The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery
Abstract
1. Introduction
2. Extracellular Matrix (ECM) and Integrins Influence Prostate Cancer Cell Fate
2.1. The Role of ECM Proteins in Prostate Cancer
2.2. The Role of Integrins in Prostate Cancer
Extracellular Vesicles (EVs)-Derived Integrins Contribute to Prostate Cancer Cell Communication within Its Own Surroundings but also with Other Cells and Tissues within the Body
3. Increased Tumor Stiffness Enables Prostate Cancer Detection Via Physical Palpation
4. Bone Turnover: ECM Biomarkers in Prostate Cancer Bone Metastasis Diagnosis and Prognosis
5. The Biomarker Potential of EVs-Derived Integrins in Prostate Cancer
6. ECM- and Integrin Adhesion Complex (IAC)-Related Single Genes and Proteins as Prostate Cancer Biomarkers
| Gene/Protein | ECM Category | Type of Molecule Studied | Compartment Used | Biomarker Type | References |
|---|---|---|---|---|---|
| Type I collagen | Collagen | mRNA and protein | CT | D | [188] |
| Type III collagen | Collagen | mRNA, protein, and autoantibodies | CT, S | D | [188,189] |
| Type VII collagen | Collagen | Protein | CT | D | [190] |
| Type VIII collagen | Collagen | Protein | S | D | [191] |
| Type XX collagen | Collagen | Protein | S | D | [192] |
| Type XXIII collagen | Collagen | Protein | CT, M, U | D, PRO | [193] |
| COL4A1 (collagen type IV alpha 1 chain) | Collagen | mRNA | CT, M | D, RS | [194] |
| COL4A6 | Collagen | mRNA | CT | D, PRO, RS | [195,196] |
| COL10A1 | Collagen | mRNA and protein | CT | D, PRO | [197] |
| ASPN (asporin) | Proteoglycans | Gene, mRNA, and protein | CT, B | D, PRO | [198,199,200,201,202] |
| BGN (biglycan) | Proteoglycans | mRNA and protein | CT | D, PRO, RS | [199,203,204] |
| ESM1 (endocan) | Proteoglycans | Protein | CT, S | D, PRO, RS | [205,206,207] |
| FMOD (fibromodulin) | Proteoglycans | Gene, mRNA, and protein | CT | D, PRO, RS, R | [208,209,210,211] |
| HSPG2 (perlecan) | Proteoglycans | Protein | CT, S, U | D | [212,213] |
| SPOCK1 (testican-1) | Proteoglycans | mRNA and protein | CT | D, PRO, RS | [214,215,216] |
| SPOCK3 (testican-3) | Proteoglycans | mRNA | CT | D, PRO, RS | [217,218] |
| VCAN (versican) | Proteoglycans | mRNA and protein | CT | D, PRO, RS | [219,220] |
| ADIPOQ (adiponectin) | Glycoproteins | Gene and protein | CT, S | D, PRO, RS, R | [221,222,223,224,225,226,227,228,229,230] |
| COMP (cartilage oligomeric matrix protein) | Glycoproteins | mRNA and protein | CT, M, B | D, RS | [202,231,232] |
| CTHRC1 (collagen triple helix repeat containing 1) | Glycoproteins | mRNA and protein | CT | PRO | [233] |
| CYR61 (cysteine-rich heparin-binding protein 61) | Glycoproteins | mRNA and protein | CT, S | D, PRO, PRE, RS | [162,163,164,165,234] |
| DSPP (dentin sialophosphoprotein) | Glycoproteins | mRNA and protein | CT, S | D, RS | [235,236] |
| EDIL3 (EGF like repeats and discoidin domains 3) | Glycoproteins | Protein | CT, S | D, RS | [237] |
| EFEMP1 (EGF containing fibulin ECM protein 1) | Glycoproteins | mRNA, protein, and DNA methylation | CT, S, U | D, RS | [238,239,240] |
| EFEMP2 | Glycoproteins | mRNA | CT | D | [216] |
| FBLN1 (fibulin 1) | Glycoproteins | mRNA and protein | CT | D | [216] |
| FBLN5 | Glycoproteins | mRNA and protein | CT | D | [216] |
| FBN1 (fibrillin 1) | Glycoproteins | Protein | U | D, RS | [241] |
| FGG (fibrinogen gamma chain) | Glycoproteins | Protein | S, U | D | [242,243] |
| FN1 (fibronectin 1) | Glycoproteins | Protein | B | D | [169] |
| HMCN2 (hemicentin 2) | Glycoproteins | Protein | U | D, RS | [241] |
| IBSP (integrin-binding sialoprotein) | Glycoproteins | mRNA and protein | CT, M, S | D, PRO, RS | [235,244,245,246,247,248,249,250,251] |
| IGFBP1 (insulin like growth factor binding protein 1) | Glycoproteins | Gene and protein | S, P | PRO, RS, R | [252,253,254,255,256,257] |
| IGFBP2 | Glycoproteins | mRNA and protein | CT, S | D, PRO, PRE, R? | [161,258,259,260,261,262,263,264,265,266,267] |
| IGFBP3 | Glycoproteins | Gene and protein | CT, S, P, U | D, PRO, RS, R | [268,269,270,271,272,273,274,275,276,277] [261,278,279,280,281,282,283] |
| IGFBP6 | Glycoproteins | Protein | S | D | [284] |
| LAMA1 (laminin subunit alpha 1) | Glycoproteins | Protein | CT | D | [285] |
| LAMA3 | Glycoproteins | Protein | CT | D | [285] |
| LAMA5 | Glycoproteins | Protein | CT | D | [286] |
| LAMB1 | Glycoproteins | mRNA | CT, M | D, RS | [194] |
| LAMB3 | Glycoproteins | mRNA and protein | CT | D | [287,288] |
| LAMC2 | Glycoproteins | mRNA and protein | CT | D | [190,287,288] |
| LRG1 (leucine rich alpha-2-glycoprotein 1) | Glycoproteins | Protein | CT, B, P (EVs), U (EVs) | D, PRO, PRE, RS | [146,147,289,290] |
| NTN1 (netrin 1) | Glycoproteins | mRNA and protein | CT, P | D | [291,292] |
| POSTN (periostin) | Glycoproteins | mRNA and protein | CT, M, B, P | D, PRO, RS | [166,167,168,169,170,171,174,175,176,232,293,294] |
| RELN (reelin) | Glycoproteins | Protein | CT | D, RS | [295] |
| RSPO3 (R-spondin 3) | Glycoproteins | mRNA | CT | D, PRO | [296] |
| SLIT2 (slit guidance ligand 2) | Glycoproteins | Protein | CT, M | D | [297] |
| SPARC (osteonectin) | Glycoproteins | mRNA, protein, and DNA methylation | CT, M | D, PRO, RS | [298,299,300,301,302] |
| SPON1 (spondin 1) | Glycoproteins | mRNA | CT | D, PRO, RS | [217] |
| SPON2 (spondin 2) | Glycoproteins | Protein and DNA methylation | CT, S | D, RS | [245,303,304,305,306] |
| SPP1 (osteopontin) | Glycoproteins | mRNA and protein | CT, M, S, P, U | D, PRO, PRE, RS | [246,275,307,308,309,310,311,312,313] [235,250,314,315,316,317,318] |
| THBS1 (thrombospondin 1) | Glycoproteins | Protein | CT, S, S (EVs) | D, PRO | [319,320,321,322] |
| THBS2 | Glycoproteins | mRNA and protein | CT | D, PRO | [323] |
| TNC (tenascin C) | Glycoproteins | Gene and protein | CT | D, PRO, RS | [286,324,325,326,327] |
| VTN (vitronectin) | Glycoproteins | Protein | CT, S, U | D, RS | [241,328] |
| WISP1 (WNT1 induced secreted protein 1) | Glycoproteins | mRNA and protein | CT | PRO | [329] |
| ADAM9 (a disintegrin and metalloproteinase domain 9) | ECM regulators | mRNA and protein | CT | D, PRO | [330,331,332,333] |
| ADAM10 | ECM regulators | Protein | CT | D, RS | [334] |
| ADAM12 | ECM regulators | Protein | B, U | D | [335] |
| ADAM15 | ECM regulators | Protein | CT | D, PRO, RS | [336,337] |
| ADAM19 | ECM regulators | mRNA and protein | CT | D, PRO, RS | [338] |
| ADAM28 | ECM regulators | Protein | CT | D | [339] |
| CTSB (cathepsin B) | ECM regulators | Gene and protein | CT, S | D, RS, R | [340,341,342,343] |
| CTSB/CSTA (cystatin A) ratio | ECM regulators | mRNA and protein | CT | D, PRO, RS | [344,345,346,347,348] |
| CTSD | ECM regulators | Protein and protein activity | CT, M, S | D, PRO, PRE, RS | [176,349,350,351,352,353,354,355] |
| CTSK | ECM regulators | mRNA and protein | CT, M | D, PRE | [356,357] |
| CTSL | ECM regulators | Protein | CT | RS | [293] |
| CTSZ | ECM regulators | mRNA and protein | CT, B | D, PRO | [358,359] |
| LOX (lysyl oxidase) | ECM regulators | Gene, mRNA, and protein | CT, M | D, PRO, PRE, RS | [182,183,360,361,362] |
| MMP1 (matrix metalloproteinase 1) | ECM regulators | Protein | CT | D, PRO, RS | [363,364] |
| MMP2 | ECM regulators | Gene, mRNA, protein, and protein activity | CT, S, P, U | D, PRO, RS, R | [365,366,367,368,369,370,371,372,373,374,375] |
| MMP7 | ECM regulators | Gene and protein | S | PRO, PRE, R | [376,377,378] |
| MMP9 | ECM regulators | mRNA, protein, and protein activity | CT, B, P | D, PRO, RS | [178,179,366,369,371,375,379,380,381,382,383,384,385] |
| MMP11 | ECM regulators | Gene and protein | CT | D, PRO, RS | [386,387,388] |
| MMP13 | ECM regulators | Protein | CT | D, PRO, RS | [386,389] |
| Gene/Protein | Category | Type of Molecule Studied | Compartment Used | Biomarker Type | References |
|---|---|---|---|---|---|
| ITGA2 (integrin subunit alpha 2) | Integrin | mRNA and protein | CT, BM, M | D, PRO | [406,407,408] |
| ITGA3 | Integrin | Protein | CT, U (EVs) | D, PRO, RS | [150,409,410] |
| ITGA5 | Integrin | Protein | CT, M | D, RS | [91,411] |
| ITGA6 | Integrin | Protein | CT, BM | PRO, RS | [407,408,409,412,413] |
| ITGA7 | Integrin | Gene and protein | CT | D, PRO, RS | [411,414] |
| ITGAV | Integrin | Gene and protein | CT, M, U | D, PRO, R, RS | [91,415,416,417] |
| ITGB1 | Integrin | Protein | CT, U (EVs) | D, PRO, RS | [150,412,418] |
| ITGB4 | Integrin | Gene, mRNA, and protein | CT | D, PRO | [391,419,420] |
| ITGB5 | Integrin | Protein | CT, S (EVs) | D, RS | [148,421] |
| α3β1 integrin | Integrin | Protein | CT | PRO | [410] |
| α6β4 integrin | Integrin | Protein | CT | D | [190,391] |
| αVβ5 integrin | Integrin | Protein | CT | PRO, RS | [415] |
| ACTN1 (actinin alpha 1) | Consensus (cons.) and αVβ5 adhesome | Protein | CT | PRO, RS | [422] |
| ANXA1 (annexin A1) | Cons. adhesome | mRNA and protein | CT, U | D | [243,420] |
| FEN1 (flap endonuclease 1) | Cons. adhesome | Protein | CT | D, RS | [423] |
| FHL2 (four and a half LIM domains 2) | Cons. adhesome | Protein (nuclear) | CT | D, PRO, RS | [424,425] |
| FLNA (filamin-A) | αVβ5 adhesome | Protein | CT, S | D | [395,426] |
| FLNA, FLNB correlation | αVβ5 adhesome | Protein | S | D | [427] |
| ILK (integrin linked kinase) | Cons. adhesome | Protein | CT | D, PRO, RS | [428] |
| IQGAP1 (IQ motif containing GTPase activating protein 1) | Cons. adhesome | mRNA and protein | CT | D, PRO, RS | [429] |
| P4HB (prolyl 4-hydroxylase subunit beta) | Cons. adhesome | mRNA | CT | D, PRO, PRE, RS | [430] |
| PDLIM5 (PDZ and LIM domain 5) | Cons. adhesome | Gene, mRNA, and protein | CT | D, PRO, PRE, RS | [431,432,433] |
| PLEC (plectin) | αVβ5 adhesome | Protein | CT, M | D | [400] |
| PXN (paxillin) | Cons. adhesome | Protein | CT, M | D, PRE, RS | [434,435] |
| SORBS1(sorbin and SH3 domain containing 1) | Cons. adhesome | mRNA | CT | D, PRO | [436,437] |
| TGM2 (transglutaminase 2) | Cons. adhesome | mRNA and protein | CT | D, PRO, RS | [438,439] |
| TLN1 (talin 1) | Consensus and αVβ5 adhesome | Protein | CT, M | D, PRE, RS | [404,440] |
| VCL (vinculin) | Consensus and αVβ5 adhesome | Gene and protein | CT, U | D | [441,442] |
| ZYX (zyxin) | Cons. adhesome | Protein | CT | D | [170,443] |
ECM- and IACs-Related Genes and Proteins Taking a Part in Prostate Cancer Gene and Protein Signatures with Biomarker Potential
7. Conclusions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Samaržija, I.; Dekanić, A.; Humphries, J.D.; Paradžik, M.; Stojanović, N.; Humphries, M.J.; Ambriović-Ristov, A. Integrin Crosstalk Contributes to the Complexity of Signalling and Unpredictable Cancer Cell Fates. Cancers 2020, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Chastney, M.R.; Conway, J.R.W.; Ivaska, J. Integrin Adhesion Complexes. Curr. Biol. 2021, 31, R496–R552. [Google Scholar] [CrossRef]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional Atlas of the Integrin Adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar] [CrossRef]
- Winograd-Katz, S.E.; Fässler, R.; Geiger, B.; Legate, K.R. The Integrin Adhesome: From Genes and Proteins to Human Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Frémillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a Consensus Integrin Adhesome and Its Dynamics during Adhesion Complex Assembly and Disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.J.; Gontarczyk, A.M.; Alghamdi, A.A.; Ellison, T.S.; Johnson, R.T.; Fowler, W.J.; Kirkup, B.M.; Silva, B.C.; Harry, B.E.; Schneider, J.G.; et al. The β3-integrin Endothelial Adhesome Regulates Microtubule-dependent Cell Migration. EMBO Rep. 2018, 19, e44578. [Google Scholar] [CrossRef] [PubMed]
- Paradžik, M.; Humphries, J.D.; Stojanović, N.; Nestić, D.; Majhen, D.; Dekanić, A.; Samaržija, I.; Sedda, D.; Weber, I.; Humphries, M.J.; et al. KANK2 Links αVβ5 Focal Adhesions to Microtubules and Regulates Sensitivity to Microtubule Poisons and Cell Migration. Front. Cell Dev. Biol. 2020, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Humphries, J.D.; Samaržija, I.; Stojanović, N.; Zha, J.; Čuljak, K.; Tomić, M.; Paradžik, M.; Nestić, D.; Kang, H.; et al. The Tongue Squamous Carcinoma Cell Line Cal27 Primarily Employs Integrin α6β4-Containing Type II Hemidesmosomes for Adhesion Which Contribute to Anticancer Drug Sensitivity. Front. Cell Dev. Biol. 2021, 9, 786758. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Samaržija, I.; Humphries, J.D.; Humphries, M.J.; Ambriović-Ristov, A. KANK Family Proteins in Cancer. Int. J. Biochem. Cell Biol. 2021, 131, 105903. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhu, L.; Bromberger, T.; Yang, J.; Yang, Q.; Liu, J.; Plow, E.F.; Moser, M.; Qin, J. Mechanism of Integrin Activation by Talin and Its Cooperation with Kindlin. Nat. Commun. 2022, 13, 2362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, F.; Ithychanda, S.S.; Apostol, M.; Das, M.; Deshpande, G.; Plow, E.F.; Qin, J. A Mechanism of Platelet Integrin αIIbβ3 Outside-in Signaling through a Novel Integrin αIIb Subunit–Filamin–Actin Linkage. Blood 2023, 141, 2629–2641. [Google Scholar] [CrossRef]
- Lončarić, M.; Stojanović, N.; Rac-Justament, A.; Coopmans, K.; Majhen, D.; Humphries, J.D.; Humphries, M.J.; Ambriović-Ristov, A. Talin2 and KANK2 Functionally Interact to Regulate Microtubule Dynamics, Paclitaxel Sensitivity and Cell Migration in the MDA-MB-435S Melanoma Cell Line. Cell. Mol. Biol. Lett. 2023, 28, 56. [Google Scholar] [CrossRef]
- Hanash, S.M. Why Have Protein Biomarkers Not Reached the Clinic? Genome Med. 2011, 3, 66. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the Matrisome-An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lee, B.; Jiang, Y. Extracellular Matrix in Cancer Progression and Therapy. Med. Rev. 2022, 2, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.E.; Weinberg, S.H.; Lemmon, C.A. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2019, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, G.; Kavasi, R.M.; Voudouri, K.; Berdiaki, A.; Spyridaki, I.; Tsatsakis, A.; Nikitovic, D. Role of the Extracellular Matrix in Cancer-Associated Epithelial to Mesenchymal Transition Phenomenon. Dev. Dyn. 2018, 247, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Kai, F.B.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting Extracellular Matrix Stiffness and Mechanotransducers to Improve Cancer Therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Jurj, A.; Ionescu, C.; Berindan-Neagoe, I.; Braicu, C. The Extracellular Matrix Alteration, Implication in Modulation of Drug Resistance Mechanism: Friends or Foes? J. Exp. Clin. Cancer Res. 2022, 41, 276. [Google Scholar] [CrossRef]
- Leight, J.L.; Drain, A.P.; Weaver, V.M. Extracellular Matrix Remodeling and Stiffening Modulate Tumor Phenotype and Treatment Response. Annu. Rev. Cancer Biol. 2017, 1, 313–334. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Liao, X.; Li, X.; Liu, R. Extracellular-Matrix Mechanics Regulate Cellular Metabolism: A Ninja Warrior behind Mechano-Chemo Signaling Crosstalk. Rev. Endocr. Metab. Disord. 2023, 24, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 631991. [Google Scholar] [CrossRef]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. Biomed Res. Int. 2014, 2014, 756078. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Necula, L.; Matei, L.; Dragu, D.; Pitica, I.; Neagu, A.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Collagen Family as Promising Biomarkers and Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2022, 23, 12415. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M. The Laminin Family. Cell Adh. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef]
- Yap, L.; Tay, H.G.; Nguyen, M.T.X.; Tjin, M.S.; Tryggvason, K. Laminins in Cellular Differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef]
- Rousselle, P.; Scoazec, J.Y. Laminin 332 in Cancer: When the Extracellular Matrix Turns Signals from Cell Anchorage to Cell Movement. Semin. Cancer Biol. 2020, 62, 149–165. [Google Scholar] [CrossRef]
- Navdaev, A.; Eble, J.A. Components of Cell-Matrix Linkage as Potential New Markers for Prostate Cancer. Cancers 2011, 3, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.V.; Rodin, S.A. Laminins in Metastatic Cancer. Mol. Biol. 2018, 52, 350–371. [Google Scholar] [CrossRef]
- Graf, F.; Horn, P.; Ho, A.D.; Boutros, M.; Maercker, C. The Extracellular Matrix Proteins Type I Collagen, Type III Collagen, Fibronectin, and Laminin 421 Stimulate Migration of Cancer Cells. FASEB J. 2021, 35, e21692. [Google Scholar] [CrossRef] [PubMed]
- Girigoswami, K.; Saini, D.; Girigoswami, A. Extracellular Matrix Remodeling and Development of Cancer. Stem Cell Rev. Rep. 2021, 17, 739–747. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Koistinen, H.; Kovanen, R.M.; Hollenberg, M.D.; Dufour, A.; Radisky, E.S.; Stenman, U.H.; Batra, J.; Clements, J.; Hooper, J.D.; Diamandis, E.; et al. The Roles of Proteases in Prostate Cancer. IUBMB Life 2023, 75, 493–513. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kryza, T.; Batra, J.; Clements, J. Remodelling of the Tumour Microenvironment by the Kallikrein-Related Peptidases. Nat. Rev. Cancer 2022, 22, 223–238. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A Challenging Paradigm of Cancer Management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and Their Roles in Human Cancer. Anat. Rec. 2020, 303, 1557–1572. [Google Scholar] [CrossRef]
