Q.; Wang, Y.; Su, J.; Sun, M.; et al. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct. Target. Ther. 2023, 8, 62. [Google Scholar] [CrossRef]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering angiopoietin-2. Stem Cell Rev. Rep. 2021, 17, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, Y.; Meyer, D.S.; Zhang, Z.; Shokat, K.M.; Akhurst, R.J.; Miyazono, K.; Derynck, R. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 2019, 12, eaau8544. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Sampogna, G.; Cocozza, G.; Guraya, S.Y.; Forgione, A. Regenerative medicine: Clinical applications and future perspectives. J. Microsc. Ultrastruct. 2017, 5, 1–8. [Google Scholar] [PubMed]
- Mirershadi, F.; Ahmadi, M.; Rezabakhsh, A.; Rajabi, H.; Rahbarghazi, R.; Keyhanmanesh, R. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res. Ther. 2020, 11, 400. [Google Scholar] [CrossRef]
- Ma, H.; Siu, W.S.; Leung, P.C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing—A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [CrossRef]
- Jia, Q.; Zhao, H.; Wang, Y.; Cen, Y.; Zhang, Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front. Immunol. 2023, 14, 1214757. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Mohanty, S. Are graphene and graphene-derived products capable of preventing COVID-19 infection? Med. Hypotheses 2020, 144, 110031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
| miRNAs/ Growth Factors/ Cytokines | Function | PMID |
|---|---|---|
| Wound healing enhancing miRNAs | ||
| miR-17-92 | Displays pro-angiogenic potential through TSP-1 and CTGF. | 26233958; 17379831; 18779589; 16878133 |
| miR-21 | Enhances wound healing by re-epithelialization by enhancing keratinocyte migration and proliferation through TIMP3 and TIAM1 molecules. Additionally, it also has anti-inflammatory activity via PDCD4 and PTEN. | 32233440; 21647251; 18539147; 29786478; 20959495; 19946272 |
| miR-23a | Induces angiogenesis by increasing vascular permeability and cellular migration by PHD1 and PHD2. | 29743543; 28436951 |
| miR-31 | Enhances wound healing by re-epithelialization by enhancing keratinocyte migration and proliferation through EMP-1. It also has a pro-angiogenic action through FIH-1 and Spred1. | 31357577; 25685928; 25685928; 11641274; 25728779; 26657862 |
| miR-99 | Enhances proliferation by regulating IGF1R, mTOR, and AKT1 levels. | 23724047 |
| miR-105 | Shows anti-inflammatory action through TLR2. | 29662176; 19509287 |
| miR-125b | Possesses anti-inflammatory potential through TNF-α. | 28672982; 21412257; 18419608 |
| miR-130a | Induces angiogenesis by GAX and HOXA5. | 28849155; 17957028 |
| miR-155 | Enhances cellular proliferation via KGF and FGF-7, as well as exhibits pro-inflammatory activity by modulating SOCS1, SHIP1, and IL-12. | 29893326; 20959495; 19144316; 19701459 |
| miR-205 | Enhances wound healing by re-epithelialization, by enhancing keratinocyte migration and proliferation through SHIP2 and Rho-ROCK1. | 23372341; 20530248; 19033458 |
| miR-210 | Enhances migration, proliferation, and tube formation of endothelial cells through EFNA3. | 20837903; 30939341; 28249798; 18417479; 18539147 |
| miR-223 | Possesses anti-inflammatory potential by regulating MEF2c. | 23895238; 18278031 |
| miR-378 | Promotes angiogenesis through Fus-1 and Sufu. | 28902356; 18077375 |
| miR-17-5p | Promotes angiogenesis through TIMP1. | 19033458 |
| miR-126 | Enhances migration and repair of endothelial cells by SPRED1 and PIK2R2 expression. | 30360780; 18227515; 18987025 |
| miR-140 | Displays pro-inflammatory action by PDGF expression. | 18264099 |
| miR-146a | Enhances new blood vessel formation by VEGF and Pak1. | 28407626 |
| miR-184 | Induces angiogenesis via AKT. | 19033458; 20530248; 18227515; 16651380 |
| miR-198 | Promotes proliferation by regulating DIAPH1, PLAU, and LAMC2 levels. | 23395958 |
| miR-203 | Possesses anti-inflammatory potential via TNF-α and IL-24. | 22917968 |
| miR-296 | Displays pro-angiogenic activity via HGS. | 18977327 |
| miR-424 | Displays pro-angiogenic activity via CUL2. | 20972335 |
| miR-483-3p | Induces angiogenesis through MK2, MK167, and YAP1. | 21676945 |
| miR-4530 | Promotes angiogenesis by VASH1. | 28693142 |
| Wound healing inhibiting miRNAs | ||
| miR-1 | Promotes angiogenesis. Inhibits tube formation and endothelial cell proliferation via VEGF-A. | 28493075 |
