Exosomes as Regulators of Macrophages in Cardiovascular Diseases
Abstract
1. Introduction
2. Atherosclerosis and Myocardial Infarction
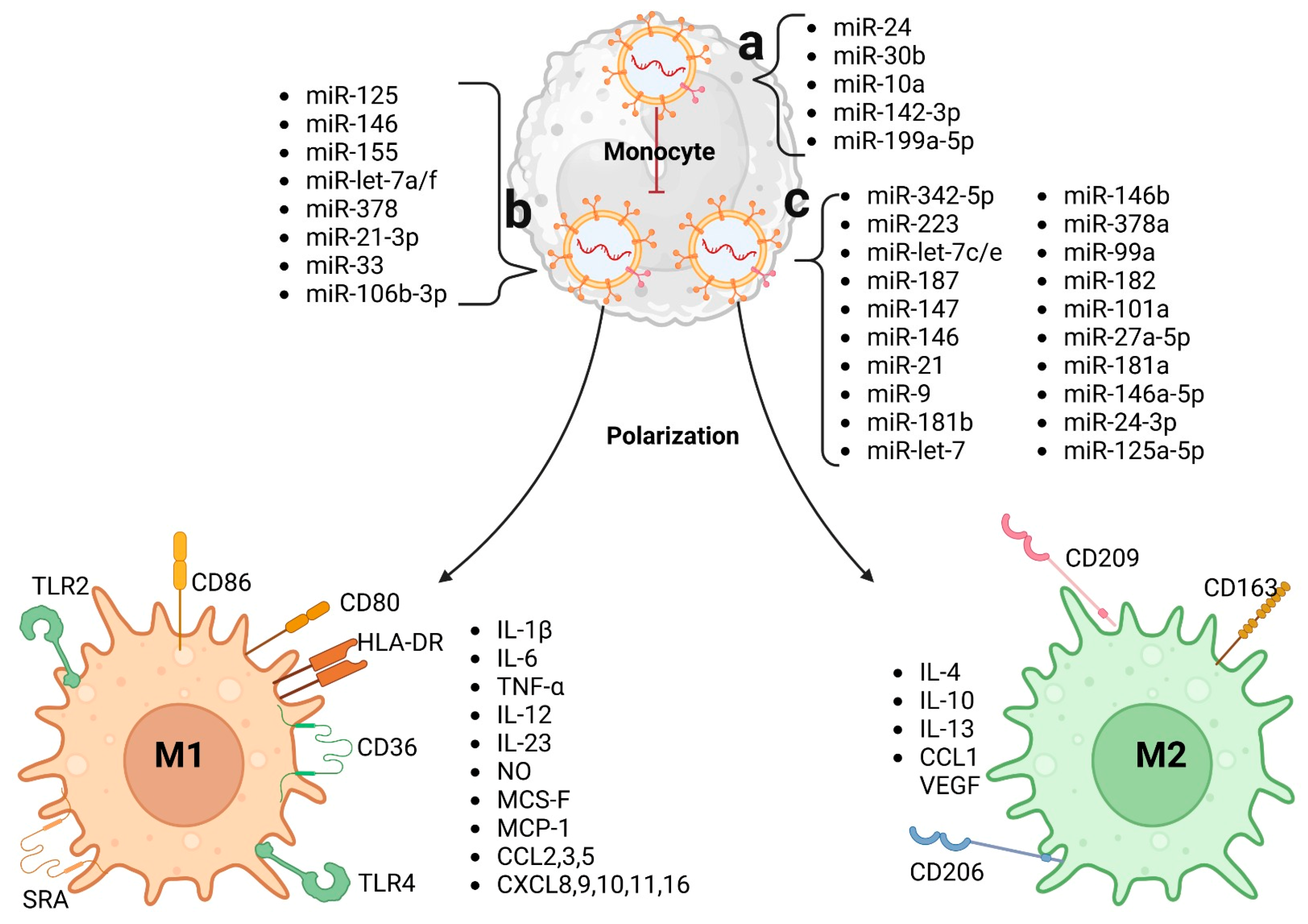
3. Macrophages
4. Macrophages in Atherosclerosis and Myocardial Infarction
5. Origin and Function of Exosomes
6. Effects of Exosomes on the Regulation of Macrophages
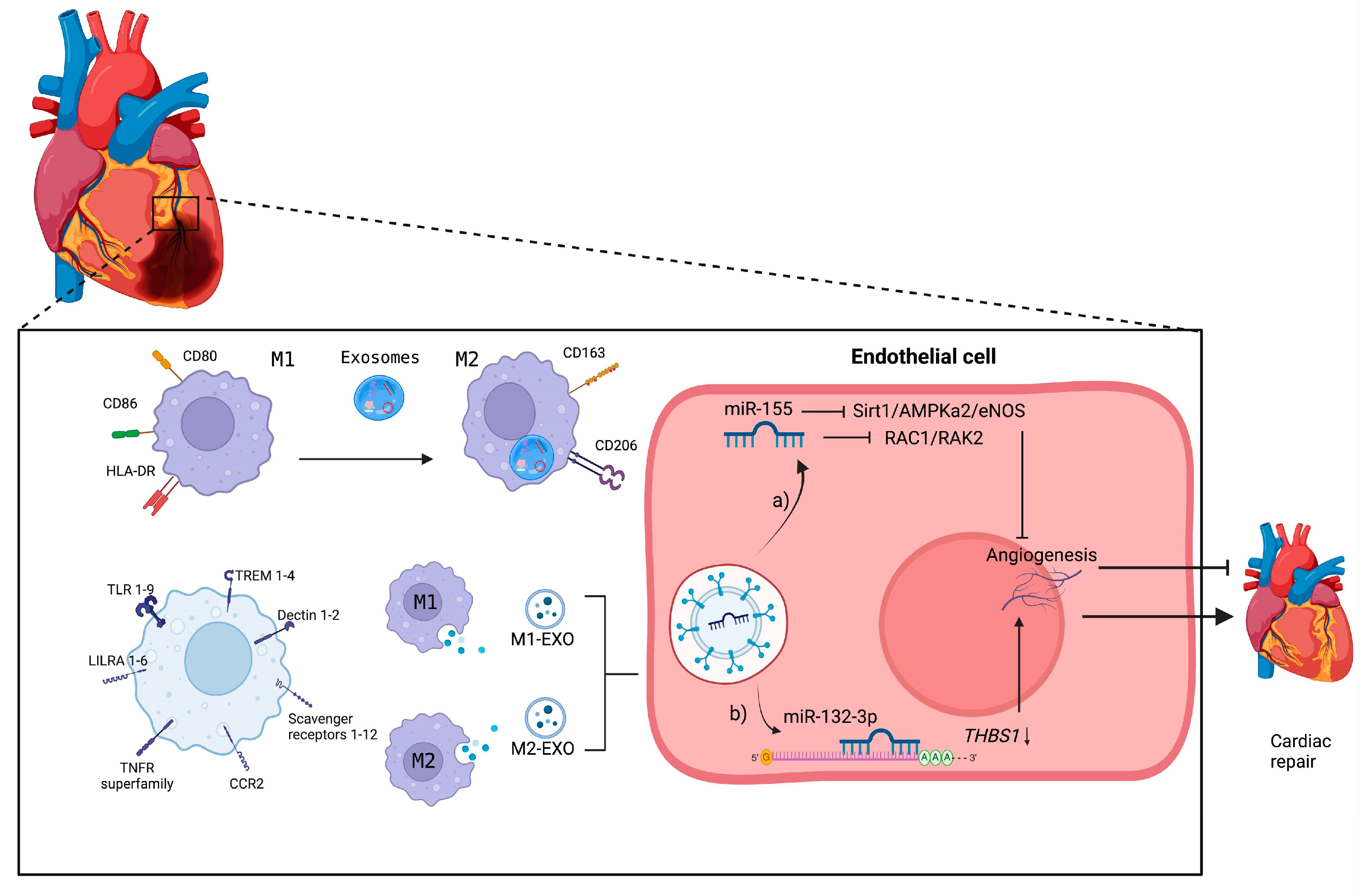
7. Exosome–Macrophage Communication in Atherosclerosis
8. Exosome–Macrophage Communication in Ischemia–Reperfusion
9. Exosome–Macrophage Communication in Myocardial Infarction
10. Macrophages as Therapeutic Targets
11. Therapeutic Potential of Exosomes
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. 2024. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 18 November 2024).
