Critical Analysis of Cytoplasmic Progression of Inflammatory Signaling Suggests Potential Pharmacologic Targets for Wound Healing and Fibrotic Disorders
Abstract
1. Introduction
2. Aberrant Wound Healing
2.1. Wound Healing Gone Wrong
2.2. Systemic Sclerosis—Scleroderma
2.3. Care Cost Impacts of Deficient Wound Healing and Fibrotic Disorders
2.4. Consideration of Novel Therapeutics
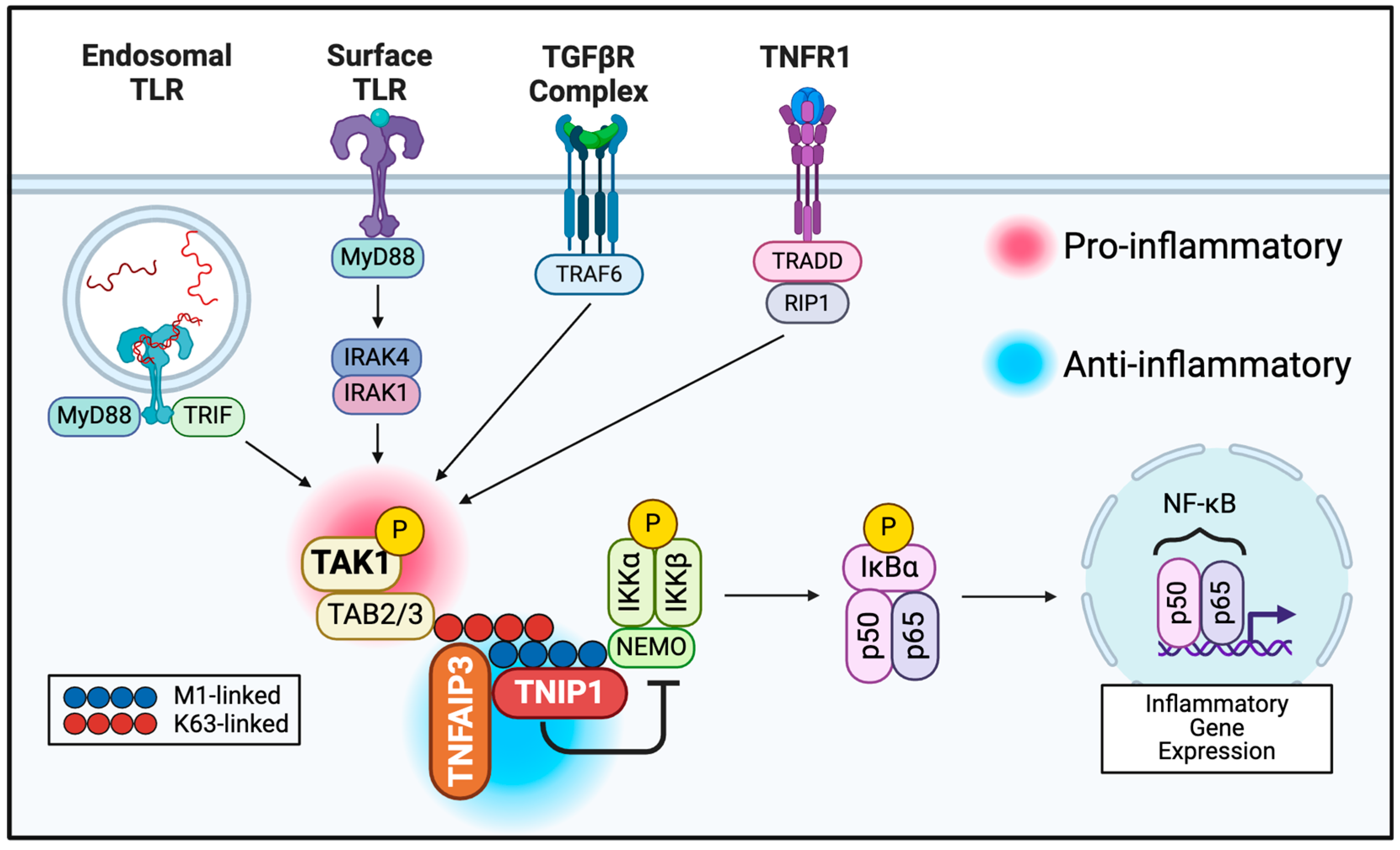
2.5. Cytoplasmic Regulation of Post-Receptor Signaling May Provide New Opportunities
3. Association of NF-κB Signal Repressing Proteins with SSc
3.1. TAK1
3.2. TNFAIP3
3.3. TNIP1
4. Concluding Observations
5. Prospectus
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stroncek, J.D.; Reichert, W.M. Frontiers in Neuroengineering. Overview of Wound Healing in Different Tissue Types. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- McCoy, S.S.; Reed, T.J.; Berthier, C.C.; Tsou, P.S.; Liu, J.; Gudjonsson, J.E.; Khanna, D.; Kahlenberg, J.M. Scleroderma keratinocytes promote fibroblast activation independent of transforming growth factor beta. Rheumatology 2017, 56, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Chen, V.; Burgess, J.; Pannu, S.; Tomic-Canic, M. Genomics of Human Fibrotic Diseases: Disordered Wound Healing Response. Int. J. Mol. Sci. 2020, 21, 8590. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair. Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Rai, V. Novel Factors Regulating Proliferation, Migration, and Differentiation of Fibroblasts, Keratinocytes, and Vascular Smooth Muscle Cells during Wound Healing. Biomedicines 2024, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Golin, A.P.; Jalili, R.B.; Ghahary, A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp. Dermatol. 2022, 31, 475–484. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 262–283. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Barmore, W.; Bajwa, T.; Burns, B. Biochemistry, Clotting Factors. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chaudhry, R.; Usama, S.M.; Babiker, H.M. Physiology, Coagulation Pathways. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Carman, L.E.; Samulevich, M.L.; Aneskievich, B.J. Repressive Control of Keratinocyte Cytoplasmic Inflammatory Signaling. Int. J. Mol. Sci. 2023, 24, 1943. [Google Scholar] [CrossRef]
- Jacinto, A.; Martinez-Arias, A.; Martin, P. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 2001, 3, E117–E123. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wen, D.; Xu, X.; Zhao, R.; Jiang, F.; Yuan, S.; Zhang, Y.; Gao, Y.; Li, Q. Extracellular matrix stiffness-The central cue for skin fibrosis. Front. Mol. Biosci. 2023, 10, 1132353. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.G.; Deinsberger, J.; Oszwald, A.; Weber, B. The Histopathology of Leg Ulcers. Dermatopathology 2024, 11, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Lim, J.Z.; Ng, N.S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Al-Rikabi, A.H.A.; Tobin, D.J.; Riches-Suman, K.; Thornton, M.J. Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses. Sci. Rep. 2021, 11, 1474. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. The role of CXCL8 in chronic nonhealing diabetic foot ulcers and phenotypic changes in fibroblasts: A molecular perspective. Mol. Biol. Rep. 2022, 49, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- desJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From Chronic Wounds to Scarring: The Growing Health Care Burden of Under- and Over-Healing Wounds. Adv. Wound Care 2022, 11, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.E.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Tredget, E.E. The molecular basis of hypertrophic scars. Burns Trauma 2016, 4, 2. [Google Scholar] [CrossRef]
- Mony, M.P.; Harmon, K.A.; Hess, R.; Dorafshar, A.H.; Shafikhani, S.H. An Updated Review of Hypertrophic Scarring. Cells 2023, 12, 678. [Google Scholar] [CrossRef]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.G.; Jiang, D.; et al. Fibroblasts-the cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef]
- Chiavegato, A.; Bochaton-Piallat, M.L.; D’Amore, E.; Sartore, S.; Gabbiani, G. Expression of myosin heavy chain isoforms in mammary epithelial cells and in myofibroblasts from different fibrotic settings during neoplasia. Virchows Arch. 1995, 426, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Schuster, R.; Younesi, F.; Ezzo, M.; Hinz, B. The Role of Myofibroblasts in Physiological and Pathological Tissue Repair. Cold Spring Harb. Perspect. Biol. 2023, 15, a041231. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Rudraiah, S.; Shamilov, R.; Aneskievich, B.J. TNIP1 reduction sensitizes keratinocytes to post-receptor signalling following exposure to TLR agonists. Cell. Signal. 2018, 45, 81–92. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Simmons, J.; Gallo, R.L. The Central Roles of Keratinocytes in Coordinating Skin Immunity. J. Investig. Dermatol. 2024, 144, 2377–2398. [Google Scholar] [CrossRef]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef]
