Club Cells—A Guardian against Occupational Hazards
Abstract
1. Introduction
2. General Characteristics of Club Cells
3. Functions of Club Cells Related to the Pathogenesis of Interstitial Lung Diseases
3.1. Progenitor and Repair Function
3.2. Metabolism of Xenobiotics
3.3. Secretion of Club Cells and Their Relation to the Fibrotic Process
3.3.1. Modulation of the Inflammatory Process
3.3.2. Modulation of the Fibrotic Process
4. Club Cell Potential Biomarkers in Occupational Interstitial Lung Diseases
4.1. Inorganic Particles
4.1.1. Silica
4.1.2. Asbestos
4.1.3. Nanomaterials
4.1.4. Coal
4.2. Organic Particles
4.2.1. Bioaerosols
4.2.2. Organic Dust
4.3. Mixture Exposure (Vapors, Gazes, Dust and Fumes VGDF)
4.3.1. Smoke Exposure during Firefighting
4.3.2. Coke Ovens
4.3.3. Sulfur Dioxide in a Non-Ferrous Smelter
4.3.4. Ozone Exposure
4.3.5. Hydrogen Peroxide
4.3.6. Diesel Exhaust
4.3.7. Welding Dusts and Fumes
4.4. Chemicals
4.4.1. Isocyanates
4.4.2. Pesticides
5. Study Limitations
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celli, B.R.; Owen, C.A. The club cell and its protein, CC16: Time to shine. Lancet Respir. Med. 2013, 1, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Dierynck, I.; Bernard, A.; Roels, H.; DeLey, M. The human Clara cell protein: Biochemical and biological characterisation of a natural immunosuppressor. MSJ 1996, 1, 385–387. [Google Scholar] [CrossRef]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Sack, C.S.; Doney, B.C.; Podolanczuk, A.J.; Hooper, L.G.; Seixas, N.S.; Hoffman, E.A.; Kawut, S.M.; Vedal, S.; Raghu, G.; Barr, R.G.; et al. Occupational Exposures and Subclinical Interstitial Lung Disease. The MESA (Multi-Ethnic Study of Atherosclerosis) Air and Lung Studies. Am. J. Respir. Crit. Care Med. 2017, 196, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Blackley, D.J.; Halldin, C.N.; Cohen, R.A.; Cummings, K.J.; Storey, E.; Laney, A.S. Misclassification of Occupational Lung Disease in a U.S. Organ Transplant Registry. Am. J. Respir. Crit. Care Med. 2017, 195, A3846. [Google Scholar]
- Kuroda, A. Recent progress and perspectives on the mechanisms underlying Asbestos toxicity. Genes Environ. 2021, 43, 46. [Google Scholar] [CrossRef]
- Dörger, M.; Münzing, S.; Allmeling, A.M.; Messmer, K.; Krombach, F. Differential responses of rat alveolar and peritoneal macrophages to man-made vitreous fibers in vitro. Environ. Res. 2001, 85, 207–214. [Google Scholar] [CrossRef]
- Wong, A.P.; Keating, A.; Waddell, T.K. Airway regeneration: The role of the Clara cell secretory protein and the cells that express it. Cytotherapy 2009, 11, 676–687. [Google Scholar] [CrossRef]
- Zuo, W.L.; Shenoy, S.A.; Li, S.; O’Beirne, S.L.; Strulovici-Barel, Y.; Leopold, P.L.; Wang, G.; Staudt, M.R.; Walters, M.S.; Mason, C.; et al. Ontogeny and Biology of Human Small Airway Epithelial Club Cells. Am. J. Respir. Crit. Care Med. 2018, 198, 1375–1388. [Google Scholar] [CrossRef]
- Plopper, C.G.; Hill, L.H.; Mariassy, A.T. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp. Lung Res. 1980, 1, 171–180. [Google Scholar] [CrossRef]
- Bernard, A.; Dumont, X.; Roels, H.; Lauwerys, R.; Dierynck, I.; De Ley, M.; Stroobant, V.; de Hoffmann, E. The molecular mass and concentrations of protein 1 or Clara cell protein in biological fluids: A reappraisal. Clin. Chim. Acta 1993, 223, 189–191. [Google Scholar] [CrossRef]
- Hermans, C.; Bernard, A. Lung epithelium-specific proteins: Characteristics and potential applications as markers. Am. J. Respir. Crit. Care Med. 1999, 159, 646–678. [Google Scholar] [CrossRef]
- Peri, A.; Cordella-Miele, E.; Miele, L.; Mukherjee, A.B. Tissue-specific expression of the gene coding for human Clara cell 10-kD protein, a phospholipase A2-inhibitory protein. J. Clin. Investig. 1993, 92, 2099–2109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doyle, I.R.; Hermans, C.; Bernard, A.; Nicholas, T.E.; Bersten, A.D. Clearance of Clara cell secretory protein 16 (CC16) and surfactant proteins A and B from blood in acute respiratory failure. Am. J. Respir. Crit. Care Med. 1998, 158 Pt 1, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Yokoyama, S.; Pilon, A.L.; Kurotani, R. Emerging role of an immunomodulatory protein secretoglobin 3A2 in human diseases. Pharmacol. Ther. 2022, 236, 108112. [Google Scholar] [CrossRef] [PubMed]
- Laucho-Contreras, M.E.; Polverino, F.; Tesfaigzi, Y.; Pilon, A.; Celli, B.R.; Owen, C.A. Club Cell Protein 16 (CC16) Augmentation: A Potential Disease-modifying Approach for Chronic Obstructive Pulmonary Disease (COPD). Expert. Opin. Ther. Targets 2016, 20, 869–883. [Google Scholar] [CrossRef]
- Fukumoto, J.; Soundararajan, R.; Leung, J.; Cox, R.; Mahendrasah, S.; Muthavarapu, N.; Herrin, T.; Czachor, A.; Tan, L.C.; Hosseinian, N.; et al. The role of club cell phenoconversion and migration in idiopathic pulmonary fibrosis. Aging 2016, 29, 3091–3109. [Google Scholar] [CrossRef]
