Thermophysical Insights into the Anti-Inflammatory Potential of Magnetic Fields
Abstract
1. Introduction
- Engagement of Receptor-Operated Calcium Entry (ROCE), modulated by auto- or paracrine adenosine triphosphate (ATP), adenosine diphosphate ribose (ADPR), and multiple other chemical ligands or physical stimuli. In this context, the inositol-phosphate 3 (IP3) receptor channel, which allows calcium fluxes into the cytoplasm, is activated by phospholipases and is paired with the production of diacylglycerol (DAG), which is a ligand for some receptors and channels. Intracellular phospholipases, modulated by magnesium or zinc interchanges, determine signal cascades downstream of the B- and T-cell receptor (BcR and TcR);
- Premature release of calcium from Store-Operated Ca2+ Entry (SOCE);
- Variations in Voltage-Operated Ca2+ Entry (VOCE [20]), and
- Variations in Na+ driving effects.
- Energy processes involve mitochondria activities; as such, the control of energy fluxes must be considered as a pillar of any approach to inflammation;
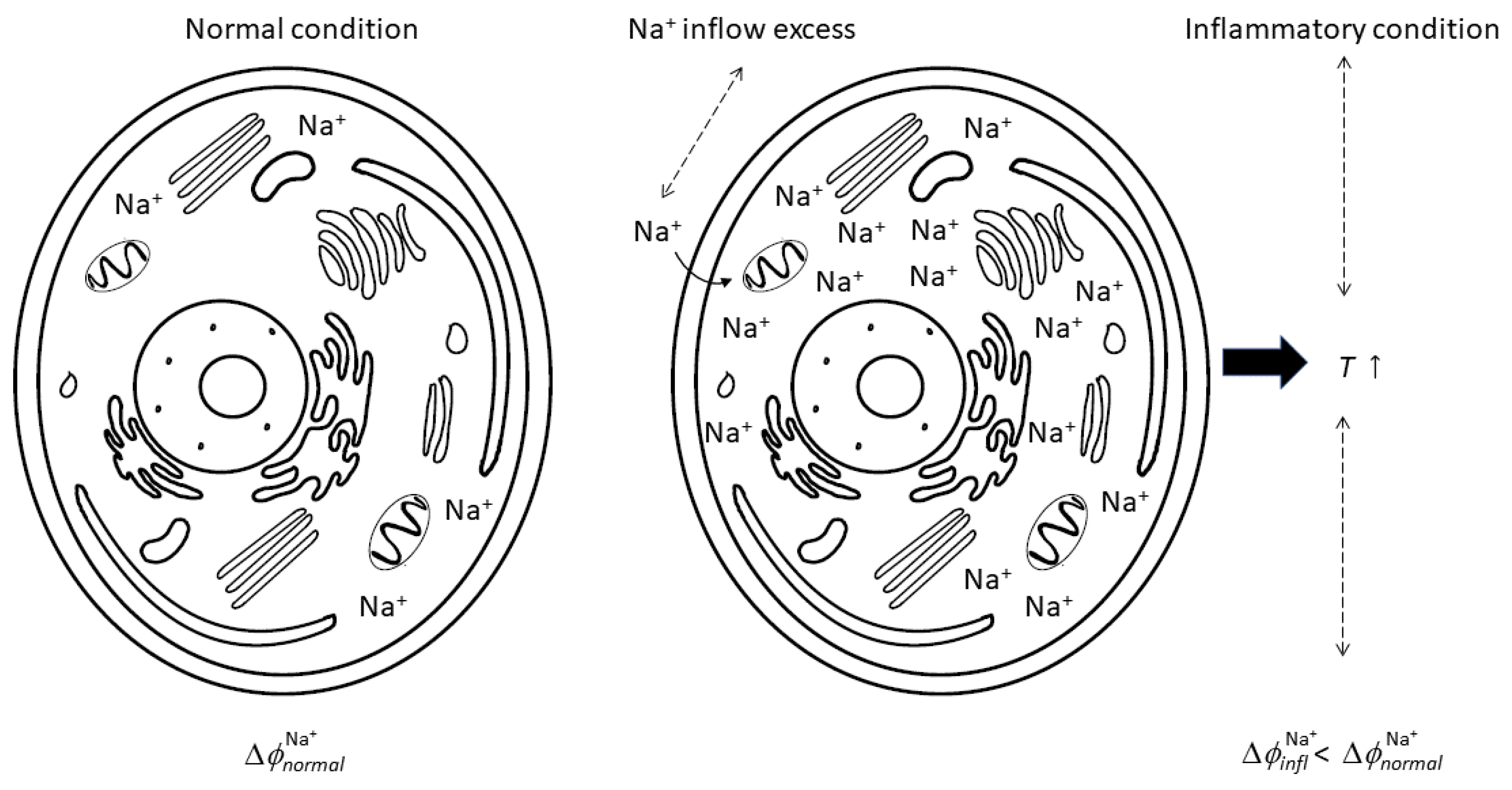
- Na+ flux is at the inception of inflammation; as such, a decrease in Na+ inner concentration can represent a fundamental therapeutic strategy to more effectively manage if not reduce inflammation. In this context, the role of Ca2+ appears to be important.
