Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives
Abstract
1. Introduction
2. Principles of Operation and Comparison of Techniques
| Technique | Description | Reference |
|---|---|---|
| Vibro-acoustography | Employs two focused ultrasound beams with slightly different frequencies to induce tissue vibrations. A surface probe detects these vibrations to assess tissue stiffness on a point-by-point basis. | [9] |
| Acoustic Radiation Force Impulse (ARFI) | Utilizes focused ultrasound pulses to generate and measure shear waves within the tissue. The propagation speed of these waves is analyzed to determine tissue stiffness. | [10] |
| 1D Transient Elastography (1D-TE) | Employs a single transducer to generate low-frequency mechanical waves that propagate through the tissue. Tissue stiffness is assessed by analyzing the propagation speed of these waves. | [11] |
| 2D Transient Elastography (2D-TE) | Extends the 1D-TE technique by using multiple vibrating sources to generate shear waves across a larger area, enabling the simultaneous analysis of multiple tissue points. | [12] |
| Shear Wave Elastography (SWEI) | Utilizes a fixed excitation beam and a moving sensor to monitor tissue deformation along the wave propagation path, providing a quantitative assessment of tissue stiffness. | [13] |
| Supersonic Shear Imaging (SSI) | Employs a rapidly moving focal point to generate shear waves, enabling real-time visualization of tissue stiffness. | [14] |
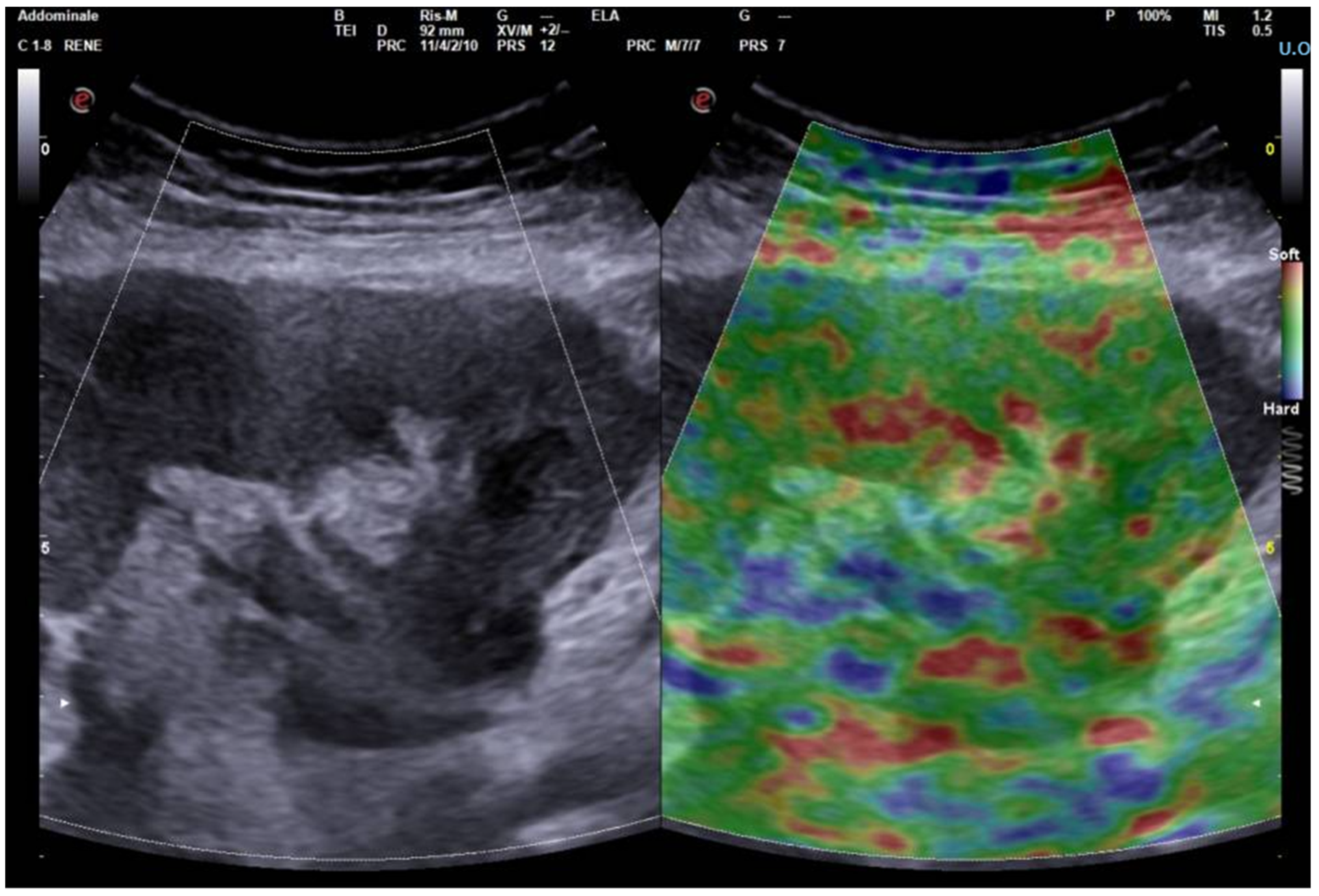
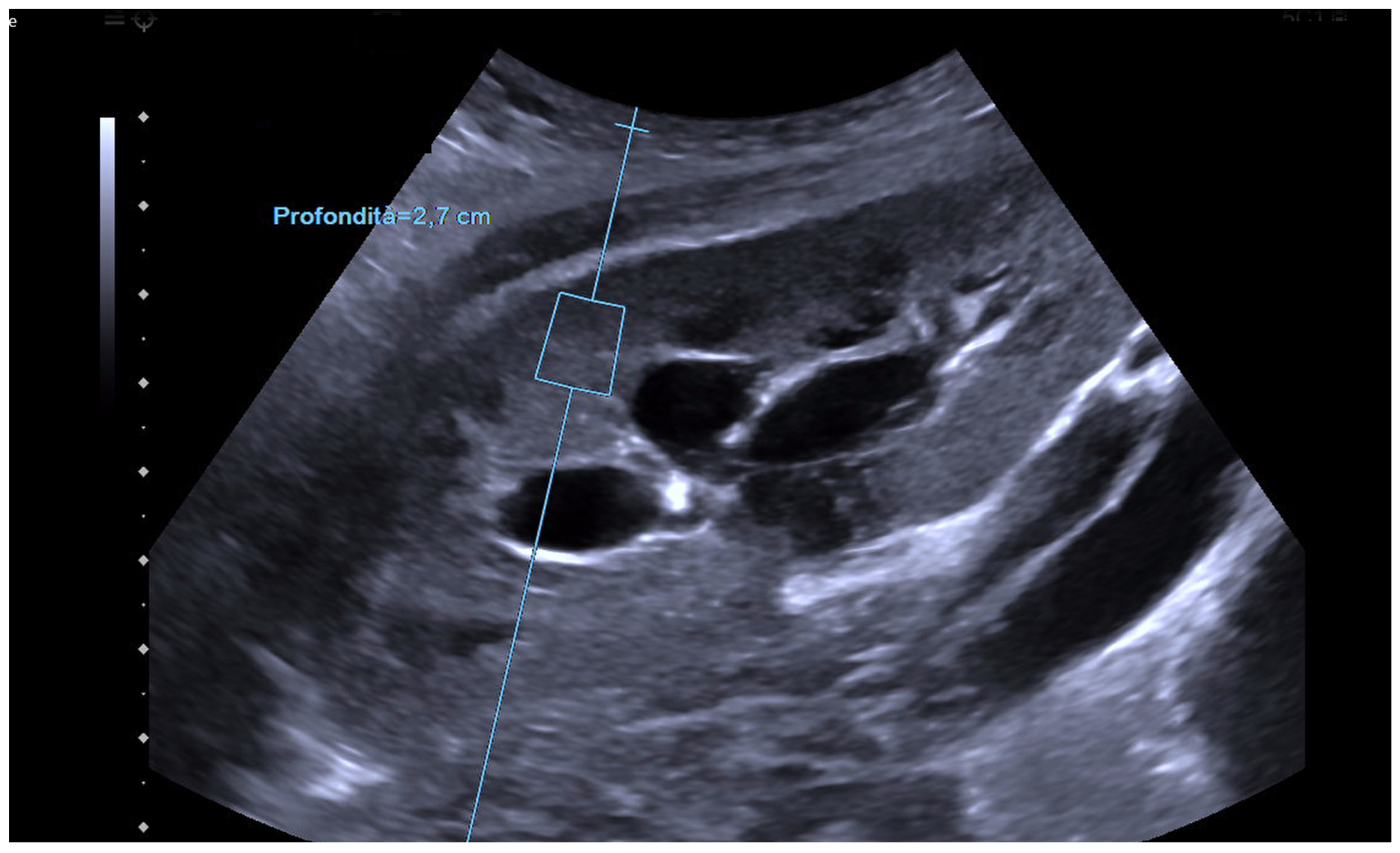
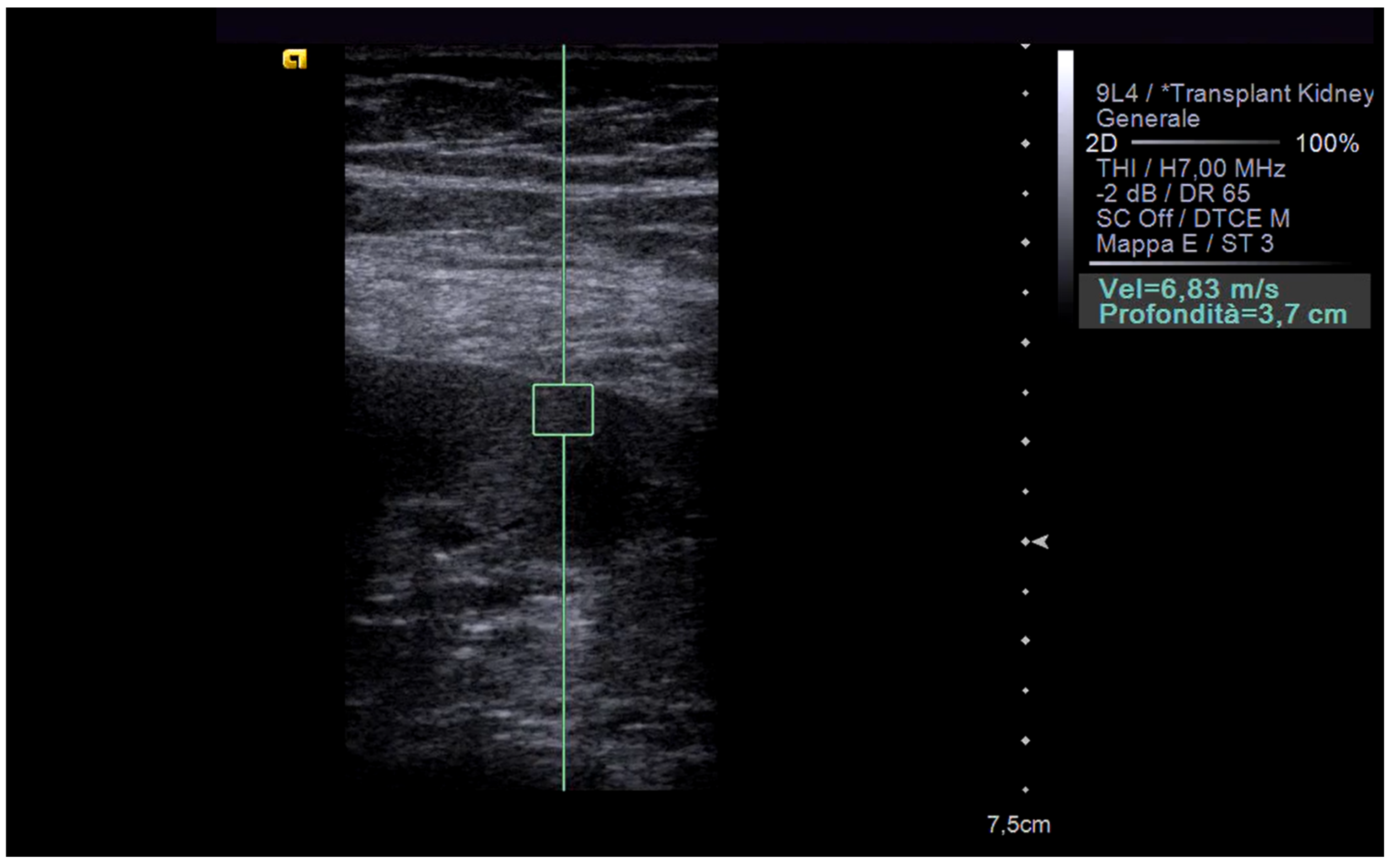
3. Elastography in the Kidney: Basic Technique and Limiting Factors
| Shear Wave Speed (m/s, Mean and SD) or Young Modules | Authors | Reference | |
|---|---|---|---|
| 1.82 ± 0.63 | Bota et al. | [19] | |
| Native Kidney | 1.49 ± 0.19 (right kidney) | Singh et al. | [20] |
| 1.54 ± 0.19 (left kidney) | |||
| Transplant Kidney | <30.95 kPa | Yang et al. | [15] |
| <2.625 m/s | He et al. | [21] | |
| <2.83 m/s | Liu et al. | [22] |
4. Elastography in Chronic Dysfunction of the Transplanted Kidney: State of the Art
| Authors | Reference | Year | Patient Number | Elastographic Assessment | Region Explored | Bioptic Assessment | Main Conclusions |
|---|---|---|---|---|---|---|---|