| miR-15b | Displays angiogenic effect through VEGF. | 17205120 |
| miR-16 | Displays angiogenic effect through VEGF. | 29399181; 18854308; 17205120 |
| miR-17 | Displays angiogenic effect through JAK1. | 33300674; 20299512 |
| miR-20b | Displays angiogenic effect through HIF-1a, VEGF | 17205120; 19893619 |
| miR-29a | Conducts remodeling of type I and type II collagen by inhibiting collagen synthesis and angiogenesis through HSP47. | 29156819; 28092445; 20201077 |
| miR-29b | Shows an anti-fibrotic effect and inhibits neovascularization through VEGF, STAT3, Smad, and β-catenin. It is also a potent post-transcriptional repressor of collagen 1 in skin fibroblasts, and its deregulation might be implicated in scar formation. | 28365400; 28122338; 18723672; 19342382; 28584629 |
| miR-92a | Exhibits anti-angiogenic activity through integrin-a5. | 31180538; 20959495; 19460962 |
| miR-218 | Inhibits neovascularization via ROBO1. | 25170221; 27623390 |
| miR-221 | Inhibits neovascularization via c-Kit. | 23895238; 16330772; 16849646 |
| miR-222 | Inhibits neovascularization via c-Kit. | 18977327; 18077375 |
| miR-503 | Inhibits neovascularization via CCNE1 and cdc25A. | 21220732 |
| miR-939 | Disrupts vascular integrity and inhibits angiogenesis through γ-catenin. | 28115160 |
| miR-20a | Disrupts vascular integrity and inhibits angiogenesis through MKK3 and VEGF. | 17205120; 22696064; 17205120; 19893619 |
| miR-21 | Suppresses angiogenesis by inhibiting proliferation and migration of endothelial cells via PTEN, SMAD7. | 23313253; 26266258 |
| miR-29c | Displays an anti-fibrotic effect. Inhibits neovascularization through VEGF, STAT3, Smad, and β-catenin. It is also a potent post-transcriptional repressor of collagen 1 in skin fibroblasts, and its deregulation might be implicated in scar formation. | 28365400; 28122338; 18723672; 19342382; 28584629 |
| miR-98 | Decreases cellular viability and increases apoptosis of fibroblast by regulating Col1α1. | 28629444 |
| miR-203 | Enhances wound healing by re-epithelialization, by enhancing keratinocyte migration and proliferation through RAN, RAPH1, and p63. | 23190607; 26383628; 23190607 |
| miR-141-3p | Inhibits cell proliferation, migration of fibroblast and enhances cell apoptosis. | 28619509 |
| miR-143 | Supports remodeling and suppresses wound healing by IRS1, PDGFD, and αSMA. | 21673106; 24690171 |
| miR-185 | Inhibits growth of fibroblasts by modulating TGF-β1 and Col-1. | 28259900 |
| miR-198 | Restrains cell proliferation via FSTL1 and CCND2. | 23395958; 23395958; 23989979; 21658389; 21789031; 26225959 |
| miR-204 | Enhances wound healing by re-epithelialization, enhancing keratinocyte migration and proliferation through SMAD4 and SIRT1. | 23661372; 26047168 |
| miR-210 | Supports re-epithelialization and inhibits proliferation of epithelial cells through E2F3. | 18059191; 20308562 |
| miR-320 | Supports re-epithelialization and inhibits proliferation of epithelial cells through IGF-1. | 18068232; 18986336 |
| miR-377 | Inhibits angiogenesis via CD133, VEGF. | 28288140; 28122338 |
| Growth factors | ||
| Angiopoietins (ANGPT) | ANGPT-1 is responsible for the stabilization of blood vessels and promotes wound closure. ANGPT-2 causes vessel destabilization and remodeling. | 18382669; 19128254; 12843410 |
| Connective tissue growth factors (CTGF) | Stimulates chemotaxis, proliferation of fibroblasts, and induction of extracellular matrix proteins, including fibronectin and collagen type I. It also promotes endothelial angiogenesis, survival, migration, proliferation, and adhesion. | 12843410; 10393331; 27734381 |
| Epidermal growth factor (EGF) | Promotes wound closure by re-epithelialization of skin wounds. | 12843410; 19128254 |
| Fibroblast growth factors (FGF) | Exerts a cytoprotective function in would repair, supporting cell survival under stress conditions. It also promotes mitogenic activity for keratinocytes and fibroblasts at the wound site. bFGF increases smooth muscle cells and endothelial cell proliferation, while FGF1 and FGF2 stimulate angiogenesis. | 11276432; 22911722 |
| Insulin-like growth factors (IGF) | IGF is associated with heparin binding-EGF (HB-EGF) and enhances the proliferation of keratinocytes in vitro. It supports the mitogenesis and survival of many cells stimulated by IGF-I and IGF-II and promotes wound closure. | 12843410 |
