Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles Preparation and Characterization
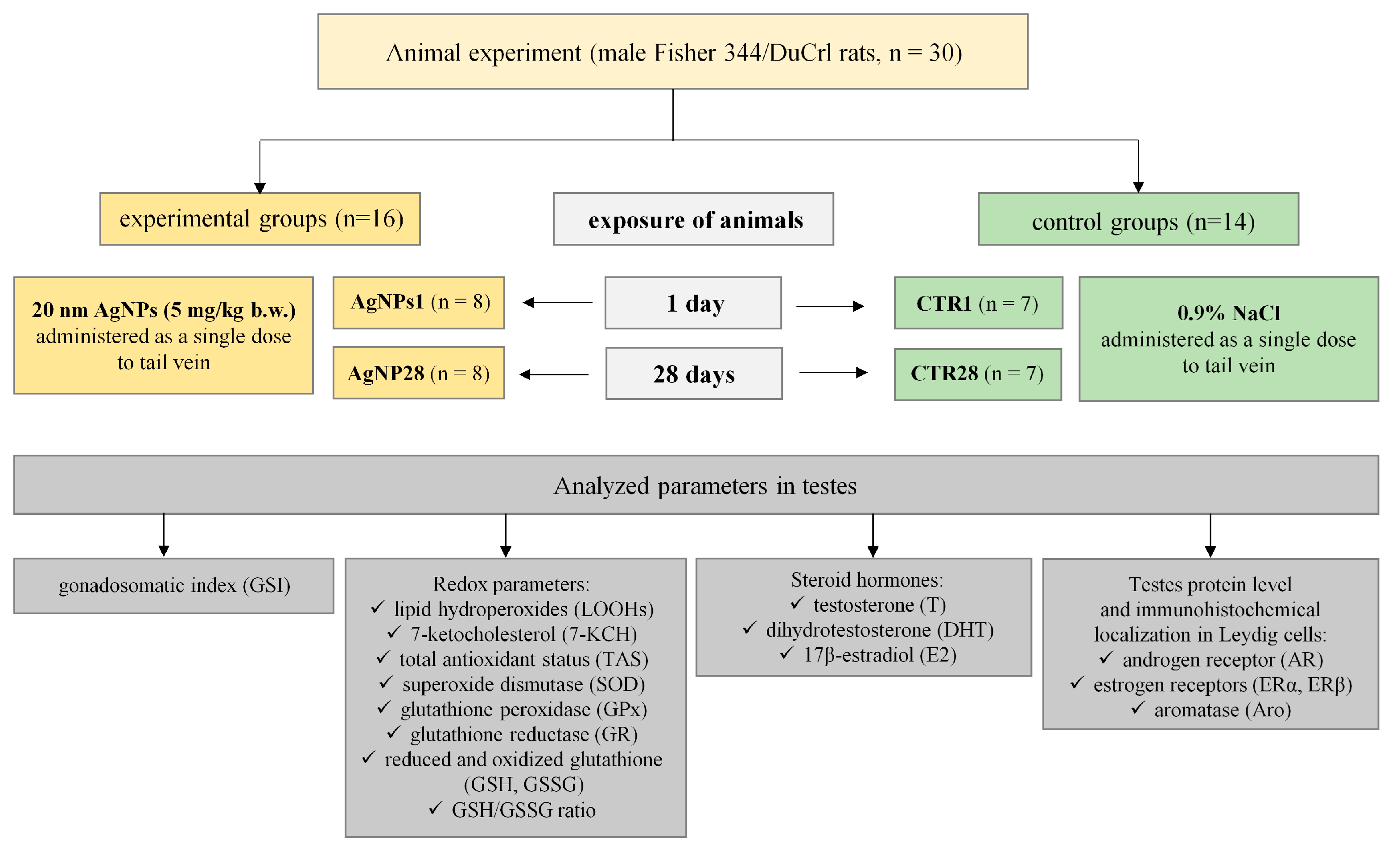
2.2. Animals and Treatment
2.3. Determination of Intratesticular Redox Parameters
2.4. Determination of Concentration of Testicular Steroid Hormones
2.5. Determination of Estrogen Receptors (ERα ERβ), Androgen