| Stock et al. | [23] | 2011 | 18 | Yes | Cortical | Yes | Moderate correlation between SWS and degree of fibrosis, no correlation with intraparenchymal IR |
| Syersveen et al. | [24] | 2012 | 31 | Yes | - | Yes | No correlation between SWS and fibrosis grading |
| Ren et al. | [25] | 2013 | 74 | Yes | Cortical, medullary, renal sinus | No | Significant correlation between intraparenchymal SER and IR vs. renal function indices |
| He et al. | [21] | 2013 | 102 | Yes | - | No | Inverse correlation between SWE and eGFR, SWE > 2.625 m/s proposed as a cutoff value to define chronic dysfunction |
| Ozkan et al. | [26] | 2013 | 42 | Yes | - | No | Significativa correlazione tra kPa rilevata e IR, elevata variabilità inter-osservatore |
| Lukenda et al. | [27] | 2014 | 52 | Yes | - | No | Significant correlation between detected kPa and IR, high inter-observer variability |
| Dai et al. | [28] | 2014 | 54 | Yes | - | Yes | Significant correlation between tissue stiffness assessed with ARFI and fibrosis grading in biopsy |
| Liu et al. | [22] | 2013 | 28 | Yes | - | No | SWE > 2.83 m/s results in a diagnostic accuracy of 78.7% in predicting chronic dysfunction |
| Gokalp et al. | [29] | 2020 | 34 | Yes | - | Yes | Positive correlation between SWE variation and inflammatory infiltrate |
| Bolboaca et al. | [30] | 2020 | 83 | Yes | Cortical, medullary | No | Positive correlation between stiffness of cortical and medullary regions and proteinuria/creatinuria ratio, significant variability in intra-operator observations |
| Chhajer et al. | [31] | 2021 | 172 | Yes | Upper, middle, lower pole | Yes | Significant correlation between SWE and Banff grade, no correlation between IR and Banff score |
| Barsoum et al. | [32] | 2022 | 36 | Yes | - | Yes | Positive correlation between SWE and time since transplant, and between SWE and Banff score |
| Eisingergy et al. | [33] | 2023 | 10 | Yes | - | Yes | Positive correlation between SWE and biochemical signs of organ dysfunction and between SWE and Banff score in patients undergoing biopsy |
| Zhang et al. | [34] | 2023 | 161 | Yes | Cortical, medullary | No | Renal medullary region stiffness predictive of primary study outcome (>25% reduction in eGFR or all-cause mortality) |