- Adigun, R.; Goyal, A.; Hariz, A. Systemic Sclerosis (Scleroderma). In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef]
- Bale, S.; Verma, P.; Varga, J.; Bhattacharyya, S. Extracellular Matrix-Derived Damage-Associated Molecular Patterns (DAMP): Implications in Systemic Sclerosis and Fibrosis. J. Investig. Dermatol. 2023, 143, 1877–1885. [Google Scholar] [CrossRef]
- Tsou, P.S.; Varga, J.; O’Reilly, S. Advances in epigenetics in systemic sclerosis: Molecular mechanisms and therapeutic potential. Nat. Rev. Rheumatol. 2021, 17, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Denton, C.P.; Johnson, S.R.; Fernandez-Codina, A.; Hudson, M.; Nevskaya, T. State-of-the-art evidence in the treatment of systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 212–226. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Varga, J. Endogenous ligands of TLR4 promote unresolving tissue fibrosis: Implications for systemic sclerosis and its targeted therapy. Immunol. Lett. 2018, 195, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Moezinia, C.; Wong, V.; Watson, J.; Nagib, L.; Lopez Garces, S.; Zhang, S.; Ahmed Abdi, B.; Newton, F.; Abraham, D.; Stratton, R. Autoantibodies Which Bind to and Activate Keratinocytes in Systemic Sclerosis. Cells 2023, 12, 2490. [Google Scholar] [CrossRef]
- Zeng, C.; Kahlenberg, J.M.; Gudjonsson, J.E. IL-17A Softens the Skin: Antifibrotic Properties of IL-17A in Systemic Sclerosis. J. Investig. Dermatol. 2020, 140, 13–14. [Google Scholar] [CrossRef]
- Campochiaro, C.; Allanore, Y. An update on targeted therapies in systemic sclerosis based on a systematic review from the last 3 years. Arthritis Res. Ther. 2021, 23, 155. [Google Scholar] [CrossRef]
- Roofeh, D.; Lescoat, A.; Khanna, D. Emerging drugs for the treatment of scleroderma: A review of recent phase 2 and 3 trials. Expert. Opin. Emerg. Drugs 2020, 25, 455–466. [Google Scholar] [CrossRef]
- Connolly, M.K. Systemic sclerosis (scleroderma): Remaining challenges. Ann. Transl. Med. 2021, 9, 438. [Google Scholar] [CrossRef]
- Bukiri, H.; Volkmann, E.R. Current advances in the treatment of systemic sclerosis. Curr. Opin. Pharmacol. 2022, 64, 102211. [Google Scholar] [CrossRef]
- Ebata, S.; Yoshizaki-Ogawa, A.; Sato, S.; Yoshizaki, A. New Era in Systemic Sclerosis Treatment: Recently Approved Therapeutics. J. Clin. Med. 2022, 11, 4631. [Google Scholar] [CrossRef]
- Papadimitriou, T.I.; van Caam, A.; van der Kraan, P.M.; Thurlings, R.M. Therapeutic Options for Systemic Sclerosis: Current and Future Perspectives in Tackling Immune-Mediated Fibrosis. Biomedicines 2022, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Xue, E.; Minniti, A.; Alexander, T.; Del Papa, N.; Greco, R.; On Behalf of the Autoimmune Diseases Working Party (ADWP) of The European Society for Blood and Marrow Transplantation (EMBT). Cellular-Based Therapies in Systemic Sclerosis: From Hematopoietic Stem Cell Transplant to Innovative Approaches. Cells 2022, 11, 3346. [Google Scholar] [CrossRef]
- Farrell, J.; Ho, L. Management of Patients with Systemic Sclerosis-Associated Interstitial Lung Disease: A Focus on the Role of the Pharmacist. Integr. Pharm. Res. Pract. 2023, 12, 101–112. [Google Scholar] [CrossRef]
- Assassi, S.; Tumuluri, S.; Levin, R.W. Interstitial lung disease in patients with systemic sclerosis: What can we learn from the SENSCIS trial? Clin. Exp. Rheumatol. 2023, 41, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Roofeh, D.; Kuwana, M.; Lafyatis, R.; Allanore, Y.; Khanna, D. Therapeutic Approaches to Systemic Sclerosis: Recent Approvals and Future Candidate Therapies. Clin. Rev. Allergy Immunol. 2023, 64, 239–261. [Google Scholar] [CrossRef]
- Mulcaire-Jones, E.; Low, A.H.L.; Domsic, R.; Whitfield, M.L.; Khanna, D. Advances in biological and targeted therapies for systemic sclerosis. Expert. Opin. Biol. Ther. 2023, 23, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cintina, I.; Pariser, A.; Oehrlein, E.; Sullivan, J.; Kennedy, A. The national economic burden of rare disease in the United States in 2019. Orphanet J. Rare Dis. 2022, 17, 163. [Google Scholar] [CrossRef]
- García-Pérez, L.; Linertová, R.; Valcárcel-Nazco, C.; Posada, M.; Gorostiza, I.; Serrano-Aguilar, P. Cost-of-illness studies in rare diseases: A scoping review. Orphanet J. Rare Dis. 2021, 16, 178. [Google Scholar] [CrossRef]
