Comparative Ten-Year Outcomes in Chronic and Acute Coronary Syndrome Patients Undergoing Invasive Diagnostics—Insights from the KORONEF Registry
Abstract
:1. Introduction
2. Materials and Methods
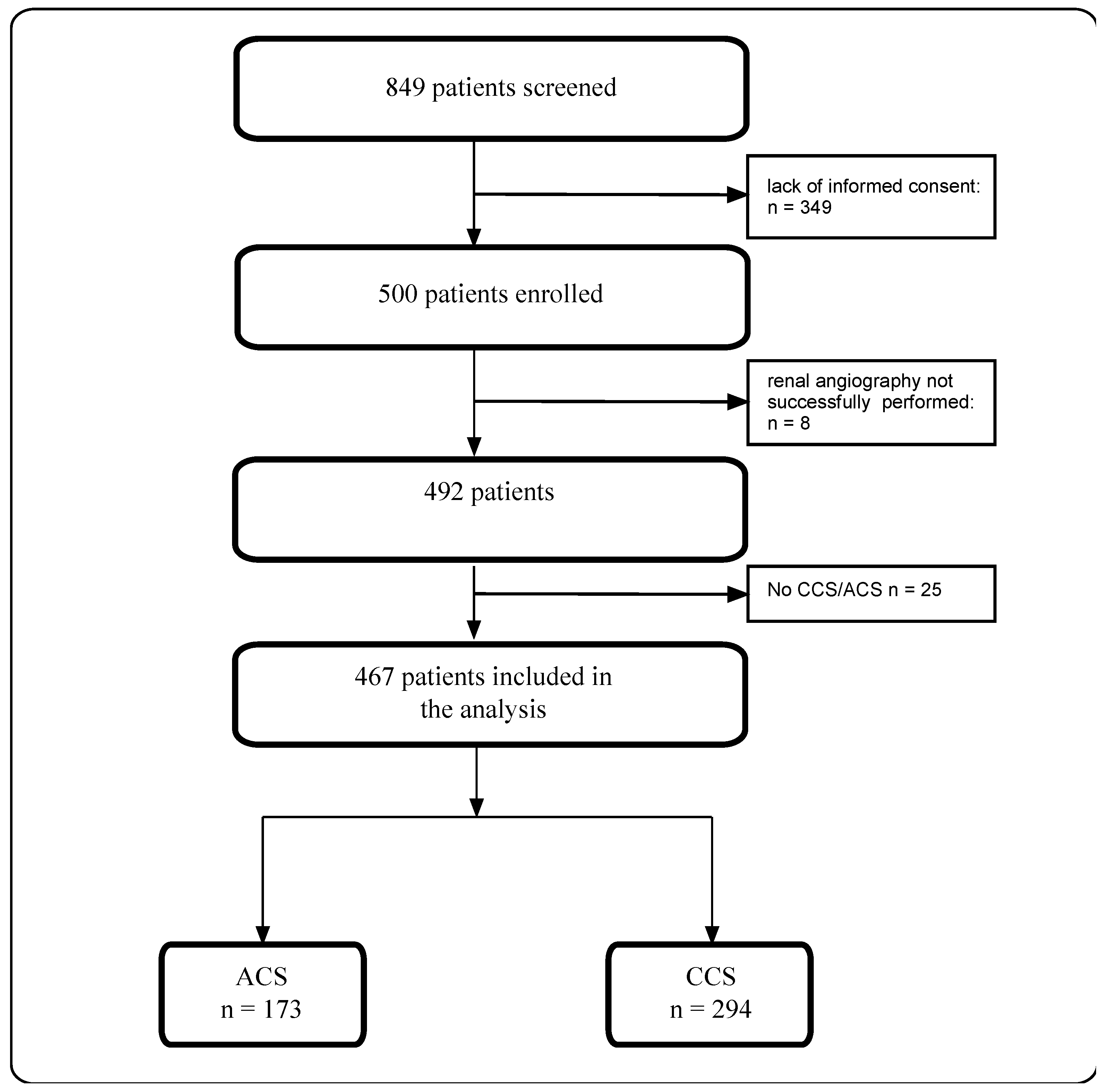
2.1. Study Design and Participants
2.2. Data Collection
2.3. Procedure Characteristics
2.4. Study Endpoints
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Periprocedural and Discharge Characteristics
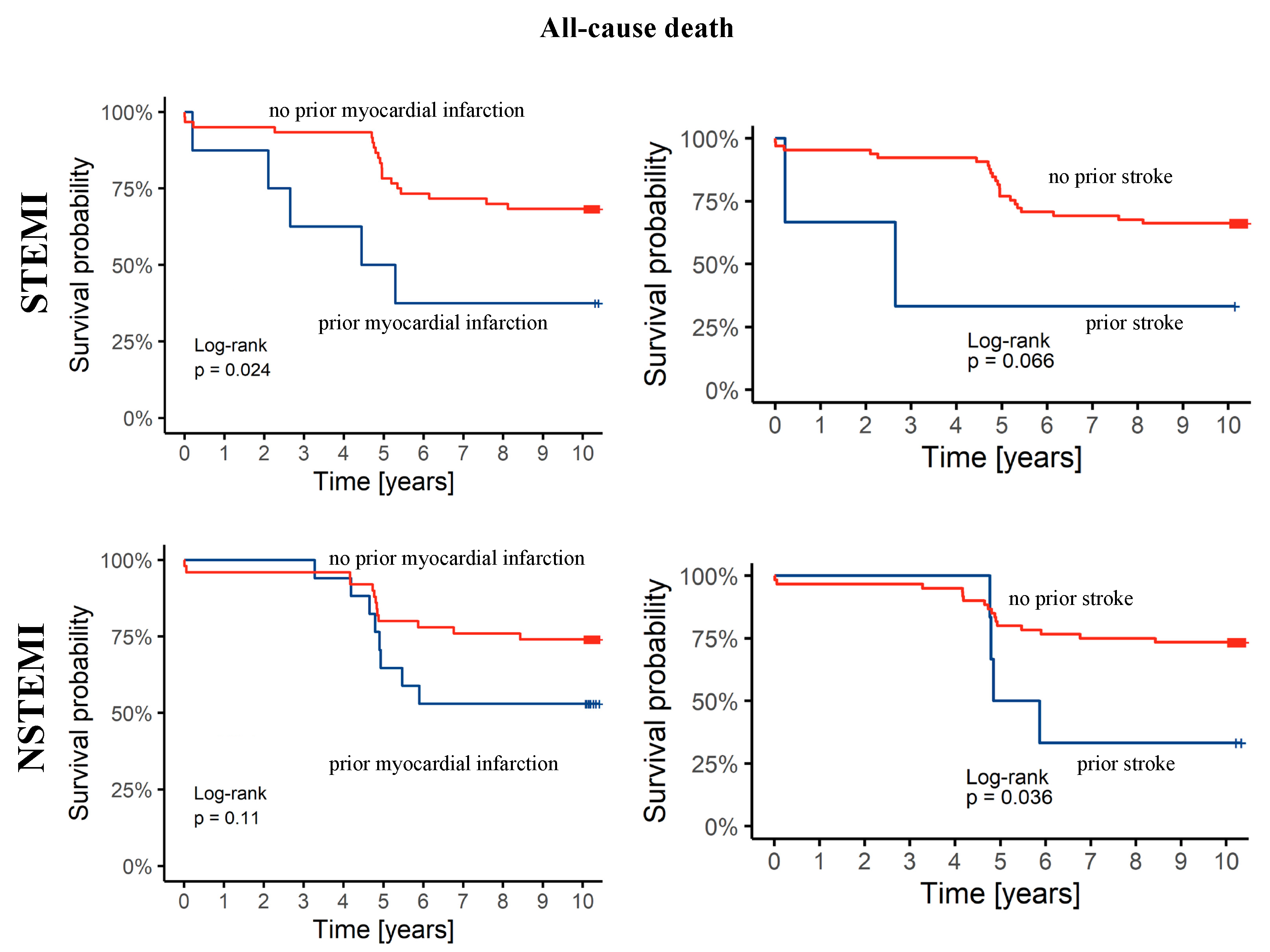
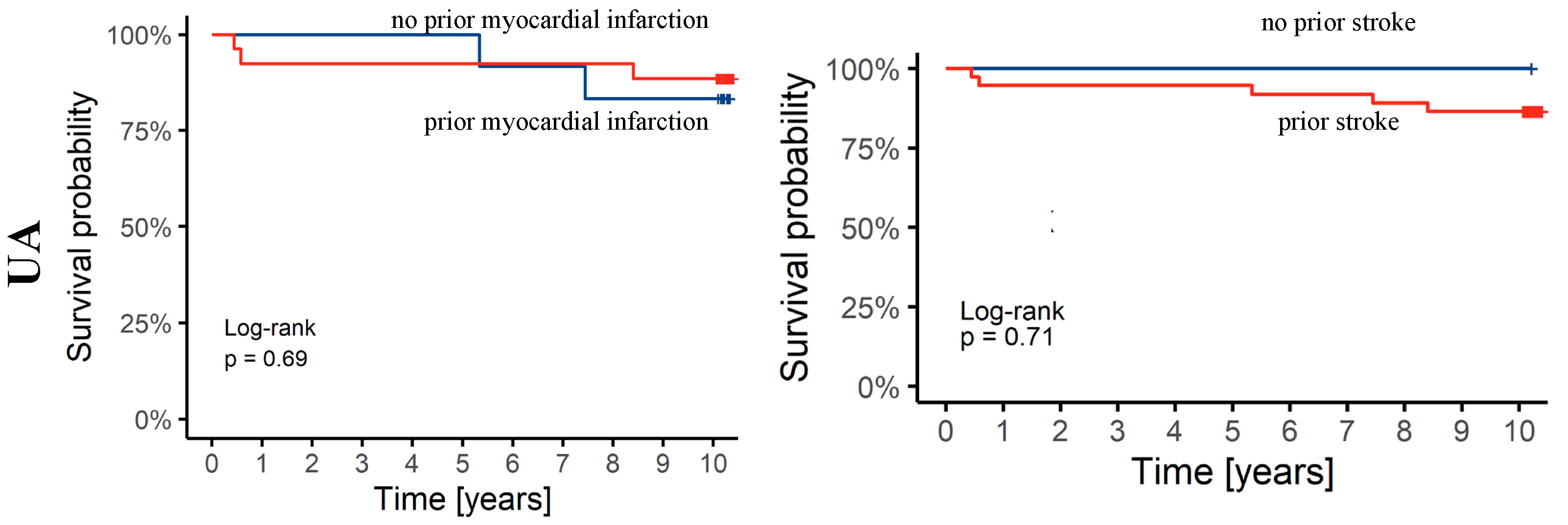
3.3. Ten-Year Follow-Up Data
3.4. Cox Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meda, J.R.; Kusima, H.L.; Magitta, N.W.F. Angiographic characteristics of coronary artery disease in patients undergoing diagnostic coronary angiography at a tertiary hospital in Tanzania. BMC Cardiovasc. Disord. 2024, 24, 125. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. Int. J. Cardiol. 2016, 215, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Winchester, D.; Jansen, M.; Lee, A.; Silverstein, B.; Stalvey, C.; Khuddus, M.; Mazza, J.; Yale, S. Assessing Prognosis of Acute Coronary Syndrome in Recent Clinical Trials: A Systematic Review. Clin. Med. Res. 2019, 17, 11–19. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Qureshi, W.T.; Kakouros, N.; Fahed, J.; Rade, J.J. Comparison of Prevalence, Presentation, and Prognosis of Acute Coronary Syndromes in ≤ 35 years, 36–54 years, and ≥ 55 years Patients. Am. J. Cardiol. 2021, 140, 1–6. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Scanlon, P.J.; Faxon, D.P.; Audet, A.-M.; Carabello, B.; Dehmer, G.J.; Eagle, K.A.; Legako, R.D.; Leon, D.F.; Murray, J.A.; Nissen, S.E.; et al. ACC/AHA Guidelines for Coronary Angiography: Executive Summary and Recommendations. Circulation 1999, 99, 2345–2357. [Google Scholar] [CrossRef]
- Lancellotti, P.; Zamorano, J.; Habib, G.; Badano, L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Bil, J.; MoZeNska, O.; Segiet-SwiEcicka, A.; Gil, R.J. Revisiting the use of the provocative acetylcholine test in patients with chest pain and nonobstructive coronary arteries: A five-year follow-up of the AChPOL registry, with special focus on patients with MINOCA. Transl. Res. 2021, 231, 64–75. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schutt, K.; Muller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Cerci, R.; Matheson, M.B.; Magalhaes, T.; Kishi, S.; Brinker, J.; Clouse, M.E.; Rochitte, C.E.; Cox, C.; Lima, J.A.C.; et al. Predicting Significant Coronary Obstruction in a Population with Suspected Coronary Disease and Absence of Coronary Calcium: CORE-64/CORE320 Studies. Arq. Bras. Cardiol. 