2. Methods
3. Results
- Diameter in the range of 20–100 µm
- Membrane depth in the range of 5–10 nm
- 2 Hz affects inflammation by downregulating TNF- and IL-1
- 4 Hz reduces oxidative stress;
- 12 Hz improves local microcirculation;
- 15 Hz increases alkaline phosphatase activity (ALP) and chondrogenesis;
- 30 Hz affects inflammation by downregulating IL-10;
- 50 Hz impacts inflammation by reducing chemokine production;
- 75 Hz upregulates A2A and A3 adenosine receptors and induces anti-inflammatory effects.
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blank, M.; Goodman, R. Do electromagnetic ields interact directly with DNA? Bioelectromagnetics 1997, 18, 111–115. [Google Scholar] [CrossRef]
- Markov, M.; Nindl, G.; Haslewood, C.; Cuppen, J. Interactions between electromagnetic fields and immune system: Possible mechanism for pain control. In Bioelectromagnetics Current Concepts; Ayrapetyan, S.N., Markov, M.S., Eds.; Springer: Berlin, Germany, 2006; pp. 213–225. [Google Scholar]
- Saino, E.; Fassina, L.; Van Vlierberghe, S.; Avanzini, M.A.; Dubruel, P.; Magenes, G.; Visai, L.; Benazzo, F. Efects of electromagnetic stimulation on osteogenic diferentiation of human mesenchymal stromal cells seeded onto gelatin cryogel. Int. J. Immunopathol. Pharmacol. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.W.; Pennison, M.J.; Brezovich, I.; Yi, N.; Yang, C.T.; Ramaker, R.; Absher, D.; Myers, R.M.; Kuster, N.; Costa, F.P.; et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer 2012, 106, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Barbault, A.; Costa, F.P.; Bottger, B.; Munden, R.F.; Bomholt, F.; Kuster, N.; Pasche, B. Amplitude-modulated electromagnetic fields for the treatment of cancer: Discovery of tumor-speciic frequencies and assessment of a novel therapeutic approach. J. Exp. Clin. Cancer Res. 2009, 28, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.P.; de Oliveira, A.C.; Meirelles, R.; Machado, M.C.C.; Zanesco, T.; Surjan, R.; Chammas, M.C.; de Souza Rocha, M.; Morgan, D.; Cantor, A.; et al. Treatment of advanced hepatocellular carcinoma with very low levels of amplitude-modulated electromagnetic fields. Br. J. Cancer 2011, 105, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Harrison, B.S. The use of magnetic field for the reduction of inflammation: A review of the history and therapeutic results. Altern. Ther. Health Med. 2013, 19, 47–54. [Google Scholar] [PubMed]
- Ganesan, K.; Gengadharan, A.C.; Balachandran, C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency pulsed eletromagnetic field—A viable alternative for arthritis. Indian J. Exp. Biol. 2009, 47, 939–948. [Google Scholar]
- Markov, M. Expanding use of pulsed electromagnetic field therapies. Electromagn. Biol. Med. 2007, 26, 257–274. [Google Scholar] [CrossRef]
- Gordon, G. Designed electromagnetic pulsed therapy: Clinical applications. J. Cell. Physiol. 2007, 212, 579–582. [Google Scholar] [CrossRef]
- Walleczek, J. Electromagnetic field efects on cells of the immune system: The role of calcium signaling. FASEB J. 1992, 6, 3177–3185. [Google Scholar] [CrossRef]
- Kindzelskii, A.; Petty, H. Ion channel clustering enhances weak electric ield detection by neutrophils: Apparent roles of SKF96365-sensitive cation channels and myeloperoxidase traicking in cellular responses. Eur. Biophys. J. 2005, 35, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, J.; Lekhraj, R.; White, N.M.; Flamm, E.S.; Pilla, A.A.; Strauch, B.; Casper, D. Attenuation of interleukin-1beta by pulsed electromagnetic fields after traumatic brain injury. Neurosci. Lett. 2012, 519, 4–8. [Google Scholar] [CrossRef]