- Binder, M.J.; Ward, A.C. The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-like Review. Int. J. Mol. Sci. 2021, 22, 3608. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.; Larsson, P.; Sarwar, M.; Semenas, J.; Syed Khaja, A.S.; Persson, J.L. The Interplay between AR, EGF Receptor and MMP-9 Signaling Pathways in Invasive Prostate Cancer. Mol. Med. 2018, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Grindel, B.J.; Martinez, J.R.; Pennington, C.L.; Muldoon, M.; Stave, J.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/Matrix Metalloproteinase-7(MMP7) Cleavage of Perlecan/HSPG2 Creates a Molecular Switch to Alter Prostate Cancer Cell Behavior. Matrix Biol. 2014, 36, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Grindel, B.J.; Martinez, J.R.; Tellman, T.V.; Harrington, D.A.; Zafar, H.; Nakhleh, L.; Chung, L.W.; Farach-Carson, M.C. Matrilysin/MMP-7 Cleavage of Perlecan/HSPG2 Complexed with Semaphorin 3A Supports FAK-Mediated Stromal Invasion by Prostate Cancer Cells. Sci. Rep. 2018, 8, 7262. [Google Scholar] [CrossRef]
- Gupta, A.; Cao, W.; Sadashivaiah, K.; Chen, W.; Schneider, A.; Chellaiah, M.A. Promising Noninvasive Cellular Phenotype in Prostate Cancer Cells Knockdown of Matrix Metalloproteinase 9. Sci. World J. 2013, 2013, 493689. [Google Scholar] [CrossRef]
- Tellman, T.V.; Cruz, L.A.; Grindel, B.J.; Farach-Carson, M.C. Cleavage of the Perlecan-Semaphorin 3a-Plexin A1-Neuropilin-1 (Pspn) Complex by Matrix Metalloproteinase 7/Matrilysin Triggers Prostate Cancer Cell Dyscohesion and Migration. Int. J. Mol. Sci. 2021, 22, 3218. [Google Scholar] [CrossRef]
- Bruni-Cardoso, A.; Johnson, L.C.; Vessella, R.L.; Peterson, T.E.; Lynch, C.C. Osteoclast-Derived Matrix Metalloproteinase-9 Directly Affects Angiogenesis in the Prostate Tumor-Bone Microenvironment. Mol. Cancer Res. 2010, 8, 459–470. [Google Scholar] [CrossRef]
- Littlepage, L.E.; Sternlicht, M.D.; Rougier, N.; Phillips, J.; Gallo, E.; Yu, Y.; Williams, K.; Brenot, A.; Gordon, J.I.; Werb, Z. Matrix Metalloproteinases Contribute Distinct Roles in Neuroendocrine Prostate Carcinogenesis, Metastasis, and Angiogenesis Progression. Cancer Res. 2010, 70, 2224–2234. [Google Scholar] [CrossRef]
- Di Donato, M.; Zamagni, A.; Galasso, G.; Di Zazzo, E.; Giovannelli, P.; Barone, M.V.; Zanoni, M.; Gunelli, R.; Costantini, M.; Auricchio, F.; et al. The Androgen Receptor/Filamin A Complex as a Target in Prostate Cancer Microenvironment. Cell Death Dis. 2021, 12, 127. [Google Scholar] [CrossRef]
- Li, T.; Wu, C.; Gao, L.; Qin, F.; Wei, Q.; Yuan, J. Lysyl Oxidase Family Members in Urological Tumorigenesis and Fibrosis. Oncotarget 2018, 9, 20156–20164. [Google Scholar] [CrossRef]
- Chen, X.; Shao, Y.; Wei, W.; Shen, H.; Li, Y.; Chen, Y.; Ma, Q.; Li, H.; Yang, Z.; Niu, Y.; et al. Downregulation of LOX Promotes Castration-Resistant Prostate Cancer Progression via IGFBP3. J. Cancer 2021, 12, 7349–7357. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Adamo, H.; Bergh, A.; Halin Bergström, S. Inhibition of Lysyl Oxidase and Lysyl Oxidase-Like Enzymes Has Tumour-Promoting and Tumour-Suppressing Roles in Experimental Prostate Cancer. Sci. Rep. 2016, 6, 19608. [Google Scholar] [CrossRef] [PubMed]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Vizovišek, M.; Fonović, M.; Turk, B. Cysteine Cathepsins in Extracellular Matrix Remodeling: Extracellular Matrix Degradation and Beyond. Matrix Biol. 2019, 75–76, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kwon, W.; Park, J.K.; Baek, S.M.; Lee, S.W.; Cho, G.J.; Ha, Y.S.; Lee, J.N.; Kwon, T.G.; Kim, M.O.; et al. Suppression of Cathepsin a Inhibits Growth, Migration, and Invasion by Inhibiting the P38 MAPK Signaling Pathway in Prostate Cancer. Arch. Biochem. Biophys. 2020, 688, 108407. [Google Scholar] [CrossRef] [PubMed]
- Nalla, A.K.; Gorantla, B.; Gondi, C.S.; Lakka, S.S.; Rao, J.S. Targeting MMP-9, UPAR, and Cathepsin B Inhibits Invasion, Migration and Activates Apoptosis in Prostate Cancer Cells. Cancer Gene Ther. 2010, 17, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, F.L.; He, Y.; Franco, O.E.; Jiang, M.; Cates, J.M.; Hayward, S.W. Cathepsin D Acts as an Essential Mediator to Promote Malignancy of Benign Prostatic Epithelium. Prostate 2013, 73, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, T.; Okamoto, K.; Iwata, J.I.; Shin, M.; Okamoto, Y.; Yasukochi, A.; Nakayama, K.I.; Kadowaki, T.; Tsukuba, T.; Yamamoto, K. Cathepsin E Prevents Tumor Growth and Metastasis by Catalyzing the Proteolytic Release of Soluble TRAIL from Tumor Cell Surface. Cancer Res. 2007, 67, 10869–10878. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Rojnik, M.; Jamnik, P.; Doljak, B.; Fonović, U.P.; Kos, J. Cathepsin H Mediates the Processing of Talin and Regulates Migration of Prostate Cancer Cells. J. Biol. Chem. 2013, 288, 2201–2209. [Google Scholar] [CrossRef]
- Le Gall, C.; Bonnelye, E.; Clézardin, P. Cathepsin K Inhibitors as Treatment of Bone Metastasis. Curr. Opin. Support. Palliat. Care 2008, 2, 218–222. [Google Scholar] [CrossRef]
- Herroon, M.K.; Rajagurubandara, E.; Rudy, D.L.; Chalasani, A.; Hardaway, A.L.; Podgorski, I. Macrophage Cathepsin K Promotes Prostate Tumor Progression in Bone. Oncogene 2013, 32, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, F.; Chen, Q.; Dai, J.; Escara-Wilke, J.; Keller, E.T.; Zimmermann, J.; Hong, N.; Lu, Y.; Zhang, J. Targeting Cathepsin K Diminishes Prostate Cancer Establishment and Growth in Murine Bone. J. Cancer Res. Clin. Oncol. 2019, 145, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, Y.Z.; Wang, K.K.; Zhong, B.Q.; Liao, Y.H.; Liang, J.M.; Jiang, N. Cathepsin K Regulates the Tumor Growth and Metastasis by IL-17/CTSK/EMT Axis and Mediates M2 Macrophage Polarization in Castration-Resistant Prostate Cancer. Cell Death Dis. 2022, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L Inhibition by the Small Molecule KGP94 Suppresses Tumor Microenvironment Enhanced Metastasis Associated Cell Functions of Prostate and Breast Cancer Cells. Clin. Exp. Metastasis 2013, 30, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Sudhan, D.R.; Pampo, C.; Rice, L.; Siemann, D.W. Cathepsin L Inactivation Leads to Multimodal Inhibition of Prostate Cancer Cell Dissemination in a Preclinical Bone Metastasis Model. Int. J. Cancer 2016, 138, 2665–2677. [Google Scholar] [CrossRef] [PubMed]
- Fitchev, P.P.; Wcislak, S.M.; Lee, C.; Bergh, A.; Brendler, C.B.; Stellmach, V.M.; Crawford, S.E.; Mavroudis, C.D.; Cornwell, M.L.; Doll, J.A. Thrombospondin-1 Regulates the Normal Prostate in Vivo through Angiogenesis and TGF-Β Activation. Lab. Investig. 2010, 90, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sakai, H. Thrombospondin-1 in urological cancer: Pathological role, clinical significance, and therapeutic prospects. Int. J. Mol. Sci. 2013, 14, 12249–12272. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Tang, C.H.; Lin, L.W.; Tsai, C.H.; Chu, C.Y.; Lin, T.H.; Huang, Y.L. Thrombospondin-2 Promotes Prostate Cancer Bone Metastasis by the up-Regulation of Matrix Metalloproteinase-2 through down-Regulating MiR-376c Expression. J. Hematol. Oncol. 2017, 10, 33. [Google Scholar] [CrossRef]
- Hou, Y.; Li, H.; Huo, W. THBS4 Silencing Regulates the Cancer Stem Cell-like Properties in Prostate Cancer via Blocking the PI3K/Akt Pathway. Prostate 2020, 80, 753–763. [Google Scholar] [CrossRef]
- Moran-Jones, K.; Ledger, A.; Naylor, M.J. β1 Integrin Deletion Enhances Progression of Prostate Cancer in the TRAMP Mouse Model. Sci. Rep. 2012, 2, 526. [Google Scholar] [CrossRef]
- Pellinen, T.; Blom, S.; Sánchez, S.; Välimäki, K.; Mpindi, J.P.; Azegrouz, H.; Strippoli, R.; Nieto, R.; Vitón, M.; Palacios, I.; et al. ITGB1-Dependent Upregulation of Caveolin-1 Switches TGFβ Signalling from Tumour-Suppressive to Oncogenic in Prostate Cancer. Sci. Rep. 2018, 8, 2338. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, I.; Smeland, H.Y.H.; Skogstrand, T.; Sortland, K.; Schmid, M.C.; Reed, R.K.; Stuhr, L. Stromal Integrin α11β1 Affects RM11 Prostate and 4T1 Breast Xenograft Tumors Differently. PLoS ONE 2016, 11, e0151663. [Google Scholar] [CrossRef] [PubMed]
- Ojalill, M.; Parikainen, M.; Rappu, P.; Aalto, E.; Jokinen, J.; Virtanen, N.; Siljamäki, E.; Heino, J. Integrin α2β1 Decelerates Proliferation, but Promotes Survival and Invasion of Prostate Cancer Cells. Oncotarget 2018, 9, 32435–32447. [Google Scholar] [CrossRef] [PubMed]
- Salemi, Z.; Azizi, R.; Fallahian, F.; Aghaei, M. Integrin α2β1 Inhibition Attenuates Prostate Cancer Cell Proliferation by Cell Cycle Arrest, Promoting Apoptosis and Reducing Epithelial–Mesenchymal Transition. J. Cell. Physiol. 2021, 236, 4954–4965. [Google Scholar] [CrossRef] [PubMed]
- Dodagatta-Marri, E.; Ma, H.Y.; Liang, B.; Li, J.; Meyer, D.S.; Chen, S.Y.; Sun, K.H.; Ren, X.; Zivak, B.; Rosenblum, M.D.; et al. Integrin αvβ8 on T Cells Suppresses Anti-Tumor Immunity in Multiple Models and Is a Promising Target for Tumor Immunotherapy. Cell Rep. 2021, 36, 109309. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, C.; Van Der Horst, G.; Cheung, H.; Buijs, J.T.; Pelger, R.C.M.; Van Der Pluijm, G. Integrin αv Expression Is Required for the Acquisition of a Metastatic Stem/Progenitor Cell Phenotype in Human Prostate Cancer. Am. J. Pathol. 2011, 179, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Otero, J.; Chen, Y.; Kim, Y.M.; Koutcher, J.A.; Satagopan, J.; Reuter, V.; Carver, B.; De Stanchina, E.; Enomoto, K.; et al. β4 Integrin Signaling Induces Expansion of Prostate Tumor Progenitors. J. Clin. Investig. 2013, 123, 682–699. [Google Scholar] [CrossRef]
- Koivusalo, S.; Schmidt, A.; Manninen, A.; Wenta, T. Regulation of Kinase Signaling Pathways by α6β4-Integrins and Plectin in Prostate Cancer. Cancers 2023, 15, 149. [Google Scholar] [CrossRef]
- Schmidt, A.; Kaakinen, M.; Wenta, T.; Manninen, A. Loss of α6β4 Integrin-Mediated Hemidesmosomes Promotes Prostate Epithelial Cell Migration by Stimulating Focal Adhesion Dynamics. Front. Cell Dev. Biol. 2022, 10, 886569. [Google Scholar] [CrossRef]
- Samaržija, I. Site-Specific and Common Prostate Cancer Metastasis Genes as Suggested by Meta-Analysis of Gene Expression Data. Life 2021, 11, 636. [Google Scholar] [CrossRef]
- Connell, B.; Kopach, P.; Ren, W.; Joshi, R.; Naber, S.; Zhou, M.; Mathew, P. Aberrant Integrin αv and α5 Expression in Prostate Adenocarcinomas and Bone-Metastases Is Consistent with a Bone-Colonizing Phenotype. Transl. Androl. Urol. 2020, 9, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, E.; Luo, L.; Zhao, S.; Liu, L.; Wang, J.; Kang, R.; Luo, J. A PSCA/PGRN-NF-ΚB-Integrin-α4 Axis Promotes Prostate Cancer Cell Adhesion to Bone Marrow Endothelium and Enhances Metastatic Potential. Mol. Cancer Res. 2020, 18, 501–513. [Google Scholar] [CrossRef]
- Chang, A.C.; Chen, P.C.; Lin, Y.F.; Su, C.M.; Liu, J.F.; Lin, T.H.; Chuang, S.M.; Tang, C.H. Osteoblast-Secreted WISP-1 Promotes Adherence of Prostate Cancer Cells to Bone via the VCAM-1/Integrin α4β1 System. Cancer Lett. 2018, 426, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Goihberg, E.; Ren, W.; Pilichowska, M.; Mathew, P. Proteolytic Fragments of Fibronectin Function as Matrikines Driving the Chemotactic Affinity of Prostate Cancer Cells to Human Bone Marrow Mesenchymal Stromal Cells via the α5β1 Integrin. Cell Adhes. Migr. 2017, 11, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.S.; Pathak, R.; Jain, A.; Jung, S.Y.; Hilsenbeck, S.G.; Piña-Barba, M.C.; Sikora, A.G.; Pienta, K.J.; Rowley, D.R. Tenascin-C and Integrin α9 Mediate Interactions of Prostate Cancer with the Bone Microenvironment. Cancer Res. 2017, 77, 5977–5988. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Li, J.; Lu, H.; Akech, J.; Pratap, J.; Wang, T.; Zerlanko, B.J.; Gerald, T.J.F.; Jiang, Z.; Birbe, R.; et al. Integrin αvb6 Promotes an Osteolytic Program in Cancer Cells by Upregulating MMP2. Cancer Res. 2014, 74, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cao, W.; Chellaiah, M.A. Integrin αvβ3 and CD44 Pathways in Metastatic Prostate Cancer Cells Support Osteoclastogenesis via a Runx2/Smad 5/Receptor Activator of NF-ΚB Ligand Signaling Axis. Mol. Cancer 2012, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Landowski, T.H.; Gard, J.; Pond, E.; Pond, G.D.; Nagle, R.B.; Geffre, C.P.; Cress, A.E. Targeting Integrin α6 Stimulates Curative-Type Bone Metastasis Lesions in a Xenograft Model. Mol. Cancer Ther. 2014, 13, 1558–1566. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Daignault-Newton, S.; Zhang, X.; Morrissey, C.; Hussain, M.H.; Keller, E.T.; Hall, C.L. Integrin Alpha2beta1 (α2β 1) Promotes Prostate Cancer Skeletal Metastasis. Clin. Exp. Metastasis 2013, 30, 569–578. [Google Scholar] [CrossRef]
- Jin, J.K.; Tien, P.C.; Cheng, C.J.; Song, J.H.; Huang, C.; Lin, S.H.; Gallick, G.E. Talin1 Phosphorylation Activates β1 Integrins: A Novel Mechanism to Promote Prostate Cancer Bone Metastasis. Oncogene 2014, 34, 1811–1821. [Google Scholar] [CrossRef]
- Ruppender, N.; Larson, S.; Lakely, B.; Kollath, L.; Brown, L.; Coleman, I.; Coleman, R.; Nguyen, H.; Nelson, P.S.; Corey, E.; et al. Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy. PLoS ONE 2015, 10, e0130565. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Singh, A.; Zerlanko, B.J.; Iozzo, R.V.; Languino, L.R. The αVβ6 Integrin Is Transferred Intercellularly via Exosomes. J. Biol. Chem. 2015, 290, 4545–4551. [Google Scholar] [CrossRef]
- Lu, H.; Bowler, N.; Harshyne, L.A.; Craig Hooper, D.; Krishn, S.R.; Kurtoglu, S.; Fedele, C.; Liu, Q.; Tang, H.Y.; Kossenkov, A.V.; et al. Exosomal αvβ6 Integrin Is Required for Monocyte M2 Polarization in Prostate Cancer. Matrix Biol. 2018, 70, 20–35. [Google Scholar] [CrossRef]
- Krishn, S.R.; Salem, I.; Quaglia, F.; Naranjo, N.M.; Agarwal, E.; Liu, Q.; Sarker, S.; Kopenhaver, J.; McCue, P.A.; Weinreb, P.H.; et al. The αvβ6 Integrin in Cancer Cell-Derived Small Extracellular Vesicles Enhances Angiogenesis. J. Extracell. Vesicles 2020, 9, 1763594. [Google Scholar] [CrossRef]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-Mediated Transfer of αvβ3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef]
- Quaglia, F.; Krishn, S.R.; Daaboul, G.G.; Sarker, S.; Pippa, R.; Domingo-Domenech, J.; Kumar, G.; Fortina, P.; McCue, P.; Kelly, W.K.; et al. Small Extracellular Vesicles Modulated by αVβ3 Integrin Induce Neuroendocrine Differentiation in Recipient Cancer Cells. J. Extracell. Vesicles 2020, 9, 1761072. [Google Scholar] [CrossRef]
- Krishn, S.R.; Singh, A.; Bowler, N.; Duffy, A.N.; Friedman, A.; Fedele, C.; Kurtoglu, S.; Tripathi, S.K.; Wang, K.; Hawkins, A.; et al. Prostate Cancer Sheds the αvβ3 Integrin in Vivo through Exosomes. Matrix Biol. 2019, 77, 41–57. [Google Scholar] [CrossRef]
- DeRita, R.M.; Sayeed, A.; Garcia, V.; Krishn, S.R.; Shields, C.D.; Sarker, S.; Friedman, A.; McCue, P.; Molugu, S.K.; Rodeck, U.; et al. Tumor-Derived Extracellular Vesicles Require β1 Integrins to Promote Anchorage-Independent Growth. iScience 2019, 14, 199–209. [Google Scholar] [CrossRef]
- Ciardiello, C.; Leone, A.; Lanuti, P.; Roca, M.S.; Moccia, T.; Minciacchi, V.R.; Minopoli, M.; Gigantino, V.; De Cecio, R.; Rippa, M.; et al. Large Oncosomes Overexpressing Integrin Alpha-V Promote Prostate Cancer Adhesion and Invasion via AKT Activation. J. Exp. Clin. Cancer Res. 2019, 38, 317. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, R.; Ali, H.E.A.; Mahmoud, M.O.; Rhim, J.S.; Ali, H.I.; Salem, H.F.; Saleem, M.; Kandeil, M.A.; Ambs, S.; Abd Elmageed, Z.Y. Exosomes-Mediated Transfer of ITGA2 Promotes Migration and Invasion of Prostate Cancer Cells by Inducing Epithelial-Mesenchymal Transition. Cancers 2020, 12, 2300. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Luthold, C.; Hallal, T.; Labbé, D.P.; Bordeleau, F. The Extracellular Matrix Stiffening: A Trigger of Prostate Cancer Progression and Castration Resistance? Cancers 2022, 14, 2887. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cavestany, M.; Bin Hahn, S.; Hope, J.M.; Reckhorn, N.T.; Greenlee, J.D.; Schwager, S.C.; VanderBurgh, J.A.; Reinhart-King, C.A.; King, M.R. Matrix Stiffness Induces Epithelial-to-Mesenchymal Transition via Piezo1-Regulated Calcium Flux in Prostate Cancer Cells. iScience 2023, 26, 106275. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.E.; Kay, E.J.; Neilson, L.J.; Henze, A.; Serneels, J.; McGhee, E.J.; Dhayade, S.; Nixon, C.; Mackey, J.B.; Santi, A.; et al. Tumor Matrix Stiffness Promotes Metastatic Cancer Cell Interaction with the Endothelium. EMBO J. 2017, 36, 2373–2389. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Mao, J.; Wang, Y.; Zhang, Z.; Wang, Z.; Yang, H. Effects of Substrate Stiffness on the Viscoelasticity and Migration of Prostate Cancer Cells Examined by Atomic Force Microscopy. Beilstein J. Nanotechnol. 2022, 13, 560–569. [Google Scholar] [CrossRef]
- Molter, C.W.; Muszynski, E.F.; Tao, Y.; Trivedi, T.; Clouvel, A.; Ehrlicher, A.J. Prostate Cancer Cells of Increasing Metastatic Potential Exhibit Diverse Contractile Forces, Cell Stiffness, and Motility in a Microenvironment Stiffness-Dependent Manner. Front. Cell Dev. Biol. 2022, 10, 932510. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerlerr, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023. 2023. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 18 November 2023).