- Coffey, C.M.; Sandhu, A.S.; Crowson, C.S.; Achenbach, S.J.; Matteson, E.L.; Osborn, T.G.; Warrington, K.J.; Makol, A. Hospitalization Rates Are Highest in the First 5 Years of Systemic Sclerosis: Results from a Population-based Cohort (1980–2016). J. Rheumatol. 2021, 48, 877–882. [Google Scholar] [CrossRef]
- Fan, Y.; Bender, S.; Shi, W.; Zoz, D. Incidence and prevalence of systemic sclerosis and systemic sclerosis with interstitial lung disease in the United States. J. Manag. Care Spec. Pharm. 2020, 26, 1539–1547. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Li, J.W.; Meng, Q.; Yuan, Y.; Lesperance, T.; Peoples, K.; Ali, F.; LaMoreaux, B.; Taylor, S.D. Economic and Health Care Resource Use Burden of Systemic Sclerosis. ACR Open Rheumatol. 2023, 5, 677–684. [Google Scholar] [CrossRef]
- Morrisroe, K.; Sandorfi, N.; Barron, M. Health Care Utilization: What Is the Cost of Caring for Scleroderma? Rheum. Dis. Clin. N. Am. 2023, 49, 359–375. [Google Scholar] [CrossRef]
- Colic, J.; Campochiaro, C.; Hughes, M.; Matucci Cerinic, M.; Dagna, L. Investigational drugs for the treatment of scleroderma: What’s new? Expert. Opin. Investig. Drugs 2023, 32, 601–614. [Google Scholar] [CrossRef]
- Farina, N.; Campochiaro, C.; Lescoat, A.; Benanti, G.; De Luca, G.; Khanna, D.; Dagna, L.; Matucci-Cerinic, M. Drug development and novel therapeutics to ensure a personalized approach in the treatment of systemic sclerosis. Expert. Rev. Clin. Immunol. 2023, 19, 1131–1142. [Google Scholar] [CrossRef]
- Komura, K.; Yanaba, K.; Bouaziz, J.D.; Yoshizaki, A.; Hasegawa, M.; Varga, J.; Takehara, K.; Matsushita, T. Perspective to precision medicine in scleroderma. Front. Immunol. 2023, 14, 1298665. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Kato, H.; Varga, J. Emerging cellular and immunotherapies for systemic sclerosis: From mesenchymal stromal cells to CAR-T cells and vaccine-based approaches. Curr. Opin. Rheumatol. 2023, 35, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Guardino, N.J.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. Modulating Cellular Responses to Mechanical Forces to Promote Wound Regeneration. Adv. Wound Care 2022, 11, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, J.; Chen, Z.; Chen, L.; Lu, H.B.; Zhang, Q.; Yang, X.; Qi, Z. The Role of Verteporfin in Prevention of Periprosthetic Capsular Fibrosis: An Experimental Study. Aesthet. Surg. J. 2022, 42, 820–829. [Google Scholar] [CrossRef]
- Wang, P.; Peng, Z.; Yu, L.; Liu, Y.; Wang, H.; Zhou, Z.; Liu, H.; Hong, S.; Nie, Y.; Deng, Y.; et al. Verteporfin-Loaded Bioadhesive Nanoparticles for the Prevention of Hypertrophic Scar. Small Methods 2024, 8, e2301295. [Google Scholar] [CrossRef]
- Berry, C.E.; Brenac, C.; Gonzalez, C.E.; Kendig, C.B.; Le, T.; An, N.; Griffin, M.F. Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing. Int. J. Mol. Sci. 2024, 25, 3274. [Google Scholar] [CrossRef]
- Cao, X.; Wu, X.; Zhang, Y.; Qian, X.; Sun, W.; Zhao, Y. Emerging biomedical technologies for scarless wound healing. Bioact. Mater. 2024, 42, 449–477. [Google Scholar] [CrossRef]
- Aebisher, D.; Szpara, J.; Bartusik-Aebisher, D. Advances in Medicine: Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 8258. [Google Scholar] [CrossRef]
- Nong, J.; Shen, S.; Hong, F.; Xiao, F.; Meng, L.; Li, P.; Lei, X.; Chen, Y.G. Verteporfin inhibits TGF-β signaling by disrupting the Smad2/3-Smad4 interaction. Mol. Biol. Cell 2024, 35, ar95. [Google Scholar] [CrossRef]
- Chen, K.; Kwon, S.H.; Henn, D.; Kuehlmann, B.A.; Tevlin, R.; Bonham, C.A.; Griffin, M.; Trotsyuk, A.A.; Borrelli, M.R.; Noishiki, C.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 5256. [Google Scholar] [CrossRef]
- Bellamri, N.; Lelong, M.; Joannes, A.; Le Tallec, E.; Jouneau, S.; Vernhet, L.; Lescoat, A.; Lecureur, V. Effects of Ruxolitinib on fibrosis in preclinical models of systemic sclerosis. Int. Immunopharmacol. 2023, 116, 109723. [Google Scholar] [CrossRef]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun. Rev. 2022, 21, 103168. [Google Scholar] [CrossRef]
- Spiera, R.; Kuwana, M.; Khanna, D.; Hummers, L.; Frech, T.M.; Stevens, W.; Matucci-Cerinic, M.; Kafaja, S.; Distler, O.; Jun, J.B.; et al. Efficacy and Safety of Lenabasum, a Cannabinoid Type 2 Receptor Agonist, in a Phase 3 Randomized Trial in Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Molecular Mechanisms for the Inflammation-Resolving Actions of Lenabasum. Mol. Pharmacol. 2021, 99, 125–132. [Google Scholar] [CrossRef]
