Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease
Abstract
1. Introduction
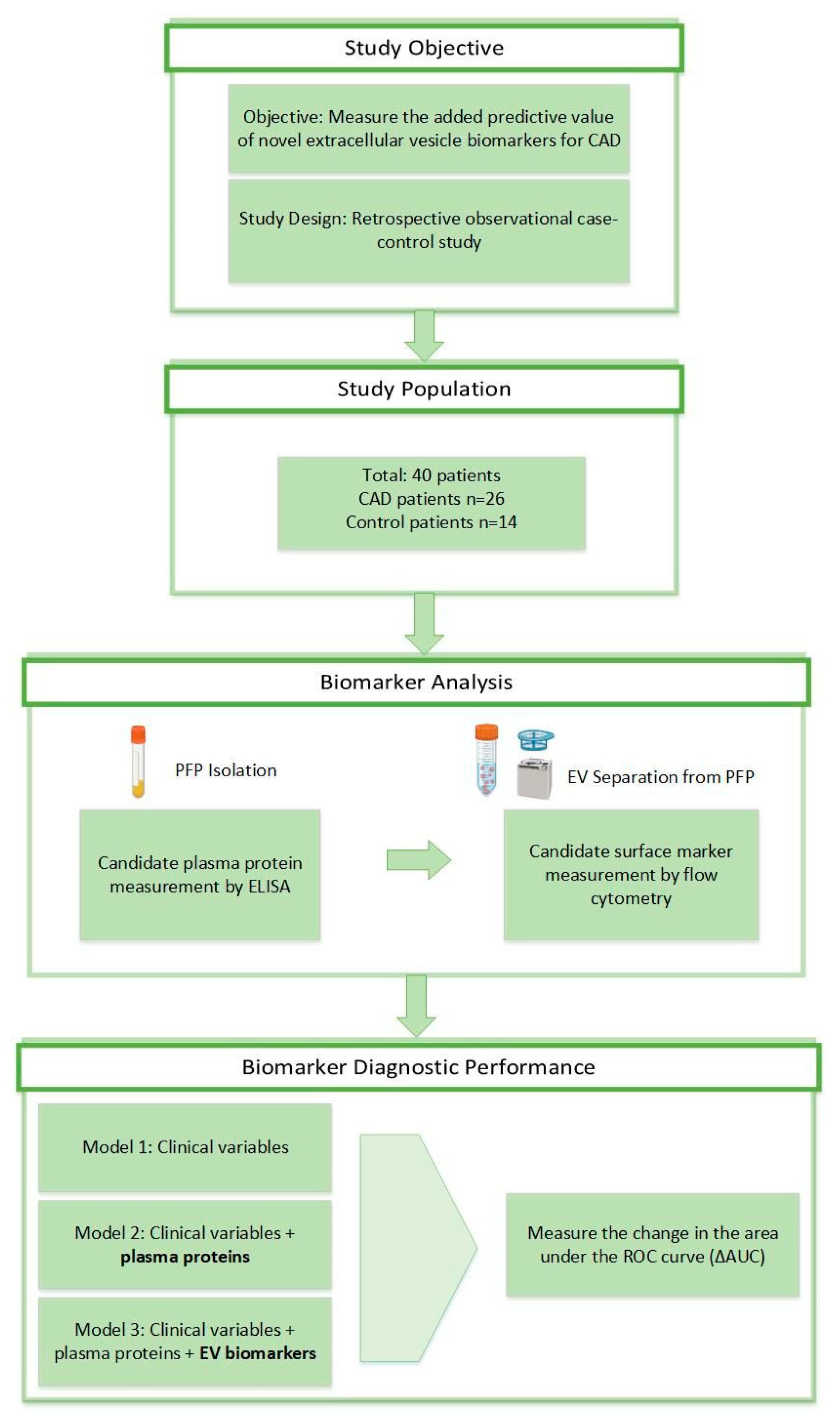
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Flow Cytometry
2.3. Identification of the Cellular Origin of EVs Using Flow Cytometry
2.4. Flow Cytometry Multiplexed Bead-Based Immunoassays
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Circulating Plasma Proteins
3.3. Circulating EV Pattern in Human Plasma Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Martinelli, N.; Peyvandi, F.; Olivieri, O. Genetic architecture of coronary artery disease in the genome-wide era: Implications for the emerging “golden dozen” loci. Semin. Thromb. Hemost. 2009, 35, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Granados, C.; Campisi, E.; Barchitta, M.; Agodi, A. Genetic, lifestyle and metabolic factors contributing to cardiovascular disease in the Italian population: A literature review. Front. Nutr. 2024, 11, 1379785. [Google Scholar] [CrossRef] [PubMed]
- Yucel, C. Cardiac biomarkers: Definition, detection, diagnostic use, and efficiency. In The Detection of Biomarkers; Ozkan, S.A., Bakirhan, N.K., Mollarasouli, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 113–130. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Somiya, M. Where does the cargo go? Solutions to provide experimental support for the extracellular vesicle cargo transfer hypothesis. J. Cell Commun. Signal. 2020, 14, 135–146. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, 968–977. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Lee, J.; Kim, S.R.; Choi, D.-S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.H.; Akbas, F.; Aral, H. Relationship between circulating microparticles and hypertension and other cardiac disease biomarkers in the elderly. BMC Cardiovasc. Disord. 2019, 19, 164. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular vesicles in cardiovascular diseases: Alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular vesicles in cardiovascular disease; potential applications in diagnosis, prognosis, and epidemiology. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Németh, A.; Sódar, B.W.; Vukman, K.V.; Buzás, E.I. Extracellular vesicles in cardiovascular disease: Are they Jedi or Sith? J. Physiol. 2016, 594, 2881–2894. [Google Scholar] [CrossRef]
- Verwer, M.C.; Mekke, J.; Timmerman, N.; Waissi, F.; Boltjes, A.; Pasterkamp, G.; de Borst, G.J.; de Kleijn, D.P.V. Comparison of cardiovascular biomarker expression in extracellular vesicles, plasma and carotid plaque for the prediction of MACE in CEA patients. Sci. Rep. 2023, 13, 1010. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Zhou, J.; Su, C. Extracellular vesicles for drug delivery in cancer treatment. Biol. Proced. Online 2023, 25, 28. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New strategies of neurodegenerative disease treatment with extracellular vesicles derived from mesenchymal stem cells. Theranostics 2023, 13, 4138–4165. [Google Scholar] [CrossRef]