- Darouiche, R. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Harris, L.; Richards, R. Staphylococci and implant surfaces: A review. Injury 2006, 37, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Coletto, L.A.; Sciorati, C.; Bozzolo, E.P.; Manunta, P.; Rovere-Querini, P.; Manfredi, A.A. Ion Channels and Transporters in Inflammation: Special Focus on TRP Channels and TRPC6. Cells 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Argalski, S.; Schäfers, M.; Hagenacker, T. Modulation of Voltage-Gated Sodium Channels by Activation of Tumor Necrosis Factor Receptor-1 and Receptor-2 in Small DRG Neurons of Rats. Mediat. Inflamm. 2015, 2015, 24942. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M. Changes in ion transport in inflammatory disease. J. Inflamm. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Bennett, M.V.L.; Pelegrin, P.; Fernandez, R. Ion Channels in Inflammatory Processes: What Is Known and What Is Next? Mediat. Inflamm. 2016, 2016, 6245731. [Google Scholar] [CrossRef][Green Version]
- Rajagopal, S. Modulatory Action of Voltage-gated Ion Channels in Inflammation and Inflammatory Pain. Open Neurol. J. 2023, 17, e1874205X2212290. [Google Scholar] [CrossRef]
- Pitake, S.; Abdus-Saboor, L.J.M.I.; Mishra, S.K. Inflammation induced sensory nerve growth and pain hypersensitivity requires the N-type calcium channel Cav2.2. Front. Neurosci. 2019, 13, 1009–1012. [Google Scholar] [CrossRef]
- Krampert, L.; Ossner, T.; Schröder, A.; Jantsch, V.S.J. Simultaneous Increases in Intracellular Sodium and Tonicity Boost Antimicrobial Activity of Macrophages. Cells 2023, 12, 2816. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Altier, C.; Hildebrand, M.E.; Trang, T.; Salter, M.W.; Zamponi, G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014, 94, 81–140. [Google Scholar] [CrossRef] [PubMed]
- Clemens, R.A.; Lowell, C.A. Store-operated calcium signaling in neutrophils. J. Leukoc. Biol. 2015, 98, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Salao, K.; Jiang, L.; Li, H.; Tsai, V.W.; Husaini, Y.; Curmi, P.M.; Brown, L.J.; Brown, D.A.; Breit, S.N. Clic1 regulates dendritic cell antigen processing and presentation by modulating phagosome acidification and proteolysis. Biol. Open 2016, 5, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Harr, M.W.; Rong, Y.; Bootman, M.D.; Roderick, H.L.; Distelhorst, C.W. Glucocorticoid-mediated inhibition of LCK modulates the pattern of T cell receptor-induced calcium signals by down-regulating inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2009, 284, 31860–31871. [Google Scholar] [CrossRef] [PubMed]
- Vaeth, M.; Feske, S. Ion channelopathies of the immune system. Curr. Opin. Immunol. 2018, 52, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Goldman, D.E. Potential impedance, and rectification in membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef]
- Grabe, M.; Wang, H.; Oster, G. The mechanochemistry of V-ATPase proton pumps. Biophs. J. 2000, 78, 2798–2813. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Elsevier Inc.: Philadelphia, PA, USA, 1960. [Google Scholar]
- Tuszynski, J.A. Molecular and Cellular Biophysics; CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics; John Wiley: New York, NY, USA, 2006. [Google Scholar]
- Binggelli, R.; Cameron, I.L. Cellular Potential of Normal and Cancerous Fibroblasts and Hepatocytes. Cancer Res. 1980, 40, 1830–1835. [Google Scholar]
- Lucia, U.; Grisolia, G. Constructal law and ion transfer in normal and cancer cells. Proc. Rom. Acad. Ser. A 2018, 19, 213–218. [Google Scholar]