2023, 120, e20220183. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijic, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Wara-Aswapati, S.; Kaewkes, D.; Chotmongkol, V.; Sawanyawisuth, K. Clinical predictive factors of coronary stenosis in patients with high-risk valvular heart disease who received diagnostic coronary angiography prior to cardiac valve surgery. Biomed. Rep. 2024, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Sorbets, E.; Greenlaw, N.; Ferrari, R.; Ford, I.; Fox, K.M.; Tardif, J.C.; Tendera, M.; Steg, P.G. Rationale, design, and baseline characteristics of the CLARIFY registry of outpatients with stable coronary artery disease. Clin. Cardiol. 2017, 40, 797–806. [Google Scholar] [CrossRef]
- Kite, T.A.; Ludman, P.F.; Gale, C.P.; Wu, J.; Caixeta, A.; Mansourati, J.; Sabate, M.; Jimenez-Quevedo, P.; Candilio, L.; Sadeghipour, P.; et al. International Prospective Registry of Acute Coronary Syndromes in Patients With COVID-19. J. Am. Coll. Cardiol. 2021, 77, 2466–2476. [Google Scholar] [CrossRef]
- Grinberg, T.; Hammer, Y.; Wiessman, M.; Perl, L.; Ovdat, T.; Tsafrir, O.; Kogan, Y.; Beigel, R.; Orvin, K.; Kornowski, R.; et al. Management and outcomes over time of acute coronary syndrome patients at particularly high cardiovascular risk: The ACSIS registry-based retrospective study. BMJ Open 2022, 12, e060953. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Hamza, M.; Mahmoud, N.; Elgendy, I.Y. A Randomized Trial of Complete Versus Culprit-Only Revascularization During Primary Percutaneous Coronary Intervention in Diabetic Patients With Acute ST Elevation Myocardial Infarction and Multi Vessel Disease. J. Interv. Cardiol. 2016, 29, 241–247. [Google Scholar] [CrossRef]
- Toma, A.; Stähli, B.E.; Gick, M.; Gebhard, C.; Nührenberg, T.; Mashayekhi, K.; Ferenc, M.; Neumann, F.J.; Buettner, H.J. Impact of multi-vessel versus single-vessel disease on outcomes after percutaneous coronary interventions for chronic total occlusions. Clin. Res. Cardiol. 2017, 106, 428–435. [Google Scholar] [CrossRef]