- Das, D.; Jothimani, G.; Banerjee, A.; Dey, A.; Duttaroy, A.K.; Pathak, S. A brief review on recent advances in diagnostic and therapeutic applications of extracellular vesicles in cardiovascular disease. Int. J. Biochem. Cell Biol. 2024, 173, 106616. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, J.; Tan, W.L.W.; Jiang, Y.; Wang, S.; Li, Q.; Yu, X.; Tan, J.; Liu, S.; Zhang, P.; et al. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death Dis. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Zhang, X.; Dutta, P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol. Res. 2021, 170, 105692. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Z.; Huang, X.; Xue, J.; Zhang, S.; Guo, X.; Zhou, S. Bacteria-Derived Outer-Membrane Vesicles Hitchhike Neutrophils to Enhance Ischemic Stroke Therapy. Adv. Mater. 2023, 35, 2301779. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Madaan, T.; Kamble, N.S.; Siddiqui, N.A.; Pauletti, G.M.; Kotagiri, N. Engineered Bacteria Enhance Immunotherapy and Targeted Therapy through Stromal Remodeling of Tumors. Adv. Healthc. Mater. 2022, 11, 2101487. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; Carroll, D.; Chen, J.; Chaiswing, L.; Zhao, Y.; Noel, T.; Alstott, M.; Bae, Y.; Dressler, E.V.; Moscow, J.A.; et al. Extracellular Vesicles Released by Cardiomyocytes in a Doxorubicin-Induced Cardiac Injury Mouse Model Contain Protein Biomarkers of Early Cardiac Injury. Clin. Cancer Res. 2018, 24, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Broadwin, M.; Harris, D.D.; Sabe, S.A.; Sengun, E.; Sylvestre, A.J.; Alexandrov, B.S.; Sellke, F.W.; Usheva, A. Impaired cardiac glycolysis and glycogen depletion are linked to poor myocardial outcomes in juvenile male swine with metabolic syndrome and ischemia. Physiol. Rep. 2023, 11, e15742. [Google Scholar] [CrossRef]
- Clavier, A.; Arnold, M.; Segiser, A.; Mendez-Carmona, N.; Wyss, R.; Heller, M.; Uldry, A.C.; Siepe, M.; Longnus, S. Perfusate Biomarkers of DCD Cardiac Graft Quality Identified with Proteomics: Studies in an Isolated Rat Heart Model. Transplantation 2023, 11, e15742. [Google Scholar] [CrossRef]
- Bradley, D.; Blaszczak, A.; Yin, Z.; Liu, J.; Joseph, J.J.; Wright, V.; Anandani, K.; Needleman, B.; Noria, S.; Renton, D.; et al. Clusterin impairs hepatic insulin sensitivity and adipocyte clusterin associates with cardiometabolic risk. Diabetes Care 2019, 42, 466–475. [Google Scholar] [CrossRef]
- Park, S.; Mathis, K.W.; Lee, I.K. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 45–53. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, H.; Zhang, W.; Wang, H.; Jin, X.; Ma, X.; Ma, Y. Comparative Proteomic Investigation of Plasma Reveals Novel Potential Biomarker Groups for Acute Aortic Dissection. Dis. Markers 2020, 2020, 4785068. [Google Scholar] [CrossRef]