| Yang et al. | [15] | 2023 | 101 | Yes | Cortical | No | Young’s modulus strongly correlates with decreased eGFR, while IR shows a weaker negative correlation. A 30.95% cut-off value accurately diagnoses CAN (biochemically suspected) with high sensitivity and specificity |
| Jesrani et al. | [35] | 2024 | 154 | Yes | - | Yes | High diagnostic accuracy of SWE for chronic changes |
5. Considerations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Oberbauer, R. Progression of Interstitial Fibrosis in Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2016, 11, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Lawitschka, A.; Böhmig, G.A.; Dauber, E.M.; Greinix, H.; Kozakowski, N.; Mühlbacher, F.; Berlakovich, G.A.; Wekerle, T. Kidney transplantation with corticosteroids alone after haploidentical HSCT from the same donor. Transplantation 2016, 100, 2219–2221. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Suo, C.; Wei, J.; Tan, R.; Gu, M. Application of Ultrasound Elastography for Chronic Allograft Dysfunction in Kidney Transplantation. J. Ultrasound Med. 2017, 36, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Del Gaudio, G.; Drudi, F.M.; Dolcetti, V.; Pacini, P.; Granata, A.; Pretagostini, R.; Garofalo, M.; Basile, A.; Bellini, M.I.; et al. Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications. Tomography 2022, 8, 1704–1715. [Google Scholar] [CrossRef]
- Derieppe, M.; Delmas, Y.; Gennisson, J.L.; Deminière, C.; Placier, S.; Tanter, M.; Combe, C.; Grenier, N. Detection of intrarenal microstructural changes with supersonic shear wave elastography in rats. Eur. Radiol. 2012, 22, 243–250. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Granata, A.; Distefano, G.; Maccarrone, R.; Pesce, F.; Costanza, G.; Digiacomo, A.; Basile, A.; Romano, M.; Cantisani, V. Quantitative imaging in nephrology: Limits and potentials of elastosonography. Glob. Ital. Nefrol. 2022, 39, 2022-vol4. [Google Scholar]
- Urban, M.W.; Alizad, A.; Aquino, W.; Greenleaf, J.F.; Fatemi, M. A Review of vibro-acoustography and its applications in medicine. Curr. Med. Imaging Rev. 2011, 7, 350–359. [Google Scholar] [CrossRef]
- Melodelima, D.; Bamber, J.C.; Duck, F.A.; Shipley, J.A. Transient elastography using impulsive ultrasound radiation force: A preliminary comparison with surface palpation elastography. Ultrasound Med. Biol. 2007, 33, 959–969. [Google Scholar] [CrossRef]