- Maroszyńska-Dmoch, E.M.; Wożakowska-Kapłon, B. Clinical and angiographic characteristics of coronary artery disease in young adults: A single centre study. Kardiol. Pol. 2016, 74, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, S.; Sakuma, M.; Abe, S.; Inoue, T.; Nakao, K.; Ozaki, Y.; Kimura, K.; Ako, J.; Noguchi, T.; Suwa, S.; et al. Prediction of Long-Term Outcomes in ST-Elevation Myocardial Infarction and Non-ST Elevation Myocardial Infarction with and without Creatinine Kinase Elevation-Post-Hoc Analysis of the J-MINUET Study. J. Clin. Med. 2020, 9, 2667. [Google Scholar] [CrossRef] [PubMed]
- Piątek, Ł.; Janion-Sadowska, A.; Piątek, K.; Zandecki, Ł.; Zabojszcz, M.; Siudak, Z.; Sadowski, M. Long-term clinical outcomes in patients with unstable angina undergoing percutaneous coronary interventions in a contemporary registry data from Poland. Coron. Artery Dis. 2020, 31, 215–221. [Google Scholar] [CrossRef]
- D’Souza, M.; Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Larsen, T.B.; Diederichsen, A.C.; Jangaard, N.; Diederichsen, S.Z.; Hosbond, S.; et al. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am. J. Med. 2015, 128, 852–860. [Google Scholar] [CrossRef]
- Eggers, K.M.; Jernberg, T.; Lindahl, B. Unstable Angina in the Era of Cardiac Troponin Assays with Improved Sensitivity-A Clinical Dilemma. Am. J. Med. 2017, 130, 1423–1430.e1425. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Twerenbold, R.; Reiter, M.; Steuer, S.; Bassetti, S.; Balmelli, C.; Winkler, K.; Kurz, S.; Stelzig, C.; Freese, M.; et al. Introduction of high-sensitivity troponin assays: Impact on myocardial infarction incidence and prognosis. Am. J. Med. 2012, 125, 1205–1213.e1. [Google Scholar] [CrossRef]
- Buller, P.; Kern, A.; Tyczynski, M.; Rosiak, W.; Figatowski, W.; Gil, R.J.; Bil, J. The Comparison of Predicting Factors and Outcomes of MINOCA and STEMI Patients in the 5-Year Follow-Up. J. Pers. Med. 2023, 13, 856. [Google Scholar] [CrossRef]
- Muller, O.; Mangiacapra, F.; Ntalianis, A.; Verhamme, K.M.; Trana, C.; Hamilos, M.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; Heyndrickx, G.R.; et al. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc. Interv. 2011, 4, 1175–1182. [Google Scholar] [CrossRef]
- Yamashita, J.; Tanaka, N.; Shindo, N.; Ogawa, M.; Kimura, Y.; Sakoda, K.; Murata, N.; Hokama, Y.; Hoshino, K.; Ikeda, S.; et al. Seven-year clinical outcomes of patients with moderate coronary artery stenosis after deferral of revascularization based on gray-zone fractional flow reserve. Cardiovasc. Interv. Ther. 2015, 30, 209–215. [Google Scholar] [CrossRef]