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Mao, J.; Sheng, L.; Wu, H.; Bai, G.; Zhong, Z.; Pan, Z. Biomarkers for Prostate Cancer Bone Metastasis Detection and Prediction. J. Pers. Med. 2023, 13, 705. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Brown, J.; Coleman, R. The Role of Biomarkers in the Management of Bone-Homing Malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Alho, I.; Casimiro, S.; Costa, L. Bone Remodeling Markers and Bone Metastases: From Cancer Research to Clinical Implications. Bonekey Rep. 2015, 4, 668. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Suzuki, H.; Endo, T.; Yano, M.; Naoi, M.; Nishimi, D.; Kawamura, K.; Imamoto, T.; Ichikawa, T. Clinical Usefulness of Bone Markers in Prostate Cancer with Bone Metastasis. Int. J. Urol. 2012, 19, 968–979. [Google Scholar] [CrossRef]
- Joerger, M.; Huober, J. Diagnostic and Prognostic Use of Bone Turnover Markers. Recent Results Cancer Res. 2012, 192, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Eastham, J.A.; Smith, M.R. Biochemical Markers of Bone Turnover and Clinical Outcomes in Men with Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 369–378. [Google Scholar] [CrossRef]
- Koopmans, N.; de Jong, I.J.; Breeuwsma, A.J.; van der Veer, E. Serum Bone Turnover Markers (PINP and ICTP) for the Early Detection of Bone Metastases in Patients with Prostate Cancer: A Longitudinal Approach. J. Urol. 2007, 178, 849–853. [Google Scholar] [CrossRef]
- Brasso, K.; Christensen, I.J.; Johansen, J.S.; Teisner, B.; Garnero, P.; Price, P.A.; Iversen, P. Prognostic Value of PINP, Bone Alkaline Phosphatase, CTX-I, and YKL-40 in Patients with Metastatic Prostate Carcinoma. Prostate 2006, 66, 503–513. [Google Scholar] [CrossRef]
- Jung, K.; Miller, K.; Wirth, M.; Albrecht, M.; Lein, M. Bone Turnover Markers as Predictors of Mortality Risk in Prostate Cancer Patients with Bone Metastases Following Treatment with Zoledronic Acid. Eur. Urol. 2011, 59, 604–612. [Google Scholar] [CrossRef]
- Lara, P.N.; Ely, B.; Quinn, D.I.; Mack, P.C.; Tangen, C.; Gertz, E.; Twardowski, P.W.; Goldkorn, A.; Hussain, M.; Vogelzang, N.J.; et al. Serum Biomarkers of Bone Metabolism in Castration-Resistant Prostate Cancer Patients with Skeletal Metastases: Results from SWOG 0421. J. Natl. Cancer Inst. 2014, 106, dju013. [Google Scholar] [CrossRef]
- Kataoka, A.; Yuasa, T.; Kageyama, S.; Tsuchiya, N.; Habuchi, T.; Iwaki, H.; Narita, M.; Okada, Y.; Yoshiki, T. Diagnosis of Bone Metastasis in Men with Prostate Cancer by Measurement of Serum ICTP in Combination with Alkali Phosphatase and Prostate-Specific Antigen. Clin. Oncol. 2006, 18, 480–484. [Google Scholar] [CrossRef]
- Jung, K.; Lein, M.; Stephan, C.; Von Hösslin, K.; Semjonow, A.; Sinha, P.; Loening, S.A.; Schnorr, D. Comparison of 10 Serum Bone Turnover Markers in Prostate Carcinoma Patients with Bone Metastatic Spread: Diagnostic and Prognostic Implications. Int. J. Cancer 2004, 111, 783–791. [Google Scholar] [CrossRef]
- Li, L.; Shen, X.; Liang, Y.; Li, B.; Si, Y.; Ma, R. N-Telopeptide as a Potential Diagnostic and Prognostic Marker for Bone Metastasis in Human Cancers: A Meta-Analysis. Heliyon 2023, 9, e15980. [Google Scholar] [CrossRef]
- Lara, P.N.; Mayerson, E.; Gertz, E.; Tangen, C.; Goldkorn, A.; van Loan, M.; Hussain, M.; Gupta, S.; Zhang, J.; Parikh, M.; et al. Bone Biomarkers and Subsequent Survival in Men with Hormone-Sensitive Prostate Cancer: Results from the SWOG S1216 Phase 3 Trial of Androgen Deprivation Therapy with or without Orteronel. Eur. Urol. 2023. [Google Scholar] [CrossRef]
- Akoto, T.; Saini, S. Role of Exosomes in Prostate Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3528. [Google Scholar] [CrossRef]
- Bamankar, S.; Londhe, V.Y. The Rise of Extracellular Vesicles as New Age Biomarkers in Cancer Diagnosis: Promises and Pitfalls. Technol. Cancer Res. Treat. 2023, 22, 15330338221149266. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Hill, M.M.; Trau, M. Extracellular Vesicles as Circulating Cancer Biomarkers: Opportunities and Challenges. Clin. Transl. Med. 2018, 7, 14. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liao, Y.; Hosseinifard, H.; Imani, S.; Wen, Q.L. Diagnostic Role of Extracellular Vesicles in Cancer: A Comprehensive Systematic Review and Meta-Analysis. Front. Cell Dev. Biol. 2021, 9, 705791. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular Vesicles as Biomarkers and Therapeutic Targets for Cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Zhou, E.; Li, Y.; Wu, F.; Guo, M.; Xu, J.; Wang, S.; Tan, Q.; Ma, P.; Song, S.; Jin, Y. Circulating Extracellular Vesicles Are Effective Biomarkers for Predicting Response to Cancer Therapy. EBioMedicine 2021, 67, 103365. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef]
- Ramirez-Garrastacho, M.; Bajo-Santos, C.; Line, A.; Martens-Uzunova, E.S.; de la Fuente, J.M.; Moros, M.; Soekmadji, C.; Tasken, K.A.; Llorente, A. Extracellular Vesicles as a Source of Prostate Cancer Biomarkers in Liquid Biopsies: A Decade of Research. Br. J. Cancer 2021, 126, 331–350. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.Y.; Su, W.C.; Chen, Y.T.; Liu, Y.F.; Wei, D.; Zhang, Y.X.; Tang, Q.Y.; Liu, Y.X.; Wang, S.Z.; et al. High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis. Molecules 2022, 27, 8155. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Wang, F.; Fan, J.; Guo, J.; Wu, T.; Lu, D.; Zhou, Q.; Liu, Z.; Wang, Y.; et al. Alterations of Plasma Exosomal Proteins and Motabolies Are Associated with the Progression of Castration-Resistant Prostate Cancer. J. Transl. Med. 2023, 21, 40. [Google Scholar] [CrossRef]
- Signore, M.; Alfonsi, R.; Federici, G.; Nanni, S.; Addario, A.; Bertuccini, L.; Aiello, A.; Di Pace, A.L.; Sperduti, I.; Muto, G.; et al. Diagnostic and Prognostic Potential of the Proteomic Profiling of Serum-Derived Extracellular Vesicles in Prostate Cancer. Cell Death Dis. 2021, 12, 636. [Google Scholar] [CrossRef]
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin β4 and Vinculin Contained in Exosomes Are Potential Markers for Progression of Prostate Cancer Associated with Taxane-Resistance. Int. J. Oncol. 2015, 47, 384–390. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Geldof, A.A.; Lavaei, M.; Piersma, S.R.; van Moorselaar, R.J.A.; Jimenez, C.R. Exosomal ITGA3 Interferes with Non-Cancerous Prostate Cell Functions and Is Increased in Urine Exosomes of Metastatic Prostate Cancer Patients. J. Extracell. Vesicles 2013, 2, 22097. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There so Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Russo, V.C.; Schütt, B.S.; Andaloro, E.; Ymer, S.I.; Hoeflich, A.; Ranke, M.B.; Bach, L.A.; Werther, G.A. Insulin-like Growth Factor Binding Protein-2 Binding to Extracellular Matrix Plays a Critical Role in Neuroblastoma Cell Proliferation, Migration, and Invasion. Endocrinology 2005, 146, 4445–4455. [Google Scholar] [CrossRef]
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Czernichow, S.; Hercberg, S. A Prospective Study of the Insulin-like Growth Factor Axis in Relation with Prostate Cancer in the SU.VI.MAX Trial. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2269–2272. [Google Scholar] [CrossRef][Green Version]
- Matuschek, C.; Rudoy, M.; Peiper, M.; Gerber, P.A.; Hoff, N.P.; Buhren, B.A.; Flehmig, B.; Budach, W.; Knoefel, W.T.; Bojar, H.; et al. Do Insulin-like Growth Factor Associated Proteins Qualify as a Tumor Marker? Results of a Prospective Study in 163 Cancer Patients. Eur. J. Med. Res. 2011, 16, 451–456. [Google Scholar] [CrossRef]
- Capoun, O.; Soukup, V.; Kalousova, M.; Sobotka, R.; Pesl, M.; Zima, T.; Hanus, T. Diagnostic Importance of Selected Protein Serum Markers in the Primary Diagnostics of Prostate Cancer. Urol. Int. 2015, 95, 429–435. [Google Scholar] [CrossRef]
- Borugian, M.J.; Spinelli, J.J.; Sun, Z.; Kolonel, L.N.; Oakley-Girvan, I.; Pollak, M.D.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P. Prostate Cancer Risk in Relation to Insulin-like Growth Factor (IGF)-I and IGF-Binding Protein-3: A Prospective Multiethnic Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 252–254. [Google Scholar] [CrossRef]
- Hong, S.K.; Han, B.K.; Jeong, J.S.; Jeong, S.J.; Moon, K.H.; Byun, S.S.; Lee, S.E. Serum Measurements of Testosterone, Insulin-like Growth Factor 1, and Insulin-like Growth Factor Binding Protein-3 in the Diagnosis of Prostate Cancer among Korean Men. Asian J. Androl. 2008, 10, 207–213. [Google Scholar] [CrossRef]
- Rainato, G.; Fabricio, A.S.C.; Zancan, M.; Peloso, L.; Dittadi, R.; Barichello, M.; Fandella, A.; Scattoni, V.; Gion, M. Evaluating Serum Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 3 as Markers in Prostate Cancer Diagnosis. Int. J. Biol. Markers 2016, 31, 317–323. [Google Scholar] [CrossRef]
- Roddam, A.W.; Allen, N.E.; Appleby, P.; Key, T.J.; Ferrucci, L.; Carter, H.B.; Metter, E.J.; Chen, C.; Weiss, N.S.; Fitzpatrick, A.; et al. Insulin-like Growth Factors, Their Binding Proteins, and Prostate Cancer Risk: Analysis of Individual Patient Data from 12 Prospective Studies. Ann. Intern. Med. 2008, 149, 461–471. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Cheng, I.; Freedman, M.L.; Mucci, L.; Allen, N.E.; Pollak, M.N.; Hayes, R.B.; Stram, D.O.; Canzian, F.; Henderson, B.E.; et al. A Comprehensive Analysis of Common IGF1, IGFBP1 and IGFBP3 Genetic Variation with Prospective IGF-I and IGFBP-3 Blood Levels and Prostate Cancer Risk among Caucasians. Hum. Mol. Genet. 2010, 19, 3089–3101. [Google Scholar] [CrossRef]
- Rowlands, M.A.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.P.; Martin, R.M. Circulating Insulin-like Growth Factor Peptides and Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Int. J. Cancer 2009, 124, 2416–2429. [Google Scholar] [CrossRef]
- D’Antonio, K.B.; Toubaji, A.; Albadine, R.; Mondul, A.M.; Platz, E.A.; Netto, G.J.; Getzenberg, R.H. Extracellular Matrix Associated Protein CYR61 Is Linked to Prostate Cancer Development. J. Urol. 2010, 183, 1604–1610. [Google Scholar] [CrossRef]
- Terada, N.; Shiraishi, T.; Zeng, Y.; Mooney, S.M.; Yeater, D.B.; Mangold, L.A.; Partin, A.W.; Kulkarni, P.; Getzenberg, R.H. Cyr61 Is Regulated by CAMP-Dependent Protein Kinase with Serum Levels Correlating with Prostate Cancer Aggressiveness. Prostate 2012, 72, 966–976. [Google Scholar] [CrossRef]
- D’Antonio, K.B.; Schultz, L.; Albadine, R.; Mondul, A.M.; Platz, E.A.; Netto, G.J.; Getzenberg, R.H. Decreased Expression of Cyr61 Is Associated with Prostate Cancer Recurrence after Surgical Treatment. Clin. Cancer Res. 2010, 16, 5908–5913. [Google Scholar] [CrossRef]
- Terada, N.; Kulkarni, P.; Getzenberg, R.H. Cyr61 Is a Potential Prognostic Marker for Prostate Cancer. Asian J. Androl. 2012, 14, 405–408. [Google Scholar] [CrossRef]
- Tsunoda, T.; Furusato, B.; Takashima, Y.; Ravulapalli, S.; Dobi, A.; Srivastava, S.; McLeod, D.G.; Sesterhenn, I.A.; Ornstein, D.K.; Shirasawa, S. The Increased Expression of Periostin during Early Stages of Prostate Cancer and Advanced Stages of Cancer Stroma. Prostate 2009, 69, 1398–1403. [Google Scholar] [CrossRef]
- Tischler, V.; Fritzsche, F.R.; Wild, P.J.; Stephan, C.; Seifert, H.H.; Riener, M.O.; Hermanns, T.; Mortezavi, A.; Gerhardt, J.; Schraml, P.; et al. Periostin Is Up-Regulated in High Grade and High Stage Prostate Cancer. BMC Cancer 2010, 10, 273. [Google Scholar] [CrossRef]
- Tian, Y.; Choi, C.H.; Li, Q.K.; Rahmatpanah, F.B.; Chen, X.; Kim, S.R.; Veltri, R.; Chia, D.; Zhang, Z.; Mercola, D.; et al. Overexpression of Periostin in Stroma Positively Associated with Aggressive Prostate Cancer. PLoS ONE 2015, 10, e0121502. [Google Scholar] [CrossRef]
- Konac, E.; Kiliccioglu, I.; Sogutdelen, E.; Dikmen, A.U.; Albayrak, G.; Bilen, C.Y. Do the Expressions of Epithelial–Mesenchymal Transition Proteins, Periostin, Integrin-A4 and Fibronectin Correlate with Clinico-Pathological Features and Prognosis of Metastatic Castration-Resistant Prostate Cancer? Exp. Biol. Med. 2017, 242, 1795–1801. [Google Scholar] [CrossRef]
- Sun, C.; Song, C.; Ma, Z.; Xu, K.; Zhang, Y.; Jin, H.; Tong, S.; Ding, W.; Xia, G.; Ding, Q. Periostin Identified as a Potential Biomarker of Prostate Cancer by ITRAQ-Proteomics Analysis of Prostate Biopsy. Proteome Sci. 2011, 9, 22. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Rubagotti, A.; Zinoli, L.; Ricci, F.; Salvi, S.; Boccardo, S.; Boccardo, F. Prognostic Value of Stromal and Epithelial Periostin Expression in Human Prostate Cancer: Correlation with Clinical Pathological Features and the Risk of Biochemical Relapse or Death. BMC Cancer 2012, 12, 625. [Google Scholar] [CrossRef]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein with Multiple Functions in Cancer Development and Progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and Function in Cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef]
- Cattrini, C.; Rubagotti, A.; Nuzzo, P.V.; Zinoli, L.; Salvi, S.; Boccardo, S.; Perachino, M.; Cerbone, L.; Vallome, G.; Latocca, M.M.; et al. Overexpression of Periostin in Tumor Biopsy Samples Is Associated with Prostate Cancer Phenotype and Clinical Outcome. Clin. Genitourin. Cancer 2018, 16, e1257–e1265. [Google Scholar] [CrossRef]
- Doldi, V.; Lecchi, M.; Ljevar, S.; Colecchia, M.; Campi, E.; Centonze, G.; Marenghi, C.; Rancati, T.; Miceli, R.; Verderio, P.; et al. Potential of the Stromal Matricellular Protein Periostin as a Biomarker to Improve Risk Assessment in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 7987. [Google Scholar] [CrossRef]
- Garcia-Marques, F.; Liu, S.; Totten, S.M.; Bermudez, A.; Tanimoto, C.; Hsu, E.C.; Nolley, R.; Hembree, A.; Stoyanova, T.; Brooks, J.D.; et al. Protein Signatures to Distinguish Aggressive from Indolent Prostate Cancer. Prostate 2022, 82, 605–616. [Google Scholar] [CrossRef]
- Murray, N.P.; Reyes, E.; Salazar, A.; Lopez, M.A.; Orrego, S.; Guzman, E. The Expression of Matrix-Metalloproteinase-2 in Bone Marrow Micro-Metastasis Is Associated with the Presence of Circulating Prostate Cells and a Worse Prognosis in Men Treated with Radical Prostatectomy for Prostate Cancer. Turk. J. Urol. 2020, 46, 186–195. [Google Scholar] [CrossRef]
- Reis, S.T.; Pontes-Junior, J.; Antunes, A.A.; de Sousa-Canavez, J.M.; Dall’Oglio, M.F.; Passerotti, C.C.; Abe, D.K.; Crippa, A.; da Cruz, J.A.S.; Timoszczuk, L.M.S.; et al. MMP-9 Overexpression Due to TIMP-1 and RECK Underexpression Is Associated with Prognosis in Prostate Cancer. Int. J. Biol. Markers 2011, 26, 255–261. [Google Scholar] [CrossRef]
- Trudel, D.; Fradet, Y.; Meyer, F.; Têtu, B. Matrix Metalloproteinase 9 Is Associated with Gleason Score in Prostate Cancer but Not with Prognosis. Hum. Pathol. 2010, 41, 1694–1701. [Google Scholar] [CrossRef]
- Geng, X.; Chen, C.; Huang, Y.; Hou, J. The Prognostic Value and Potential Mechanism of Matrix Metalloproteinases among Prostate Cancer. Int. J. Med. Sci. 2020, 17, 1550–1560. [Google Scholar] [CrossRef]
- Eiro, N.; Medina, A.; Gonzalez, L.O.; Fraile, M.; Palacios, A.; Escaf, S.; Fernández-Gómez, J.M.; Vizoso, F.J. Evaluation of Matrix Metalloproteases by Artificial Intelligence Techniques in Negative Biopsies as New Diagnostic Strategy in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 7022. [Google Scholar] [CrossRef]
- Nilsson, M.; Hägglöf, C.; Hammarsten, P.; Thysell, E.; Stattin, P.; Egevad, L.; Granfors, T.; Jernberg, E.; Wikstrom, P.; Halin Bergström, S.; et al. High Lysyl Oxidase (LOX) in the Non-Malignant Prostate Epithelium Predicts a Poor Outcome in Prostate Cancer Patient Managed by Watchful Waiting. PLoS ONE 2015, 10, e0140985. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Chen, Q.; Fang, K.; Wang, L.; Chen, F.; Li, X.; Li, Z.; Wang, J.; Liu, Y.; et al. Low Extracellular Lysyl Oxidase Expression Is Associated with Poor Prognosis in Patients with Prostate Cancer. Oncol. Lett. 2016, 12, 3161–3166. [Google Scholar] [CrossRef]
- Kim, M.S.; Ha, S.E.; Wu, M.; Zogg, H.; Ronkon, C.F.; Lee, M.Y.; Ro, S. Extracellular Matrix Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 9185. [Google Scholar] [CrossRef]
- Sathyanarayana, U.G.; Padar, A.; Suzuki, M.; Maruyama, R.; Shigematsu, H.; Hsieh, J.T.; Frenkel, E.P.; Gazdar, A.F. Aberrant Promoter Methylation of Laminin-5-Encoding Genes in Prostate Cancers and Its Relationship to Clinicopathological Features. Clin. Cancer Res. 2003, 9, 6395–6400. [Google Scholar]
- Suhovskih, A.V.; Mostovich, L.A.; Kunin, I.S.; Boboev, M.M.; Nepomnyashchikh, G.I.; Aidagulova, S.V.; Grigorieva, E.V. Proteoglycan Expression in Normal Human Prostate Tissue and Prostate Cancer. ISRN Oncol. 2013, 2013, 680136. [Google Scholar] [CrossRef]
- Ageeli, W.; Zhang, X.; Ogbonnaya, C.N.; Ling, Y.; Wilson, J.; Li, C.; Nabi, G. Characterisation of Collagen Re-modelling in Localised Prostate Cancer Using Second-generation Harmonic Imaging and Transrectal Ultrasound Shear Wave Elastography. Cancers 2021, 13, 5553. [Google Scholar] [CrossRef]