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Ling, H.; Guo, Z.; Tan, L.; Cao, Q.; Song, C. Stem cell-derived exosomes: Role in the pathogenesis and treatment of atherosclerosis. Int. J. Biochem. Cell Biol. 2021, 130, 105884. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Sánchez, L.; Espinosa-Luna, J.E.; Chávez-Rueda, K.; Legorreta-Haquet, M.V.; Montoya-Díaz, E.; Blanco-Favela, F. Innate Immune System Cells in Atherosclerosis. Arch. Med. Res. 2014, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Xing, Z.; Zhao, C.; Liu, H.; Fan, Y. Endothelial Progenitor Cell-Derived Extracellular Vesicles: A Novel Candidate for Regenerative Medicine and Disease Treatment. Adv. Healthc. Mater. 2020, 9, 2000255. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Peled, M.; Fisher, E.A. Dynamic Aspects of Macrophage Polarization during Atherosclerosis Progression and Regression. Front. Immunol. 2014, 5, 579. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Song, Z.; Chen, S.; Lin, X.; Sun, H.; Zhou, P.; Peng, Q.; Du, S.; Zheng, S.; et al. Macrophage polarization states in atherosclerosis. Front. Immunol. 2023, 14, 1664–3224. [Google Scholar] [CrossRef]
- Müller, E.; Christopoulos, P.F.; Halder, S.; Lunde, A.; Beraki, K.; Speth, M.; Øynebråten, I.; Corthay, A. Toll-Like Receptor Ligands and Interferon-γ Synergize for Induction of Antitumor M1 Macrophages. Front. Immunol. 2017, 8, 1383. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Eshghjoo, S.; Kim, D.M.; Jayaraman, A.; Sun, Y.; Alaniz, R.C. Macrophage Polarization in Atherosclerosis. Genes 2022, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Khallou-Laschet, J.; Varthaman, A.; Fornasa, G.; Compain, C.; Gaston, A.T.; Clement, M.; Dussiot, M.; Levillain, O.; Graff-Dubois, S.; Nicoletti, A.; et al. Macrophage plasticity in experimental atherosclerosis. PLoS ONE 2010, 5, e8852. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Sánchez, L.; Garza-Reyes, M.G.; Espinosa-Luna, J.E.; Chávez-Rueda, K.; Legorreta-Haquet, M.V.; Blanco-Favela, F. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum. Immunol. 2014, 75, 322–329. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ley, K. Atherosclerosis: A chronic inflammatory disease with an autoimmune component. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Isa, S.A.; Ruffino, J.S.; Ahluwalia, M.; Thomas, A.W.; Morris, K.; Webb, R. M2 macrophages exhibit higher sensitivity to oxLDL-induced lipotoxicity than other monocyte/macrophage subtypes. Lipids Health Dis. 2011, 10, 229. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef]
- Stögera, J.L.; Gijbelsa, M.J.J.; van der Velden, S.; Manca, M.; van der Loosd, C.M.; Biessen, E.A.L.; Daemen, M.J.A.P.; Lutgens, E.; de Winther, M.P.J. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.M.; Weinheimer, C.; et al. Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, H.; Dai, R.; Shang, L.; Zhang, X.; Wen, H. Macrophage polarization in tissue fibrosis. PeerJ 2023, 11, e16092. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Lozano-Gerona, J.; Vanapruks, S.; Bishop, T.; Boisvert, W.A. Macrophages in cardiac remodelling after myocardial infarction. Nat. Rev. Cardiol. 2023, 20, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 2–18. [Google Scholar] [CrossRef]
- Hessvik, P.N.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.-I.; Spell, M.L.; Wang, Y.; Chanda, D.; Dager, J.R.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef]