- Kempf, T.; Wollert, K.C. Growth differentiation factor-15: A new biomarker in cardiovascular disease. Herz 2009, 34, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, L.; Yang, X.; Zhong, J. Roles of Growth Differentiation Factor 15 in Atherosclerosis and Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012826. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; van der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MI-FlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Módos, K.; Pállinger, É.; Pálóczi, K.; Pásztói, M.; Misják, P.; Deli, M.A.; Sipos, Á.; Szalai, A.; Voszka, I.; et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011, 117, e48. [Google Scholar] [CrossRef] [PubMed]
- French, S.L.; Butov, K.R.; Allaeys, I.; Canas, J.; Morad, G.; Davenport, P.; Laroche, A.; Trubina, N.M.; Italiano, J.E., Jr.; Moses, M.A.; et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020, 4, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Castelló, A. Principal components analysis in clinical studies. Ann. Transl. Med. 2017, 5, 351. [Google Scholar] [CrossRef]
- Cassar, A.; Holmes DRJr Rihal, C.S.; Gersh, B.J. Chronic coronary artery disease: Diagnosis and management. Mayo Clin. Proc. 2009, 84, 1130–1146. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 30, 68. [Google Scholar] [CrossRef]
- Hegyesi, H.; Pallinger, S.; Mecsei, S.; Hornyák, B.; Kovácsházi, C.; Brenner, G.B.; Giricz, Z.; Pálóczi, K.; Kittel, A.; Tóvári, J.; et al. Circulating cardiomyocyte-derived extracellular vesicles reflect cardiac injury during systemic inflammatory response syndrome in mice. Cell. Mol. Life Sci. 2022, 79, 84. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGranaghan, P.; Pallinger, É.; Fekete, N.; Maurovich-Horvát, P.; Drobni, Z.; Merkely, B.; Menna, L.; Buzás, E.I.; Hegyesi, H. Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease. Biomedicines 2024, 12, 2682. https://doi.org/10.3390/biomedicines12122682
McGranaghan P, Pallinger É, Fekete N, Maurovich-Horvát P, Drobni Z, Merkely B, Menna L, Buzás EI, Hegyesi H. Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease. Biomedicines. 2024; 12(12):2682. https://doi.org/10.3390/biomedicines12122682
Chicago/Turabian StyleMcGranaghan, Peter, Éva Pallinger, Nóra Fekete, Pál Maurovich-Horvát, Zsófia Drobni, Béla Merkely, Luigi Menna, Edit I. Buzás, and Hargita Hegyesi. 2024. "Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease" Biomedicines 12, no. 12: 2682. https://doi.org/10.3390/biomedicines12122682
APA StyleMcGranaghan, P., Pallinger, É., Fekete, N., Maurovich-Horvát, P., Drobni, Z., Merkely, B., Menna, L., Buzás, E. I., & Hegyesi, H. (2024). Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease. Biomedicines, 12(12), 2682. https://doi.org/10.3390/biomedicines12122682