- Aono, Y.; Ledford, J.G.; Mukherjee, S.; Ogawa, H.; Nishioka, Y.; Sone, S.; Beers, M.F.; Noble, P.W.; Wright, J.R. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am. J. Respir. Crit. Care Med. 2012, 185, 525–536. [Google Scholar] [CrossRef]
- van de Wetering, J.K.; van Golde, L.M.; Batenburg, J.J. Collectins: Players of the innate immune system. Eur. J. Biochem. 2004, 271, 1229–1249. [Google Scholar] [CrossRef]
- Stripp, B.R. Hierarchical organization of lung progenitor cells: Is there an adult lung tissue stem cell? Proc. Am. Thorac. Soc. 2008, 15, 695–698. [Google Scholar] [CrossRef]
- Chen, F.; Fine, A. Stem Cells in Lung Injury and Repair. Am. J. Pathol. 2016, 186, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Tata, P.R.; Rajagopal, J. Plasticity in the lung: Making and breaking cell identity. Development 2017, 144, 755–766. [Google Scholar] [CrossRef]
- Stripp, B.R.; Reynolds, S.D. Maintenance and repair of the bronchiolar epithelium. Proc. Am. Thorac. Soc. 2008, 5, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999, 159, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Katyal, S.L. Clara cells and Clara cell 10 kD protein (CC10). Am. J. Respir. Cell Mol. Biol. 1997, 17, 141–143. [Google Scholar] [CrossRef]
- Redente, E.F.; Black, B.P.; Backos, D.S.; Bahadur, A.N.; Humphries, S.M.; Lynch, D.A.; Tuder, R.M.; Zemans, R.L.; Riches, D.W.H. Persistent, Progressive Pulmonary Fibrosis and Epithelial Remodeling in Mice. Am. J. Respir. Cell Mol. Biol. 2021, 64, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc. Am. Thorac. Soc. 2008, 5, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef]
- Greene, K.E.; King, T.E.; Kuroki, Y.; Bucher-Bartelson, B.; Hunninghake, G.W.; Newman, L.S.; Nagae, H.; Mason, R.J. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur. Respir. J. 2002, 19, 439–446. [Google Scholar] [CrossRef]
- Hukkanen, J.; Pelkonen, O.; Hakkola, J.; Raunio, H. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Crit. Rev. Toxicol. 2002, 32, 391–411. [Google Scholar] [CrossRef]
- Lautt, W.W.; Greenway, C.V. Conceptual review of the hepatic vascular bed. Hepatology 1987, 7, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Hynes, D.E.; DeNicola, D.B.; Carlson, G.P. Metabolism of styrene by mouse and rat isolated lung cells. Toxicol. Sci. 1999, 51, 195–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rokicki, W.; Rokicki, M.; Wojtacha, J.; Dżeljijli, A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir. Torakochirurgia. Pol. 2016, 13, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.; Chang, A.; Weir, A.; Nishio, S.; Hyde, D.; Philpot, R.; Buckpitt, A.; Plopper, C. Cellular and metabolic basis of Clara cell tolerance to multiple doses of cytochrome P450-activated cytotoxicants. I: Bronchiolar epithelial reorganization and expression of cytochrome P450 monooxygenases in mice exposed to multiple doses of naphthalene. J. Pharmacol. Exp. Ther. 1996, 278, 1408–1418. [Google Scholar]
- Townsend, D.M. S-glutathionylation: Indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.H.; van der Velden, J.L.; Lahue, K.G.; Qian, X.; Schneider, R.W.; Iberg, M.S.; Nolin, J.D.; Abdalla, S.; Casey, D.T.; Tew, K.D.; et al. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase π. JCI Insight 2016, 1, e85717. [Google Scholar] [CrossRef]
- Harju, T.; Mazur, W.; Merikallio, H.; Soini, Y.; Kinnula, V.L. Glutathione-S-transferases in lung and sputum specimens, effects of smoking and COPD severity. Respir. Res. 2008, 9, 80. [Google Scholar] [CrossRef]
- Devereux, T.R. Alveolar type II and Clara cells: Isolation and xenobiotic metabolism. Environ. Health Perspect. 1984, 56, 95–101. [Google Scholar] [CrossRef][Green Version]
- Adair-Kirk, T.L.; Atkinson, J.J.; Griffin, G.L.; Watson, M.A.; Kelley, D.G.; DeMello, D.; Senior, R.M.; Betsuyaku, T. Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in Clara cells. Am. J. Respir. Cell Mol. Biol. 2008, 39, 400–411. [Google Scholar] [CrossRef]
- Stripp, B.R.; Reynolds, S.D.; Boe, I.M.; Lund, J.; Power, J.H.; Coppens, J.T.; Wong, V.; Reynolds, P.R.; Plopper, C.G. Clara cell secretory protein deficiency alters clara cell secretory apparatus and the protein composition of airway lining fluid. Am. J. Respir. Cell Mol. Biol. 2002, 27, 170–178. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Long, X.B.; Hu, S.; Wang, N.; Zhen, H.T.; Cui, Y.H.; Liu, Z. Clara cell 10-kDa protein gene transfection inhibits NF-κB activity in airway epithelial cells. PLoS ONE 2012, 7, e35960. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Yuan, Y.; Wang, D.; Li, T.; Wang, D.; Shi, X.; Guo, M.; Wang, C.; Zhang, X.; Zheng, G.; et al. Recombinant CC16 protein inhibits the production of pro-inflammatory cytokines via NF-κB and p38 MAPK pathways in LPS-activated RAW264.7 macrophages. Acta Biochim. Biophys. Sin. 2017, 49, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Uozumi, N.; Ishii, S.; Kita, Y.; Yamamoto, H.; Ohga, E.; Ouchi, Y.; Shimizu, T. A pivotal role of cytosolic phospholipase A(2) in bleomycin-induced pulmonary fibrosis. Nat. Med. 2002, 8, 480–484. [Google Scholar] [CrossRef]