- Babu, A.S.; Wells, M.L.; Teytelboym, O.M.; Mackey, J.E.; Miller, F.H.; Yeh, B.M.; Ehman, R.L.; Venkatesh, S.K. Elastography in chronic liver disease: Modalities, techniques, limitations and future directions. Radiographics 2016, 36, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Tanter, M.; Catheline, S.; Fink, M. Shear modulus imaging with 2-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, R.; Li, S.; Dunsby, C.; Eckersley, R.J.; Elson, D.S.; Tang, M.-X. Shear wave elasticity imaging based on acoustic radiation force and optical detection. Ultrasound Med. Biol. 2012, 38, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; La, Q.; Ding, X.M.; Song, Y. Application of Real-Time Sound Touch Elastography for Evaluating Chronic Kidney Disease of Transplanted Kidneys. Transplant. Proc. 2023, 55, 2095–2101. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Dahl, J.J.; Frinkley, K.D.; Wang, M. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Grenier, N.; Combe, C.; Tanter, M. Supersonic shear wave elastography of in vivo pig kidney: Influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med. Biol. 2012, 38, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ogata, A.; Tanaka, K.; Ide, Y.; Sankoda, A.; Kawakita, C.; Nishikawa, M.; Ohmori, K.; Kinomura, M.; Shimada, N.; et al. Acoustic radiation force impulse elastography of the kidneys: Is shear wave velocity affected by tissue fibrosis or renal blood flow? J. Ultrasound Med. 2014, 33, 793–801. [Google Scholar] [CrossRef]
- Bota, S.; Bob, F.; Sporea, I.; Şirli, R.; Popescu, A. Factors that influence kidney shear wave speed assessed by acoustic radiation force impulse elastography in patients without kidney pathology. Ultrasound Med. Biol. 2015, 41, 1–6. [Google Scholar] [CrossRef]
- Singh, H.; Panta, O.B.; Khanal, U.; Ghimire, R.K. Renal cortical elastography: Normal values and variations. J. Med. Ultrasound 2017, 25, 215–220. [Google Scholar] [CrossRef]