| Keratinocyte growth factor (KGF) | Promotes wound closure either by acting as a transporter for alveolar epithelial fluid or by playing a role in tissue remodeling. | 18382669; 19128254; 22911722; 23197761 |
| Nerve growth factor (NGF) | Promotes fibroblast migration, increasing actin expression by smooth muscle, and collagen gel contraction by these cells. It also stimulates the proliferation of keratinocytes and inhibits apoptosis in vitro, and also supports the proliferation of human dermal growth by enhancing microvascular endothelial cells and their adherence molecule expression. | 11344264; 12843410 |
| Platelet-derived growth factor (PDGF) | Stimulates DNA synthesis, attracts fibroblasts to wound sites and enhances collagenase, collagen, and glycosaminoglycan production. It acts as one of the first chemotactic growth factors in the migration of fibroblasts, monocytes, and neutrophils into the skin wound, subsequently stimulating the production of extracellular matrix and the induction of a myofibroblast phenotype. | 3499612; 30265575 |
| Hepatocyte growth factor (HGF) or Plasminogen-related growth factor-1 (PRGF-1) | It inhibits fibrosis and promotes re-epithelialization. Also, it enhances keratinocytes to migrate, proliferate and produce matrix metalloproteinase and stimulates new blood vessel formation. | 22935176; 25835956; 23861688; 27641068 |
| Macrophage-stimulating protein (MSP) or Scatter factor-2 (SF-2) or Hepatocyte growth factor-like protein (HGFL) | Accelerates wound healing by regulating proliferation and differentiation of keratinocytes and macrophages; plays an integral role in inflammation, proliferation, and the remodeling phases of the healing process. | 12843410; 11702235 |
| Transforming growth factor (TGF) | Enhances the proliferation of epithelial cells, expression of antimicrobial peptides, and release of chemotactic cytokines, hence stimulating remodeling and wound repair. It activates keratinocytes and macrophages while suppressing T-lymphocytes. Members of this family, Activins, enhance granulation of tissue fibroblasts, and induce extracellular matrix deposition. | 12843410; 22911722; 23197761; 12843410; 18382669; 19128254 |
| Vascular endothelial growth factor (VEGF) | Regulates angiogenesis by promoting the proliferation of endothelial cells by VEGF-α, which leads to wound closure. | 19023885; 12843410; 18382669; 19128254; 23197761 |
| Pro-inflammatory cytokines | ||
| MCP-1 | Involved in macrophage infiltration and acts as inflammation regulatory chemokine in the wound-healing process. | 19654931; 22913454; 12843410 |
| MIP-1 | MIP-1α and MIP-1β promote wound closure and increase macrophage trafficking. | 18382669; 19128254; 22913454 |
| IL-1α | Influences the inflammatory phase. | 12477628; 23582261; 21954847 |
| IL-1β IL-6 TNF-β | Promote wound healing by controlling fibroblast and keratinocyte proliferation and regulating the synthesis and breakdown of extracellular matrix proteins. They also control fibroblast chemotaxis and regulate the immune response. | 12843410 |
| Anti-inflammatory cytokines | ||
| PGE2, IL-1, IL-4 | They play a primary role in the limitation and termination of inflammatory responses. | 23197761; 12843410; 23582261; 21954847; 17569781; 22913454; 19098906 |
| LL-37 | Acts as an antimicrobial peptide and reduces inflammation. | 20945332 |
| Proliferative cytokines | ||
| IL-6 | It plays an axial role in wound healing by regulating cellular responses such as epithelial cell migration, angiogenesis, leukocyte recruitment, infiltration to the inflammatory sites, and regulating collagen deposition. It also possesses both pro-inflammatory and anti-inflammatory activities depending on the phase of wound healing. | 24527299; 20471978; 24527301; 24527299; 24527301; 12773503; 12773503; 24527301; 22913454 |
| IL-10 | Regulates differentiation and growth of keratinocytes, endothelial and various immune cells, including infiltration of macrophage-derived neutrophils into the wound site, promoting the expression of pro-inflammatory cytokines and reducing matrix deposition and thereby inhibiting scar formation. | 11244051; 12843410 |
| GM-CSF | Enhances wound healing, either indirectly via stimulating secondary cytokines such as TGF-β1. It also stimulates endothelial cell proliferation and migration. Regulating angiogenesis formation, cellular responses, and tissue remodeling. | 11886498; 12843410; 24527299 |