- Lee, Y.; EL Andaloussi, S.; Wood, M.J. Wood Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.Y.; Xu, J.; Liu, X.; Lu, Y.Q. Cardioprotective Roles of Endothelial Progenitor Cell-Derived Exosomes. Front. Cardiovasc. Med. 2021, 8, 717536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, F.; Zhang, D.; Qi, S.; Liu, Y. Metabolites as extracellular vesicle cargo in health, cancer, pleural effusion, and cardiovascular diseases: An emerging field of study to diagnostic and therapeutic purposes. Biomed. Pharmacother. 2023, 157, 114046. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Purghè, B.; Manfredi, M.; Ragñoli, B.; Baldanzi, G.; Malerba, M. Exosome in chronic respiratory diseases. Biomed. Pharmacother. 2021, 144, 112270. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef]
- Anakor, E.; Le Gall, L.; Dumonceaux, J.; Duddy, W.J.; Duguez, S. Exosomes in Ageing and Motor Neurone Disease: Biogenesis, Uptake Mechanisms, Modifications in Disease and Uses in the Development of Biomarkers and Therapeutics. Cells 2021, 10, 2930. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.G.; Wang, Z.; DiVito, S.J.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Larregina, A.T.; et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J. Immunol. 2008, 180, 3081–3090. [Google Scholar] [CrossRef]
- Noonin, C.; Thongboonkerd, V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 2021, 11, 4436–4451. [Google Scholar] [CrossRef]
- Deng, C.; Huo, M.; Chu, H.; Zhuang, X.; Deng, G.; Li, W.; Wei, H.; Zeng, L.; He, Y.; Liu, H.; et al. Exosome circATP8A1 induces macrophage M2 polarization by regulating the miR-1-3p/STAT6 axis to promote gastric cancer progression. Mol. Cancer 2024, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Dutta, B.; Tse, S.W.; Gupta, N.; Tan, C.F.; Low, J.K.; Yeoh, K.W.; Kon, O.L.; Tam, J.P.; Sze, S.K. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene 2019, 38, 5158–5173. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Liu, W.; Wang, X.; Feng, Y.; Guan, H.; Chen, Z. Plasma exosomes from patients with acute myocardial infarction alleviate myocardial injury by inhibiting ferroptosis through miR-26b-5p/SLC7A11 axis. Life Sci. 2023, 322, 121649. [Google Scholar] [CrossRef]
- Olona, A.; Hateley, C.; Muralidharan, S.; Wenk, M.R.; Torta, F.; Behmoaras, J. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br. J. Farm. 2021, 178, 4575–4587. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Zhang, H.; Wang, S.; Cui, J.; Hu, Y.; Liu, J.; Li, M.; Zhang, K.; Zhou, F.; et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact. Mater. 2023, 28, 273–283. [Google Scholar] [CrossRef]
- Pan, X.; Yang, L.; Wang, S.; Liu, Y.; Yue, L.; Chen, S. Semaglutide alleviates inflammation-Induced endothelial progenitor cells injury by inhibiting MiR-155 expression in macrophage exosomes. Int. Immunopharmacol. 2023, 119, 110196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Karunakaran, D.; Geoffrion, M.; Cheng, H.S.; Tandoc, K.; Matic, L.P.; Hedin, U.; Maegdefessel, L.; Fish, J.E.; Rayner, K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2017, 38, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, Y.; Zhou, Y.; Nie, W.; Pu, X.; Xu, X.; Zhu, J. Exosomes derived from oxidized LDL-stimulated macrophages attenuate the growth and tube formation of endothelial cells. Mol. Med. Rep. 2018, 17, 4605–4610. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE−/− mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.-S.; Xu, L.; Wang, X.; Li, X.-L.; Yang, X.-C. Endothelium-specific endothelin-1 expression promotes pro-inflammatory macrophage activation by regulating miR-33/NR4A axis. Exp. Cell Res. 2021, 399, 112443. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic. Res. Cardiol. 2020, 115, 22. [Google Scholar] [CrossRef] [PubMed]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Guo, L.; Yuan, S.; Sun, J.; Zhao, K.; Wang, J.; An, R. Exosomal Lnc NEAT1 from endothelial cells promote bone regeneration by regulating macrophage polarization via DDX3X/NLRP3 axis. J. Nanobiotechnol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Njock, M.S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Su, M.; Qian, W.; Xuan, Y.; Chen, T.; Zhou, R.; Jiang, T. CXCR4-overexpressed exosomes from cardiosphere-derived cells attenuate myocardial ischemia/reperfusion injury by transferring miRNA to macrophages and regulating macrophage polarization. Cell. Mol. Biol. 2023, 69, 98–103. [Google Scholar] [PubMed]
- Tan, W.; Li, Y.; Ma, L.; Fu, X.; Long, Q.; Yan, F.; Li, W.; Liu, X.; Ding, H.; Wang, Y.; et al. Exosomes of endothelial progenitor cells repair injured vascular endothelial cells through the Bcl2/Bax/Caspase-3 pathway. Open Access 2024, 14, 4465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Johnson, T.K.; Wang, Y.; Thomas, M.; Huynh, K.; Yang, Q.; Bond, V.C.; Chen, Y.E.; Liu, D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res. Ther. 2020, 11, 162. [Google Scholar] [CrossRef]
- Chen, C.; Cai, S.; Wu, M.; Wang, R.; Liu, M.; Cao, G.; Dong, M.; Yiu, K.-H. Role of Cardiomyocyte-Derived Exosomal MicroRNA-146a-5p in Macrophage Polarization and Activation. Dis. Markers 2022, 2022, 2948578. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Cao, C.; Wang, B.; Guo, J.; Qin, Z.; Lu, Y.; Zhang, J.; Zhang, L.; Wang, W.; et al. Exosomes as a messager to regulate the crosstalk between macrophages and cardiomyocytes under hypoxia conditions. J. Cell Mol. Med. 2022, 26, 1486–1500. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Maimaitijiang, A.; Huang, Q.; Chen, Q. Ferroptotic cardiomyocyte-derived exosomes promote cardiac macrophage M1 polarization during myocardial infarction. PeerJ 2022, 10, e13717. [Google Scholar] [CrossRef]
- Wei, Z.; Qiao, S.; Zhao, J.; Liu, Y.; Li, Q.; Wei, Z.; Dai, Q.; Kang, L.; Xu, B. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019, 232, 116632. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Z.; Yuan, J.; Li, C.; Zhao, R.; Liu, W.; Deng, W.; Gu, N.; Zhang, W.; Hu, S.; et al. Hypoxia-reoxygenation induces macrophage polarization and causes the release of exosomal miR-29a to mediate cardiomyocyte pyroptosis. Vitr. Cell Dev. Biol. Anim. 2021, 57, 30–41. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Xiao, B.; Huang, R. M2 macrophage-derived exosomes promote angiogenesis and improve cardiac function after myocardial infarction. Open Access 2024, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.; Liu, L.; A, X.; Chen, B.; Li, Y.; Du, J. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol. Ter. 2017, 25, 192–204. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Nature 2024, 9, 130. [Google Scholar] [CrossRef]