- Lucia, U.; Deisboeck, T.S. The importance of ion fluxes for cancer proliferation and metastasis: A thermodynamic analysis. J. Theor. Biol. 2018, 445, 1–8. [Google Scholar] [CrossRef]
- Nakanishi-Matsui, M.; Sekiya, M.; Futai, R.K.N.M. The mechanism of rotating proton pumping ATPases. BBA—Bioenerg. 2010, 1797, 1343–1352. [Google Scholar] [CrossRef]
- Stevens, T.H.; Forgac, M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell. Dev. Biol. 1997, 13, 779–808. [Google Scholar] [CrossRef]
- Tuszynski, J.A.; Kurzynski, M. Introduction to Molecular Biophysics; CRC Press: Boca Raton, FL, USA, 2003; pp. 383–392. [Google Scholar]
- Lucia, U.; Ponzetto, A.; Deisboeck, T.S. A thermo-physical analysis of the proton pump vacuolar-ATPase: The constructal approach. Sci. Rep. 2014, 4, 1. [Google Scholar] [CrossRef]
- Rudolph, M.G.; Stanfield, R.L.; Wilson, I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006, 24, 419–466. [Google Scholar] [CrossRef]
- Strong, R.K. Asymmetric ligand recognition by the activating natural killer cell receptor NKG2D, a symmetric homodimer. Mol. Immunol. 2002, 38, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U.; Grisolia, G. Thermal Resonance and Cell Behavior. Entropy 2020, 22, 774. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G.; Dolcino, D.; Astori, M.R.; Massa, E.; Ponzetto, A. Constructal approach to bio-engineering: The ocular anterior chamber temperature. Sci. Rep. 2016, 6, 31099. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G.; Astori, M.R. Constructal law analysis of Cl− transport in eyes aqueous humor. Sci. Rep. 2017, 7, 6856. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U.; Grisolia, G.; Francia, S.; Astori, M.R. Theoretical biophysical approach to cross-linking effects on eyes pressure. Phys. A 2019, 534, 122163. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G. Resonance in Thermal Fluxes Through Cancer Membrane. Atti Dell’Accademia Peloritana Dei Pericolanti 2020, 98, SC1–SC6. [Google Scholar] [CrossRef]
- Ruff, R.L. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am. J. Physiol. 1999, 277, C937–C947. [Google Scholar] [CrossRef] [PubMed]
- Duzhyy, D.E.; Voitenko, N.V.; Belan, P.V. Peripheral Inflammation Results in Increased Excitability of Capsaicin-Insensitive Nociceptive DRG Neurons Mediated by Upregulation of ASICs and Voltage-Gated Ion Channels. Front. Cell. Neurosci. 2021, 15, 723295. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures on Physics, Volume II; Addison-Wesley Publishing Company: Boston, MA, USA, 1963. [Google Scholar]
- Lucia, U. Thermodynamic approach to nano-properties of cell membrane. Physical A 2014, 407, 185–191. [Google Scholar] [CrossRef]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Valentine, J.S.; Gimzewski, J.K. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 2004, 305, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Markus, I.; Ohayon, E.; Constantini, K.; Geva-Kleinberger, K.; Ibrahim, R.; Ruban, A.; Gepner, Y. The Effect of Extremely Low-Frequency Electromagnetic Fields on Inflammation and Performance-Related Indices in Trained Athletes: A Double-Blinded Crossover Study. Int. J. Mol. Sci. 2023, 24, 13463. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U.; Deisboeck, T.S.; Ponzetto, A.; Grisolia, G. A Thermodynamic Approach to the Metaboloepigenetics of Cancer. Int. J. Mol. Sci. 2023, 24, 3337. [Google Scholar] [CrossRef]
- Jayaram, D.T.; Luo, Q.; Thourson, S.B.; Finlay, A.H.; Payne, C.K. Controlling the Resting Membrane Potential of Cells with Conducting Polymer Microwires. Small 2017, 13, 1700789. [Google Scholar] [CrossRef] [PubMed]