- Goy, J.J.; Kaufmann, U.; Hurni, M.; Cook, S.; Versaci, F.; Ruchat, P.; Bertel, O.; Pieper, M.; Meier, B.; Chiarello, L.; et al. 10-year follow-up of a prospective randomized trial comparing bare-metal stenting with internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis the SIMA (Stenting versus Internal Mammary Artery grafting) trial. J. Am. Coll. Cardiol. 2008, 52, 815–817. [Google Scholar] [CrossRef]
| Parameter | Total Study Population N = 467 (%) | Patients with CCS N = 294 (%) | Patients with ACS N = 173 (%) | p |
|---|---|---|---|---|
| Females | 174 (37.3%) | 111 (37.8%) | 63 (36.4%) | 0.773 |
| Age (years) | 64.4 ± 9.9 | 64.9 ± 9.5 | 63.7 ± 10.3 | 0.106 |
| BMI (kg/m2) | 28.0 ± 4.3 | 28.3 ± 4.4 | 27.4 ± 4.0 | 0.077 |
| Arterial hypertension | 350 (74.9%) | 239 (81.3%) | 111 (64.2%) | <0.001 |
| Hyperlipidemia | 220 (47.1%) | 147 (50.0%) | 73 (42.2%) | 0.103 |
| Diabetes | 124 (26.6%) | 85 (28.9%) | 39 (22.5%) | 0.132 |
| Obesity | 143 (30.6%) | 102 (34.7%) | 41 (23.7%) | 0.013 |
| Nicotine addiction | 134 (28.7%) | 71 (24.1%) | 63 (36.4%) | 0.005 |
| Positive family history | 72 (15.4%) | 46 (15.6%) | 26 (15.0%) | 0.858 |
| Previous MI | 152 (32.5%) | 115 (39.1%) | 37 (21.4%) | <0.001 |
| Previous stroke | 31 (6.7%) | 21 (7.1%) | 10 (5.8%) | 0.579 |
| Peripheral artery disease | 25 (5.1%) | 23 (5.0%) | 2 (5.7%) | 0.696 |
| Dialysis | 4 (0.9%) | 4 (1.4%) | 0 (0.0%) | 0.302 |
| Chronic kidney disease | 45 (9.6%) | 31 (10.5%) | 14 (8.1%) | 0.386 |
| Aortic aneurysm | 3 (0.6%) | 2 (0.7%) | 1 (0.6%) | >0.999 |
| Renal artery stenosis | 59 (12.6%) | 36 (12.2%) | 23 (13.3%) | 0.742 |
| Previous heart valve surgery | 3 (0.6%) | 2 (0.7%) | 1 (0.6%) | >0.999 |
| Previous CABG | 20 (4.3%) | 17 (5.8%) | 3 (1.7%) | 0.037 |
| Previous PCI | 105 (22.5%) | 69 (23.5%) | 36 (20.8%) | 0.506 |
| Echocardiography Results | ||||
| EF (%) | 52.6 ± 11.2 | 53.7 ± 11.8 | 51.1 ± 10.0 | 0.013 |
| TAPSE (mm) | 17.4 [12.0–23.0] | 20.5 [18.0–23.0] | 16.2 [12.0–22.0] | 0.329 |
| Parameter | Total Study Population N = 467 (%) | Patients with CCS N = 294 (%) | Patients with ACS N = 173 (%) | p |
|---|---|---|---|---|
| Erythrocytes (1012/L) | 4.7 [4.3, 5.0] | 4.7 [4.3, 5.0] | 4.6 [4.4, 5.0] | 0.753 |
| Hemoglobin (g/dL) | 13.9 [5.9–18.2] | 13.9 [5.9–18.2] | 13.8 [8.6–16.6] | 0.997 |
| Hematocrit (%) | 40.8 [14.6–52.7] | 41.0 [14.6–52.7] | 40.5 [27.8–48.9] | 0.298 |
| Fibrinogen (mg/dL) | 398.5 [104.0–834.0] | 389.4 [104.0–834.0] | 419.6 [198.0–809.0] | 0.012 |