- Duarte, A.H.; Colli, S.; Alves-Pereira, J.L.; Martins, M.P.; Sampaio, F.J.B.; Ramos, C.F. Collagen I and III and Metalloproteinase Gene and Protein Expression in Prostate Cancer in Relation to Gleason Score. Int. Braz. J. Urol. 2012, 38, 341–355. [Google Scholar] [CrossRef]
- Jensen, C.; Drobinski, P.; Thorlacius-Ussing, J.; Karsdal, M.A.; Bay-Jensen, A.C.; Willumsen, N. Autoreactivity against Denatured Type III Collagen Is Significantly Decreased in Serum from Patients with Cancer Compared to Healthy Controls. Int. J. Mol. Sci. 2023, 24, 7067. [Google Scholar] [CrossRef]
- Nagle, R.B.; Hao, J.; Knox, J.D.; Dalkin, B.L.; Clark, V.; Cress, A.E. Expression of Hemidesmosomal and Extracellular Matrix Proteins by Normal and Malignant Human Prostate Tissue. Am. J. Pathol. 1995, 146, 1498–1507. [Google Scholar]
- Hansen, N.U.B.; Willumsen, N.; Sand, J.M.B.; Larsen, L.; Karsdal, M.A.; Leeming, D.J. Type VIII Collagen Is Elevated in Diseases Associated with Angiogenesis and Vascular Remodeling. Clin. Biochem. 2016, 49, 903–908. [Google Scholar] [CrossRef]
- Thorlacius-ussing, J.; Jensen, C.; Madsen, E.A.; Nissen, N.I.; Manon-jensen, T.; Chen, I.M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Karsdal, M.A.; et al. Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors. Int. J. Mol. Sci. 2022, 23, 4144. [Google Scholar] [CrossRef]
- Banyard, J.; Bao, L.; Hofer, M.D.; Zurakowski, D.; Spivey, K.A.; Feldman, A.S.; Hutchinson, L.M.; Kuefer, R.; Rubin, M.A.; Zetter, B.R. Collagen XXIII Expression Is Associated with Prostate Cancer Recurrence and Distant Metastases. Clin. Cancer Res. 2007, 13, 2634–2642. [Google Scholar] [CrossRef]
- Pföhler, C.; Fixemer, T.; Jung, V.; Dooley, S.; Remberger, K.; Bonkhoff, H. In Situ Hybridization Analysis of Genes Coding Collagen IV A1 Chain, Laminin Β1 Chain, and S-Laminin in Prostate Tissue and Prostate Cancer: Increased Basement Membrane Gene Expression in High-Grade and Metastatic Lesions. Prostate 1998, 36, 143–150. [Google Scholar] [CrossRef]
- Varisli, L. Identification of New Genes Downregulated in Prostate Cancer and Investigation of Their Effects on Prognosis. Genet. Test. Mol. Biomarkers 2013, 17, 562–566. [Google Scholar] [CrossRef]
- Ma, J.-B.; Bai, J.Y.; Zhang, H.B.; Gu, L.; He, D.; Guo, P. Downregulation of Collagen COL4A6 Is Associated with Prostate Cancer Progression and Metastasis. Genet. Test. Mol. Biomarkers 2020, 24, 399–408. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Chen, S.; Li, K.; Wan, S.; Yang, L. COL10A1 as a Prognostic Biomarker in Association with Immune Infiltration in Prostate Cancer. Curr. Cancer Drug Targets 2023. ahead of print. [Google Scholar] [CrossRef]
- Rochette, A.; Boufaied, N.; Scarlata, E.; Hamel, L.; Brimo, F.; Whitaker, H.C.; Ramos-Montoya, A.; Neal, D.E.; Dragomir, A.; Aprikian, A.; et al. Asporin Is a Stromally Expressed Marker Associated with Prostate Cancer Progression. Br. J. Cancer 2017, 116, 775–784. [Google Scholar] [CrossRef]
- Gerke, J.S.; Orth, M.F.; Tolkach, Y.; Romero-Pérez, L.; Wehweck, F.S.; Stein, S.; Musa, J.; Knott, M.M.L.; Hölting, T.L.B.; Li, J.; et al. Integrative Clinical Transcriptome Analysis Reveals TMPRSS2-ERG Dependency of Prognostic Biomarkers in Prostate Adenocarcinoma. Int. J. Cancer 2020, 146, 2036–2046. [Google Scholar] [CrossRef]
- Zhang, P.; Qian, B.; Liu, Z.; Wang, D.; Lv, F.; Xing, Y.; Xiao, Y. Identification of Novel Biomarkers of Prostate Cancer through Integrated Analysis. Transl. Androl. Urol. 2021, 10, 3239–3254. [Google Scholar] [CrossRef]
- Hurley, P.J.; Sundi, D.; Shinder, B.; Simons, B.W.; Hughes, R.M.; Miller, R.M.; Benzon, B.; Faraj, S.F.; Netto, G.J.; Vergara, I.A.; et al. Germline Variants in Asporin Vary by Race, Modulate the Tumor Microenvironment, and Are Differentially Associated with Metastatic Prostate Cancer. Clin. Cancer Res. 2016, 22, 448–458. [Google Scholar] [CrossRef]
- Klee, E.W.; Bondar, O.P.; Goodmanson, M.K.; Dyer, R.B.; Erdogan, S.; Bergstralh, E.J.; Bergen, H.R.; Sebo, T.J.; Klee, G.G. Candidate Serum Biomarkers for Prostate Adenocarcinoma Identified by mRNA Differences in Prostate Tissue and Verified with Protein Measurements in Tissue and Blood. Clin. Chem. 2012, 58, 599–609. [Google Scholar] [CrossRef]
- Jacobsen, F.; Kraft, J.; Schroeder, C.; Hube-Magg, C.; Kluth, M.; Lang, D.S.; Simon, R.; Sauter, G.; Izbicki, J.R.; Clauditz, T.S.; et al. Up-Regulation of Biglycan Is Associated with Poor Prognosis and PTEN Deletion in Patients with Prostate Cancer. Neoplasia 2017, 19, 707–715. [Google Scholar] [CrossRef]
- Furumido, J.; Maishi, N.; Yanagawa-Matsuda, A.; Kikuchi, H.; Matsumoto, R.; Osawa, T.; Abe, T.; Matsuno, Y.; Shinohara, N.; Hida, Y.; et al. Stroma Biglycan Expression Can Be a Prognostic Factor in Prostate Cancers. Int. J. Urol. 2023, 30, 147–154. [Google Scholar] [CrossRef]
- Arslan, B.; Onuk, Ö.; Hazar, I.; Aydin, M.; Çilesiz, N.C.; Eroglu, A.; Nuhoglu, B. Prognostic Value of Endocan in Prostate Cancer: Clinicopathologic Association between Serum Endocan Levels and Biochemical Recurrence after Radical Prostatectomy. Tumori 2017, 103, 204–208. [Google Scholar] [CrossRef]
- Lai, C.Y.; Chen, C.M.; Hsu, W.H.; Hsieh, Y.H.; Liu, C.J. Overexpression of Endothelial Cell-Specific Molecule 1 Correlates with Gleason Score and Expression of Androgen Receptor in Prostate Carcinoma. Int. J. Med. Sci. 2017, 14, 1263–1267. [Google Scholar] [CrossRef]
- Dadali, M.; Tad, M.; Bagbanci, M.S. Expression of Endocan in Tissue Samples from Prostate Adenocarcinoma and Prostate Hyperplasia: A Comparative Retrospective Study. Urol. J. 2021, 18, 530–536. [Google Scholar] [CrossRef]
- Bettin, A.; Reyes, I.; Reyes, N. Gene Expression Profiling of Prostate Cancer-Associated Genes Identifies Fibromodulin as Potential Novel Biomarker for Prostate Cancer. Int. J. Biol. Markers 2016, 31, 153–162. [Google Scholar] [CrossRef]
- Silva, T.; Gomes, C.P.; Voigt, D.D.; De Souza, R.B.; Medeiros, K.; Cosentino, N.L.; Fonseca, A.C.P.; Tilli, T.M.; Loayza, E.A.C.; Ramos, V.G.; et al. Fibromodulin Gene Variants (FMOD) as Potential Biomarkers for Prostate Cancer and Benign Prostatic Hyperplasia. Dis. Markers 2022, 2022, 5215247. [Google Scholar] [CrossRef]
- Reyes, N.; Benedetti, I.; Bettin, A.; Rebollo, J.; Geliebter, J. The Small Leucine Rich Proteoglycan Fibromodulin Is Overexpressed in Human Prostate Epithelial Cancer Cell Lines in Culture and Human Prostate Cancer Tissue. Cancer Biomark. 2016, 16, 191–202. [Google Scholar] [CrossRef]
- Samaržija, I.; Konjevoda, P. Extracellular Matrix- and Integrin Adhesion Complexes-Related Genes in the Prognosis of Prostate Cancer Patients’ Progression-Free Survival. Biomedicines 2023, 11, 2006. [Google Scholar] [CrossRef]
- Grindel, B.; Li, Q.; Arnold, R.; Petros, J.; Zayzafoon, M.; Muldoon, M.; Stave, J.; Chung, L.W.K.; Farach-Carson, M.C. Perlecan/HSPG2 and Matrilysin/MMP-7 as Indices of Tissue Invasion: Tissue Localization and Circulating Perlecan Fragments in a Cohort of 288 Radical Prostatectomy Patients. Oncotarget 2016, 7, 10433–10447. [Google Scholar] [CrossRef]
- Lima, T.; Barros, A.S.; Trindade, F.; Ferreira, R.; Leite-Moreira, A.; Barros-Silva, D.; Jerónimo, C.; Araújo, L.; Henrique, R.; Vitorino, R.; et al. Application of Proteogenomics to Urine Analysis towards the Identification of Novel Biomarkers of Prostate Cancer: An Exploratory Study. Cancers 2022, 14, 2001. [Google Scholar] [CrossRef]
- Chien, M.H.; Lin, Y.W.; Wen, Y.C.; Yang, Y.C.; Hsiao, M.; Chang, J.L.; Huang, H.C.; Lee, W.J. Targeting the SPOCK1-Snail/Slug Axis-Mediated Epithelial-to-Mesenchymal Transition by Apigenin Contributes to Repression of Prostate Cancer Metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef]
- Chen, M.L.; Ho, C.J.; Yeh, C.M.; Chen, S.L.; Sung, W.W.; Wang, S.C.; Chen, C.J. High SPOCK1 Expression Is Associated with Advanced Stage, T Value, and Gleason Grade in Prostate Cancer. Medicina 2019, 55, 343. [Google Scholar] [CrossRef]
- Wlazlinski, A.; Engers, R.; Hoffmann, M.J.; Hader, C.; Jung, V.; Müller, M.; Schulz, W.A. Downregulation of Several Fibulin Genes in Prostate Cancer. Prostate 2007, 67, 1770–1780. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cui, J.J.; Zhu, T.; Shao, W.H.; Zhao, Y.; Wang, S.; Zhang, Y.P.; Wu, J.C.; Zhang, L. Biomarkers Identified for Prostate Cancer Patients through Genome-Scale Screening. Oncotarget 2017, 8, 92055–92063. [Google Scholar] [CrossRef]
- Luo, J.; Lai, C.; Xu, X.; Shi, J.; Hu, J.; Guo, K.; Mulati, Y.; Xiao, Y.; Kong, D.; Liu, C.; et al. Mechanism of Prognostic Marker SPOCK3 Affecting Malignant Progression of Prostate Cancer and Construction of Prognostic Model. BMC Cancer 2023, 23, 741. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Mayne, K.; Sykes, P.J.; Raymond, W.A.; McCaul, K.; Marshall, V.R.; Horsfall, D.J. Elevated Levels of Versican but Not Decorin Predict Disease Progression in Early-Stage Prostate Cancer. Clin. Cancer Res. 1998, 4, 963–971. [Google Scholar]
- Lygirou, V.; Fasoulakis, K.; Stroggilos, R.; Makridakis, M.; Latosinska, A.; Frantzi, M.; Katafigiotis, I.; Alamanis, C.; Stravodimos, K.G.; Constantinides, C.A.; et al. Proteomic Analysis of Prostate Cancer FFPE Samples Reveals Markers of Disease Progression and Aggressiveness. Cancers 2022, 14, 3765. [Google Scholar] [CrossRef]
- Housa, D.; Vernerová, Z.; Heráček, J.; Procházka, B.; Čechák, P.; Kuncová, J.; Haluzík, M. Adiponectin as a Potential Marker of Prostate Cancer Progression: Studies in Organ-Confined and Locally Advanced Prostate Cancer. Physiol. Res. 2008, 57, 451–458. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Penney, K.L.; Schumacher, F.; Rider, J.R.; Sesso, H.D.; Pollak, M.; Fiorentino, M.; Finn, S.; Loda, M.; Rifai, N.; et al. Common Polymorphisms in the Adiponectin and Its Receptor Genes, Adiponectin Levels and the Risk of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2618–2627. [Google Scholar] [CrossRef]
- Gu, C.; Qu, Y.; Zhang, G.; Sun, L.J.; Zhu, Y.; Ye, D. A Single Nucleotide Polymorphism in ADIPOQ Predicts Biochemical Recurrence after Radical Prostatectomy in Localized Prostate Cancer. Oncotarget 2015, 6, 32205–32211. [Google Scholar] [CrossRef]
- Freedland, S.J.; Sokoll, L.J.; Platz, E.A.; Mangold, L.A.; Bruzek, D.J.; Mohr, P.; Yiu, S.K.; Partin, A.W. Association between Serum Adiponectin, and Pathological Stage and Grade in Men Undergoing Radical Prostatectomy. J. Urol. 2005, 174, 1266–1270. [Google Scholar] [CrossRef]
- Michalakis, K.; Williams, C.J.; Mitsiades, N.; Blakeman, J.; Balafouta-Tselenis, S.; Giannopoulos, A.; Mantzoros, C.S. Serum Adiponectin Concentrations and Tissue Expression of Adiponectin Receptors Are Reduced in Patients with Prostate Cancer: A Case Control Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 308–313. [Google Scholar] [CrossRef]
- Kaklamani, V.; Yi, N.; Zhang, K.; Sadim, M.; Offit, K.; Oddoux, C.; Ostrer, H.; Mantzoros, C.; Pasche, B. Polymorphisms of ADIPOQ and ADIPOR1 and Prostate Cancer Risk. Metabolism 2011, 60, 1234–1243. [Google Scholar] [CrossRef]
- Nishimura, K.; Soda, T.; Nakazawa, S.; Yamanaka, K.; Hirai, T.; Kishikawa, H.; Ichikawa, Y. Serum Adiponectin and Leptin Levels Are Useful Markers for Prostate Cancer Screening after Adjustments for Age, Obesity-Related Factors, and Prostate Volume. Minerva Urol. E Nefrol. 2012, 64, 199–208. [Google Scholar]
- Liao, Q.; Long, C.; Deng, Z.; Bi, X.; Hu, J. The Role of Circulating Adiponectin in Prostate Cancer: A Meta-Analysis. Int. J. Biol. Markers 2015, 30, 22–31. [Google Scholar] [CrossRef]
- Hu, M.B.; Xu, H.; Hu, J.M.; Zhu, W.H.; Yang, T.; Jiang, H.W.; Ding, Q. Genetic Polymorphisms in Leptin, Adiponectin and Their Receptors Affect Risk and Aggressiveness of Prostate Cancer: Evidence from a Meta-Analysis and Pooled-Review. Oncotarget 2016, 7, 81049–81061. [Google Scholar] [CrossRef]
- Burton, A.J.; Gilbert, R.; Tilling, K.; Langdon, R.; Donovan, J.L.; Holly, J.M.P.; Martin, R.M. Circulating Adiponectin and Leptin and Risk of Overall and Aggressive Prostate Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 320. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Liu, W.; Zhang, S. Cartilage Oligomeric Matrix Protein Acts as a Molecular Biomarker in Multiple Cancer Types. Clin. Transl. Oncol. 2023, 25, 535–554. [Google Scholar] [CrossRef]
- Chen, J.; Xi, J.; Tian, Y.; Bova, G.S.; Zhang, H. Identification, Prioritization, and Evaluation of Glycoproteins for Aggressive Prostate Cancer Using Quantitative Glycoproteomics and Antibody-Based Assays on Tissue Specimens. Proteomics 2013, 13, 2268–2277. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiong, W.; Zhou, X.; Gao, R.S.; Lin, Q.F.; Liu, H.Y.; Li, J.N.; Tian, X.F. CTHRC1 and PD-1/PD-L1 Expression Predicts Tumor Recurrence in Prostate Cancer. Mol. Med. Rep. 2019, 20, 4244–4252. [Google Scholar] [CrossRef]
- Lv, H.; Fan, E.; Sun, S.; Ma, X.; Zhang, X.; Han, D.M.K.; Cong, Y.S. Cyr61 Is Up-Regulated in Prostate Cancer and Associated with the P53 Gene Status. J. Cell. Biochem. 2009, 106, 738–744. [Google Scholar] [CrossRef]
- Jain, A.; McKnight, D.A.; Fisher, L.W.; Humphreys, E.B.; Mangold, L.A.; Partin, A.W.; Fedarko, N.S. Small Integrin-Binding Proteins as Serum Markers for Prostate Cancer Detection. Clin. Cancer Res. 2009, 15, 5199–5207. [Google Scholar] [CrossRef]
- Chaplet, M.; Waltregny, D.; Detry, C.; Fisher, L.W.; Castronovo, V.; Bellahcène, A. Expression of Dentin Sialophosphoprotein in Human Prostate Cancer and Its Correlation with Tumor Aggressiveness. Int. J. Cancer 2006, 118, 850–856. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, H.T.; Ha, Y.S.; Lee, E.H.; Chun, S.Y.; Lee, C.H.; Byeon, K.H.; Choi, S.H.; Lee, J.N.; Kim, B.S.; et al. Identification of a Novel Non-Invasive Biological Marker to Overcome the Shortcomings of PSA in Diagnosis and Risk Stratification for Prostate Cancer: Initial Prospective Study of Developmental Endothelial Locus-1 Protein. PLoS ONE 2021, 16, e0250254. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Zhou, J.; Chen, Z.; Yang, G.; Liao, Y.; Zhu, M. Epidermal Growth Factor-Containing Fibulin-like Extracellular Matrix Protein 1 (EFEMP1) Acts as a Potential Diagnostic Biomarker for Prostate Cancer. Med. Sci. Monit. 2017, 23, 216–222. [Google Scholar] [CrossRef]
- Almeida, M.; Costa, V.L.; Costa, N.R.; Ramalho-Carvalho, J.; Baptista, T.; Ribeiro, F.R.; Paulo, P.; Teixeira, M.R.; Oliveira, J.; Lothe, R.A.; et al. Epigenetic Regulation of EFEMP1 in Prostate Cancer: Biological Relevance and Clinical Potential. J. Cell. Mol. Med. 2014, 18, 2287–2297. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, H.Y.; Kim, S.K.; Kim, Y.W.; Kim, E.J.; Kim, I.Y.; Kim, W.J. EFEMP1 as a Novel DNA Methylation Marker for Prostate Cancer: Array-Based DNA Methylation and Expression Profiling. Clin. Cancer Res. 2011, 17, 4523–4530. [Google Scholar] [CrossRef]
- Wang, Y.; Lih, T.S.M.; Höti, N.; Sokoll, L.J.; Chesnut, G.; Petrovics, G.; Kohaar, I.; Zhang, H. Differentially Expressed Glycoproteins in Pre- and Post-Digital Rectal Examination Urine Samples for Detecting Aggressive Prostate Cancer. Proteomics 2023, 23, e2200023. [Google Scholar] [CrossRef]
- Peng, H.H.; Wang, J.N.; Xiao, L.F.; Yan, M.; Chen, S.P.; Wang, L.; Yang, K. Elevated Serum FGG Levels Prognosticate and Promote the Disease Progression in Prostate Cancer. Front. Genet. 2021, 12, 651647. [Google Scholar] [CrossRef]
- Davalieva, K.; Kiprijanovska, S.; Kostovska, I.M.; Stavridis, S.; Stankov, O.; Komina, S.; Petrusevska, G.; Polenakovic, M. Comparative Proteomics Analysis of Urine Reveals Down-Regulation of Acute Phase Response Signaling and LXR/RXR Activation Pathways in Prostate Cancer. Proteomes 2018, 6, 1. [Google Scholar] [CrossRef]
- Wei, R.J.; Li, T.Y.; Yang, X.C.; Jia, N.; Yang, X.L.; Song, H.B. Serum Levels of PSA, ALP, ICTP, and BSP in Prostate Cancer Patients and the Significance of ROC Curve in the Diagnosis of Prostate Cancer Bone Metastases. Genet. Mol. Res. 2016, 15, 15027707. [Google Scholar] [CrossRef]
- Zhu, B.P.; Guo, Z.Q.; Lin, L.; Liu, Q. Serum BSP, PSADT, and Spondin-2 Levels in Prostate Cancer and the Diagnostic Significance of Their ROC Curves in Bone Metastasis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 61–67. [Google Scholar]
- Fedarko, N.S.; Jain, A.; Karadag, A.; van Eman, M.R.; Fisher, L.W. Elevated Serum Bone Sialoprotein and Osteopontin in Colon, Breast, Prostate, and Lung Cancer. Clin. Cancer Res. 2001, 7, 4060–4066. [Google Scholar]
- Waltregny, D.; Bellahcène, A.; Van Riet, I.; Fisher, L.W.; Young, M.; Fernandez, P.; Dewé, W.; De Leval, J.; Castronovo, V. Prognostic Value of Bone Sialoprotein Expression in Clinically Localized Human Prostate Cancer. J. Natl. Cancer Inst. 1998, 90, 1000–1008. [Google Scholar] [CrossRef]
- Waltregny, D.; Bellahcène, A.; De Leval, X.; Florkin, B.; Weidle, U.; Castronovo, V. Increased Expression of Bone Sialoprotein in Bone Metastases Compared with Visceral Metastases in Human Breast and Prostate Cancers. J. Bone Miner. Res. 2000, 15, 834–843. [Google Scholar] [CrossRef]