- Werth, V.P.; Hejazi, E.; Pena, S.M.; Haber, J.; Zeidi, M.; Reddy, N.; Okawa, J.; Feng, R.; Bashir, M.M.; Gebre, K.; et al. Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: A Randomized Clinical Trial. J. Investig. Dermatol. 2022, 142, 2651–2659.e2651. [Google Scholar] [CrossRef]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults with Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef]
- Tian, N.; Cheng, H.; Du, Y.; Wang, X.; Lei, Y.; Liu, X.; Chen, M.; Xu, Z.; Wang, L.; Yin, H.; et al. Cannabinoid receptor 2 selective agonist alleviates systemic sclerosis by inhibiting Th2 differentiation through JAK/SOCS3 signaling. J. Autoimmun. 2024, 147, 103233. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; JHernández-Muñoz, J.; JMiller, M.; Pope, M.; Huyan, Y.; Zhong, L. The economic burden of systemic sclerosis—A systematic review. Int. J. Rheum. Dis. 2022, 25, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Fullard, N.; Moles, A.; O’Reilly, S.; van Laar, J.M.; Faini, D.; Diboll, J.; Reynolds, N.J.; Mann, D.A.; Reichelt, J.; Oakley, F. The c-Rel subunit of NF-κB regulates epidermal homeostasis and promotes skin fibrosis in mice. Am. J. Pathol. 2013, 182, 2109–2120. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Micus, L.C.; Trautschold-Krause, F.S.; Jelit, A.L.; Schön, M.P.; Lorenz, V.N. NF-κB c-Rel modulates pre-fibrotic changes in human fibroblasts. Arch. Dermatol. Res. 2022, 314, 943–951. [Google Scholar] [CrossRef]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- Heissmeyer, V.; Krappmann, D.; Hatada, E.N.; Scheidereit, C. Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol. Cell Biol. 2001, 21, 1024–1035. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Gilmore, T.; Gapuzan, M.E.; Kalaitzidis, D.; Starczynowski, D. Rel/NF-kappa B/I kappa B signal transduction in the generation and treatment of human cancer. Cancer Lett. 2002, 181, 1–9. [Google Scholar] [CrossRef]
- Sun, S.C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef]
- Moorthy, A.K.; Savinova, O.V.; Ho, J.Q.; Wang, V.Y.; Vu, D.; Ghosh, G. The 20S proteasome processes NF-kappaB1 p105 into p50 in a translation-independent manner. EMBO J. 2006, 25, 1945–1956. [Google Scholar] [CrossRef]
- Brignall, R.; Moody, A.T.; Mathew, S.; Gaudet, S. Considering Abundance, Affinity, and Binding Site Availability in the NF-κB Target Selection Puzzle. Front. Immunol. 2019, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Kumase, F.; Takeuchi, K.; Morizane, Y.; Suzuki, J.; Matsumoto, H.; Kataoka, K.; Al-Moujahed, A.; Maidana, D.E.; Miller, J.W.; Vavvas, D.G. AMPK-Activated Protein Kinase Suppresses Ccr2 Expression by Inhibiting the NF-κB Pathway in RAW264.7 Macrophages. PLoS ONE 2016, 11, e0147279. [Google Scholar] [CrossRef] [PubMed]
- Szołtysek, K.; Janus, P.; Zając, G.; Stokowy, T.; Walaszczyk, A.; Widłak, W.; Wojtaś, B.; Gielniewski, B.; Cockell, S.; Perkins, N.D.; et al. RRAD, IL4I1, CDKN1A, and SERPINE1 genes are potentially co-regulated by NF-κB and p53 transcription factors in cells exposed to high doses of ionizing radiation. BMC Genom. 2018, 19, 813. [Google Scholar] [CrossRef] [PubMed]
- Van Ginderachter, J.A. The wound healing chronicles. Blood 2012, 120, 499–500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaffrick, L.; Ding, J.; Kwan, P.; Tredget, E. The dynamic changes of monocytes and cytokines during wound healing post-burn injury. Cytokine 2023, 168, 156231. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Joshi, A.; Schaller, M.; Davis, F.M.; denDekker, A.; Obi, A.T.; Moore, B.B.; Kunkel, S.L.; Gallagher, K.A. Murine macrophage chemokine receptor CCR2 plays a crucial role in macrophage recruitment and regulated inflammation in wound healing. Eur. J. Immunol. 2018, 48, 1445–1455. [Google Scholar] [CrossRef]
- Ågren, M.S.; Litman, T.; Eriksen, J.O.; Schjerling, P.; Bzorek, M.; Gjerdrum, L.M.R. Gene Expression Linked to Reepithelialization of Human Skin Wounds. Int. J. Mol. Sci. 2022, 23, 5746. [Google Scholar] [CrossRef]
- Kida, M.; Fatima, I.; Rozhkova, E.; Otero-Viñas, M.; Wu, M.; Kalin, J.H.; Cole, P.A.; Falanga, V.; Alani, R.M.; Sharov, A.A. Inhibition of the CoREST Repressor Complex Promotes Wound Re-Epithelialization through the Regulation of Keratinocyte Migration. J. Investig. Dermatol. 2024, 144, 378–386.e372. [Google Scholar] [CrossRef]
- Simone, T.M.; Higgins, C.E.; Czekay, R.P.; Law, B.K.; Higgins, S.P.; Archambeault, J.; Kutz, S.M.; Higgins, P.J. SERPINE1: A Molecular Switch in the Proliferation-Migration Dichotomy in Wound-”Activated” Keratinocytes. Adv. Wound Care 2014, 3, 281–290. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Frank, J.; Born, K.; Barker, J.H.; Marzi, I. In Vivo Effect of Tumor Necrosis Factor Alpha on Wound Angiogenesis and Epithelialization. Eur. J. Trauma. 2003, 29, 208–219. [Google Scholar] [CrossRef]