- He, W.Y.; Jin, Y.J.; Wang, W.P.; Li, C.-L.; Ji, Z.-B.; Yang, C. Tissue elasticity quantification by acoustic radiation force impulse for the assessment of renal allograft function. Ultrasound Med. Biol. 2014, 40, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Shi, D.; Sun, X.; Li, J. Value of virtual touch tissue quantification in the diagnosis of chronic allograft nephropathy after renal transplantation. J. Shandong Univ. (Health Sci.) 2013, 51, 78–82. [Google Scholar]
- Stock, K.F.; Klein, B.S.; Cong, M.T.; Regenbogen, C.; Kemmner, S.; Büttner, M.; Wagenpfeil, S.; Matevossian, E.; Renders, L.; Heemann, U.; et al. ARFI-based tissue elasticity quantification and kidney graft dysfunction: First clinical experiences. Clin. Hemorheol. Microcirc. 2011, 49, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Syversveen, T.; Midtvedt, K.; Berstad, A.E.; Brabrand, K.; Strøm, E.H.; Abildgaard, A. Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: An experimental study in kidney transplant patients. Eur. Radiol. 2012, 22, 2130–2137. [Google Scholar] [CrossRef]
- Ren, X.Z.; Li, C.L.; Xu, H.; Zhang, Y.; Niu, H.; Li, X.M. The primary application of acoustic radiation force impulse in transplanted kidneys. Chin. J. Med. Ultrasound 2013, 10, 227–230. [Google Scholar]
- Ozkan, F.; Yavuz, Y.C.; Inci, M.F.; Altunoluk, B.; Ozcan, N.; Yuksel, M.; Sayarlioglu, H.; Dogan, E. Interobserver variability of ultrasound elastography in transplant kidneys: Correlations with clinical-Doppler parameters. Ultrasound Med. Biol. 2013, 39, 4–9. [Google Scholar] [CrossRef]
- Lukenda, V.; Mikolasevic, I.; Racki, S.; Jelic, I.; Stimac, D.; Orlic, L. Transient elastography: A new noninvasive diagnostic tool for assessment of chronic allograft nephropathy. Int. Urol. Nephrol. 2014, 46, 1435–1440. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Guo, Y.; Zhao, B.; Tan, Y.; Xiang, F. Noninvasive evaluation of renal allograft fibrosis by virtual touch tissue quantification. Zhong Nan Da Xue Bao Yi Xue Ban 2014, 39, 173–177. (In Chinese) [Google Scholar]
- Gokalp, C.; Oytun, M.G.; Gunay, E.; Tamsel, S.; Sen, S.; Sezer, T.O.; Toz, H. Acoustic Radiation Force Impulse Elastography May Predict Acute Rejection in Kidney Transplantation. Transplant. Proc. 2020, 52, 3097–3102. [Google Scholar] [CrossRef]
- Bolboaca, S.D.; Elec, F.I.; Elec, A.D.; Muntean, A.M.; Socaciu, M.A.; Iacob, G.; Zaro, R.; Andries, A.I.; Badulescu, R.M.; Ignat, R.M.; et al. Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study. Diagnostics 2020, 10, 41. [Google Scholar] [CrossRef]