| Glucose (mg/dL) | 117.3 [74.0–406.0] | 111.2 [74.0–275.0] | 127.7 [75.0–406.0] | <0.001 |
| Creatinine (mg/dL) | 1.0 [0.3–9.1] | 1.0 [0.5–9.1] | 0.9 [0.3–2.5] | 0.322 |
| Blood urea nitrogen (mg/dL) | 38.4 [13.0–161.0] | 38.5 [16.0–138.0] | 38.2 [13.0–161.0] | 0.356 |
| K+ (mmol/L) | 4.4 [2.8–6.5] | 4.5 [3.1–6.0] | 4.2 [2.8–6.5] | <0.001 |
| Na+ (mmol/L) | 140.7 [131.0–152.0] | 141.3 [131.0–152.0] | 139.6 [131.0–146.0] | <0.001 |
| Cl− (mmol/L) | 103.4 [5.9–196.1] | 103.9 [92.2–196.1] | 102.5 [5.9–114.0] | 0.087 |
| Total cholesterol (mg/dL) | 183.7 [70.0–607.0] | 175.4 [70.0–320.0] | 198.3 [79.0–607.0] | <0.001 |
| LDL (mg/dL) | 110.1 [28.0–465.0] | 103.2 [28.0–241.0] | 122.1 [34.0–465.0] | <0.001 |
| HDL (mg/dL) | 53.3 [17.0–183.0] | 52.8 [17.0–132.0] | 54.0 [22.0–183.0] | 0.589 |
| Triglycerides (mg/dL) | 118.0 [86.0,163.8] | 119.0 [87.0,161.0] | 116.0 [86.0,169.0] | 0.945 |
| TSH (μIU/mL) | 2.0 [0.0–58.6] | 1.8 [0.0–11.1] | 2.6 [0.0–58.6] | 0.052 |
| NT-proBNP1 (pg/mL) | 1622.5 [0.0–70,000] | 1480.0 [0.0–70,000] | 1762.0 [0.5–33,029] | 0.003 |
| hs-CRP (mg/L) | 1.2 [0.0–82.0] | 0.4 [0.0–10.2] | 2.5 [0.0–82.0] | <0.001 |
| Parameter | Whole Study Population N = 467 (%) | Patients with CCS N = 294 (%) | Patients with ACS N = 173 (%) | p |
|---|---|---|---|---|
| Coronary angiography results | ||||
| One-vessel disease | 135 (37.3%) | 66 (32.5%) | 69 (43.4%) | <0.001 |
| Two-vessel disease | 136 (37.6%) | 73 (36.0%) | 63 (39.6%) | |
| Three-vessel disease | 66 (18.2%) | 46 (22.7%) | 20 (12.6%) | |
| Left main stem | 22 (6.1%) | 18 (8.9%) | 4 (2.5%) | |
| Qualification for revascularization | ||||
| Pharmacological treatment | 134 (30.9%) | 119 (43.0%) | 15 (9.6%) | <0.001 |
| PCI | 211 (48.6%) | 80 (28.9%) | 131 (83.4%) | |
| CABG | 89 (20.5%) | 78 (28.2%) | 11 (7.0%) | |
| Location of lesions treated by PCI | ||||
| Bypass | 2 (0.8%) | 2 (2.1%) | 0 (0.0%) | 0.017 |
| Left circumflex artery/marginal branch | 59 (24.7%) | 28 (29.8%) | 31 (21.5%) | |
| Intermediate artery | 4 (1.7%) | 4 (4.3%) | 0 (0.0%) | |
| Left anterior descending artery/diagonal branch | 89 (37.3%) | 28 (29.9%) | 61 (42.4%) | |
| Left main stem | 1 (0.4%) | 0 (0.0%) | 1 (0.7%) | |
| Right coronary artery | 83 (34.9%) | 32 (34.0%) | 51 (35.4%) | |
| Number of implanted bare metal stents | ||||
| 0 | 321 (68.7%) | 240 (81.6%) | 81 (46.8%) | <0.001 |
| 1 | 121 (25.9%) | 48 (16.3%) | 73 (42.2%) | |
| 2 | 21 (4.5%) | 6 (2.0%) | 15 (8.7%) | |
| 3 | 4 (0.9%) | 0 (0.0%) | 4 (2.3%) | |