- Nagase, T.; Uozumi, N.; Ishii, S.; Kume, K.; Izumi, T.; Ouchi, Y.; Shimizu, T. Acute lung injury by sepsis and acid aspiration: A key role for cytosolic phospholipase A2. Nat. Immunol. 2000, 1, 42–46. [Google Scholar] [CrossRef]
- Suryadevara, V.; Ramchandran, R.; Kamp, D.W.; Natarajan, V. Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4257. [Google Scholar] [CrossRef]
- Bozyk, P.D.; Moore, B.B. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 45–52. [Google Scholar] [CrossRef]
- Coward, W.R.; Watts, K.; Feghali-Bostwick, C.A.; Knox, A.; Pang, L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol. Cell Biol. 2009, 9, 4325–4339. [Google Scholar] [CrossRef]
- Chen, L.C.; Huang, J.L.; Huang, S.K.; Kuo, M.L.; Wu, A.H. Clara Cell 10-kd Protein (CC10) Modulates Superoxide Generation and Arachidonic Acid Metabolism via Formyl Peptide Receptor-Like 1 (FPRL1). J. Allergy Clin. Immunol. 2019, 143 (Suppl. S2), AB190. [Google Scholar] [CrossRef]
- Lesur, O.; Bernard, A.; Arsalane, K.; Lauwerys, R.; Bégin, R.; Cantin, A.; Lane, D. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am. J. Respir. Crit. Care Med. 1995, 152, 290–297. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Medjane, S.; Chabot, S.; Kubrusly, F.S.; Raw, I.; Chignard, M.; Touqui, L. Surfactant protein-A and phosphatidylglycerol suppress type IIA phospholipase A2 synthesis via nuclear factor-kappaB. Am. J. Respir. Crit. Care Med. 2003, 168, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chabot, S.; Koumanov, K.; Lambeau, G.; Gelb, M.H.; Balloy, V.; Chignard, M.; Whitsett, J.A.; Touqui, L. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J. Immunol. 2003, 171, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Hu, Y. TGF-β1: Gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int. J. Mol. Med. 2021, 48, 132. [Google Scholar] [CrossRef]
- Yokoyama, T.; Yanagihara, T.; Suzuki, K.; Hamada, N.; Tsubouchi, K.; Ogata-Suetsugu, S.; Mikumo, H.; Ikeda-Harada, C.; Maeyama, T.; Kuwano, K.; et al. Depletion of club cells attenuates bleomycin-induced lung injury and fibrosis in mice. J. Inflamm. 2017, 14, 20. [Google Scholar] [CrossRef]
- Park, S.Y.; Hong, J.Y.; Lee, S.Y.; Lee, S.H.; Kim, M.J.; Kim, S.Y.; Kim, K.W.; Shim, H.S.; Park, M.S.; Lee, C.G.; et al. Club cell-specific role of programmed cell death 5 in pulmonary fibrosis. Nat. Commun. 2021, 12, 2923. [Google Scholar] [CrossRef]
- Van Winkle, L.S.; Isaac, J.M.; Plopper, C.G. Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am. J. Pathol. 1997, 151, 443–459. [Google Scholar]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef]
- Hancock, L.A.; Hennessy, C.E.; Solomon, G.M.; Dobrinskikh, E.; Estrella, A.; Hara, N.; Hill, D.B.; Kissner, W.J.; Markovetz, M.R.; Grove Villalon, D.E.; et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 2018, 9, 5363. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011, 364, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Na, H.G.; Choi, Y.S.; Bae, C.H.; Song, S.Y.; Kim, Y.D. Saponin attenuates diesel exhaust particle (DEP)-induced MUC5AC expression and pro-inflammatory cytokine upregulation via TLR4/TRIF/NF-κB signaling pathway in airway epithelium and ovalbumin (OVA)-sensitized mice. J. Ginseng Res. 2022, 46, 801–808. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Tang, X.X. Irreversibility of Pulmonary Fibrosis. Aging Dis. 2022, 13, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Montero-Fernandez, A.; Borg, E.; Osadolor, T.; Viola, P.; De Lauretis, A.; Stock, C.J.; Bonifazi, M.; Bonini, M.; Caramori, G.; et al. Mucins MUC5B and MUC5AC in Distal Airways and Honeycomb Spaces: Comparison among Idiopathic Pulmonary Fibrosis/Usual Interstitial Pneumonia, Fibrotic Nonspecific Interstitial Pneumonitis, and Control Lungs. Am. J. Respir. Crit. Care Med. 2016, 193, 462–464. [Google Scholar] [CrossRef]
- Tokita, E.; Tanabe, T.; Asano, K.; Suzaki, H.; Rubin, B.K. Club cell 10-kDa protein attenuates airway mucus hypersecretion and inflammation. Eur. Respir. J. 2014, 44, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, P.; Ahmed, E.; Serre, I.; Knabe, L.; Bommart, S.; Suehs, C.; Vachier, I.; Berthet, J.P.; Romagnoli, M.; Vernisse, C.; et al. Club Cell Loss as a Feature of Bronchiolization in ILD. Front. Immunol. 2021, 12, 630096. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Lomas, N.J.; Spiteri, M.A.; Forsyth, N.R. Club cells inhibit alveolar epithelial wound repair via TRAIL-dependent apoptosis. Eur. Respir. J. 2013, 41, 683–694. [Google Scholar] [CrossRef]
- Kim, K.K.; Dotson, M.R.; Agarwal, M.; Yang, J.; Bradley, P.B.; Subbotina, N.; Osterholzer, J.J.; Sisson, T.H. Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell Death Dis. 2018, 9, 1056. [Google Scholar] [CrossRef]