- Cao, Y.; Harvey, B.P.; Jin, L.; Westmoreland, S.; Wang, J.; Puri, M.; Yang, Y.; Robb, H.M.; Tanriverdi, S.; Hu, C.; et al. Therapeutic TNF Inhibitors Exhibit Differential Levels of Efficacy in Accelerating Cutaneous Wound Healing. JID Innov. 2024, 4, 100250. [Google Scholar] [CrossRef] [PubMed]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 control of cell death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; van Loo, G. A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity. Cold Spring Harb. Perspect. Biol. 2020, 12, a036418. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, R.; Aneskievich, B.J. TNIP1 in Autoimmune Diseases: Regulation of Toll-like Receptor Signaling. J. Immunol. Res. 2018, 2018, 3491269. [Google Scholar] [CrossRef]
- Zhou, J.; Rasmussen, N.L.; Olsvik, H.L.; Akimov, V.; Hu, Z.; Evjen, G.; Kaeser-Pebernard, S.; Sankar, D.S.; Roubaty, C.; Verlhac, P.; et al. TBK1 phosphorylation activates LIR-dependent degradation of the inflammation repressor TNIP1. J. Cell Biol. 2023, 222, e202108144. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef]
- Lee, J.; Mira-Arbibe, L.; Ulevitch, R.J. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 2000, 68, 909–915. [Google Scholar] [CrossRef]
- Wang, W.; Gao, W.; Zhu, Q.; Alasbahi, A.; Seki, E.; Yang, L. TAK1: A Molecular Link Between Liver Inflammation, Fibrosis, Steatosis, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 734749. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.R.; Lei, C.Q. TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses. Front. Immunol. 2020, 11, 608976. [Google Scholar] [CrossRef]
- Leask, A.; Naik, A.; Stratton, R.J. Back to the future: Targeting the extracellular matrix to treat systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 713–723. [Google Scholar] [CrossRef]
- Landström, M. The TAK1-TRAF6 signalling pathway. Int. J. Biochem. Cell Biol. 2010, 42, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, L.; Lyu, L.; Qi, W.; Yang, W.; Ren, R.; Kao, C.; Zhang, Y.; Zhang, C.; Zhang, M. Regulation of TAK-TAB Complex Activation through Ubiquitylation. Front. Biosci. 2024, 29, 169. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, A.; Seth, R.B.; Sun, L.; Ea, C.K.; Hong, M.; Shaito, A.; Chiu, Y.H.; Deng, L.; Chen, Z.J. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 2004, 15, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Gram, H.; Di Padova, F.; Huang, B.; New, L.; Ulevitch, R.J.; Luo, Y.; Han, J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 2002, 295, 1291–1294. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Li, J.; Yin, H.; Guo, X.; Doan, J.; Molkentin, J.D.; Liu, Q. TAK1 Regulates Myocardial Response to Pathological Stress via NFAT, NFκB, and Bnip3 Pathways. Sci. Rep. 2015, 5, 16626. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Hutchenreuther, J.; Carter, D.E.; Leask, A. TAK1 is required for dermal wound healing and homeostasis. J. Investig. Dermatol. 2013, 133, 1646–1654. [Google Scholar] [CrossRef]
- Maddali, P.; Ambesi, A.; McKeown-Longo, P.J. Induction of pro-inflammatory genes by fibronectin DAMPs in three fibroblast cell lines: Role of TAK1 and MAP kinases. PLoS ONE 2023, 18, e0286390. [Google Scholar] [CrossRef]
- Bale, S.; Verma, P.; Yalavarthi, B.; Scarneo, S.A.; Hughes, P.; Amin, M.A.; Tsou, P.S.; Khanna, D.; Haystead, T.A.; Bhattacharyya, S.; et al. Pharmacological inhibition of TAK1 prevents and induces regression of experimental organ fibrosis. JCI Insight 2023, 8, e165358. [Google Scholar] [CrossRef]
- Freeze, R.; Yang, K.W.; Haystead, T.; Hughes, P.; Scarneo, S. Delineation of the distinct inflammatory signaling roles of TAK1 and JAK1/3 in the CIA model of rheumatoid arthritis. Pharmacol. Res. Perspect. 2023, 11, e01124. [Google Scholar] [CrossRef]
- Asano, Y.; Varga, J. Rationally-based therapeutic disease modification in systemic sclerosis: Novel strategies. Semin. Cell Dev. Biol. 2020, 101, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Totzke, J.; Scarneo, S.A.; Yang, K.W.; Haystead, T.A.J. TAK1: A potent tumour necrosis factor inhibitor for the treatment of inflammatory diseases. Open Biol. 2020, 10, 200099. [Google Scholar] [CrossRef] [PubMed]
- Scarneo, S.; Hughes, P.; Freeze, R.; Yang, K.; Totzke, J.; Haystead, T. Development and Efficacy of an Orally Bioavailable Selective TAK1 Inhibitor for the Treatment of Inflammatory Arthritis. ACS Chem. Biol. 2022, 17, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, N. EydisBio’s Systemic Sclerosis Treatment Receives FDA Orphan Drug Designation. Available online: https://www.ncbiotech.org/news/eydisbios-systemic-sclerosis-treatment-receives-fda-orphan-drug-designation (accessed on 20 November 2024).