| Number of implanted drug-eluting stents | ||||
| 0 | 380 (81.5%) | 258 (87.8%) | 122 (70.9%) | <0.001 |
| 1 | 77 (16.5%) | 31 (10.5%) | 46 (26.7%) | |
| 2 | 8 (1.7%) | 4 (1.4%) | 4 (2.3%) | |
| 3 | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | |
| TIMI after PCI | ||||
| 0 | 11 (4.6%) | 3 (3.2%) | 8 (5.5%) | 0.876 |
| 1 | 3 (1.3%) | 1 (1.1%) | 2 (1.4%) | |
| 2 | 1 (0.4%) | 0 (0.0%) | 1 (0.7%) | |
| 3 | 225 (93.8%) | 90 (95.7%) | 135 (92.5%) | |
| Periprocedural complications (PCI) | ||||
| No reflow/slow reflow | 9 (2.1%) | 3 (1.1%) | 6 (3.6%) | 0.120 |
| Stent thrombosis | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) | |
| Parameter | Total Study Population N = 467 (%) | Patients with CCS N = 294 (%) | Patients with ACS N = 173 (%) | p |
|---|---|---|---|---|
| Acetylsalicylic acid | 429 (91.9%) | 263 (89.5%) | 166 (96.0%) | 0.013 |
| Clopidogrel | 307 (65.7%) | 151 (51.4%) | 156 (90.2%) | <0.001 |
| ACE inhibitor | 406 (86.9%) | 247 (84.0%) | 159 (91.9%) | 0.014 |
| Angiotensin receptor antagonist | 20 (4.3%) | 17 (5.8%) | 3 (1.7%) | 0.037 |
| Beta-blocker | 434 (92.9%) | 268 (91.2%) | 166 (96.0%) | 0.051 |
| Ca-blocker | 119 (25.5%) | 89 (30.3%) | 30 (17.3%) | 0.002 |
| Statins | 443 (94.9%) | 277 (94.2%) | 166 (96.0%) | 0.412 |
| Fibrates | 15 (3.2%) | 10 (3.4%) | 5 (2.9%) | 0.762 |
| Loop diuretic | 80 (17.1%) | 51 (17.3%) | 29 (16.8%) | 0.872 |
| Thiazide | 48 (10.3%) | 38 (12.9%) | 10 (5.8%) | 0.014 |
| Mineralcorticoid receptor antagonist | 51 (10.9%) | 30 (10.2%) | 21 (12.1%) | 0.517 |
| Alpha-blocker | 12 (2.6%) | 9 (3.1%) | 3 (1.7%) | 0.548 |
| Oral anticoagulation | 33 (7.1%) | 23 (7.8%) | 10 (5.8%) | 0.405 |
| Insulin | 44 (9.4%) | 28 (9.5%) | 16 (9.2%) | 0.922 |
| Nitrates | 84 (18.0%) | 64 (21.8%) | 20 (11.6%) | 0.006 |
| Endpoint | CAD Study Population N = 467 (%) | Patients with CCS N = 294 (%) | Patients with ACS N = 173 (%) | p |
|---|---|---|---|---|
| Death | 139 (29.8%) | 89 (30.4%) | 50 (28.9%) | 0.737 |
| MI | 56 (12.0%) | 29 (9.9%) | 27 (15.6%) | 0.067 |
| Stroke | 21 (4.5%) | 12 (4.1%) | 9 (5.2%) | 0.578 |
| CABG | 37 (7.9%) | 29 (9.9%) | 8 (4.6%) | 0.042 |
| PCI | 79 (17.0%) | 50 (17.1%) | 29 (16.8%) | 0.933 |
| Study Population | Patients with CCS | Patients with ACS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | |||||||||
| [30, 55] | — | — | — | — | — | — | — | — | — |
| [55, 60] | 2.12 | 0.92, 4.86 | 0.076 | 5.29 | 0.63, 44.2 | 0.12 | 0.94 | 0.30, 2.97 | >0.9 |