- Bartram, U.; Speer, C.P. The role of transforming growth factor beta in lung development and disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef]
- Kuwano, K. Epithelial cell apoptosis and lung remodeling. Cell Mol. Immunol. 2007, 4, 419–429. [Google Scholar]
- Zuo, W.-L.; Rostami, M.R.; LeBlanc, M.; Kaner, R.J.; O’Beirne, S.L.; Mezey, J.G.; Leopold, P.L.; Quast, K.; Visvanathan, S.; Fine, J.S.; et al. Dysregulation of club cell biology in idiopathic pulmonary fibrosis. PLoS ONE 2020, 15, e0237529. [Google Scholar] [CrossRef] [PubMed]
- Bolton, S.J.; Pinnion, K.; Marshall, C.V.; Wilson, E.; Barker, J.E.; Oreffo, V.; Foster, M.L. Changes in Clara cell 10 kDa protein (CC10)-positive cell distribution in acute lung injury following repeated lipopolysaccharide challenge in the rat. Toxicol. Pathol. 2008, 36, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Roldán, I.; Ruiz, V.; Sierra, P.; Montes, E.; Ramírez, R.; Vega, A.; Salgado, A.; Vargas, M.H.; Mejía, M.; Pardo, A.; et al. Increased Expression of CC16 in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0168552. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Levine, S.J.; Cowan, M.J.; Logun, C.; Shelhamer, J.H. Tumor necrosis factor-alpha stimulates human Clara cell secretory protein production by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Sun, F.; Yu, X.; Li, Q.; Zhao, L.; Hao, W.; Han, W. CC16-TNF-α negative feedback loop formed between Clara cells and normal airway epithelial cells protects against diesel exhaust particles exposure-induced inflammation. Aging 2021, 13, 19442–19459. [Google Scholar] [CrossRef] [PubMed]
- Nance, S.C.; Yi, A.K.; Re, F.C.; Fitzpatrick, E.A. MyD88 is necessary for neutrophil recruitment in hypersensitivity pneumonitis. J. Leukoc. Biol. 2008, 83, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, G.; Hunninghake, G.W. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J. Clin. Investig. 1997, 99, 2386–2390. [Google Scholar] [CrossRef]
- Nance, S.; Cross, R.; Fitzpatrick, E. Chemokine production during hypersensitivity pneumonitis. Eur. J. Immunol. 2004, 34, 677–685. [Google Scholar] [CrossRef]
- Dierynck, I.; Bernard, A.; Roels, H.; De Ley, M. Potent inhibition of both human interferon-gamma production and biologic activity by the Clara cell protein CC16. Am. J. Respir. Cell Mol. Biol. 1995, 12, 205–210. [Google Scholar] [CrossRef]
- Chen, E.S.; Greenlee, B.M.; Wills-Karp, M.; Moller, D.R. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am. J. Respir. Cell Mol. Biol. 2001, 24, 545–555. [Google Scholar] [CrossRef]
- Segel, M.J.; Izbicki, G.; Cohen, P.Y.; Or, R.; Christensen, T.G.; Wallach-Dayan, S.B.; Breuer, R. Role of interferon-gamma in the evolution of murine bleomycin lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L1255–L1262. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G.E.; Stock, C.J.; Shi-Wen, X.; Leoni, P.; Sestini, P.; Howat, S.L.; Bou-Gharios, G.; Nicholson, A.G.; Denton, C.P.; Grutters, J.C.; et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir. Res. 2013, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Duan, M.; Tang, Y.; Wu, J.; Zhao, K.; Zhong, Y.; He, Z.; Meng, J.; Chen, F.; Xiao, X.; et al. Impaired interferon-γ signaling promotes the development of silicosis. iScience 2022, 25, 104647. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.N.; Chen, X.; Foda, H.D.; Smaldone, G.C.; Hasaneen, N.A. Interferon-γ enhances the antifibrotic effects of pirfenidone by attenuating IPF lung fibroblast activation and differentiation. Respir. Res. 2019, 20, 206. [Google Scholar] [CrossRef]
- Thongtip, S.; Siviroj, P.; Prapamontol, T.; Deesomchok, A.; Wisetborisut, A.; Nangola, S.; Khacha-Ananda, S. A suitable biomarker of effect, club cell protein 16, from crystalline silica exposure among Thai stone-carving workers. Toxicol. Ind. Health 2020, 36, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.S.; Lambe, U.P.; Sarkar, K.; Sawant, S.; Deshpande, J. A rapid point of care CC16 kit for screening of occupational silica dust exposed workers for early detection of silicosis/silico-tuberculosis. Sci. Rep. 2021, 11, 23485. [Google Scholar] [CrossRef]
- Sarkar, K.; Dhatrak, S.; Sarkar, B.; Ojha, U.C.; Raghav, P.; Pagdhune, A. Secondary prevention of silicosis and silico-tuberculosis by periodic screening of silica dust exposed workers using serum club cell protein 16 as a proxy marker. Health Sci. Rep. 2021, 4, e373. [Google Scholar] [CrossRef]
- Liu, J.; Song, H.Y.; Zhu, B.L.; Pan, L.P.; Qian, X.L. The Effect of Silica Dust Exposure on the Serum Clara Cell Protein 16 Levels in Chinese Workers. Biomed. Environ. Sci. 2019, 32, 47–50. [Google Scholar]
- Naha, N.; Muhamed, J.C.J.; Pagdhune, A.; Sarkar, B.; Sarkar, K. Club cell protein 16 as a biomarker for early detection of silicosis. Indian J. Med. Res. 2020, 151, 319–325. [Google Scholar]