- Moya-Gómez, A.; Font, L.; Burlacu, A.; Alpizar, Y.; Cardonne, M.; Brône, B.; Bronckaers, A. Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Improves Neurological Outcome and Reduces Microglial Reactivity in a Rodent Model of Global Transient Stroke. Int. J. Mol. Sci. 2023, 24, 11117. [Google Scholar] [CrossRef] [PubMed]
- Tekam, C.K.S.; Majumdar, S.; Kumari, P.; Prajapati, S.K.; Sahi, A.K.; Shinde, S.; Singh, R.; Samaiya, P.K.; Patnaik, R.; Krishnamurthy, S.; et al. Effects of ELF-PEMF exposure on spontaneous alternation, anxiety, motor co-ordination and locomotor activity of adult wistar rats and viability of C6 (Glial) cells in culture. Toxicology 2023, 485, 153409. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.B.; Kim, Y.C.; Lim, Y.J.; Lee, C.J.; Choi, S.S.; Park, S.H.; Lee, J.G.; Lee, S.C. Efficacy of pulsed electromagnetic therapy for chronic lower back pain: A randomized, double-blind, placebo-controlled study. J. Int. Med Res. 2006, 34, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.; Cole, S. A randomized controlled trial of the effects of a combination of static and dynamic fields on carpal tunnel syndrome. J. Exp. Clin. Cancer Res. 2008, 9, 493–504. [Google Scholar] [CrossRef][Green Version]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Liao, R.; Shuai, Y.; Pan, H.; Qian, G.; Peng, S.; Shuai, C. A conductive network enhances nerve cell response. Addit. Manuf. 2022, 52, 102694. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- Qi, F.; Li, H.; Chen, G.; Peng, S.; Luo, X.; Xiong, S.; Zhu, H.; Shuai, C. A CuS@g-C3N4 heterojunction endows scaffold with synergetic antibacterial effect. Colloids Surf. B Biointerfaces 2023, 230, 113512. [Google Scholar] [CrossRef]
- Costantini, E.; Aielli, L.; Gualdi, G.; Baronio, M.; Monari, P.; Amerio, P.; Reale, M. Pulsed Radiofrequency Electromagnetic Fields as Modulators of Inflammation and Wound Healing in Primary Dermal Fibroblasts of Ulcers. Bioengineering 2024, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Reddi, A.H. Pulsed Electromagnetic Fields and Tissue Engineering of the Joints. Tissue Eng. Part B Rev. 2018, 24, 144–154. [Google Scholar] [CrossRef]

| Ion | Extracellular | Intracellular | Chemical | Membrane |
|---|---|---|---|---|
| Species | Concentration | Concentration | Potential | Potential |
| [M] | [M] | [J mol−1] | [V] | |
| Na+ | 18 | 150 | −261.89 | +56 |
| K+ | 140 | 5 | −283.26 | −89 |
| Cl− | 120 | 7 | −131.26 | −76 |
| Ca2+ | 1.2 | 0.1 | −553.04 | +125 |
| Disease | Frequency [Hz] | Key Finding | Reference |
|---|---|---|---|
| Arthritis | 60 | Reduction in pain and inflammation | [8] |
| Back pain | 64 | Statistically significant for reducing pain | [60] |
| Carpal tunnel | 20 | Statistically significant pain reduction * | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia, U.; Grisolia, G.; Ponzetto, A.; Deisboeck, T.S. Thermophysical Insights into the Anti-Inflammatory Potential of Magnetic Fields. Biomedicines 2024, 12, 2534. https://doi.org/10.3390/biomedicines12112534
Lucia U, Grisolia G, Ponzetto A, Deisboeck TS. Thermophysical Insights into the Anti-Inflammatory Potential of Magnetic Fields. Biomedicines. 2024; 12(11):2534. https://doi.org/10.3390/biomedicines12112534
Chicago/Turabian StyleLucia, Umberto, Giulia Grisolia, Antonio Ponzetto, and Thomas S. Deisboeck. 2024. "Thermophysical Insights into the Anti-Inflammatory Potential of Magnetic Fields" Biomedicines 12, no. 11: 2534. https://doi.org/10.3390/biomedicines12112534
APA StyleLucia, U., Grisolia, G., Ponzetto, A., & Deisboeck, T. S. (2024). Thermophysical Insights into the Anti-Inflammatory Potential of Magnetic Fields. Biomedicines, 12(11), 2534. https://doi.org/10.3390/biomedicines12112534