| [60, 65] | 1.24 | 0.48, 3.16 | 0.7 | 3.04 | 0.33, 27.7 | 0.3 | 0.54 | 0.13, 2.34 | 0.4 |
| [65, 75] | 2.88 | 1.31, 6.34 | 0.008 | 3.97 | 0.48, 32.6 | 0.2 | 1.60 | 0.54, 4.69 | 0.4 |
| [75, 90] | 8.07 | 3.65, 17.8 | <0.001 | 13.3 | 1.68, 106 | 0.014 | 4.99 | 1.70, 14.7 | 0.003 |
| Diabetes | |||||||||
| no | 0.63 | 0.42, 0.95 | 0.028 | 0.41 | 0.19, 0.87 | 0.021 | — | — | — |
| Previous myocardial infarction | |||||||||
| no | 0.61 | 0.41, 0.92 | 0.017 | 0.46 | 0.22, 0.98 | 0.045 | — | — | — |
| Previous stroke | |||||||||
| no | — | — | — | — | — | — | 0.27 | 0.11, 0.68 | 0.005 |
| Cardiac arrest | |||||||||
| no | — | — | — | — | — | — | 0.20 | 0.04, 0.95 | 0.044 |
| Atrial fibrillation | |||||||||
| no | 0.49 | 0.28, 0.85 | 0.011 | 0.04 | 0.01, 0.25 | <0.001 | 0.45 | 0.20, 1.03 | 0.059 |
| Chronic kidney disease | |||||||||
| no | 0.45 | 0.27, 0.75 | 0.002 | — | — | — | — | — | — |
| TIMI after PCI | |||||||||
| 0 | — | — | — | — | — | — | — | — | — |
| 1 | — | — | — | 1.42 | 0.11, 18.1 | 0.8 | — | — | — |
| 2 | — | — | — | — | — | — | — | — | — |
| 3 | — | — | — | 0.20 | 0.05, 0.81 | 0.024 | — | — | — |
| Clinical status | |||||||||
| CCS | — | — | — | — | — | — | — | — | — |
| NSTEMI | 0.93 | 0.53, 1.61 | 0.8 | — | — | — | — | — | — |
| STEMI | 1.73 | 0.99, 3.02 | 0.052 | — | — | — | — | — | — |
| UA | 0.37 | 0.15, 0.93 | 0.034 | — | — | — | — | — | — |
| Left ventricular ejection fraction | |||||||||
| ≤40 | — | — | — | — | — | — | — | — | — |
| [40, 50] | 0.53 | 0.32, 0.87 | 0.011 | — | — | — | 0.46 | 0.22, 0.98 | 0.044 |
| [50, 60] | 0.51 | 0.29, 0.90 | 0.020 | — | — | — | 0.24 | 0.09, 0.65 | 0.005 |
| >60 | 0.43 | 0.23, 0.78 | 0.006 | — | — | — | 0.23 | 0.08, 0.69 | 0.008 |
| LDL cholesterol | |||||||||
| ≤100 | — | — | — | — | — | — | — | — | — |
| [100, 129] | 0.61 | 0.35, 1.06 | 0.080 | — | — | — | — | — | — |
| [129, 159] | 0.77 | 0.46, 1.29 | 0.3 | — | — | — | — | — | — |
| [159, 465] | 0.96 | 0.51, 1.81 | >0.9 | — | — | — | — | — | — |
| Study | No of Patients | Comorbidities | Treatment | Outcomes |
|---|---|---|---|---|
| Grinberg et al. [18] High/very high/extremely high risk | 5359 STEMI 39.1/32.8/23.9% | Hypertension: 87.7/92.4/97.3% DM: 57.1/69.4/80.7% Dyslipidemia: 74.1/77.5/82.3% Prior MI: 38.1/51.9/65.5% Prior PCI: 35.9/41.6/52.3% | PCI: 59.3/50.4/40.8% Based on time: 2002–2008 vs. 2010–2018: 52.6 vs. 66.5%/41.7 vs. 59.1%/34.7 vs. 47.2% | 1-year mortality: 12.8/18.9/28.8% |