| IL-8 | Increases keratinocyte proliferation and stimulates re-epithelialization in human skin grafts, both in vitro and in vivo. IL-8 and its receptor (CXCL8) act as a chemoattractant for neutrophils and enhance the migration of epithelial cells in vitro. | 10945942; 27651560; 25244101; 24527301; 21176394 |
| SDF-1 | Plays a role in regulating skin homeostasis and tissue remodeling, promoting wound closure and inducing cell migration. | 12843410; 18382669; 19128254; 23577036 |
| Study Title | Condition | Study Type | Phase | Status | Clinical Trials |
|---|---|---|---|---|---|
| Effect of Plasma-Derived Exosomes on Cutaneous Wound Healing | Ulcer | Interventional | Early Phase 1 | Enrolling by invitation | NCT02565264 |
| A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS | Acute respiratory distress syndrome | Interventional | Phase 1 Phase 2 | Not yet recruiting | NCT04602104 |
| MSC EVs in Dystrophic Epidermolysis Bullosa | Dystrophic epidermolysis bullosa | Interventional | Phase 1 Phase 2 | Not yet recruiting | NCT04173650 |
| Therapeutic Potential of Stem Cell Conditioned Medium on Chronic Ulcer Wounds | Chronic Ulcer | Interventional | Phase 1 | Completed | NCT04134676 |
| Circulating Extracellular Vesicles Released by Human Islets of Langerhans | Type 1 Diabetes Mellitus Type 2 Diabetes Islet | Observational | - | Unknown | NCT03106246 |
| Tyrosine Kinase Inhibitor (TKI) + Anti-PD-1 Antibody in TKI-responded Microsatellite Stability/Proficient Mismatch Repair (MSS/pMMR) Metastatic Colorectal Adenocarcinoma. | MSS pMMR Metastatic colorectal adenocarcinoma | Interventional | Phase 2 | Recruiting | NCT04483219 |
| Expanded Access to Zofin™ (Organicell™ Flow) for Patients With COVID-19 | COVID19 SARS Infection | Expanded accesstreatment of IND/Protocol | - | Available | NCT04657406 |
| Zofin (Organicell Flow) for Patients With COVID-19 | COVID19 SARS Infection | Interventional | Phase 1 Phase 2 | Recruiting | NCT04384445 |
| Pilot Study of Human Adipose Tissue Derived Exosomes Promoting Wound Healing | Wounds and injuries | Interventional | Not Applicable | Completed | NCT05475418 |
| Evaluation of Personalized Nutritional Intervention on Wound Healing of Cutaneous Ulcers in Diabetics | Diabetic foot wound | Interventional | Not Applicable | Recruiting | NCT05243368 |
| The Role of Mesenchymal Stem Cell and Exosome in Treating Pilonidal Sinus Disease in Children | Pilonidal sinus disease | Interventional | Not Applicable | Recruiting | NCT06391307 |
| Effect of Plasma Derived Exosomes on Cutaneous Wound Healing | Ulcer | Interventional | Early Phase 1 | Unknown | NCT02565264 |
| Clinical Efficacy of Exosome in Degenerative Meniscal Injury | Knee and Meniscus Injury | Interventional | Phase 2 | Recruiting | NCT05261360 |
| Phase 2a Multi-Center Prospective, Randomized Trial to Evaluate the Safety & Efficacy of Topical PEP-TISSEEL for Diabetic Foot Ulcers (DFU) | Diabetic foot ulcer | Interventional | Phase 2 | Recruiting | NCT06319287 |
| Omics Sequencing of Exosomes in Body Fluids of Patients With Acute Lung Injury | Acute lung injury | Observational | Not Available | Recruiting | NCT05058768 |
| The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19 | COVID19 caused pneumonia | Interventional | Phase 1 Phase 2 | Unknown | NCT04798716 |
| PEP on a Skin Graft Donor Site Wound | Skin graft | Interventional | Phase 1 | Active, not recruiting | NCT04664738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghav, P.K.; Mann, Z. Nano-Delivery Revolution: Harnessing Mesenchymal Stem Cell-Derived Exosomes’ Potential for Wound Healing. Biomedicines 2024, 12, 2791. https://doi.org/10.3390/biomedicines12122791
Raghav PK, Mann Z. Nano-Delivery Revolution: Harnessing Mesenchymal Stem Cell-Derived Exosomes’ Potential for Wound Healing. Biomedicines. 2024; 12(12):2791. https://doi.org/10.3390/biomedicines12122791
Chicago/Turabian StyleRaghav, Pawan Kumar, and Zoya Mann. 2024. "Nano-Delivery Revolution: Harnessing Mesenchymal Stem Cell-Derived Exosomes’ Potential for Wound Healing" Biomedicines 12, no. 12: 2791. https://doi.org/10.3390/biomedicines12122791
APA StyleRaghav, P. K., & Mann, Z. (2024). Nano-Delivery Revolution: Harnessing Mesenchymal Stem Cell-Derived Exosomes’ Potential for Wound Healing. Biomedicines, 12(12), 2791. https://doi.org/10.3390/biomedicines12122791