- De Pinieux, G.; Flam, T.; Zerbib, M.; Taupin, P.; Bellahcène, A.; Waltregny, D.; Vieillefond, A.; Poupon, M.F. Bone Sialoprotein, Bone Morphogenetic Protein 6 and Thymidine Phosphorylase Expression in Localized Human Prostatic Adenocarcinoma as Predictors of Clinical Outcome: A Clinicopathological and Immunohistochemical Study of 43 Cases. J. Urol. 2001, 166, 1924–1930. [Google Scholar] [CrossRef]
- Carlinfante, G.; Vassiliou, D.; Svensson, O.; Wendel, M.; Heinegård, D.; Andersson, G. Differential Expression of Osteopontin and Bone Sialoprotein in Bone Metastasis of Breast and Prostate Carcinoma. Clin. Exp. Metastasis 2003, 20, 437–444. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.F.; Dai, J.; Zheng, Y.C.; Zhang, M.G.; He, J.J. Predictive Value of Serum Bone Sialoprotein and Prostate-Specific Antigen Doubling Time in Patients with Bone Metastasis of Prostate Cancer. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2013, 33, 559–562. [Google Scholar] [CrossRef]
- Sharma, J.; Gray, K.P.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated Insulin-like Growth Factor Binding Protein-1 (IGFBP-1) in Men with Metastatic Prostate Cancer Starting Androgen Deprivation Therapy (ADT) Is Associated with Shorter Time to Castration Resistance and Overall Survival. Prostate 2014, 74, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Nimptsch, K.; Shui, I.M.; Platz, E.A.; Wu, K.; Pollak, M.N.; Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.L. Prediagnostic Plasma IGFBP-1, IGF-1 and Risk of Prostate Cancer. Int. J. Cancer 2015, 136, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.; Lewis, S.J.; Rowlands, M.A.; Gaunt, T.R.; Davey Smith, G.; Gunnell, D.; Palmer, T.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; et al. Assessing the Role of Insulin-like Growth Factors and Binding Proteins in Prostate Cancer Using Mendelian Randomization: Genetic Variants as Instruments for Circulating Levels. Int. J. Cancer 2016, 139, 1520–1533. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.L.; Perez-Cornago, A.; Fensom, G.K.; Smith-Byrne, K.; Noor, U.; Andrews, C.D.; Gunter, M.J.; Holmes, M.V.; Martin, R.M.; Tsilidis, K.K.; et al. Circulating Insulin-like Growth Factors and Risks of Overall, Aggressive and Early-Onset Prostate Cancer: A Collaborative Analysis of 20 Prospective Studies and Mendelian Randomization Analysis. Int. J. Epidemiol. 2023, 52, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Penney, K.L.; Stram, D.O.; Le Marchand, L.; Giorgi, E.; Haiman, C.A.; Kolonel, L.N.; Pike, M.; Hirschhorn, J.; Henderson, B.E.; et al. Haplotype-Based Association Studies of IGFBP1 and IGFBP3 with Prostate and Breast Cancer Risk: The Multiethnic Cohort. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hong, C.Q.; Lin, Y.W.; Luo, Y.; Ding, T.Y.; Xu, Y.W.; Peng, Y.H.; Wu, F.C. Association between IGFBP1 Expression and Cancer Risk: A Systematic Review and Meta-Analysis. Heliyon 2023, 9, e16470. [Google Scholar] [CrossRef]
- Inman, B.A.; Harel, F.; Audet, J.F.; Meyer, F.; Douville, P.; Fradet, Y.; Lacombe, L. Insulin-like Growth Factor Binding Protein 2: An Androgen-Dependent Predictor of Prostate Cancer Survival. Eur. Urol. 2005, 47, 695–702. [Google Scholar] [CrossRef]
- Mehrian-Shai, R.; Chen, C.D.; Shi, T.; Horvath, S.; Nelson, S.F.; Reichardt, J.K.V.; Sawyers, C.L. Insulin Growth Factor-Binding Protein 2 Is a Candidate Biomarker for PTEN Status and PI3K/Akt Pathway Activation in Glioblastoma and Prostate Cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 5563–5568. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Farnedi, A.; Montironi, R.; Foschini, M.P. IGFBP2 as an Immunohistochemical Marker for Prostatic Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 318–328. [Google Scholar] [CrossRef]
- Rowlands, M.A.; Holly, J.M.P.; Gunnell, D.; Donovan, J.; Lane, J.A.; Hamdy, F.; Neal, D.E.; Oliver, S.; Smith, G.D.; Martin, R.M. Circulating Insulin-like Growth Factors and IGF-Binding Proteins in PSA-Detected Prostate Cancer: The Large Case-Control Study ProtecT. Cancer Res. 2012, 72, 503–515. [Google Scholar] [CrossRef]
- Tennant, M.K.; Thrasher, J.B.; Twomey, P.A.; Birnbaum, R.S.; Plymate, S.R. Insulin-like Growth Factor-Binding Protein-2 and -3 Expression in Benign Human Prostate Epithelium, Prostate Intraepithelial Neoplasia, and Adenocarcinoma of the Prostate. J. Clin. Endocrinol. Metab. 1996, 81, 411–420. [Google Scholar] [CrossRef]
- Ho, P.J.; Baxter, R.C. Insulin-like Growth Factor-Binding Protein-2 in Patients with Prostate Carcinoma and Benign Prostatic Hyperplasia. Clin. Endocrinol. 1997, 46, 145–154. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Platz, E.A.; Till, C.; Tangen, C.M.; Goodman, P.J.; Kristal, A.; Parnes, H.L.; Tao, Y.; Figg, W.D.; Lucia, M.S.; et al. Insulin-like Growth Factors and Insulin-like Growth Factor-Binding Proteins and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Cancer Prev. Res. 2013, 6, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Appleby, P.N.; Martin, R.M.; Holly, J.M.P.; Albanes, D.; Black, A.; Bueno-De-Mesquita, H.B.; Chan, J.M.; Chen, C.; Chirlaque, M.D.; et al. A Meta-Analysis of Individual Participant Data Reveals an Association between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Res. 2016, 76, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Till, C.; Price, D.K.; Goodman, P.J.; Neuhouser, M.L.; Pollak, M.N.; Thompson, I.M.; Figg, W.D. Serum Markers, Obesity and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Endocr. Relat. Cancer 2022, 29, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Borziak, K.; Finkelstein, J. Gene Expression Markers of Prognostic Importance for Prostate Cancer Risk in Patients with Benign Prostate Hyperplasia. In Proceedings of the Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Glasgow, Scotland, 11–15 July 2022. [Google Scholar]
- Miyata, Y.; Sakai, H.; Hayashi, T.; Kanetake, H. Serum Insulin-like Growth Factor Binding Protein-3/Prostate-Specific Antigen Ratio Is a Useful Predictive Marker in Patients with Advanced Prostate Cancer. Prostate 2003, 54, 125–132. [Google Scholar] [CrossRef]
- Platz, E.A.; Pollak, M.N.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Plasma Insulin-like Growth Factor-1 and Binding Protein-3 and Subsequent Risk of Prostate Cancer in the PSA Era. Cancer Causes Control. 2005, 16, 255–262. [Google Scholar] [CrossRef]
- Severi, G.; Morris, H.A.; MacInnis, R.J.; English, D.R.; Tilley, W.D.; Hopper, J.L.; Boyle, P.; Giles, G.G. Circulating Insulin-like Growth Factor-I and Binding Protein-3 and Risk of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1137–1141. [Google Scholar] [CrossRef][Green Version]
- Hernandez, W.; Grenade, C.; Santos, E.R.; Bonilla, C.; Ahaghotu, C.; Kittles, R.A. IGF-1 and IGFBP-3 Gene Variants Influence on Serum Levels and Prostate Cancer Risk in African-Americans. Carcinogenesis 2007, 28, 2154–2159. [Google Scholar] [CrossRef]
- Tajtakova, M.; Pidanicova, A.; Valansky, L.; Lachvac; Nagy, V.; Sivonova, M.; Dobrota, D.; Kliment, J.; Petrovicova, J. Serum Level of IGFBP3 and IGF1/IGFBP3 Molar Ratio in Addition to PSA and Single Nucleotide Polymorphism in PSA and CYP17 Gene May Contribute to Early Diagnostics of Prostate Cancer. Neoplasma 2010, 57, 118–122. [Google Scholar] [CrossRef][Green Version]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Relationship of Insulin-like Growth Factor (IGF) Binding Protein-3 (IGFBP-3) Gene Polymorphism with the Susceptibility to Development of Prostate Cancer and Influence on Serum Levels of IGF-I, and IGFBP-3. Growth Horm. IGF Res. 2011, 21, 146–154. [Google Scholar] [CrossRef]
- Terracciano, D.; Bruzzese, D.; Ferro, M.; Mazzarella, C.; Di Lorenzo, G.; Altieri, V.; Mariano, A.; MacChia, V.; Di Carlo, A. Preoperative Insulin-like Growth Factor-Binding Protein-3 (IGFBP-3) Blood Level Predicts Gleason Sum Upgrading. Prostate 2012, 72, 100–107. [Google Scholar] [CrossRef]
- Prager, A.J.; Peng, C.R.; Lita, E.; Mcnally, D.; Kaushal, A.; Sproull, M.; Compton, K.; Dahut, W.L.; Figg, W.D.; Citrin, D.; et al. Urinary AHGF, IGFBP3 and OPN as Diagnostic and Prognostic Biomarkers for Prostate Cancer. Biomark. Med. 2013, 7, 831–841. [Google Scholar] [CrossRef]
- Corrêa de Mello, L.L.; Neto, L.V.; Balarini Lima, G.A.; Gabrich, R.; de Miranda, L.C.D.; Gadelha, M.R. Insulin-like Growth Factor (IgF)-I, IgF Binding Protein-3, and Prostate Cancer: Correlation with Gleason Score. Int. Braz. J. Urol. 2015, 41, 110–115. [Google Scholar] [CrossRef]
- Tan, V.Y.; Biernacka, K.M.; Dudding, T.; Bonilla, C.; Gilbert, R.; Kaplan, R.C.; Qibin, Q.; Teumer, A.; Martin, R.M.; Perks, C.M.; et al. Reassessing the Association between Circulating Vitamin D and IGFBP-3: Observational and Mendelian Randomization Estimates from Independent Sources. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1462–1471. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Schenk, J.; Yoon, J.S.; Tangen, C.M.; Goodman, P.J.; Pollak, M.; Penson, D.F.; Thompson, I.M.; Kristal, A.R. Insulin-like Growth Factor-I, Insulin-like Growth Factor Binding Protein-3 and Risk of Benign Prostate Hyperplasia in the Prostate Cancer Prevention Trial. Prostate 2008, 68, 1477–1486. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.H.; Jeon, H.G.; Byun, S.S.; Lee, E. Influence of IGFBP3 Gene Polymorphisms on IGFBP3 Serum Levels and the Risk of Prostate Cancer in Low-Risk Korean Men. Urology 2010, 75, 1516.e1–1516.e7. [Google Scholar] [CrossRef]
- Rowlands, M.A.; Holly, J.M.P.; Hamdy, F.; Phillips, J.; Goodwin, L.; Marsden, G.; Gunnell, D.; Donovan, J.; Neal, D.E.; Martin, R.M. Serum Insulin-like Growth Factors and Mortality in Localised and Advanced Clinically Detected Prostate Cancer. Cancer Causes Control. 2012, 23, 347–354. [Google Scholar] [CrossRef]
- Seligson, D.B.; Yu, H.; Tze, S.; Said, J.; Pantuck, A.J.; Cohen, P.; Lee, K.W. IGFBP-3 Nuclear Localization Predicts Human Prostate Cancer Recurrence. Horm. Cancer 2013, 4, 12–23. [Google Scholar] [CrossRef]
- Ding, Q.; Shi, Y.; Fan, B.; Fan, Z.; Wang, J. IGFBP-3 Promoter Polymorphism-202A>C (Rs2854774) Contributes to Prostate Cancer Risk: Evidence Based on 9482 Subjects. Urol. Int. 2014, 93, 100–107. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Y.; Liu, F.; Gu, C.; Chen, H.; Xu, J.; Ye, D. Genetic Variants in Insulin-like Growth Factor Binding Protein-3 Are Associated with Prostate Cancer Susceptibility in Eastern Chinese Han Men. Onco Targets Ther. 2015, 9, 61–66. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Sun, S.K.; Zhang, X. CC Chemokine Ligand 18 and IGF-Binding Protein 6 as Potential Serum Biomarkers for Prostate Cancer. Tohoku J. Exp. Med. 2014, 233, 25–31. [Google Scholar] [CrossRef]
- Brar, P.K.; Dalkin, B.L.; Weyer, C.; Sallam, K.; Virtanen, I.; Nagle, R.B. Laminin Alpha-1, Alpha-3, and Alpha-5 Chain Expression in Human Prepubetal Benign Prostate Glands and Adult Benign and Malignant Prostate Glands. Prostate 2003, 55, 65–70. [Google Scholar] [CrossRef]
- Tomas, D.; Ulamec, M.; Hudolin, T.; Bulimbašić, S.; Belicza, M.; Krušlin, B. Myofibroblastic Stromal Reaction and Expression of Tenascin-C and Laminin in Prostate Adenocarcinoma. Prostate Cancer Prostatic Dis. 2006, 9, 414–419. [Google Scholar] [CrossRef]
- Hao, J.; Yang, Y.; McDaniel, K.M.; Dalkin, B.L.; Cress, A.E.; Nagle, R.B. Differential Expression of Laminin 5 (α3β3γ2) by Human Malignant and Normal Prostate. Am. J. Pathol. 1996, 149, 1341. [Google Scholar]
- Hao, J.; Jackson, L.; Calaluce, R.; McDaniel, K.; Dalkin, B.L.; Nagle, R.B. Investigation into the Mechanism of the Loss of Laminin 5 (α3β3γ2) Expression in Prostate Cancer. Am. J. Pathol. 2001, 158, 1129–1135. [Google Scholar] [CrossRef]
- Guldvik, I.J.; Zuber, V.; Braadland, P.R.; Grytli, H.H.; Ramberg, H.; Lilleby, W.; Thiede, B.; Zucknick, M.; Saatcioglu, F.; Gislefoss, R.; et al. Identification and Validation of Leucine-Rich α-2-Glycoprotein 1 as a Noninvasive Biomarker for Improved Precision in Prostate Cancer Risk Stratification. Eur. Urol. Open Sci. 2020, 21, 51–60. [Google Scholar] [CrossRef]
- Guldvik, I.J.; Braadland, P.R.; Sivanesan, S.; Ramberg, H.; Kristensen, G.; Tennstedt, P.; Røder, A.; Schlomm, T.; Berge, V.; Eri, L.M.; et al. Low Blood Levels of LRG1 Before Radical Prostatectomy Identify Patients with High Risk of Progression to Castration-Resistant Prostate Cancer. Eur. Urol. Open Sci. 2022, 45, 68–75. [Google Scholar] [CrossRef]
- Ramesh, G.; Berg, A.; Jayakumar, C. Plasma Netrin-1 Is a Diagnostic Biomarker of Human Cancers. Biomarkers 2011, 16, 172–180. [Google Scholar] [CrossRef]
- Latil, A.; Chêne, L.; Cochant-Priollet, B.; Mangin, P.; Fournier, G.; Berthon, P.; Cussenot, O. Quantification of Expression of Netrins, Slits and Their Receptors in Human Prostate Tumors. Int. J. Cancer 2003, 103, 306–315. [Google Scholar] [CrossRef]
- Tian, Y.; Bova, G.S.; Zhang, H. Quantitative Glycoproteomic Analysis of Optimal Cutting Temperature-Embedded Frozen Tissues Identifying Glycoproteins Associated with Aggressive Prostate Cancer. Anal. Chem. 2011, 83, 7013–7019. [Google Scholar] [CrossRef]
- Cattrini, C.; Barboro, P.; Rubagotti, A.; Zinoli, L.; Zanardi, E.; Capaia, M.; Boccardo, F. Integrative Analysis of Periostin in Primary and Advanced Prostate Cancer. Transl. Oncol. 2020, 13, 100789. [Google Scholar] [CrossRef]
- Perrone, G.; Vincenzi, B.; Zagami, M.; Santini, D.; Panteri, R.; Flammia, G.; Verzí, A.; Lepanto, D.; Morini, S.; Russo, A.; et al. Reelin Expression in Human Prostate Cancer: A Marker of Tumor Aggressiveness Based on Correlation with Grade. Mod. Pathol. 2007, 20, 344–351. [Google Scholar] [CrossRef]
- Mesci, A.; Lucien, F.; Huang, X.; Wang, E.H.; Shin, D.; Meringer, M.; Hoey, C.; Ray, J.; Boutros, P.C.; Leong, H.S.; et al. RSPO3 Is a Prognostic Biomarker and Mediator of Invasiveness in Prostate Cancer. J. Transl. Med. 2019, 17, 125. [Google Scholar] [CrossRef]
- Bartholow, T.L.; Becich, M.J.; Chandran, U.R.; Parwani, A.V. Immunohistochemical Staining of Slit2 in Primary and Metastatic Prostatic Adenocarcinoma. Transl. Oncol. 2011, 4, 314–320. [Google Scholar] [CrossRef]
- DeRosa, C.A.; Furusato, B.; Shaheduzzaman, S.; Srikantan, V.; Wang, Z.; Chen, Y.; Siefert, M.; Ravindranath, L.; Young, D.; Nau, M.; et al. Elevated Osteonectin/SPARC Expression in Primary Prostate Cancer Predicts Metastatic Progression. Prostate Cancer Prostatic Dis. 2012, 15, 150–156. [Google Scholar] [CrossRef]
- Thomas, R.; True, L.D.; Bassuk, J.A.; Lange, P.H.; Vessella, R.L. Differential Expression of Osteonectin/SPARC during Human Prostate Cancer Progression. Clin. Cancer Res. 2000, 6, 1140–1149. [Google Scholar]
- Liu, T.; Qiu, X.; Zhao, X.; Yang, R.; Lian, H.; Qu, F.; Li, X.; Guo, H. Hypermethylation of the SPARC Promoter and Its Prognostic Value for Prostate Cancer. Oncol. Rep. 2018, 39, 659–666. [Google Scholar] [CrossRef]
- López-Moncada, F.; Torres, M.; Castellón, E.; Contreras, H.R. Secreted Protein Acidic and Rich in Cysteine (SPARC) Induces Epithelial-Mesenchymal Transition, Enhancing Migration and Invasion, and Is Associated with High Gleason Score in Prostate Cancer. Asian J. Androl. 2019, 21, 557–564. [Google Scholar] [CrossRef]
- Enriquez, C.; Cancila, V.; Ferri, R.; Sulsenti, R.; Fischetti, I.; Milani, M.; Ostano, P.; Gregnanin, I.; Mello-Grand, M.; Berrino, E.; et al. Castration-Induced Downregulation of SPARC in Stromal Cells Drives Neuroendocrine Differentiation of Prostate Cancer. Cancer Res. 2021, 81, 4257–4274. [Google Scholar] [CrossRef]
- Hanousková, L.; Řezáč, J.; Veselý, Š.; Průša, R.; Kotaška, K. Diagnostic Benefits of Mindin as a Prostate Cancer Biomarker. J. Med. Biochem. 2019, 39, 108–111. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Bettocchi, C.; Palazzo, S.; Vavallo, A.; Galleggiante, V.; Trabucco, S.; Di Clemente, D.; Selvaggi, F.P.; Battaglia, M.; et al. Spondin-2, a Secreted Extracellular Matrix Protein, Is a Novel Diagnostic Biomarker for Prostate Cancer. J. Urol. 2013, 190, 2271–2277. [Google Scholar] [CrossRef]
- Qian, X.; Li, C.; Pang, B.; Xue, M.; Wang, J.; Zhou, J. Spondin-2 (SPON2), a More Prostate-Cancer-Specific Diagnostic Biomarker. PLoS ONE 2012, 7, e37225. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.T.; Turner, A.R.; Young, T.; Smith, S.; Liu, W.; Lindberg, J.; Egevad, L.; Gronberg, H.; Isaacs, W.B.; et al. Identification of New Differentially Methylated Genes That Have Potential Functional Consequences in Prostate Cancer. PLoS ONE 2012, 7, e48455. [Google Scholar] [CrossRef]
- Pang, X.; Xie, R.; Zhang, Z.; Liu, Q.; Wu, S.; Cui, Y. Identification of SPP1 as an Extracellular Matrix Signature for Metastatic Castration-Resistant Prostate Cancer. Front. Oncol. 2019, 9, 924. [Google Scholar] [CrossRef]
- Yu, A.; Guo, K.; Qin, Q.; Xing, C.; Zu, X. Clinicopathological and Prognostic Significance of Osteopontin Expression in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Biosci. Rep. 2021, 41, BSR20203531. [Google Scholar] [CrossRef]
- Wang, C.; Liu, G.; Liu, Y.; Yang, Z.; Xin, W.; Wang, M.; Li, Y.; Yang, L.; Mu, H.; Zhou, C. Novel Serum Proteomic Biomarkers for Early Diagnosis and Aggressive Grade Identification of Prostate Cancer. Front. Oncol. 2022, 12, 1004015. [Google Scholar] [CrossRef]