- Chhajer, G.; Arunachalam, V.K.; Ramasamy, R.; Mehta, P.; Cherian, M. Elastography: A surrogate marker of renal allograft fibrosis—Quantification by shear-wave technique. Pol. J. Radiol. 2021, 86, e151–e156. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, N.R.; Elsisy, A.E.; Mohamed, M.F.; Hassan, A.A. Role of shear wave elastography in assessment of chronic allograft nephropathy. Egypt. J. Radiol. Nucl. Med. 2022, 53, 100. [Google Scholar] [CrossRef]
- Elsingergy, M.M.; Viteri, B.; Otero, H.J.; Bhatti, T.; Morales, T.; Roberts, T.P.L.; Amaral, S.; Hartung, E.; Serai, S.D. Imaging fibrosis in pediatric kidney transplantation: A pilot study. Pediatr. Transplant. 2023, 27, e14540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Yan, J.; Wu, J.; Yang, W.; Zhang, S.; Xia, J.; Che, X.; Li, H.; Li, D.; Ying, L.; et al. Shear wave elastography parameters adds prognostic value to adverse outcome in kidney transplantation recipients. Ren. Fail. 2023, 45, 2235015. [Google Scholar] [CrossRef]
- Jesrani, A.K.; Faiq, S.M.; Rashid, R.; Kalwar, T.A.; Mohsin, R.; Aziz, T.; Khan, N.A.; Mubarak, M. Comparison of resistive index and shear-wave elastography in the evaluation of chronic kidney allograft dysfunction. World J. Transplant. 2024, 14, 89255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distefano, G.; Granata, S.; Morale, W.; Granata, A. Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives. Biomedicines 2024, 12, 2671. https://doi.org/10.3390/biomedicines12122671
Distefano G, Granata S, Morale W, Granata A. Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives. Biomedicines. 2024; 12(12):2671. https://doi.org/10.3390/biomedicines12122671
Chicago/Turabian StyleDistefano, Giulio, Salvatore Granata, Walter Morale, and Antonio Granata. 2024. "Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives" Biomedicines 12, no. 12: 2671. https://doi.org/10.3390/biomedicines12122671
APA StyleDistefano, G., Granata, S., Morale, W., & Granata, A. (2024). Advancements in Elastography for Evaluating Fibrosis in Renal Transplants: Current Perspectives. Biomedicines, 12(12), 2671. https://doi.org/10.3390/biomedicines12122671