- Cui, H.S.; Joo, S.Y.; Cho, Y.S.; Kim, J.B.; Seo, C.H. CPEB1 or CPEB4 knockdown suppresses the TAK1 and Smad signalings in THP-1 macrophage-like cells and dermal fibroblasts. Arch. Biochem. Biophys. 2020, 683, 108322. [Google Scholar] [CrossRef] [PubMed]
- Woeller, C.F.; O’Loughlin, C.W.; Roztocil, E.; Feldon, S.E.; Phipps, R.P. Salinomycin and other polyether ionophores are a new class of antiscarring agent. J. Biol. Chem. 2015, 290, 3563–3575. [Google Scholar] [CrossRef]
- Fang, Q.Q.; Wang, X.F.; Zhao, W.Y.; Ding, S.L.; Shi, B.H.; Xia, Y.; Yang, H.; Wu, L.H.; Li, C.Y.; Tan, W.Q. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-β1 pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef]
- Cui, H.S.; Lee, Y.R.; Ro, Y.M.; Joo, S.Y.; Cho, Y.S.; Kim, J.B.; Kim, D.H.; Seo, C.H. Knockdown of CPEB1 and CPEB4 Inhibits Scar Formation via Modulation of TAK1 and SMAD Signaling. Ann. Dermatol. 2023, 35, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Kim, D.H.; Joo, S.Y.; Cho, Y.S.; Kim, J.B.; Seo, C.H. Exosomes derived from human hypertrophic scar fibroblasts induces smad and TAK1 signaling in normal dermal fibroblasts. Arch. Biochem. Biophys. 2022, 722, 109215. [Google Scholar] [CrossRef]
- Dixit, V.M.; Green, S.; Sarma, V.; Holzman, L.B.; Wolf, F.W.; O’Rourke, K.; Ward, P.A.; Prochownik, E.V.; Marks, R.M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990, 265, 2973–2978. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. A20 restricts inflammation via ubiquitin binding. Nat. Immunol. 2020, 21, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; De Valck, D.; Vanden Berghe, W.; Van Criekinge, W.; Contreras, R.; Fiers, W.; Haegeman, G.; Beyaert, R. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J. Cell Biol. 1999, 145, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Polykratis, A.; Martens, A.; Eren, R.O.; Shirasaki, Y.; Yamagishi, M.; Yamaguchi, Y.; Uemura, S.; Miura, M.; Holzmann, B.; Kollias, G.; et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 2019, 21, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.K.; Venigalla, R.K.; Ordureau, A.; Patterson-Kane, J.C.; Powell, D.W.; Toth, R.; Arthur, J.S.; Cohen, P. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J. Exp. Med. 2011, 208, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Carpentier, I.; Rogov, V.; Kreike, M.; Ikeda, F.; Löhr, F.; Wu, C.J.; Ashwell, J.D.; Dötsch, V.; Dikic, I.; et al. Ubiquitin binding mediates the NF-kappaB inhibitory potential of ABIN proteins. Oncogene 2008, 27, 3739–3745. [Google Scholar] [CrossRef] [PubMed]
- Boudra, R.; Ramsey, M.R. Understanding Transcriptional Networks Regulating Initiation of Cutaneous Wound Healing. Yale J. Biol. Med. 2020, 93, 161–173. [Google Scholar]
- Dong, J.; Ma, Q. In Vivo Activation and Pro-Fibrotic Function of NF-κB in Fibroblastic Cells During Pulmonary Inflammation and Fibrosis Induced by Carbon Nanotubes. Front. Pharmacol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar] [CrossRef]
- Wang, W.; Bale, S.; Wei, J.; Yalavarthi, B.; Bhattacharyya, D.; Yan, J.J.; Abdala-Valencia, H.; Xu, D.; Sun, H.; Marangoni, R.G.; et al. Fibroblast A20 governs fibrosis susceptibility and its repression by DREAM promotes fibrosis in multiple organs. Nat. Commun. 2022, 13, 6358. [Google Scholar] [CrossRef] [PubMed]
- Dieudé, P.; Guedj, M.; Wipff, J.; Ruiz, B.; Riemekasten, G.; Matucci-Cerinic, M.; Melchers, I.; Hachulla, E.; Airo, P.; Diot, E.; et al. Association of the TNFAIP3 rs5029939 variant with systemic sclerosis in the European Caucasian population. Ann. Rheum. Dis. 2010, 69, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Assassi, S.; Diaz-Gallo, L.M.; Broen, J.C.; Simeon, C.P.; Castellvi, I.; Vicente-Rabaneda, E.; Fonollosa, V.; Ortego-Centeno, N.; González-Gay, M.A.; et al. A systemic sclerosis and systemic lupus erythematosus pan-meta-GWAS reveals new shared susceptibility loci. Hum. Mol. Genet. 2013, 22, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Broen, J.C.; Carmona, F.D.; Teruel, M.; Simeon, C.P.; Vonk, M.C.; van ‘t Slot, R.; Rodriguez-Rodriguez, L.; Vicente, E.; Fonollosa, V.; et al. Identification of CSK as a systemic sclerosis genetic risk factor through Genome Wide Association Study follow-up. Hum. Mol. Genet. 2012, 21, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Terao, C.; Ohmura, K.; Kawaguchi, Y.; Nishimoto, T.; Kawasaki, A.; Takehara, K.; Furukawa, H.; Kochi, Y.; Ota, Y.; Ikari, K.; et al. PLD4 as a novel susceptibility gene for systemic sclerosis in a Japanese population. Arthritis Rheum. 2013, 65, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.Y.; Li, Y.; Gorlov, I.; Ying, J.; Chen, W.V.; Assassi, S.; Reveille, J.D.; Arnett, F.C.; Zhou, X.; Bossini-Castillo, L.; et al. Gene-level association analysis of systemic sclerosis: A comparison of African-Americans and White populations. PLoS ONE 2018, 13, e0189498. [Google Scholar] [CrossRef]
- Ota, Y.; Kuwana, M. Updates on genetics in systemic sclerosis. Inflamm. Regen. 2021, 41, 17. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Y.; Guo, X.; Hei, N.; Lai, S.; Assassi, S.; Liu, M.; Tan, F.; Zhou, X. Identification of an Association of TNFAIP3 Polymorphisms with Matrix Metalloproteinase Expression in Fibroblasts in an Integrative Study of Systemic Sclerosis-Associated Genetic and Environmental Factors. Arthritis Rheumatol. 2016, 68, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Malcomson, B.; Wilson, H.; Veglia, E.; Thillaiyampalam, G.; Barsden, R.; Donegan, S.; El Banna, A.; Elborn, J.S.; Ennis, M.; Kelly, C.; et al. Connectivity mapping (ssCMap) to predict A20-inducing drugs and their antiinflammatory action in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2016, 113, E3725–E3734. [Google Scholar] [CrossRef]