- Puzone, R.; Paleari, L.; Montefiore, F.; Ruggiero, L.; Puntoni, M.; Maffezzini, M.; Bobbio, B.; Marroni, P.; Libener, R.; Betta, P.G. Osteopontin Plasma Level Does Not Detect Prostate Cancer in Patients Referred for Diagnostic Prostate Biopsy. Int. J. Biol. Markers 2010, 25, 200–206. [Google Scholar] [CrossRef]
- Thoms, J.W.; Dal Pra, A.; Anborgh, P.H.; Christensen, E.; Fleshner, N.; Menard, C.; Chadwick, K.; Milosevic, M.; Catton, C.; Pintilie, M.; et al. Plasma Osteopontin as a Biomarker of Prostate Cancer Aggression: Relationship to Risk Category and Treatment Response. Br. J. Cancer 2012, 107, 840–846. [Google Scholar] [CrossRef]
- Anunobi, C.C.; Koli, K.; Saxena, G.; Banjo, A.A.; Ogbureke, K.U.E. Expression of the SIBLINGs and Their MMP Partners in Human Benign and Malignant Prostate Neoplasms. Oncotarget 2016, 7, 48038–48049. [Google Scholar] [CrossRef]
- Wiśniewski, T.; Zyromska, A.; Makarewicz, R.; Zekanowska, E. Osteopontin and Angiogenic Factors as New Biomarkers of Prostate Cancer. Urol. J. 2019, 16, 134–140. [Google Scholar]
- Tozawa, K.; Yamada, Y.; Kawai, N.; Okamura, T.; Ueda, K.; Kohri, K. Osteopontin Expression in Prostate Cancer and Benign Prostatic Hyperplasia. Urol. Int. 1999, 62, 155–158. [Google Scholar] [CrossRef]
- Hotte, S.J.; Winquist, E.W.; Stitt, L.; Wilson, S.M.; Chambers, A.F. Plasma Osteopontin: Associations with Survival and Metastasis to Bone in Men with Hormone-Refractory Prostate Carcinoma. Cancer 2002, 95, 506–512. [Google Scholar] [CrossRef]
- Forootan, S.S.; Foster, C.S.; Aachi, V.R.; Adamson, J.; Smith, P.H.; Lin, K.; Ke, Y. Prognostic Significance of Osteopontin Expression in Human Prostate Cancer. Int. J. Cancer 2006, 118, 2255–2261. [Google Scholar] [CrossRef]
- Ramankulov, A.; Lein, M.; Kristiansen, G.; Loening, S.A.; Jung, K. Plasma Osteopontin in Comparison with Bone Markers as Indicator of Bone Metastasis and Survival Outcome in Patients with Prostate Cancer. Prostate 2007, 67, 330–340. [Google Scholar] [CrossRef]
- Weber, G.F.; Lett, G.S.; Haubein, N.C. Osteopontin Is a Marker for Cancer Aggressiveness and Patient Survival. Br. J. Cancer 2010, 103, 861–869. [Google Scholar] [CrossRef]
- Vallbo, C.; Wang, W.; Damber, J.E. The Expression of Thrombospondin-1 in Benign Prostatic Hyperplasia and Prostatic Intraepithelial Neoplasia Is Decreased in Prostate Cancer. BJU Int. 2004, 93, 1339–1343. [Google Scholar] [CrossRef]
- Shafer, M.W.; Mangold, L.; Partin, A.W.; Haab, B.B. Antibody Array Profiling Reveals SerumTSP-I as a Marker to Distinguish Benign from Malignant Prostatic Disease. Prostate 2007, 67, 255–267. [Google Scholar] [CrossRef]
- Bhagirath, D.; Liston, M.; Akoto, T.; Lui, B.; Bensing, B.A.; Sharma, A.; Saini, S. Novel, Non-Invasive Markers for Detecting Therapy Induced Neuroendocrine Differentiation in Castration-Resistant Prostate Cancer Patients. Sci. Rep. 2021, 11, 8279. [Google Scholar] [CrossRef]
- Firlej, V.; Mathieu, J.R.R.; Gilbert, C.; Lemonnier, L.; Nakhlé, J.; Gallou-Kabani, C.; Guarmit, B.; Morin, A.; Prevarskaya, N.; Delongchamps, N.B.; et al. Thrombospondin-1 Triggers Cell Migration and Development of Advanced Prostate Tumors. Cancer Res. 2011, 71, 7649–7658. [Google Scholar] [CrossRef]
- Matos, A.R.; Coutinho-Camillo, C.M.; Thuler, L.C.S.; Fonseca, F.P.; Soares, F.A.; Silva, E.A.; Gimba, E.R. Expression Analysis of Thrombospondin 2 in Prostate Cancer and Benign Prostatic Hyperplasia. Exp. Mol. Pathol. 2013, 94, 438–444. [Google Scholar] [CrossRef]
- Mishra, P.; Kiebish, M.A.; Cullen, J.; Srinivasan, A.; Patterson, A.; Sarangarajan, R.; Narain, N.R.; Dobi, A. Genomic Alterations of Tenascin c in Highly Aggressive Prostate Cancer: A Meta-Analysis. Genes Cancer 2019, 10, 150–159. [Google Scholar] [CrossRef]
- Ni, W.D.; Yang, Z.T.; Cui, C.A.; Cui, Y.; Fang, L.Y.; Xuan, Y.H. Tenascin-C Is a Potential Cancer-Associated Fibroblasts Marker and Predicts Poor Prognosis in Prostate Cancer. Biochem. Biophys. Res. Commun. 2017, 486, 607–612. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, X.; Feng, Y.; Li, X.; Xuan, Y. Tenascin C Regulates Cancer Cell Glycolysis and Tumor Progression in Prostate Cancer. Int. J. Urol. 2022, 29, 578–585. [Google Scholar] [CrossRef]
- Karkampouna, S.; De Filippo, M.R.; Ng, C.K.Y.; Klima, I.; Zoni, E.; Spahn, M.; Stein, F.; Haberkant, P.; Thalmann, G.N.; de Julio, M.K. Stroma Transcriptomic and Proteomic Profile of Prostate Cancer Metastasis Xenograft Models Reveals Prognostic Value of Stroma Signatures. Cancers 2020, 12, 3786. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, L.; Bi, X.; Yuan, S.; Chen, P. Evaluation of Vitronectin Expression in Prostate Cancer and the Clinical Significance of the Association of Vitronectin Expression with Prostate Specific Antigen in Detecting Prostate Cancer. Urol. J. 2016, 13, 2527–2532. [Google Scholar]
- Gaudreau, P.O.; Clairefond, S.; Class, C.A.; Boulay, P.L.; Chrobak, P.; Allard, B.; Azzi, F.; Pommey, S.; Do, K.A.; Saad, F.; et al. WISP1 Is Associated to Advanced Disease, EMT and an Inflamed Tumor Microenvironment in Multiple Solid Tumors. Oncoimmunology 2019, 8, e1581545. [Google Scholar] [CrossRef]
- Fritzsche, F.R.; Jung, M.; Tölle, A.; Wild, P.; Hartmann, A.; Wassermann, K.; Rabien, A.; Lein, M.; Dietel, M.; Pilarsky, C.; et al. ADAM9 Expression Is a Significant and Independent Prognostic Marker of PSA Relapse in Prostate Cancer. Eur. Urol. 2008, 54, 1097–1108. [Google Scholar] [CrossRef]
- Lin, G.-W.; Yao, X.-D.; Ye, D.-W.; Zhang, S.-L.; Dai, B.; Zhang, H.-H.; Ma, C.-G. ADAM9 Decreases in Castration Resistant Prostate Cancer and Is a Prognostic Factor for Overall Survival. Chin. Med. J. 2012, 125, 3800–3805. [Google Scholar] [CrossRef]
- Pen, C.C.; Liu, C.M.; Lin, C.C.; Lin, C.C.; Hsieh, T.F.; Josson, S.; He, Y.C.; Chung, L.W.K.; Lin, K.L.; Sung, S.Y. Combined Dynamic Alterations in Urinary VEGF Levels and Tissue ADAM9 Expression as Markers for Lethal Phenotypic Progression of Prostate Cancer. Chin. J. Physiol. 2012, 55, 390–397. [Google Scholar] [CrossRef]
- Sung, S.Y.; Kubo, H.; Shigemura, K.; Arnold, R.S.; Logani, S.; Wang, R.; Konaka, H.; Nakagawa, M.; Mousses, S.; Amin, M.; et al. Oxidative Stress Induces ADAM9 Protein Expression in Human Prostate Cancer Cells. Cancer Res. 2006, 66, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Enokida, H.; Kubo, H.; Kagara, I.; Matsuda, R.; Toki, K.; Nishimura, H.; Chiyomaru, T.; Tatarano, S.; Idesako, T.; et al. Nuclear Translocation of ADAM-10 Contributes to the Pathogenesis and Progression of Human Prostate Cancer. Cancer Sci. 2007, 98, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Bilgin Doğru, E.; Dizdar, Y.; Akşit, E.; Ural, F.; Şanlı, Ö.; Yasasever, V. EMMPRIN and ADAM12 in Prostate Cancer: Preliminary Results of a Prospective Study. Tumor Biol. 2014, 35, 11647–11653. [Google Scholar] [CrossRef] [PubMed]
- Burdelski, C.; Fitzner, M.; Hube-Magg, C.; Kluth, M.; Heumann, A.; Simon, R.; Krech, T.; Clauditz, T.; Büscheck, F.; Steurer, S.; et al. Overexpression of the A Disintegrin and Metalloproteinase ADAM15 Is Linked to a Small but Highly Aggressive Subset of Prostate Cancers. Neoplasia 2017, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kuefer, R.; Day, K.C.; Kleer, C.G.; Sabel, M.S.; Hofer, M.D.; Varambally, S.; Zorn, C.S.; Chinnaiyan, A.M.; Rubin, M.A.; Day, M.L. ADAM15 Disintegrin Is Associated with Aggressive Prostate and Breast Cancer Disease. Neoplasia 2006, 8, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hoyne, G.; Rudnicka, C.; Sang, Q.X.; Roycik, M.; Howarth, S.; Leedman, P.; Schlaich, M.; Candy, P.; Matthews, V. Genetic and Cellular Studies Highlight That A Disintegrin and Metalloproteinase 19 Is a Protective Biomarker in Human Prostate Cancer. BMC Cancer 2016, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, C.; Mochizuki, S.; Okada, Y.; McLaughlin, C.; Leedman, P.J.; Stuart, L.; Epis, M.; Hoyne, G.; Boulos, S.; Johnson, L.; et al. Overexpression and Knock-down Studies Highlight That a Disintegrin and Metalloproteinase 28 Controls Proliferation and Migration in Human Prostate Cancer. Medicine 2016, 95, e5085. [Google Scholar] [CrossRef]
- Sinha, A.A.; Wilson, M.J.; Reddy, P.K.; Gleason, D.F.; Sameni, M.; Sloane, B.F. Immunohistochemical Localization of Cathepsin B in Neoplastic Human Prostate. Prostate 1995, 26, 171–178. [Google Scholar] [CrossRef]
- Sinha, A.A.; Jamuar, M.P.; Wilson, M.J.; Rozhin, J.; Sloane, B.F. Plasma Membrane Association of Cathepsin B in Human Prostate Cancer: Biochemical and Immunogold Electron Microscopic Analysis. Prostate 2001, 49, 172–184. [Google Scholar] [CrossRef]
- Miyake, H.; Hara, I.; Eto, H. Serum Level of Cathepsin B and Its Density in Men with Prostate Cancer as Novel Markers of Disease Progression. Anticancer Res. 2004, 24, 2573–2577. [Google Scholar]
- Štiblar-Martinčič, D.; Hajdinjak, T. Polymorphism L26V in the Cathepsin B Gene May Be Associated with a Risk of Prostate Cancer and Differentiation. J. Int. Med. Res. 2009, 37, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.A.; Quast, B.J.; Korkowski, J.C.; Wilson, M.J.; Reddy, P.K.; Ewing, S.L.; Sloane, B.F.; Gleason, D.F. The Relationship of Cathepsin B and Stefin A mRNA Localization Identifies a Potentially Aggressive Variant of Human Prostate Cancer within a Gleason Histologic Score. Anticancer Res. 1999, 19, 2821–2829. [Google Scholar] [PubMed]
- Sinha, A.A.; Quast, B.J.; Wilson, M.J.; Fernandes, E.T.; Reddy, P.K.; Ewing, S.L.; Sloane, B.F.; Gleason, D.F. Ratio of Cathepsin B to Stefin A Identifies Heterogeneity within Gleason Histologic Scores for Human Prostate Cancer. Prostate 2001, 48, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.A.; Quast, B.J.; Wilson, M.J.; Fernandes, E.T.; Reddy, P.K.; Ewing, S.L.; Gleason, D.F. Prediction of Pelvic Lymph Node Metastasis by the Ratio of Cathepsin B to Stefin A in Patients with Prostate Carcinoma. Cancer 2002, 94, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Bostwick, D.G.; Iczkowski, K.A.; Betre, K.; Wilson, M.J.; Le, C.; Sinha, A.A. Characterization of Prostate Cancer in Needle Biopsy by Cathepsin B, Cell Proliferation and DNA Ploidy. Anticancer Res. 2010, 30, 719–725. [Google Scholar] [PubMed]
- Sinha, A.A.; Morgan, J.L.; Wood, N.; Betre, K.; Reddy, A.; Wilson, M.J.; Ramanani, D.M. Heterogeneity of Cathepsin B and Stefin A Expression in Gleason Pattern 3 + 3 (Score 6) Prostate Cancer Needle Biopsies. Anticancer Res. 2007, 27, 1407–1413. [Google Scholar] [PubMed]
- Maygarden, S.J.; Novotny, D.B.; Moul, J.W.; Bae, V.L.; Ware, J.L. Evaluation of Cathepsin D and Epidermal Growth Factor Receptor in Prostate Carcinoma. Mod. Pathol. 1994, 7, 930–936. [Google Scholar]
- Kuczyk, M.A.; Serth, J.; Bokemeyer, C.; Hofner, K.; Allhoff, E.P.; Jonas, U. Immunohistochemical Detection of Cathepsin D Expression in Prostate Cancer. Oncol. Rep. 1994, 1, 1247–1251. [Google Scholar] [CrossRef]
- Cherry, J.P. Analysis of Cathepsin d Forms and Their Clinical Implications in Human Prostate Cancer. J. Urol. 1998, 160, 2223–2228. [Google Scholar] [CrossRef]
- Hara, I.; Miyake, H.; Yamanaka, K.; Hara, S.; Kamidono, S. Serum Cathepsin D and Its Density in Men with Prostate Cancer as New Predictors of Disease Progression. Oncol. Rep. 2002, 9, 1379–1383. [Google Scholar] [CrossRef]
- Miyake, H.; Hara, I.; Eto, H. Prediction of the Extent of Prostate Cancer by the Combined Use of Systematic Biopsy and Serum Level of Cathepsin D. Int. J. Urol. 2003, 10, 196–200. [Google Scholar] [CrossRef] [PubMed]
- El Melegy, N.T.; Aboulella, H.A.; Abul-Fadl, A.M.; Mohamed, N.A. Potential Biomarkers for Differentiation of Benign Prostatic Hyperplasia and Prostate Cancer. Br. J. Biomed. Sci. 2010, 67, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Moul, J.W.; Maygarden, S.J.; Ware, J.L.; Mohler, J.L.; Maher, P.D.; Schenkman, N.S.; Ho, C.K. Cathepsin D and Epidermal Growth Factor Receptor Immunohistochemistry Does Not Predict Recurrence of Prostate Cancer in Patients Undergoing Radical Prostatectomy. J. Urol. 1996, 155, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, K.D.; Vessella, R.L.; True, L.D.; Thomas, R.; Corey, E. Cathepsin K mRNA and Protein Expression in Prostate Cancer Progression. J. Bone Miner. Res. 2003, 18, 222–230. [Google Scholar] [CrossRef]
- Munari, E.; Cima, L.; Massari, F.; Bertoldo, F.; Porcaro, A.B.; Caliò, A.; Riva, G.; Ciocchetta, E.; Ciccarese, C.; Modena, A.; et al. Cathepsin K Expression in Castration-Resistant Prostate Carcinoma: A Therapeutical Target for Patients at Risk for Bone Metastases. Int. J. Biol. Markers 2017, 32, 243–247. [Google Scholar] [CrossRef]
- Nägler, D.K.; Krüger, S.; Kellner, A.; Ziomek, E.; Menard, R.; Buhtz, P.; Krams, M.; Roessner, A.; Kellner, U. Up-Regulation of Cathepsin X in Prostate Cancer and Prostatic Intraepithelial Neoplasia. Prostate 2004, 60, 109–119. [Google Scholar] [CrossRef]
- Batista, A.A.S.; Franco, B.M.; Perez, M.M.; Pereira, E.G.; Rodrigues, T.; Wroclawski, M.L.; Fonseca, F.L.A.; Suarez, E.R. Decreased Levels of Cathepsin Z MRNA Expressed by Immune Blood Cells: Diagnostic and Prognostic Implications in Prostate Cancer. Braz. J. Med. Biol. Res. 2021, 54, e11439. [Google Scholar] [CrossRef]
- Fraga, A.; Ribeiro, R.; Coelho, A.; Vizcaíno, J.R.; Coutinho, H.; Lopes, J.M.; Príncipe, P.; Lobato, C.; Lopes, C.; Medeiros, R. Genetic Polymorphisms in Key Hypoxia-Regulated Downstream Molecules and Phenotypic Correlation in Prostate Cancer. BMC Urol. 2017, 17, 12. [Google Scholar] [CrossRef]
- Ren, C.; Yang, G.; Timme, T.L.; Wheeler, T.M.; Thompson, T.C. Reduced Lysyl Oxidase Messenger RNA Levels in Experimental and Human Prostate Cancer. Cancer Res. 1998, 58, 1285–1290. [Google Scholar]
- Stewart, G.D.; Gray, K.; Pennington, C.J.; Edwards, D.R.; Riddick, A.C.P.; Ross, J.A.; Habib, F.K. Analysis of Hypoxia-Associated Gene Expression in Prostate Cancer: Lysyl Oxidase and Glucose Transporter-1 Expression Correlate with Gleason Score. Oncol. Rep. 2008, 20, 1561–1567. [Google Scholar] [CrossRef]
- Ozden, F.; Saygin, C.; Uzunaslan, D.; Onal, B.; Durak, H.; Aki, H. Expression of MMP-1, MMP-9 and TIMP-2 in Prostate Carcinoma and Their Influence on Prognosis and Survival. J. Cancer Res. Clin. Oncol. 2013, 139, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Zhou, W.; Wang, M.; Xia, W.; Tang, Q. Prognostic Value of Matrix Metalloprotease-1/Protease-Activated Receptor-1 Axis in Patients with Prostate Cancer. Med. Oncol. 2014, 31, 968. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Significance of MMP-2 Expression in Prostate Cancer: An Immunohistochemical Study. Cancer Res. 2003, 63, 8511–8515. [Google Scholar] [PubMed]
- Morgia, G.; Falsaperla, M.; Malaponte, G.; Madonia, M.; Indelicato, M.; Travali, S.; Mazzarino, M.C. Matrix Metalloproteinases as Diagnostic (MMP-13) and Prognostic (MMP-2, MMP-9) Markers of Prostate Cancer. Urol. Res. 2005, 33, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cicco, C.; Ravasi, L.; Zorzino, L.; Sandri, M.; Botteri, E.; Verweij, F.; Granchi, D.; Cobelli, O.; Paganelli, G. Circulating Levels of VCAM and MMP-2 May Help Identify Patients with More Aggressive Prostate Cancer. Curr. Cancer Drug Targets 2008, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Membrane-Type-1 Matrix Metalloproteinase, Matrix Metalloproteinase 2, and Tissue Inhibitor of Matrix Proteinase 2 in Prostate Cancer: Identification of Patients with Poor Prognosis by Immunohistochemistry. Hum. Pathol. 2008, 39, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Muramaki, M.; Kurahashi, T.; Takenaka, A.; Fujisawa, M. Expression of Potential Molecular Markers in Prostate Cancer: Correlation with Clinicopathological Outcomes in Patients Undergoing Radical Prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Prior, C.; Guillen-Grima, F.; Robles, J.E.; Rosell, D.; Fernandez-Montero, J.M.; Agirre, X.; Catena, R.; Calvo, A. Use of a Combination of Biomarkers in Serum and Urine to Improve Detection of Prostate Cancer. World J. Urol. 2010, 28, 681–686. [Google Scholar] [CrossRef]
- Hetzl, A.C.; Fávaro, W.J.; Billis, A.; Ferreira, U.; Cagnon, V.H.A. Steroid Hormone Receptors, Matrix Metalloproteinases, Insulin-like Growth Factor, and Dystroglycans Interactions in Prostatic Diseases in the Elderly Men. Microsc. Res. Tech. 2012, 75, 1197–1205. [Google Scholar] [CrossRef]
- Reis, S.T.; Antunes, A.A.; Pontes-Junior, J.P.; de Sousa-Canavez, J.M.; Dall’Oglio, M.F.; Piantino, C.B.; da Cruz, J.A.S.; Reis Morais, D.; Srougi, M.; Leite, K.R.M. Underexpression of MMP-2 and Its Regulators, TIMP2, MT1-MMP and IL-8, Is Associated with Prostate Cancer. Int. Braz. J. Urol. 2012, 38, 167–174. [Google Scholar] [CrossRef]