- Zhang, S.; Jia, Q.; Song, J.; Tan, Q.; Yu, G.; Guo, X.; Zhang, H. Clinical significance of CC16 and IL-12 in bronchoalveolar lavage fluid of various stages of silicosis. Ann. Palliat. Med. 2020, 9, 3848–3856. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, M.; Wang, C.; Zuo, Y.; Zhai, Z. Club cell secretory protein 16 is a potential biomarker for silica-induced pulmonary fibrosis. Acta Biochim. Pol. 2022, 69, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Lesur, O.; Bernard, A.M.; Bégin, R.O. Clara cell protein (CC-16) and surfactant-associated protein A (SP-A) in asbestos-exposed workers. Chest 1996, 109, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Petrek, M.; Hermans, C.; Kolek, V.; Fialová, J.; Bernard, A. Clara cell protein (CC16) in serum and bronchoalveolar lavage fluid of subjects exposed to asbestos. Biomarkers 2002, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Morimoto, Y.; Oyabu, T.; Kim, H.; Ohgami, A.; Yatera, K.; Hirohashi, M.; Yamato, H.; Hori, H.; Higashi, T.; et al. Gene Expression of Clara Cell Secretory Protein, Surfactant Protein-A and Thyroid Transcription Factor-1 in the Lungs of Rats Exposed to Potassium Octatitanate Whiskers in vivo. J. Occup. Health 2001, 43, 111–117. [Google Scholar] [CrossRef]
- Manning, C.B.; Sabo-Attwood, T.; Robledo, R.F.; Macpherson, M.B.; Rincón, M.; Vacek, P.; Hemenway, D.; Taatjes, D.J.; Lee, P.J.; Mossman, B.T. Targeting the MEK1 cascade in lung epithelium inhibits proliferation and fibrogenesis by asbestos. Am. J. Respir. Cell Mol. Biol. 2008, 38, 618–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borm, P.J.A.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part Fibre. Toxicol. 2006, 3, 11–45. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Mercer, R.R.; Benkovic, S.A.; Harkema, J.; Sriram, K.; Schwegler-Berry, D.; Goravanahally, M.P.; Nurkiewicz, T.R.; Castranova, V.; Sargent, L.M. Nanotoxicology—A Pathologist’s Perspective. Toxicol. Pathol. 2010, 39, 301–324. [Google Scholar] [CrossRef]
- Hesterberg, T.; Long, C.; Lapin, C.; Hamade, A.; Valberg, P. Diesel exhaust particulate (DEP) and nanoparticle exposures: What do DEP human clinical studies tell us about potential human health hazards of nanoparticles? Inhal. Toxicol. 2010, 22, 679–694. [Google Scholar] [CrossRef]
- Liao, H.Y.; Chung, Y.T.; Lai, C.H.; Wang, S.L.; Chiang, H.C.; Li, L.A.; Tsou, T.C.; Li, W.F.; Lee, H.L.; Wu, W.T.; et al. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nano-materials. Nanotoxicology 2014, 8 (Suppl. S1), 100–110. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, A.; Li, Q.; Xie, S.; Wan, E.; Zhang, S.; Tan, Y.; Li, X.; Xie, H.; Lu, W. Pulmonary functions and blood biochemical markers for workers with and without coal worker pneumoconiosis. Life Sci. J. 2009, 6, 33–39. [Google Scholar]
- Barnes, H.; Olin, A.C.; Torén, K.; McSharry, C.; Donnelly, I.; Lärstad, M.; Iribarren, C.; Quinlan, P.; Blanc, P.D. Occupation versus environmental factors in hypersensitivity pneumonitis: Population attributable fraction. ERJ Open Res. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, D.G.; Despres, V.; Frohlich-Nowoisky, J.; Psenner, R.; Ariya, P.A.; Posfai, M.; Ahern, H.E. Microbiology and atmospheric processes: Biological, physical and chemical characterization of aerosol particles. Biogeosciences. 2009, 6, 721–737. [Google Scholar] [CrossRef]
- Behbod, B.; Urch, B.; Speck, M.; Scott, J.; Liu, L.; Poon, R.; Coull, B.; Schwartz, J.; Koutrakis, P.; Silverman, F.; et al. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup. Environ. Med. 2013, 70, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Thorn, J.; Beijer, L.; Rylander, R. Work related symptoms among sewage workers: A nationwide survey in Sweden. Occup. Environ. Med. 2002, 59, 562–566. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chang, W.T.; Chuang, H.Y.; Tsai, S.S.; Wu, T.N.; Sung, F.C. Adverse health effects among household waste collectors in Taiwan. Environ. Res. 2001, 85, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Jeggli, S.; Tschopp, A.; Bernard, A.; Oppliger, A.; Hilfiker, S.; Hotz, P. Clara cell protein and surfactant protein B in garbage collectors and in wastewater workers exposed to bioaerosols. Int. Arch. Occup. Environ. Health 2005, 78, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Eduard, W.; Pearce, N.; Douwes, J. Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest 2009, 136, 716–725. [Google Scholar] [CrossRef]
- Zejda, J.E.; McDuffie, H.H.; Dosman, J.A. Epidemiology of health and safety risks in agriculture and related industries. Practical applications for rural physicians. West. J. Med. 1993, 158, 56–63. [Google Scholar]