- Reihill, J.A.; Malcomson, B.; Bertelsen, A.; Cheung, S.; Czerwiec, A.; Barsden, R.; Elborn, J.S.; Dürkop, H.; Hirsch, B.; Ennis, M.; et al. Induction of the inflammatory regulator A20 by gibberellic acid in airway epithelial cells. Br. J. Pharmacol. 2016, 173, 778–789. [Google Scholar] [CrossRef]
- Han, M.T.; Pei, H.; Sun, Q.Q.; Wang, C.L.; Li, P.; Xie, Y.Y.; Cao, L.J.; Zhang, X.X.; Sun, Z.L. ZY-444 inhibits the growth and metastasis of prostate cancer by targeting TNFAIP3 through TNF signaling pathway. Am. J. Cancer Res. 2023, 13, 1533–1546. [Google Scholar]
- Fang, F.; Liu, L.; Yang, Y.; Tamaki, Z.; Wei, J.; Marangoni, R.G.; Bhattacharyya, S.; Summer, R.S.; Ye, B.; Varga, J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: Novel target for fibrosis therapy. Arthritis Res. Ther. 2012, 14, R229. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Fang, F.; Lam, A.P.; Sargent, J.L.; Hamburg, E.; Hinchcliff, M.E.; Gottardi, C.J.; Atit, R.; Whitfield, M.L.; Varga, J. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012, 64, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.; Mennillo, E.; Bain, J.L.; Li, Z.; Sun, X.; Ly, K.M.; Rosli, Y.Y.; Naser, M.; Wang, Z.; Advincula, R.; et al. Microbial signals, MyD88, and lymphotoxin drive TNF-independent intestinal epithelial tissue damage. J. Clin. Investig. 2022, 132, e154993. [Google Scholar] [CrossRef] [PubMed]
- Harirchian, P.; Lee, J.; Hilz, S.; Sedgewick, A.J.; Perez White, B.E.; Kesling, M.J.; Mully, T.; Golovato, J.; Gray, M.; Mauro, T.M.; et al. A20 and ABIN1 Suppression of a Keratinocyte Inflammatory Program with a Shared Single-Cell Expression Signature in Diverse Human Rashes. J. Investig. Dermatol. 2019, 139, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Kattah, M.G.; Shao, L.; Rosli, Y.Y.; Shimizu, H.; Whang, M.I.; Advincula, R.; Achacoso, P.; Shah, S.; Duong, B.H.; Onizawa, M.; et al. A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival. J. Exp. Med. 2018, 215, 1839–1852. [Google Scholar] [CrossRef]
- Shamilov, R.; Ackley, T.W.; Aneskievich, B.J. Enhanced Wound Healing- and Inflammasome-Associated Gene Expression in TNFAIP3-Interacting Protein 1- (TNIP1-) Deficient HaCaT Keratinocytes Parallels Reduced Reepithelialization. Mediat. Inflamm. 2020, 2020, 5919150. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, S.A.; Su, Z.; Jean Barrett, V.; Najafov, A.; Mookhtiar, A.K.; Amin, P.; Pan, H.; Sun, L.; Zhu, H.; Ma, A.; et al. ABIN-1 regulates RIPK1 activation by linking Met1 ubiquitylation with Lys63 deubiquitylation in TNF-RSC. Nat. Cell Biol. 2018, 20, 58–68. [Google Scholar] [CrossRef]
- Kuriakose, J.; Redecke, V.; Guy, C.; Zhou, J.; Wu, R.; Ippagunta, S.K.; Tillman, H.; Walker, P.D.; Vogel, P.; Häcker, H. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J. Clin. Investig. 2019, 129, 2251–2265. [Google Scholar] [CrossRef]
- G’Sell, R.T.; Gaffney, P.M.; Powell, D.W. A20-Binding Inhibitor of NF-κB Activation 1 is a Physiologic Inhibitor of NF-κB: A Molecular Switch for Inflammation and Autoimmunity. Arthritis Rheumatol. 2015, 67, 2292–2302. [Google Scholar] [CrossRef]
- Oshima, S.; Turer, E.E.; Callahan, J.A.; Chai, S.; Advincula, R.; Barrera, J.; Shifrin, N.; Lee, B.; Benedict Yen, T.S.; Woo, T.; et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature 2009, 457, 906–909. [Google Scholar] [CrossRef]
- Verstrepen, L.; Carpentier, I.; Verhelst, K.; Beyaert, R. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem. Pharmacol. 2009, 78, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Korte, E.A.; Caster, D.J.; Barati, M.T.; Tan, M.; Zheng, S.; Berthier, C.C.; Brosius, F.C., 3rd; Vieyra, M.B.; Sheehan, R.M.; Kosiewicz, M.; et al. ABIN1 Determines Severity of Glomerulonephritis via Activation of Intrinsic Glomerular Inflammation. Am. J. Pathol. 2017, 187, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Xu, C.; Zhao, Q.; Liu, J.; Xing, M.; Li, X.; Zhang, H.; Wu, X.; Wang, L.; et al. Ubiquitin-binding domain in ABIN1 is critical for regulating cell death and inflammation during development. Cell Death Differ. 2022, 29, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Saad, M.; Dieudé, P.; Avouac, J.; Distler, J.H.; Amouyel, P.; Matucci-Cerinic, M.; Riemekasten, G.; Airo, P.; Melchers, I.; et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 2011, 7, e1002091. [Google Scholar] [CrossRef]
- Orvain, C.; Assassi, S.; Avouac, J.; Allanore, Y. Systemic sclerosis pathogenesis: Contribution of recent advances in genetics. Curr. Opin. Rheumatol. 2020, 32, 505–514. [Google Scholar] [CrossRef]
- López-Isac, E.; Acosta-Herrera, M.; Kerick, M.; Assassi, S.; Satpathy, A.T.; Granja, J.; Mumbach, M.R.; Beretta, L.; Simeón, C.P.; Carreira, P.; et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat. Commun. 2019, 10, 4955. [Google Scholar] [CrossRef]
- Bossini-Castillo, L.; Martin, J.E.; Broen, J.; Simeon, C.P.; Beretta, L.; Gorlova, O.Y.; Vonk, M.C.; Ortego-Centeno, N.; Espinosa, G.; Carreira, P.; et al. Confirmation of TNIP1 but not RHOB and PSORS1C1 as systemic sclerosis risk factors in a large independent replication study. Ann. Rheum. Dis. 2013, 72, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ortiz-Fernández, L.; Andrés-León, E.; Ciudad, L.; Javierre, B.M.; López-Isac, E.; Guillén-Del-Castillo, A.; Simeón-Aznar, C.P.; Ballestar, E.; Martin, J. Epigenomics and transcriptomics of systemic sclerosis CD4+ T cells reveal long-range dysregulation of key inflammatory pathways mediated by disease-associated susceptibility loci. Genome Med. 2020, 12, 81. [Google Scholar] [CrossRef]
- Ortíz-Fernández, L.; Martín, J.; Alarcón-Riquelme, M.E. A Summary on the Genetics of Systemic Lupus Erythematosus, Rheumatoid Arthritis, Systemic Sclerosis, and Sjögren’s Syndrome. Clin. Rev. Allergy Immunol. 2023, 64, 392–411. [Google Scholar] [CrossRef]