- Srivastava, P.; Lone, T.A.; Kapoor, R.; Mittal, R.D. Association of Promoter Polymorphisms in MMP2 and TIMP2 with Prostate Cancer Susceptibility in North India. Arch. Med. Res. 2012, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, I.E.; Aytac Vuruskan, B.; Kaygısız, O.; Egeli, U.; Tunca, B.; Kordan, Y.; Cecener, G. RNA-Based Markers in Biopsy Cores with Atypical Small Acinar Proliferation: Predictive Effect of T2E Fusion Positivity and MMP-2 Upregulation for a Subsequent Prostate Cancer Diagnosis. Prostate 2019, 79, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Medina-González, A.; Eiró-Díaz, N.; Fernández-Gómez, J.M.; Ovidio-González, L.; Jalón-Monzón, A.; Casas-Nebra, J.; Escaf-Barmadah, S. Comparative Analysis of the Expression of Metalloproteases (MMP-2, MMP-9, MMP-11 and MMP-13) and the Tissue Inhibitor of Metalloprotease 3 (TIMP-3) between Previous Negative Biopsies and Radical Prostatectomies. Actas Urol. Esp. 2020, 44, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jaboin, J.J.; Hwang, M.; Lopater, Z.; Chen, H.; Ray, G.L.; Perez, C.; Cai, Q.; Wills, M.L.; Lu, B. The Matrix Metalloproteinase-7 Polymorphism RS10895304 Is Associated with Increased Recurrence Risk in Patients with Clinically Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Jaworska-Bieniek, K.; Sukiennicki, G.; Durda, K.; Gromowski, T.; Prajzendanc, K.; et al. Association of Zinc Level and Polymorphism in MMP-7 Gene with Prostate Cancer in Polish Population. PLoS ONE 2018, 13, e0201065. [Google Scholar] [CrossRef]
- Szarvas, T.; Sevcenco, S.; Módos, O.; Keresztes, D.; Nyirády, P.; Csizmarik, A.; Ristl, R.; Puhr, M.; Hoffmann, M.J.; Niedworok, C.; et al. Matrix Metalloproteinase 7, Soluble Fas and Fas Ligand Serum Levels for Predicting Docetaxel Resistance and Survival in Castration-Resistant Prostate Cancer. BJU Int. 2018, 122, 695–704. [Google Scholar] [CrossRef]
- Boxler, S.; Djonov, V.; Kessler, T.M.; Hlushchuk, R.; Bachmann, L.M.; Held, U.; Markwalder, R.; Thalmann, G.N. Matrix Metalloproteinases and Angiogenic Factors: Predictors of Survival after Radical Prostatectomy for Clinically Organ-Confined Prostate Cancer? Am. J. Pathol. 2010, 177, 2216–2224. [Google Scholar] [CrossRef]
- Oguić, R.; Mozetič, V.; Cini Tešar, E.; Fučkar Čupić, D.; Mustać, E.; Orević, G. Matrix Metalloproteinases 2 and 9 Immunoexpression in Prostate Carcinoma at the Positive Margin of Radical Prostatectomy Specimens. Pathol. Res. Int. 2014, 2014, 262195. [Google Scholar] [CrossRef][Green Version]
- Marín-Aguilera, M.; Reig, Ò.; Lozano, J.J.; Jiménez, N.; García-Recio, S.; Erill, N.; Gaba, L.; Tagliapietra, A.; Ortega, V.; Carrera, G.; et al. Molecular Profiling of Peripheral Blood Is Associated with Circulating Tumor Cells Content and Poor Survival in Metastatic Castration-Resistant Prostate Cancer. Oncotarget 2015, 6, 10604–10616. [Google Scholar] [CrossRef]
- Pouyanfar, N.; Monabbati, A.; Sharifi, A.A.; Dianatpour, M. Expression Levels of MMP9 and PIWIL2 in Prostate Cancer: A Case-Control Study. Clin. Lab. 2016, 62, 651–657. [Google Scholar] [CrossRef]
- Baspinar, S.; Bircan, S.; Ciris, M.; Karahan, N.; Bozkurt, K.K. Expression of NGF, GDNF and MMP-9 in Prostate Carcinoma. Pathol. Res. Pract. 2017, 213, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Syed Khaja, A.S.; Semenas, J.; Wang, T.; Sarwar, M.; Dizeyi, N.; Simoulis, A.; Hedblom, A.; Wai, S.N.; Ødum, N.; et al. The Functional Interlink between AR and MMP9/VEGF Signaling Axis Is Mediated through PIP5K1α/PAKT in Prostate Cancer. Int. J. Cancer 2020, 146, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Ok Atılgan, A.; Özdemir, B.H.; Yılmaz Akçay, E.; Tepeoğlu, M.; Börcek, P.; Dirim, A. Association between Focal Adhesion Kinase and Matrix Metalloproteinase-9 Expression in Prostate Adenocarcinoma and Their Influence on the Progression of Prostatic Adenocarcinoma. Ann. Diagn. Pathol. 2020, 45, 151480. [Google Scholar] [CrossRef] [PubMed]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Study of Matrix Metalloproteinases and Their Inhibitors in Prostate Cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef]
- Nonsrijun, N.; Mitchai, J.; Brown, K.; Leksomboon, R.; Tuamsuk, P. Overexpression of Matrix Metalloproteinase 11 in Thai Prostatic Adenocarcinoma Is Associated with Poor Survival. Asian Pac. J. Cancer Prev. 2013, 14, 3331–3335. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chou, Y.E.; Lin, C.Y.; Wang, S.S.; Chien, M.H.; Tang, C.H.; Lin, J.C.; Wen, Y.C.; Yang, S.F. Impact of Matrix Metalloproteinase-11 Gene Polymorphisms on Biochemical Recurrence and Clinicopathological Characteristics of Prostate Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8603. [Google Scholar] [CrossRef]
- Kalantari, E.; Abolhasani, M.; Roudi, R.; Farajollahi, M.M.; Farhad, S.; Madjd, Z.; Askarian-Amiri, S.; Mohsenzadegan, M. Co-Expression of TLR-9 and MMP-13 Is Associated with the Degree of Tumour Differentiation in Prostate Cancer. Int. J. Exp. Pathol. 2019, 100, 123–132. [Google Scholar] [CrossRef]
- Quaglia, F.; Krishn, S.R.; Wang, Y.; Goodrich, D.W.; McCue, P.; Kossenkov, A.V.; Mandigo, A.C.; Knudsen, K.E.; Weinreb, P.H.; Corey, E.; et al. Differential Expression of αvß3 and αvß6 Integrins in Prostate Cancer Progression. PLoS ONE 2021, 16, e0244985. [Google Scholar] [CrossRef]
- Davis, T.L.; Cress, A.E.; Dalkin, B.L.; Nagle, R.B. Unique Expression Pattern of the α6β4 Integrin and Laminin-5 in Human Prostate Carcinoma. Prostate 2001, 46, 240–248. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular Architecture and Function of the Hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef]
- Park, S.; Kang, M.; Kim, S.; An, H.T.; Gettemans, J.; Ko, J. α-Actinin-4 Promotes the Progression of Prostate Cancer Through the Akt/GSK-3β/β-Catenin Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 588544. [Google Scholar] [CrossRef] [PubMed]
- Ishizuya, Y.; Uemura, M.; Narumi, R.; Tomiyama, E.; Koh, Y.; Matsushita, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Kato, T.; et al. The Role of Actinin-4 (ACTN4) in Exosomes as a Potential Novel Therapeutic Target in Castration-Resistant Prostate Cancer. Biochem. Biophys. Res. Commun. 2020, 523, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Bedolla, R.G.; Wang, Y.; Asuncion, A.; Chamie, K.; Siddiqui, S.; Mudryj, M.M.; Prihoda, T.J.; Siddiqui, J.; Chinnaiyan, A.M.; Mehra, R.; et al. Nuclear versus Cytoplasmic Localization of Filamin a in Prostate Cancer: Immunohistochemical Correlation with Metastases. Clin. Cancer Res. 2009, 15, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Mooso, B.A.; Vinall, R.L.; Tepper, C.G.; Savoy, R.M.; Cheung, J.P.; Singh, S.; Siddiqui, S.; Wang, Y.; Bedolla, R.G.; Martinez, A.; et al. Enhancing the Effectiveness of Androgen Deprivation in Prostate Cancer by Inducing Filamin A Nuclear Localization. Endocr. Relat. Cancer 2012, 19, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, D.K.; Ehrich, M.; Van Den Boom, D.; Cheville, J.C.; Karnes, R.J.; Tindall, D.J.; Cantor, C.R.; Young, C.Y.F. Hypermethylation of Genes for Diagnosis and Risk Stratification of Prostate Cancer. Cancer Investig. 2009, 27, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Klee, E.W.; Young, C.Y.F.; Sun, Z.; Jimenez, R.E.; Klee, G.G.; Tindall, D.J.; Donkena, K.V. Global Methylation Profiling for Risk Prediction of Prostate Cancer. Clin. Cancer Res. 2012, 18, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Chuang, H.C.; Salunke, S.B.; Kulp, S.K.; Chen, C.S. A Novel HIF-1α-Integrin-Linked Kinase Regulatory Loop That Facilitates Hypoxia-Induced HIF-1α Expression and Epithelial-Mesenchymal Transition in Cancer Cells. Oncotarget 2015, 6, 8271–8285. [Google Scholar] [CrossRef]
- Buckup, M.; Rice, M.A.; Hsu, E.C.; Garcia-Marques, F.; Liu, S.; Aslan, M.; Bermudez, A.; Huang, J.; Pitteri, S.J.; Stoyanova, T. Plectin Is a Regulator of Prostate Cancer Growth and Metastasis. Oncogene 2020, 40, 663–676. [Google Scholar] [CrossRef]
- Wenta, T.; Schmidt, A.; Zhang, Q.; Devarajan, R.; Singh, P.; Yang, X.; Ahtikoski, A.; Vaarala, M.; Wei, G.H.; Manninen, A. Disassembly of α6β4-Mediated Hemidesmosomal Adhesions Promotes Tumorigenesis in PTEN-Negative Prostate Cancer by Targeting Plectin to Focal Adhesions. Oncogene 2022, 41, 3804–3820. [Google Scholar] [CrossRef]
- Ma, X.; Biswas, A.; Hammes, S.R. Paxillin Regulated Genomic Networks in Prostate Cancer. Steroids 2019, 151, 108463. [Google Scholar] [CrossRef]
- Sakamoto, S.; McCann, R.; Kyprianou, N. Talin1 Promotes Prostate Cancer Invasion and Metastasis via AKT Signaling and Anoikis Resistance. Nat. Preced. 2009, 70, 1885–1895. [Google Scholar] [CrossRef]
- Sakamoto, S.; McCann, R.O.; Dhir, R.; Kyprianou, N. Talin1 Promotes Tumor Invasion and Metastasis via Focal Adhesion Signaling and Anoikis Resistance. Cancer Res. 2010, 70, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, H.; Gong, L.; Cao, D.; Jin, T.; Wang, Y.; Pi, J.; Yang, Y.; Yi, X.; Liao, D.; et al. Vinculin Orchestrates Prostate Cancer Progression by Regulating Tumor Cell Invasion, Migration, and Proliferation. Prostate 2021, 81, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.E.; Zhang, Z.; Madamanchi, A.; Boyd, K.L.; O’Rear, L.D.; Nashabi, A.; Li, Z.; Dupont, W.D.; Zijlstra, A.; Zutter, M.M. The α2β1 Integrin Is a Metastasis Suppressor in Mouse Models and Human Cancer. J. Clin. Investig. 2011, 121, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Colombel, M.; Eaton, C.L.; Hamdy, F.; Ricci, E.; Van Der Pluijm, G.; Cecchini, M.; Mege-Lechevallier, F.; Clezardin, P.; Thalmann, G. Increased Expression of Putative Cancer Stem Cell Markers in Primary Prostate Cancer Is Associated with Progression of Bone Metastases. Prostate 2012, 72, 713–720. [Google Scholar] [CrossRef]
- Ricci, E.; Mattei, E.; Dumontet, C.; Eaton, C.L.; Hamdy, F.; Van Der Pluije, G.; Cecchini, M.; Thalmann, G.; Clezardin, P.; Colombel, M. Increased Expression of Putative Cancer Stem Cell Markers in the Bone Marrow of Prostate Cancer Patients Is Associated with Bone Metastasis Progression. Prostate 2013, 73, 1738–1746. [Google Scholar] [CrossRef]
- Schmelz, M.; Cress, A.E.; Scott, K.M.; Bürgery, F.; Cui, H.; Sallam, K.; McDaniel, K.M.; Dalkin, B.L.; Nagle, R.B. Different Phenotypes in Human Prostate Cancer: α6 or α3 Integrin in Cell-Extracellular Adhesion Sites. Neoplasia 2002, 4, 243–254. [Google Scholar] [CrossRef]
- Pontes, J.; Reis, S.T.; De Oliveira, L.C.N.; Sant’Anna, A.C.; Dall’Oglio, M.F.; Antunes, A.A.; Ribeiro-Filho, L.A.; Carvalho, P.A.; Cury, J.; Srougi, M.; et al. Association between Integrin Expression and Prognosis in Localized Prostate Cancer. Prostate 2010, 70, 1189–1195. [Google Scholar] [CrossRef]
- Drivalos, A.; Chrisofos, M.; Efstathiou, E.; Kapranou, A.; Kollaitis, G.; Koutlis, G.; Antoniou, N.; Karanastasis, D.; Dimopoulos, M.A.; Bamias, A. Expression of α5-Integrin, α7-Integrin, Ε-Cadherin, and N-Cadherin in Localized Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 165.e11–165.e18. [Google Scholar] [CrossRef]
- Drivalos, A.; Emmanouil, G.; Gavriatopoulou, M.; Terpos, E.; Sergentanis, T.N.; Psaltopoulou, T. Integrin Expression in Correlation to Clinicopathological Features and Prognosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 221–232. [Google Scholar] [CrossRef]
- Hoogland, A.M.; Verhoef, E.I.; Roobol, M.J.; Schröder, F.H.; Wildhagen, M.F.; Van Der Kwast, T.H.; Jenster, G.; Van Leenders, G.J.L.H. Validation of Stem Cell Markers in Clinical Prostate Cancer: α6-Integrin Is Predictive for Non-Aggressive Disease. Prostate 2014, 74, 488–496. [Google Scholar] [CrossRef]
- Ren, B.; Yu, Y.P.; Tseng, G.C.; Wu, C.; Chen, K.; Rao, U.N.; Nelson, J.; Michalopoulos, G.K.; Luo, J.H. Analysis of Integrin α7 Mutations in Prostate Cancer, Liver Cancer, Glioblastoma Multiforme, and Leiomyosarcoma. J. Natl. Cancer Inst. 2007, 99, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Heß, K.; Böger, C.; Behrens, H.M.; Röcken, C. Correlation between the Expression of Integrins in Prostate Cancer and Clinical Outcome in 1284 Patients. Ann. Diagn. Pathol. 2014, 18, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; He, Y.; Liu, J.; Liao, B.; Liao, G. Genetic Variants in the Integrin Gene Predicted MicroRNA-Binding Sites Were Associated with the Risk of Prostate Cancer. Mol. Carcinog. 2014, 53, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zemskova, M.Y.; Marinets, M.V.; Sivkov, A.V.; Pavlova, J.V.; Shibaev, A.N.; Sorokin, K.S. Integrin Alpha V in Urine: A Novel Noninvasive Marker for Prostate Cancer Detection. Front. Oncol. 2021, 10, 610647. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.; Reis, S.T.; Bernardes, F.S.; Oliveira, L.C.N.; de Barros, É.A.F.; Dall’Oglio, M.F.; Timosczuk, L.M.S.; Ribeiro-Filho, L.A.; Srougi, M.; Leite, K.R.M. Correlation between Beta1 Integrin Expression and Prognosis in Clinically Localized Prostate Cancer. Int. Braz. J. Urol. 2013, 39, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Chen, C.; Tan, W.; Li, P.; Chen, G.; Peng, Q.; Yin, W. Integrin β4 Is an Effective and Efficient Marker in Synchronously Highlighting Lymphatic and Blood Vascular Invasion, and Perineural Aggression in Malignancy. Am. J. Surg. Pathol. 2020, 44, 681–690. [Google Scholar] [CrossRef]
- He, Z.; Tang, F.; Lu, Z.; Huang, Y.; Lei, H.; Li, Z.; Zeng, G. Analysis of Differentially Expressed Genes, Clinical Value and Biological Pathways in Prostate Cancer. Am. J. Transl. Res. 2018, 10, 1444–1456. [Google Scholar]
- Röbeck, P.; Franzén, B.; Cantera-Ahlman, R.; Dragomir, A.; Auer, G.; Jorulf, H.; Jacobsson, S.P.; Viktorsson, K.; Lewensohn, R.; Häggman, M.; et al. Multiplex Protein Analysis and Ensemble Machine Learning Methods of Fine Needle Aspirates from Prostate Cancer Patients Reveal Potential Diagnostic Signatures Associated with Tumour Grade. Cytopathology 2023, 34, 286–294. [Google Scholar] [CrossRef]
- Shipitsin, M.; Small, C.; Choudhury, S.; Giladi, E.; Friedlander, S.; Nardone, J.; Hussain, S.; Hurley, A.D.; Ernst, C.; Huang, Y.E.; et al. Identification of Proteomic Biomarkers Predicting Prostate Cancer Aggressiveness and Lethality despite Biopsy-Sampling Error. Br. J. Cancer 2014, 111, 1201–1212. [Google Scholar] [CrossRef]
- Lam, J.S.; Seligson, D.B.; Yu, H.; Li, A.; Eeva, M.; Pantuck, A.J.; Zeng, G.; Horvath, S.; Belldegrun, A.S. Flap Endonuclease 1 Is Overexpressed in Prostate Cancer and Is Associated with a High Gleason Score. BJU Int. 2006, 98, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kahl, P.; Gullotti, L.; Heukamp, L.C.; Wolf, S.; Friedrichs, N.; Vorreuther, R.; Solleder, G.; Bastian, P.J.; Ellinger, J.; Metzger, E.; et al. Androgen Receptor Coactivators Lysine-Specific Histone Demethylase 1 and Four and a Half LIM Domain Protein 2 Predict Risk of Prostate Cancer Recurrence. Cancer Res. 2006, 66, 11341–11347. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.J.; Binge, L.C.; Sriratana, A.; Wang, H.; Robinson, P.A.; Pook, D.; Fedele, C.G.; Brown, S.; Dyson, J.M.; Cottle, D.L.; et al. Regulation of the Transcriptional Coactivator FHL2 Licenses Activation of the Androgen Receptor in Castrate-Resistant Prostate Cancer. Cancer Res. 2013, 73, 5066–5079. [Google Scholar] [CrossRef] [PubMed]
- Kiebish, M.A.; Tekumalla, P.; Ravipaty, S.; Dobi, A.; Srivastava, S.; Wu, W.; Patil, S.; Friss, T.; Klotz, A.; Srinivasan, A.; et al. Clinical Utility of a Serum Biomarker Panel in Distinguishing Prostate Cancer from Benign Prostate Hyperplasia. Sci. Rep. 2021, 11, 15052. [Google Scholar] [CrossRef] [PubMed]
- Narain, N.R.; Diers, A.R.; Lee, A.; Lao, S.; Chan, J.Y.; Schofield, S.; Andreazi, J.; Ouro-Djobo, R.; Jimenez, J.J.; Friss, T.; et al. Identification of Filamin-A and -B as Potential Biomarkers for Prostate Cancer. Future Sci. OA 2017, 3, FSO161. [Google Scholar] [CrossRef]
- Graff, J.R.; Konicek, B.W.; Deddens, J.A.; Colligan, B.M.; Hurst, B.M.; Carter, H.W.; Carter, J.H. Integrin-Linked Kinase Expression Increases with Prostate Tumor Grade. Clin. Cancer Res. 2001, 7, 1987–1991. [Google Scholar]
- Gu, Y.; Lin, X.; Kapoor, A.; Li, T.; Major, P.; Tang, D. Effective Prediction of Prostate Cancer Recurrence through the Iqgap1 Network. Cancers 2021, 13, 430. [Google Scholar] [CrossRef]
- Feng, D.; Li, L.; Li, D.; Wu, R.; Zhu, W.; Wang, J.; Ye, L.; Han, P. Prolyl 4-Hydroxylase Subunit Beta (P4HB) Could Serve as a Prognostic and Radiosensitivity Biomarker for Prostate Cancer Patients. Eur. J. Med. Res. 2023, 28, 245. [Google Scholar] [CrossRef]
- Shui, I.M.; Lindström, S.; Kibel, A.S.; Berndt, S.I.; Campa, D.; Gerke, T.; Penney, K.L.; Albanes, D.; Berg, C.; Bueno-De-Mesquita, H.B.; et al. Prostate Cancer (PCa) Risk Variants and Risk of Fatal PCa in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Eur. Urol. 2014, 65, 1069–1075. [Google Scholar] [CrossRef]