- Miteva, K.; Pappritz, K.; El-Shafeey, M.; Dong, F.; Ringe, J.; Tschöpe, C.; Van Linthout, S. Mesenchymal Stromal Cells Modulate Monocytes Trafficking in Coxsackievirus B3-Induced Myocarditis. Stem Cells Transl. Med. 2017, 6, 1249–1261. [Google Scholar] [CrossRef]
- Cortés-Morales, V.A.; Vázquez-González, W.G.; Montesinos, J.J.; Moreno-Ruíz, L.; Salgado-Pastor, S.; Salinas-Arreola, P.M.; Díaz-Duarte, K.; Chávez-Rueda, A.K.; Chávez-Sánchez, L. Human Bone Marrow Mesenchymal Stem Cells Promote the M2 Phenotype in Macrophages Derived from STEMI Patients. Int. J. Mol. Sci. 2023, 24, 16257. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Guo, B.; Zhang, H.; Zhang, H.; Liu, S.; Li, Y. Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochem. Biophys. Res. Commun. 2019, 514, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Chen, Y.; Li, J.; Yang, Z.; Lin, Y.; Jiang, B.; Shao, L.; Hu, S.; Shen, Z. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Myocardial Infarction Injury via miR-24-3p-Promoted M2 Macrophage Polarization. Adv. Biol. 2022, 6, e2200074. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Izumi, Y.; Nakamura, Y.; Yamazaki, T.; Shiota, M.; Sano, S.; Tanaka, M.; Osada-Oka, M.; Shimada, K.; Miura, K.; et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int. J. Cardiol. 2015, 178, 239–246. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Shen, D.; Zhu, D.; Huang, K.; Su, T.; Dinh, P.U.; Cores, J.; Cheng, K. Exosome-eluting stents for vascular healing after ischaemic injury. Nature 2021, 5, 1174–1188. [Google Scholar] [CrossRef]
- Gong, Z.T.; Xiong, Y.Y.; Ning, Y.; Tang, R.J.; Xu, J.Y.; Jiang, W.Y.; Li, X.S.; Zhang, L.L.; Chen, C.; Pan, Q.; et al. Nicorandil-Pretreated Mesenchymal Stem Cell-Derived Exosomes Facilitate Cardiac Repair After Myocardial Infarction via Promoting Macrophage M2 Polarization by Targeting miR-125a-5p/TRAF6/IRF5 Signaling Pathway. Int. J. Nanomed. 2024, 19, 2005–2024. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Shi, Z.; Wu, P.; Fu, J.; Tang, J.; Qing, L. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp. Neurol. 2022, 356, 114139. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Huang, H.; Liu, M.; Shi, D.; Ma, X. Platelet-Derived Exosomes and Atherothrombosis. Front. Cardiovasc. Med. 2022, 9, 886132. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, S.; Li, W.; Silverstein, R.L.; McIntyre, T.M. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J. Thromb. Haemost. 2014, 12, 1906–1907. [Google Scholar] [CrossRef]
- Wei, K.; Yu, L.; Li, J.; Gao, J.; Chen, L.; Liu, M. Platelet-derived exosomes regulate endothelial cell inflammation and M1 macrophage polarization in coronary artery thrombosis via modulating miR-34a-5p expression. Sci. Rep. 2024, 14, 17429. [Google Scholar] [CrossRef]
- Qian, J.; Wang, X.; Su, G.; Shu, X.; Huang, Z.; Jiang, H.; Zhu, Q. Platelet-rich plasma-derived exosomes attenuate intervertebral disc degeneration by promoting NLRP3 autophagic degradation in macrophages. Int. Immunopharmacol. 2022, 110, 108962. [Google Scholar] [CrossRef]
- Song, N.; Pan, K.; Chen, L.; Jin, K. Platelet Derived Vesicles Enhance the TGF-beta Signaling Pathway of M1 Macrophage. Front. Endocrinol. 2022, 13, 868893. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef]