- Li, X.; Ampleford, E.J.; Howard, T.D.; Moore, W.C.; Torgerson, D.G.; Li, H.; Busse, W.W.; Castro, M.; Erzurum, S.C.; Israel, E.; et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J. Allergy Clin. Immunol. 2012, 130, 861–868.e867. [Google Scholar] [CrossRef]
- Milano, A.; Pendergrass, S.A.; Sargent, J.L.; George, L.K.; McCalmont, T.H.; Connolly, M.K.; Whitfield, M.L. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 2008, 3, e2696. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Alipanahi, B.; Lee, L.J.; Bretschneider, H.; Merico, D.; Yuen, R.K.; Hua, Y.; Gueroussov, S.; Najafabadi, H.S.; Hughes, T.R.; et al. RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science 2015, 347, 1254806. [Google Scholar] [CrossRef]
- Cruz, J.A.; Childs, E.E.; Amatya, N.; Garg, A.V.; Beyaert, R.; Kane, L.P.; Aneskievich, B.J.; Ma, A.; Gaffen, S.L. Interleukin-17 signaling triggers degradation of the constitutive NF-κB inhibitor ABIN-1. Immunohorizons 2017, 1, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, Y.; Imami, K.; Fuseya, Y.; Sasaki, K.; Ohmura, K.; Ishihama, Y.; Morinobu, A.; Iwai, K. ABIN1 is a signal-induced autophagy receptor that attenuates NF-κB activation by recognizing linear ubiquitin chains. FEBS Lett. 2022, 596, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, D.; Zhao, F. Updates on Recent Clinical Assessment of Commercial Chronic Wound Care Products. Adv. Healthc. Mater. 2023, 12, e2300556. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shiraishi, K.; Muto, J.; Mori, H.; Murakami, M.; Sayama, K. Nuclear IL-33 Plays an Important Role in EGFR-Mediated Keratinocyte Migration by Regulating the Activation of Signal Transducer and Activator of Transcription 3 and NF-κB. JID Innov. 2023, 3, 100205. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Li, G.; Xu, C.; Yang, L.; Wu, Y.; Liu, Y.; Liu, Z.; Wang, M.; Li, J.; et al. N-acetyltransferase 10 promotes cutaneous wound repair via the NF-κB-IL-6 axis. Cell Death Discov. 2023, 9, 324. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Huang, Q.; Yang, H.; Zheng, J.; Xie, R.; Han, D.; Wei, Q. RIG-I contributes to keratinocyte proliferation and wound repair by inducing TIMP-1 expression through NF-κB signaling pathway. J. Cell Physiol. 2023, 238, 1876–1890. [Google Scholar] [CrossRef]
- Dolivo, D.M.; Sun, L.S.; Rodrigues, A.E.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. Epidermal Potentiation of Dermal Fibrosis: Lessons from Occlusion and Mucosal Healing. Am. J. Pathol. 2023, 193, 510–519. [Google Scholar] [CrossRef]
- Aden, N.; Shiwen, X.; Aden, D.; Black, C.; Nuttall, A.; Denton, C.P.; Leask, A.; Abraham, D.; Stratton, R. Proteomic analysis of scleroderma lesional skin reveals activated wound healing phenotype of epidermal cell layer. Rheumatology 2008, 47, 1754–1760. [Google Scholar] [CrossRef]
- Sarate, R.M.; Hochstetter, J.; Valet, M.; Hallou, A.; Song, Y.; Bansaccal, N.; Ligare, M.; Aragona, M.; Engelman, D.; Bauduin, A.; et al. Dynamic regulation of tissue fluidity controls skin repair during wound healing. Cell 2024, 187, 5298–5315.e5219. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, X.; Zheng, S.; Lin, K.; Su, J. Aligned electrospun poly(L-lactide) nanofibers facilitate wound healing by inhibiting macrophage M1 polarization via the JAK-STAT and NF-κB pathways. J. Nanobiotechnol. 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, E.; Sharifiaghdam, M.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 2022, 29, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Tang, H.; Xu, X.; Shao, J.; Wu, G.; Cho, Y.C.; Ping, Y.; Liang, G. Inflammation conditional genome editing mediated by the CRISPR-Cas9 system. iScience 2023, 26, 106872. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, M.; Liu, X.; Xiao, S.; Cai, Y.; Li, F.; Tian, J.; Qi, F.; Xu, G.; Deng, C. The progress, prospects, and challenges of the use of non-coding RNA for diabetic wounds. Mol. Ther. Nucleic Acids 2021, 24, 554–578. [Google Scholar] [CrossRef]
- Dikic, I.; Schulman, B.A. An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol. 2023, 24, 273–287. [Google Scholar] [CrossRef]
- Akizuki, Y.; Kaypee, S.; Ohtake, F.; Ikeda, F. The emerging roles of non-canonical ubiquitination in proteostasis and beyond. J. Cell Biol. 2024, 223, e202311171. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Song, J.; An, Q.; Chen, J.; Zheng, W.; Zhang, L.; Gu, J.; Deng, C.; Yang, B. Inhibition of Ubiquitin C-Terminal Hydrolase L1 Facilitates Cutaneous Wound Healing via Activating TGF-β/Smad Signalling Pathway in Fibroblasts. Exp. Dermatol. 2024, 33, e15186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samulevich, M.L.; Carman, L.E.; Aneskievich, B.J. Critical Analysis of Cytoplasmic Progression of Inflammatory Signaling Suggests Potential Pharmacologic Targets for Wound Healing and Fibrotic Disorders. Biomedicines 2024, 12, 2723. https://doi.org/10.3390/biomedicines12122723
Samulevich ML, Carman LE, Aneskievich BJ. Critical Analysis of Cytoplasmic Progression of Inflammatory Signaling Suggests Potential Pharmacologic Targets for Wound Healing and Fibrotic Disorders. Biomedicines. 2024; 12(12):2723. https://doi.org/10.3390/biomedicines12122723
Chicago/Turabian StyleSamulevich, Michael L., Liam E. Carman, and Brian J. Aneskievich. 2024. "Critical Analysis of Cytoplasmic Progression of Inflammatory Signaling Suggests Potential Pharmacologic Targets for Wound Healing and Fibrotic Disorders" Biomedicines 12, no. 12: 2723. https://doi.org/10.3390/biomedicines12122723
APA StyleSamulevich, M. L., Carman, L. E., & Aneskievich, B. J. (2024). Critical Analysis of Cytoplasmic Progression of Inflammatory Signaling Suggests Potential Pharmacologic Targets for Wound Healing and Fibrotic Disorders. Biomedicines, 12(12), 2723. https://doi.org/10.3390/biomedicines12122723