- Piao, S.; Zheng, L.; Zheng, H.; Zhou, M.; Feng, Q.; Zhou, S.; Ke, M.; Yang, H.; Wang, X. High Expression of PDLIM2 Predicts a Poor Prognosis in Prostate Cancer and Is Correlated with Epithelial-Mesenchymal Transition and Immune Cell Infiltration. J. Immunol. Res. 2022, 2022, 2922832. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Huang, H.; Lv, J.M.; Chen, M.; Qu, F.J.; Pan, X.W.; Li, L.; Yin, L.; Cui, X.G.; et al. High Expression of PDLIM5 Facilitates Cell Tumorigenesis and Migration by Maintaining AMPK Activation in Prostate Cancer. Oncotarget 2017, 8, 98117–98134. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; De Castro, I.; DeFranco, D.B.; Deng, F.M.; Melamed, J.; Kapur, P.; Raj, G.V.; Rossi, R.; Hammes, S.R. Paxillin Mediates Extranuclear and Intranuclear Signaling in Prostate Cancer Proliferation. J. Clin. Investig. 2012, 122, 2469–2481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.S.; Chen, S.H.; Wu, Y.P.; Chen, H.J.; Chen, H.; Wei, Y.; Li, X.D.; Huang, J.B.; Xue, X.Y.; Xu, N. Increased Paxillin Expression in Prostate Cancer Is Associated with Advanced Pathological Features, Lymph Node Metastases and Biochemical Recurrence. J. Cancer 2018, 9, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, L.L.; Tao, W.; Xia, T.L.; Li, L.Y. Prediction of Potential Prognostic Biomarkers in Metastatic Prostate Cancer Based on a Circular RNA-Mediated Competing Endogenous RNA Regulatory Network. PLoS ONE 2021, 16, e0260983. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pang, L.; Jin, F.; Song, Y.; Zhou, D.; Song, Y.; Li, Y.; Jin, S.; Zhang, L.; Liang, W.; et al. Integrated Network Analysis to Determine CNN1, MYL9, TAGLN, and SORBS1 as Potential Key Genes Associated with Prostate Cancer. Clin. Lab. 2023, 69, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abu el Maaty, M.A.; Terzic, J.; Keime, C.; Rovito, D.; Lutzing, R.; Yanushko, D.; Parisotto, M.; Grelet, E.; Namer, I.J.; Lindner, V.; et al. Hypoxia-Mediated Stabilization of HIF1A in Prostatic Intraepithelial Neoplasia Promotes Cell Plasticity and Malignant Progression. Sci. Adv. 2022, 8, eabo2295. [Google Scholar] [CrossRef] [PubMed]
- Atobatele, A.G.; Tonoli, E.; Vadakekolathu, J.; Savoca, M.P.; Barr, M.; Kataria, Y.; Rossanese, M.; Burhan, I.; McArdle, S.; Caccamo, D.; et al. Canonical and Truncated Transglutaminase-2 Regulate Mucin-1 Expression and Androgen Independency in Prostate Cancer Cell Lines. Cell Death Dis. 2023, 14, 317. [Google Scholar] [CrossRef]
- Xu, N.; Chen, H.J.; Chen, S.H.; Xue, X.Y.; Chen, H.; Zheng, Q.S.; Wei, Y.; Li, X.D.; Huang, J.B.; Cai, H.; et al. Upregulation of Talin-1 Expression Associates with Advanced Pathological Features and Predicts Lymph Node Metastases and Biochemical Recurrence of Prostate Cancer. Medicine 2016, 95, e4326. [Google Scholar] [CrossRef]
- Ruiz, C.; Holz, D.R.; Oeggerli, M.; Schneider, S.; Gonzales, I.M.; Kiefer, J.M.; Zellweger, T.; Bachmann, A.; Koivisto, P.A.; Helin, H.J.; et al. Amplification and Overexpression of Vinculin Are Associated with Increased Tumour Cell Proliferation and Progression in Advanced Prostate Cancer. J. Pathol. 2011, 223, 543–552. [Google Scholar] [CrossRef]
- Geisler, C.; Gaisa, N.T.; Pfister, D.; Fuessel, S.; Kristiansen, G.; Braunschweig, T.; Gostek, S.; Beine, B.; Diehl, H.C.; Jackson, A.M.; et al. Identification and Validation of Potential New Biomarkers for Prostate Cancer Diagnosis and Prognosis Using 2D-DIGE and MS. Biomed Res. Int. 2015, 2015, 454256. [Google Scholar] [CrossRef]
- Garbis, S.D.; Tyritzis, S.I.; Roumeliotis, T.; Zerefos, P.; Giannopoulou, E.G.; Vlahou, A.; Kossida, S.; Diaz, J.; Vourekas, S.; Tamvakopoulos, C.; et al. Search for Potential Markers for Prostate Cancer Diagnosis, Prognosis and Treatment in Clinical Tissue Specimens Using Amine-Specific Isobaric Tagging (ITRAQ) with Two-Dimensional Liquid Chromatography and Tandem Mass Spectrometry. J. Proteome Res. 2008, 7, 3146–3158. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.T.; Chen, Y.; Wang, H.; Young, D.; Shi, T.; Song, Y.; Schepmoes, A.A.; Kuo, C.; Fillmore, T.L.; et al. Proteomic Tissue-Based Classifier for Early Prediction of Prostate Cancer Progression. Cancers 2020, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Han, X.; Min, Z.; He, X.; Zhu, S. Prognostic Signatures Associated with High Infiltration of Tregs in Bone Metastatic Prostate Cancer. Aging 2021, 13, 17442–17461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Zeng, Y.R.; Han, Z.D.; Zhuo, Y.J.; Liang, Y.K.; Hon, C.T.; Wan, S.; Wu, S.; Dahl, D.; De Zhong, W.; et al. Novel Immune-Related Signature for Risk Stratification and Prognosis in Prostatic Adenocarcinoma. Cancer Sci. 2021, 112, 4365–4376. [Google Scholar] [CrossRef] [PubMed]
- Treacy, P.J.; Martini, A.; Falagario, U.G.; Ratnani, P.; Wajswol, E.; Beksac, A.T.; Wiklund, P.; Nair, S.; Kyprianou, N.; Durand, M.; et al. Association between Expression of Connective Tissue Genes and Prostate Cancer Growth and Progression. Int. J. Mol. Sci. 2023, 24, 7520. [Google Scholar] [CrossRef] [PubMed]
- Söderdahl, F.; Xu, L.-D.; Bring, J.; Häggman, M. A Novel Risk Score (P-Score) Based on a Three-Gene Signature, for Estimating the Risk of Prostate Cancer-Specific Mortality. Res. Rep. Urol. 2022, 14, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Saemundsson, A.; Xu, L.-D.; Meisgen, F.; Cao, R.; Ahlgren, G. Validation of the Prognostic Value of a Three-Gene Signature and Clinical Parameters-Based Risk Score in Prostate Cancer Patients. Prostate 2023, 83, 1133–1140. [Google Scholar] [CrossRef]
- Röbeck, P.; Xu, L.; Ahmed, D.; Dragomir, A.; Dahlman, P.; Häggman, M.; Ladjevardi, S. P-Score in Preoperative Biopsies Accurately Predicts P-Score in Final Pathology at Radical Prostatectomy in Patients with Localized Prostate Cancer. Prostate 2023, 83, 831–839. [Google Scholar] [CrossRef]
- O’Rourke, D.J.; DiJohnson, D.A.; Caiazzo, R.J.; Nelson, J.C.; Ure, D.; O’Leary, M.P.; Richie, J.P.; Liu, B.C.S. Autoantibody Signatures as Biomarkers to Distinguish Prostate Cancer from Benign Prostatic Hyperplasia in Patients with Increased Serum Prostate Specific Antigen. Clin. Chim. Acta 2012, 413, 561–567. [Google Scholar] [CrossRef]
- Dayyani, F.; Zurita, A.J.; Nogueras-González, G.M.; Slack, R.; Millikan, R.E.; Araujo, J.C.; Gallick, G.E.; Logothetis, C.J.; Corn, P.G. The Combination of Serum Insulin, Osteopontin, and Hepatocyte Growth Factor Predicts Time to Castration-Resistant Progression in Androgen Dependent Metastatic Prostate Cancer—An Exploratory Study. BMC Cancer 2016, 16, 721. [Google Scholar] [CrossRef]
- Muazzam, A.; Spick, M.; Cexus, O.N.F.; Geary, B.; Azhar, F.; Pandha, H.; Michael, A.; Reed, R.; Lennon, S.; Gethings, L.A.; et al. A Novel Blood Proteomic Signature for Prostate Cancer. Cancers 2023, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Shephard, A.P.; Giles, P.; Mbengue, M.; Alraies, A.; Spary, L.K.; Kynaston, H.; Gurney, M.J.; Falcón-Pérez, J.M.; Royo, F.; Tabi, Z.; et al. Stroma-Derived Extracellular Vesicle MRNA Signatures Inform Histological Nature of Prostate Cancer. J. Extracell. Vesicles 2021, 10, e12150. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, H.Y.; Byun, Y.J.; Jeong, P.; Yoon, J.S.; Kim, D.H.; Kim, W.T.; Kim, Y.J.; Lee, S.C.; Yun, S.J.; et al. A Novel Urinary MRNA Signature Using the Droplet Digital Polymerase Chain Reaction Platform Improves Discrimination between Prostate Cancer and Benign Prostatic Hyperplasia within the Prostate-Specific Antigen Gray Zone. Investig. Clin. Urol. 2020, 61, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Prestagiacomo, L.E.; Tradigo, G.; Aracri, F.; Gabriele, C.; Rota, M.A.; Alba, S.; Cuda, G.; Damiano, R.; Veltri, P.; Gaspari, M. Data-Independent Acquisition Mass Spectrometry of EPS-Urine Coupled to Machine Learning: A Predictive Model for Prostate Cancer. ACS Omega 2023, 8, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Ravipaty, S.; Wu, W.; Dalvi, A.; Tanna, N.; Andreazi, J.; Friss, T.; Klotz, A.; Liao, C.; Garren, J.; Schofield, S.; et al. Clinical Validation of a Serum Protein Panel (FLNA, FLNB and KRT19) for Diagnosis of Prostate Cancer. J. Mol. Biomark Diagn. 2017, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Tennstedt, P.; Wittig, A.; Huber, R.; Straub, O.; Schiess, R.; Steuber, T. A Novel Serum Biomarker Quintet Reveals Added Prognostic Value When Combined with Standard Clinical Parameters in Prostate Cancer Patients by Predicting Biochemical Recurrence and Adverse Pathology. PLoS ONE 2021, 16, e0259093. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Goddard, A.D.; Natraj, N.; Cherbavaz, D.B.; Clark-Langone, K.M.; Snable, J.; Watson, D.; Falzarano, S.M.; Magi-Galluzzi, C.; Klein, E.A.; et al. Analytical Validation of the Oncotype DX Prostate Cancer Assay—A Clinical RT-PCR Assay Optimized for Prostate Needle Biopsies. BMC Genom. 2013, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Endt, K.; Goepfert, J.; Omlin, A.; Athanasiou, A.; Tennstedt, P.; Guenther, A.; Rainisio, M.; Engeler, D.S.; Steuber, T.; Gillessen, S.; et al. Development and Clinical Testing of Individual Immunoassays for the Quantification of Serum Glycoproteins to Diagnose Prostate Cancer. PLoS ONE 2017, 12, e0181557. [Google Scholar] [CrossRef]
- Steuber, T.; Tennstedt, P.; Macagno, A.; Athanasiou, A.; Wittig, A.; Huber, R.; Golding, B.; Schiess, R.; Gillessen, S. Thrombospondin 1 and Cathepsin D Improve Prostate Cancer Diagnosis by Avoiding Potentially Unnecessary Prostate Biopsies. BJU Int. 2019, 123, 826–833. [Google Scholar] [CrossRef]
- Klocker, H.; Golding, B.; Weber, S.; Steiner, E.; Tennstedt, P.; Keller, T.; Schiess, R.; Gillessen, S.; Horninger, W.; Steuber, T. Development and Validation of a Novel Multivariate Risk Score to Guide Biopsy Decision for the Diagnosis of Clinically Significant Prostate Cancer. BJUI Compass 2020, 1, 15–20. [Google Scholar] [CrossRef]
- Cima, I.; Schiess, R.; Wild, P.; Kaelin, M.; Schüffler, P.; Lange, V.; Picotti, P.; Ossola, R.; Templeton, A.; Schubert, O.; et al. Cancer Genetics-Guided Discovery of Serum Biomarker Signatures for Diagnosis and Prognosis of Prostate Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Kälin, M.; Cima, I.; Schiess, R.; Fankhauser, N.; Powles, T.; Wild, P.; Templeton, A.; Cerny, T.; Aebersold, R.; Krek, W.; et al. Novel Prognostic Markers in the Serum of Patients with Castration-Resistant Prostate Cancer Derived from Quantitative Analysis of the Pten Conditional Knockout Mouse Proteome. Eur. Urol. 2011, 60, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Macagno, A.; Athanasiou, A.; Wittig, A.; Huber, R.; Weber, S.; Keller, T.; Rhiel, M.; Golding, B.; Schiess, R. Analytical Performance of Thrombospondin-1 and Cathepsin D Immunoassays Part of a Novel CE-IVD Marked Test as an Aid in the Diagnosis of Prostate Cancer. PLoS ONE 2020, 15, e0233442. [Google Scholar] [CrossRef] [PubMed]
- Steuber, T.; Heidegger, I.; Kafka, M.; Roeder, M.A.; Chun, F.; Preisser, F.; Palisaar, R.J.; Hanske, J.; Budaeus, L.; Schiess, R.; et al. PROPOSe: A Real-Life Prospective Study of Proclarix, a Novel Blood-Based Test to Support Challenging Biopsy Decision-Making in Prostate Cancer. Eur. Urol. Oncol. 2022, 5, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Roche, S.; Mast, R.; Santamaría, A.; Planas, J.; et al. The Efficacy of Proclarix to Select Appropriate Candidates for Magnetic Resonance Imaging and Derived Prostate Biopsies in Men with Suspected Prostate Cancer. World J. Men’s Health 2022, 40, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Regis, L.; Celma, A.; de Torres, I.; Semidey, M.E.; Roche, S.; Mast, R.; Santamaria, A.; Planas, J.; et al. Who with Suspected Prostate Cancer Can Benefit from Proclarix after Multiparametric Magnetic Resonance Imaging? Int. J. Biol. Markers 2022, 37, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Campistol, M.; Triquell, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaria, A.; Planas, J.; et al. Improving the Early Detection of Clinically Significant Prostate Cancer in Men in the Challenging Prostate Imaging-Reporting and Data System 3 Category. Eur. Urol. Open Sci. 2022, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Campistol, M.; Morote, J.; Regis, L.; Celma, A.; Planas, J.; Trilla, E. Proclarix, A New Biomarker for the Diagnosis of Clinically Significant Prostate Cancer: A Systematic Review. Mol. Diagn. Ther. 2022, 26, 273–281. [Google Scholar] [CrossRef]
- Campistol, M.; Morote, J.; Triquell, M.; Regis, L.; Celma, A.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaría, A.; Planas, J.; et al. Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after MpMRI. Cancers 2022, 14, 2702. [Google Scholar] [CrossRef]
- Kaufmann, B.; Fischer, S.; Athanasiou, A.; Lautenbach, N.; Wittig, A.; Bieri, U.; Schmid, F.A.; von Stauffenberg, F.; Scherer, T.; Eberli, D.; et al. Evaluation of Proclarix in the Diagnostic Work-up of Prostate Cancer. BJUI Compass 2023, in press. [Google Scholar] [CrossRef]
- Terracciano, D.; La Civita, E.; Athanasiou, A.; Liotti, A.; Fiorenza, M.; Cennamo, M.; Crocetto, F.; Tennstedt, P.; Schiess, R.; Haese, A.; et al. New Strategy for the Identification of Prostate Cancer: The Combination of Proclarix and the Prostate Health Index. Prostate 2022, 82, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Bruzzese, D.; Crocetto, F.; Tennstedt, P.; Steuber, T.; Velotta, R.; Terracciano, D. A Neural Network Model Combining [-2]ProPSA, FreePSA, Total PSA, Cathepsin D, and Thrombospondin-1 Showed Increased Accuracy in the Identification of Clinically Significant Prostate Cancer. Cancers 2023, 15, 1355. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Pye, H.; Campistol, M.; Celma, A.; Regis, L.; Semidey, M.; de Torres, I.; Mast, R.; Planas, J.; Santamaria, A.; et al. Accurate Diagnosis of Prostate Cancer by Combining Proclarix with Magnetic Resonance Imaging. BJU Int. 2023, 132, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Campistol, M.; Triquell, M.; Regis, L.; Celma, A.; de Torres, I.; Semidey, M.E.; Mast, R.; Mendez, O.; Planas, J.; Trilla, E.; et al. Relationship between Proclarix and the Aggressiveness of Prostate Cancer. Mol. Diagn. Ther. 2023, 27, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.; Strand, S.H.; Mundbjerg, K.; Liang, G.; Gill, I.; Haldrup, C.; Borre, M.; Høyer, S.; Ørntoft, T.F.; Sørensen, K.D. Heterogeneous Patterns of DNA Methylation-Based Field Effects in Histologically Normal Prostate Tissue from Cancer Patients. Sci. Rep. 2017, 7, 40636. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, C.; Mundbjerg, K.; Vestergaard, E.M.; Lamy, P.; Wild, P.; Schulz, W.A.; Arsov, C.; Visakorpi, T.; Borre, M.; Høyer, S.; et al. DNA Methylation Signatures for Prediction of Biochemical Recurrence after Radical Prostatectomy of Clinically Localized Prostate Cancer. J. Clin. Oncol. 2013, 31, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.G.; Wessel, T.; Kawashima, A.; Okello, J.B.A.; Jamaspishvili, T.; Guérard, K.P.; Lee, L.; Lee, A.Y.W.; How, N.E.; Dion, D.; et al. A Three-Gene DNA Methylation Biomarker Accurately Classifies Early Stage Prostate Cancer. Prostate 2019, 79, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.H.; Orntoft, T.F.; Sorensen, K.D. Prognostic DNA Methylation Markers for Prostate Cancer. Int. J. Mol. Sci. 2014, 15, 16544–16576. [Google Scholar] [CrossRef]
- Bjerre, M.T.; Nørgaard, M.; Larsen, O.H.; Jensen, S.Ø.; Strand, S.H.; Østergren, P.; Fode, M.; Fredsøe, J.; Ulhøi, B.P.; Mortensen, M.M.; et al. Epigenetic Analysis of Circulating Tumor DNA in Localized and Metastatic Prostate Cancer: Evaluation of Clinical Biomarker Potential. Cells 2020, 9, 1362. [Google Scholar] [CrossRef]
- Kern, S.E. Why Your New Cancer Biomarker May Never Work: Recurrent Patterns and Remarkable Diversity in Biomarker Failures. Cancer Res. 2012, 72, 6097–6101. [Google Scholar] [CrossRef]
- Pepe, M.S.; Etzioni, R.; Feng, Z.; Potter, J.D.; Thompson, M.L.; Thornquist, M.; Winget, M.; Yasui, Y. Phases of Biomarker Development for Early Detection of Cancer. J. Natl. Cancer Inst. 2001, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Teutsch, S.M.; Bradley, L.A.; Palomaki, G.E.; Haddow, J.E.; Piper, M.; Calonge, N.; Dotson, W.D.; Douglas, M.P.; Berg, A.O. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: Methods of the EGAPP Working Group. Genet. Med. 2009, 11, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer Biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.E. Evolution of Novel Biomarkers for Detection of Prostate Cancer. J. Urol. 2013, 190, 1970–1971. [Google Scholar] [CrossRef]
- Boutros, P.C.; Fraser, M.; Harding, N.J.; De Borja, R.; Trudel, D.; Lalonde, E.; Meng, A.; Hennings-Yeomans, P.H.; McPherson, A.; Sabelnykova, V.Y.; et al. Spatial Genomic Heterogeneity within Localized, Multifocal Prostate Cancer. Nat. Genet. 2015, 47, 736–745. [Google Scholar] [CrossRef]
- Diamandis, E.P. The Failure of Protein Cancer Biomarkers to Reach the Clinic: Why, and What Can Be Done to Address the Problem? BMC Med. 2012, 10, 87. [Google Scholar] [CrossRef]
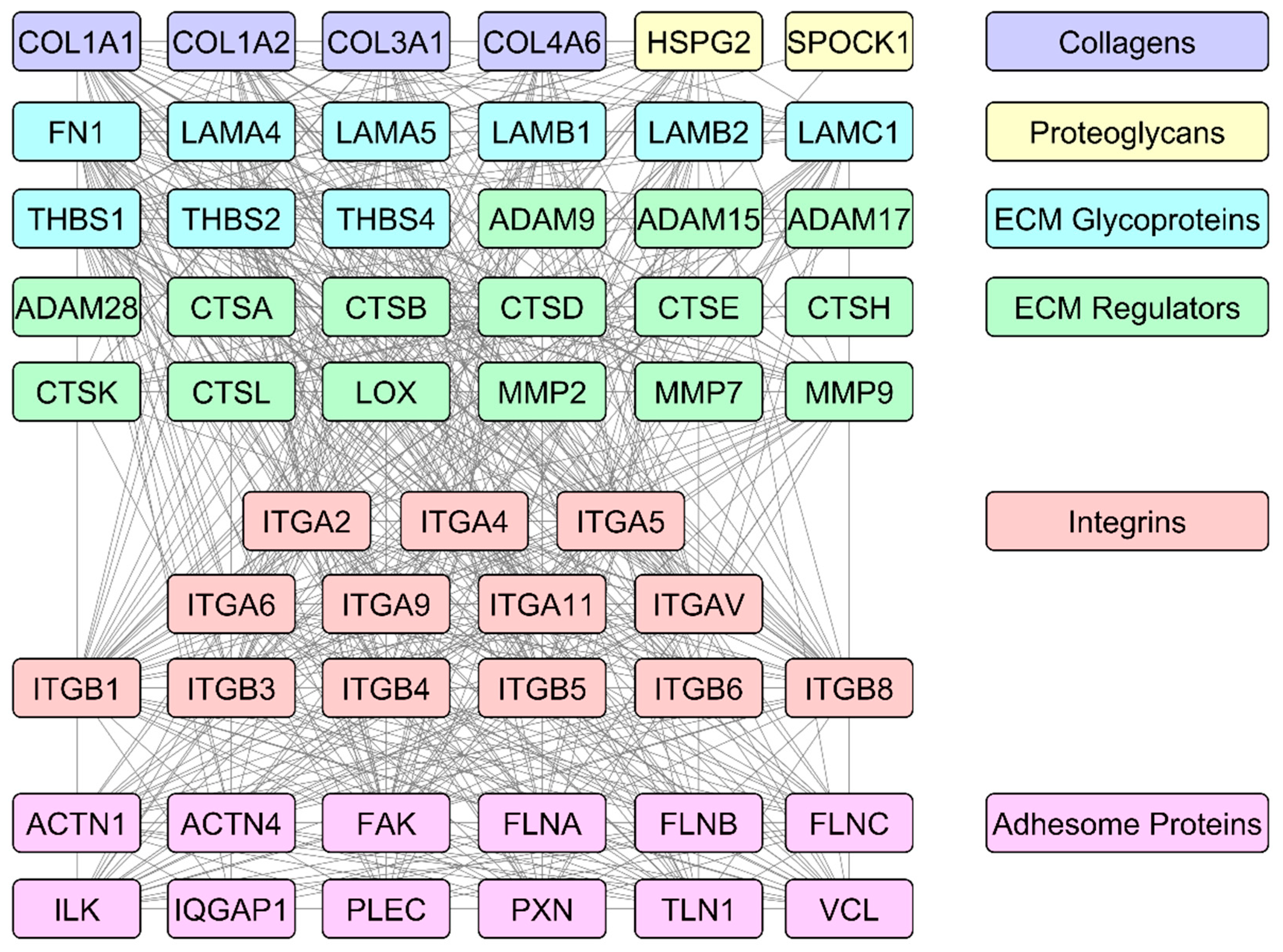
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaržija, I. The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines 2024, 12, 79. https://doi.org/10.3390/biomedicines12010079
Samaržija I. The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines. 2024; 12(1):79. https://doi.org/10.3390/biomedicines12010079
Chicago/Turabian StyleSamaržija, Ivana. 2024. "The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery" Biomedicines 12, no. 1: 79. https://doi.org/10.3390/biomedicines12010079
APA StyleSamaržija, I. (2024). The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery. Biomedicines, 12(1), 79. https://doi.org/10.3390/biomedicines12010079