- Sethi, R.; David, S.; Baljit, S. Characterization of the lung epithelium of wild-type and TLR9−/− mice after single and repeated exposures to chicken barn air. Exp. Toxicol. Pathol. 2013, 65, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Tepper, A.; Comstock, G.; Levine, M. A longitudinal study of pulmonary function in fire fighters. Am. J. Ind. Med. 1991, 20, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.S. Smoke and Combustion Products. Patty’s Toxicol. 2012, 399–418. [Google Scholar] [CrossRef]
- Bernard, A.; Hermans, C.; Van Houte, G. Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup. Environ. Med. 1997, 54, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.; Pira, E.; Romano, C.; Maiello, R.; Petyx, M.; Iavicoli, S. Evaluation of DNA damage induction on human pulmonary cells exposed to PAHs from organic extract of PM10 collected in a coke-oven plant. Acta Biomed. 2008, 79, 97–103. [Google Scholar] [PubMed]
- Van Vyve, T.; Chanez, P.; Bernard, A.; Bousquet, J.; Godard, P.; Lauwerijs, R.; Sibille, Y. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J. Allergy Clin. Immunol. 1995, 95, 60–68. [Google Scholar] [CrossRef]
- Liu, L.; Wei, J.; Wang, Y.; Feng, Q.; Guo, S.; Liu, G.; Dong, J.; Jiang, L.; Li, Q.; Nie, J.; et al. Effect of Club cell secretory proteins on the association of tobacco smoke and PAH co-exposure with lung function decline: A longitudinal observation of Chinese coke oven workers. Int. J. Hyg. Environ. Health 2023, 247, 114058. [Google Scholar] [CrossRef]
- Nurhisanah, S.; Hasyim, H. Environmental health risk assessment of sulfur dioxide (SO2) at workers around in combined cycle power plant (CCPP). Heliyon 2022, 8, e09388. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 8; 9, Sulfur Dioxide Acute Exposure Guideline Levels; National Academies Press: Washington, DC, USA, 2010; Available online: https://www.ncbi.nlm.nih.gov/books/NBK219999 (accessed on 2 November 2023).
- Haddam, N.; Samira, S.; Dumont, X.; Taleb, A.; Haufroid, V.; Lison, D.; Bernard, A. Lung epithelium injury biomarkers in workers exposed to sulphur dioxide in a non-ferrous smelter. Biomarkers 2009, 4, 292–298. [Google Scholar] [CrossRef]
- Sunil, V.; Vayas, K.; Massa, C.; Gow, A.; Laskin, J.; Laskin, D. Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics. Toxicol. Sci. 2013, 133, 309–319. [Google Scholar] [CrossRef]
- Cho, H.Y.; Zhang, L.Y.; Kleeberger, S. Ozone-induced lung inflammation and hyper-reactivity are mediated via tumor necrosis factor-alpha receptors. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L537–L546. [Google Scholar] [CrossRef] [PubMed]
- EU-RAR. Hydrogen Peroxide. European Risk Assessment Report, 2nd Priority List; European Chemicals Bureau: Helsinki, Finland, 2003; Volume 38. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC26024 (accessed on 5 November 2023).
- Ernstgård, L.; Sjögren, B.; Johanson, G. Acute effects of exposure to vapors of hydrogen peroxide in humans. Toxicol. Lett. 2012, 212, 222–227. [Google Scholar] [CrossRef] [PubMed]
- ARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels. Lyon (FR): International Agency for Research on Cancer; 1989. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 45). DIESEL FUELS. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531266/ (accessed on 7 November 2023).
- Pronk, A.; Coble, J.; Stewart, P.A. Occupational exposure to diesel engine exhaust: A literature review. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Alexis, N.E.; Carlsten, C. Interplay of air pollution and asthma immunopathogenesis: A focused review of diesel exhaust and ozone. Int. Immunopharmacol. 2014, 23, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, B.; Tam, S.; Chen, Y.; Sin, D.; Carlsten, C. Effect of controlled human exposure to diesel exhaust and allergen on airway surfactant protein D, myeloperoxidase and club (Clara) cell secretory protein 16. Clin. Exp. Allergy 2016, 46, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Antonini, J.M. Health effects of welding. Crit. Rev. Toxicol. 2003, 33, 61–103. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Criswell, S.R.; Lundin, J.I.; Hobson, A.; Seixas, N.; Kotzbauer, P.T.; Evanoff, B.A.; Perlmutter, J.S.; Zhang, J.; Sheppard, L.; et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 2012, 33, 1356–1361. [Google Scholar] [CrossRef]
- Li, H.; Hedmer, M.; Kåredal, M.; Björk, J.; Stockfelt, L.; Tinnerberg, H.; Albin, M.; Broberg, K. A cross-sectional study of the cardiovascular effects of welding fumes. PLoS ONE 2015, 10, e0131648. [Google Scholar] [CrossRef]
- Hałatek, T.; Stanisławska, M.; Świercz, R.; Domeradzka-Gajda, K.; Kuraś, R.; Wąsowicz, W. Clara cells protein, prolactin and transcription factors of protein NF-ĸB and c-Jun/AP-1 levels in rats inhaled to stainless steel welding dust and its soluble form. Int. J. Occup. Med. Environ. Health 2018, 31, 613–632. [Google Scholar] [CrossRef]
- Isocyanates. Available online: https://www.osha.gov/isocyanates (accessed on 5 November 2023).