- Sun, S.J.; Wei, R.; Li, F.; Liao, S.Y.; Tse, H.F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep. 2021, 16, 1662–1673. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Chen, S.; Wu, Y.; Geng, D.; Sun, G.; Zhang, N.; et al. Advances in the study of exosome in cardiovascular diseases. J. Adv. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wang, Y.; Feng, H.; Zhang, F.; Ji, Z.; Zhang, K.; Zhang, Q.; Jiang, L.; Qian, Y.; Fu, Y. Gene-Engineered Cerium-Exosomes Mediate Atherosclerosis Therapy Through Remodeling of the Inflammatory Microenvironment and DNA Damage Repair. Small 2024, e2404463. [Google Scholar] [CrossRef]
- Clemmens, H.; Lambert, D.W. Extracellular vesicles: Translational challenges and opportunities. Biochem. Soc. Trans. 2018, 46, 1073–1082. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as drug delivery systems: A brief overview and progress update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef]


| Exosome Type | Nucleic Acid | Related CVD | References |
|---|---|---|---|
| Macrophages-EXO | miR-155 | Atherosclerosis | [49] |
| Macrophages-EXO | miR-146a | [50,51] | |
| Macrophages-EXO | miR-21-3p | [52] | |
| mesenchymal stem cells-EXO | miR-let-7 | [53] | |
| AdET-1-HUVEC-EXO | miR-33 | [54] | |
| Monocytes-EXO | miR-4532 | [56] | |
| Macrophages-EXO | miR-146b, miR-378a, and miR-99a | [57] | |
| Endothelial cell-EXO | LncNEAT1 | [58] | |
| Endothelial cell-EXO | miR-10a | [59] | |
| mesenchymal stem cells-EXO | miR-182 | Ischemia-reperfusion | [60] |
| mesenchymal stem cells-EXO | miR-181a | [61] | |
| Cardiospheres-EXO | miR-27a-5p, miR-182, and miR-101a | [62] | |
| Plasma-EXO | miR-26b-5p | [63] | |
| stem cell-EXO | miR-21 | Ischemia | [64] |
| Macrophages-EXO | miR-29a | Hypoxia | [65] |
| Cardiomyocytes-EXO | miR-146a-5p | Myocardial infarction | [66] |
| Ferroptotic cardiomyocyte-EXO | miR-106b-3p | [67] | |
| Macrophages-EXO | miR-155 | [68] | |
| Macrophages-EXO | miR-132-3p | [69] | |
| Macrophages-EXO | miR-155 | [59] |
| Exosomes | Condition | Cargo | Phase | Number |
|---|---|---|---|---|
| MSCs | Familial hypercholesterolemia | Ldlr mRNA | I | NCT05043181 |
| MSCs | Cerebrovascular disorders | miRNA-124 | I | NCT03384433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano-Cruz, M.; Vázquez-González, W.G.; Molina-Vargas, P.; Faustino-Trejo, A.; Chávez-Rueda, A.K.; Legorreta-Haquet, M.V.; Aguilar-Ruíz, S.R.; Chávez-Sánchez, L. Exosomes as Regulators of Macrophages in Cardiovascular Diseases. Biomedicines 2024, 12, 2683. https://doi.org/10.3390/biomedicines12122683
Soriano-Cruz M, Vázquez-González WG, Molina-Vargas P, Faustino-Trejo A, Chávez-Rueda AK, Legorreta-Haquet MV, Aguilar-Ruíz SR, Chávez-Sánchez L. Exosomes as Regulators of Macrophages in Cardiovascular Diseases. Biomedicines. 2024; 12(12):2683. https://doi.org/10.3390/biomedicines12122683
Chicago/Turabian StyleSoriano-Cruz, Marina, Wendy Guadalupe Vázquez-González, Paula Molina-Vargas, Alejandro Faustino-Trejo, Adriana Karina Chávez-Rueda, María Victoria Legorreta-Haquet, Sergio Roberto Aguilar-Ruíz, and Luis Chávez-Sánchez. 2024. "Exosomes as Regulators of Macrophages in Cardiovascular Diseases" Biomedicines 12, no. 12: 2683. https://doi.org/10.3390/biomedicines12122683
APA StyleSoriano-Cruz, M., Vázquez-González, W. G., Molina-Vargas, P., Faustino-Trejo, A., Chávez-Rueda, A. K., Legorreta-Haquet, M. V., Aguilar-Ruíz, S. R., & Chávez-Sánchez, L. (2024). Exosomes as Regulators of Macrophages in Cardiovascular Diseases. Biomedicines, 12(12), 2683. https://doi.org/10.3390/biomedicines12122683