| Toyoda et al. [23] | 3283 STEMI–68.9% | - | - | 3-year mortality: STEMI: 14.9–16.9% NSTEMI: 5.6–29.6% |
| Piątek et al. [24] STEMI/NSTEMI/UA | STEMI: 2134 NSTEMI: 1162 UA: 2729 | Hypertension: 62.7/70.8/76.6% DM: 18/21.3/24.7% Prior MI: 11/18.2/23.4% Prior PCI: 9.8/13.1/22.6% | All patients underwent PCI | 3-year outcomes: Death: 15.4/15.5/10.5% MI: 3.5/2.2/1.6% PCI: 29.5/31.4/29.1% CABG: 3.2/2.5/2.0% |
| Reichlin et al. [27] | Acute MI: 242 | Hypertension: 75% DM: 24% Dyslipidemia: 51% Prior MI: 31% Prior PCI: 28% | PCI/CABG: 68% | 30-month mortality: 16.4–23.9% depending on MI size |
| Buller et al. [28] MINOCA/STEMI | MINOCA: 112 STEMI: 166 | Hypertension: 53/42% DM: 13/17% Dyslipidemia: 25/37% Prior MI: 0/5.4% Prior PCI: 0/1.8% | All patients underwent PCI | 9-year outcomes: All-cause death: 17.9/24.1% Cardiac death: 9.8/16.9% MI: 14.3/21.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kern, A.; Stompór, T.; Bojko, K.; Sienkiewicz, E.; Pawlak, S.; Pawlak, K.; Pawlak, D.; Poskrobko, G.; Andrasz, E.; Gromadziński, L.; et al. Comparative Ten-Year Outcomes in Chronic and Acute Coronary Syndrome Patients Undergoing Invasive Diagnostics—Insights from the KORONEF Registry. Biomedicines 2024, 12, 2672. https://doi.org/10.3390/biomedicines12122672
Kern A, Stompór T, Bojko K, Sienkiewicz E, Pawlak S, Pawlak K, Pawlak D, Poskrobko G, Andrasz E, Gromadziński L, et al. Comparative Ten-Year Outcomes in Chronic and Acute Coronary Syndrome Patients Undergoing Invasive Diagnostics—Insights from the KORONEF Registry. Biomedicines. 2024; 12(12):2672. https://doi.org/10.3390/biomedicines12122672
Chicago/Turabian StyleKern, Adam, Tomasz Stompór, Krystian Bojko, Ewa Sienkiewicz, Sebastian Pawlak, Krystyna Pawlak, Dariusz Pawlak, Grzegorz Poskrobko, Ewa Andrasz, Leszek Gromadziński, and et al. 2024. "Comparative Ten-Year Outcomes in Chronic and Acute Coronary Syndrome Patients Undergoing Invasive Diagnostics—Insights from the KORONEF Registry" Biomedicines 12, no. 12: 2672. https://doi.org/10.3390/biomedicines12122672
APA StyleKern, A., Stompór, T., Bojko, K., Sienkiewicz, E., Pawlak, S., Pawlak, K., Pawlak, D., Poskrobko, G., Andrasz, E., Gromadziński, L., Jalali, R., Onichimowski, D., Piwko, G., Zalewski, A., & Bil, J. (2024). Comparative Ten-Year Outcomes in Chronic and Acute Coronary Syndrome Patients Undergoing Invasive Diagnostics—Insights from the KORONEF Registry. Biomedicines, 12(12), 2672. https://doi.org/10.3390/biomedicines12122672