- Baur, X.; Marek, W.; Ammon, J.; Czuppon, A.B.; Marczynski, B.; Raulf-Heimsoth, M.; Roemmelt, H.; Fruhmann, G. Respiratory and other hazards of isocyanates. Int. Arch. Occup. Environ. Health 1994, 66, 141–152. [Google Scholar] [CrossRef]
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin exposure to isocyanates: Reasons for concern. Environ. Health Perspect. 2007, 115, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; El-Ebiary, A.; Abuelfadl, A.; El-Maddah, E.; El-Shourbagy, S. Pulmonary Toxicity in Car Spray Painters’. Eur. Respir. J. 2011, 38 (Suppl. S55), 4943. [Google Scholar]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Elia, J.; Aoki, A.; Maldonado, C. Response of bronchiolar Clara cells induced by a domestic insecticide. Analysis of CC10 kDa protein content. Histochem. Cell. Biol. 2000, 113, 125–133. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, D.S.; Kim, Y.W.; Chung, M.P.; Uh, S.T.; Park, C.S.; Jeong, S.H.; Park, Y.B.; Lee, H.L.; Song, J.S.; et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: A Korean national survey. Chest 2015, 147, 465–474. [Google Scholar] [CrossRef]
 inhibition;
inhibition;  activation. AKR—aldo-keto reductase; DH—dehydrogenase; CCSP—club cell secretory protein; GSH—glutathione S-transferase; IFNγ—interferon γ; IL-1—interleukin 1; IL-6—interleukin 6; MUC5AC—mucin 5AC; MUC5B—mucin 5B; NF-KB—nuclear factor kappa B; p38 MAPK—p38 mitogen-activated protein kinase; PLA2—phospholipase 2; SP-A—surfactant protein A; SP-D—surfactant protein D; TGFβ—transforming growth factor β; TNFα—tumor necrosis factor α; 🡣 decrease.
activation. AKR—aldo-keto reductase; DH—dehydrogenase; CCSP—club cell secretory protein; GSH—glutathione S-transferase; IFNγ—interferon γ; IL-1—interleukin 1; IL-6—interleukin 6; MUC5AC—mucin 5AC; MUC5B—mucin 5B; NF-KB—nuclear factor kappa B; p38 MAPK—p38 mitogen-activated protein kinase; PLA2—phospholipase 2; SP-A—surfactant protein A; SP-D—surfactant protein D; TGFβ—transforming growth factor β; TNFα—tumor necrosis factor α; 🡣 decrease.
 inhibition;
inhibition;  activation. AKR—aldo-keto reductase; DH—dehydrogenase; CCSP—club cell secretory protein; GSH—glutathione S-transferase; IFNγ—interferon γ; IL-1—interleukin 1; IL-6—interleukin 6; MUC5AC—mucin 5AC; MUC5B—mucin 5B; NF-KB—nuclear factor kappa B; p38 MAPK—p38 mitogen-activated protein kinase; PLA2—phospholipase 2; SP-A—surfactant protein A; SP-D—surfactant protein D; TGFβ—transforming growth factor β; TNFα—tumor necrosis factor α; 🡣 decrease.
activation. AKR—aldo-keto reductase; DH—dehydrogenase; CCSP—club cell secretory protein; GSH—glutathione S-transferase; IFNγ—interferon γ; IL-1—interleukin 1; IL-6—interleukin 6; MUC5AC—mucin 5AC; MUC5B—mucin 5B; NF-KB—nuclear factor kappa B; p38 MAPK—p38 mitogen-activated protein kinase; PLA2—phospholipase 2; SP-A—surfactant protein A; SP-D—surfactant protein D; TGFβ—transforming growth factor β; TNFα—tumor necrosis factor α; 🡣 decrease.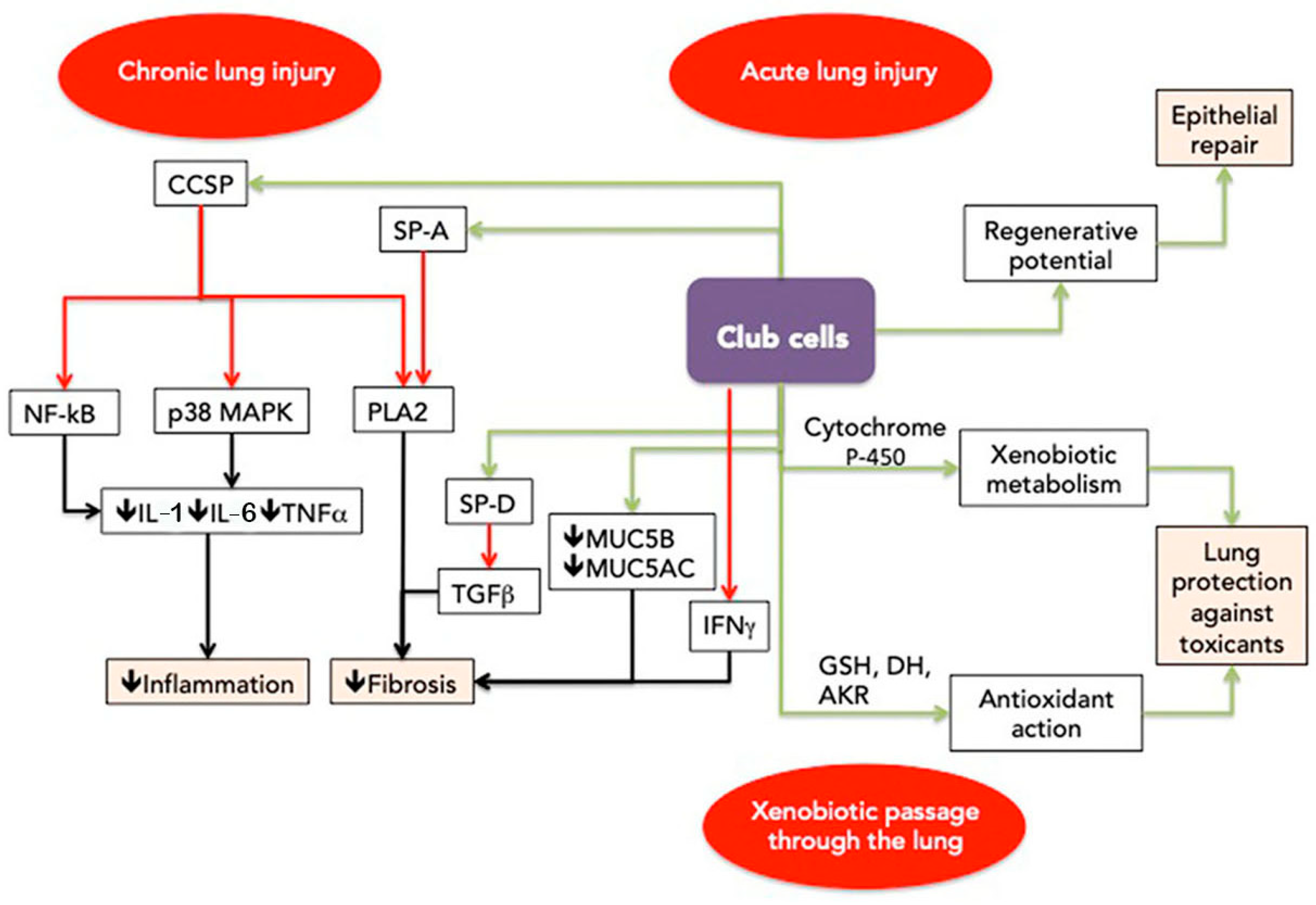
| Type of Hazard | Hazard | Interstitial Lung Disorder | Club Cells Biomarker | Reference |
|---|---|---|---|---|
| Inorganic Particles | Free silica | Silicosis | Serum CC16 🡫 | [89] |
| Serum CC16 🡫 | [86] | |||
| Serum CC16 🡫 | [87] | |||
| CC16 in BALF 🡫 | [90] | |||
| Serum CC16 🡫 | [85] | |||
| Serum CC16 🡫 | [88] | |||
| Serum and BALF CC16 🡫 SP-D * | [91] | |||
| Asbestos | Asbestosis | Serum and BALF CC 16 🡩 SP-A- BALF ~ | [92] | |
| CC 16 serum and BALF | [93] | |||
| CCSP mRNA * 🡫 | [95] | |||
| CCSP mRNA * 🡫 | [94] | |||
| Nanotubes and Nanoparticles | Lung fibrosis | Serum CC16 🡫 | [99] | |
| Carbonauceous | Coal worker pneumoconiosis | Serum CC16 🡫 | [100] | |
| Organic particles | Bioaerosol | Hypersensivity pneumonitis | Serum CC16 🡩 | [106] |
| Organic dust | Organic dust syndrome | Club cell * 🡫 CC10 🡫 | [111] | |
| Mixture | Vapours, gazes, dust and fumes (VGDF) | Lung fibrosis | ||
| Smoke | Serum CC16 🡩 transient | [114] | ||
| Polycyclic aromatic hydrocarbons (PAH) | Serum CC16 🡫 | [117] | ||
| Sulphure dioxide (SO2) | Serum CC16 🡫 Serum SP-D | [120] | ||
| Diesel engine exhaust | Serum CC16 🡫 | [128] | ||
| Hydrogen peroxide | Serum CC16 🡫 | [124] | ||
| Welding dust | BALF CC16 * 🡫 | [132] | ||
| Ozone | BALF CC16 * 🡫 | [121] | ||
| Chemicals | Isocyanates | Hypersensivity pneumonitis | Serum CC16 🡫 | [136] |
| Pesticides | Lung fibrosis | BALF CC10 * 🡫 | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otelea, M.R.; Oancea, C.; Reisz, D.; Vaida, M.A.; Maftei, A.; Popescu, F.G. Club Cells—A Guardian against Occupational Hazards. Biomedicines 2024, 12, 78. https://doi.org/10.3390/biomedicines12010078
Otelea MR, Oancea C, Reisz D, Vaida MA, Maftei A, Popescu FG. Club Cells—A Guardian against Occupational Hazards. Biomedicines. 2024; 12(1):78. https://doi.org/10.3390/biomedicines12010078
Chicago/Turabian StyleOtelea, Marina Ruxandra, Corina Oancea, Daniela Reisz, Monica Adriana Vaida, Andreea Maftei, and Florina Georgeta Popescu. 2024. "Club Cells—A Guardian against Occupational Hazards" Biomedicines 12, no. 1: 78. https://doi.org/10.3390/biomedicines12010078
APA StyleOtelea, M. R., Oancea, C., Reisz, D., Vaida, M. A., Maftei, A., & Popescu, F. G. (2024). Club Cells—A Guardian against Occupational Hazards. Biomedicines, 12(1), 78. https://doi.org/10.3390/biomedicines12010078