Receptor (AR) and Aromatase (Aro) Protein Concentrations
2.6. Immunohistological Evaluation of Estrogen Receptors (ERα, ERβ), Androgen Receptor (AR) and Aromatase (Aro) in Leydig Cells
2.7. Statistical Analysis
3. Results
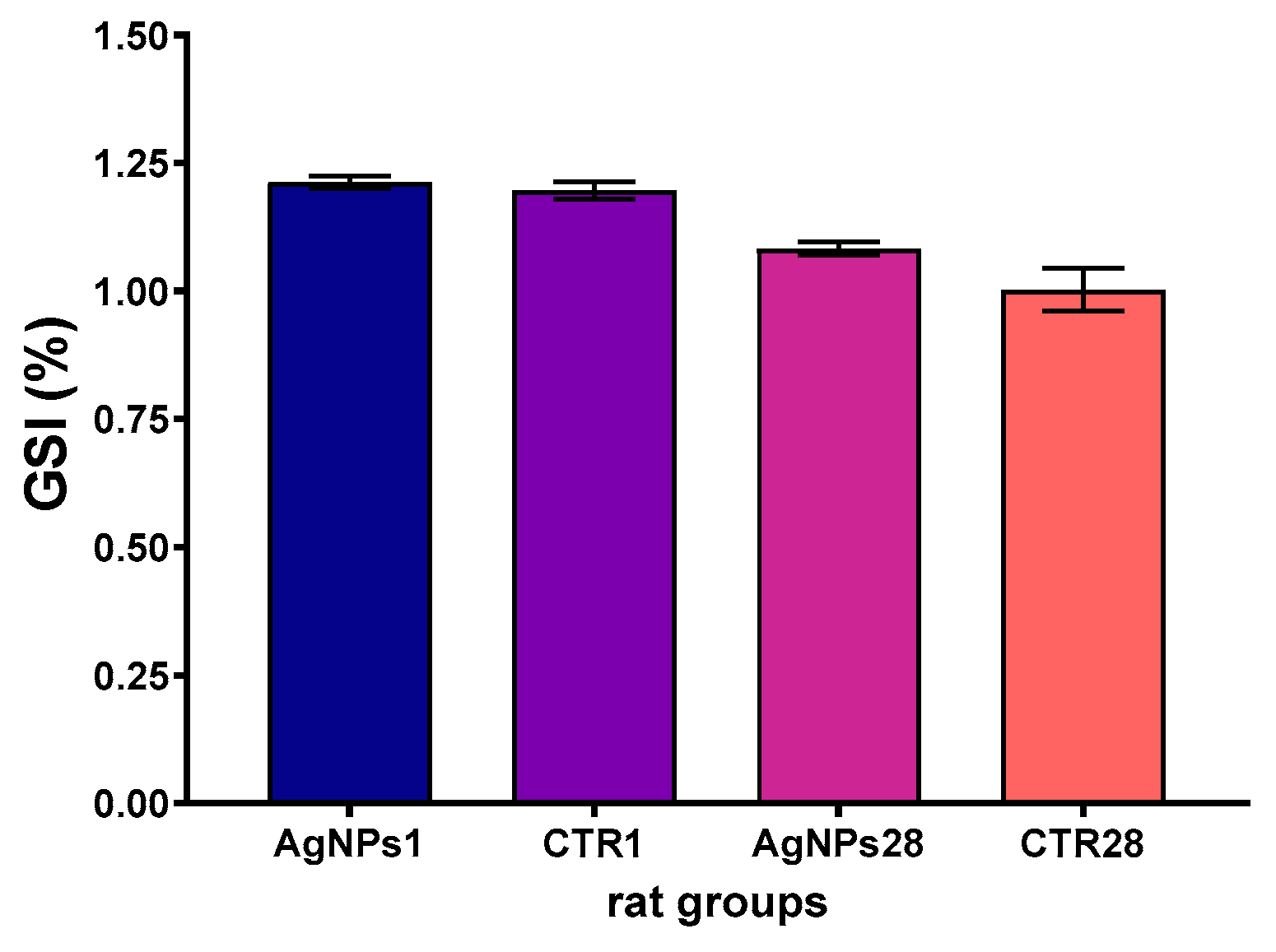
3.1. Overall Animals’ Condition, Body Weight Gain and Gonadosomatic Index
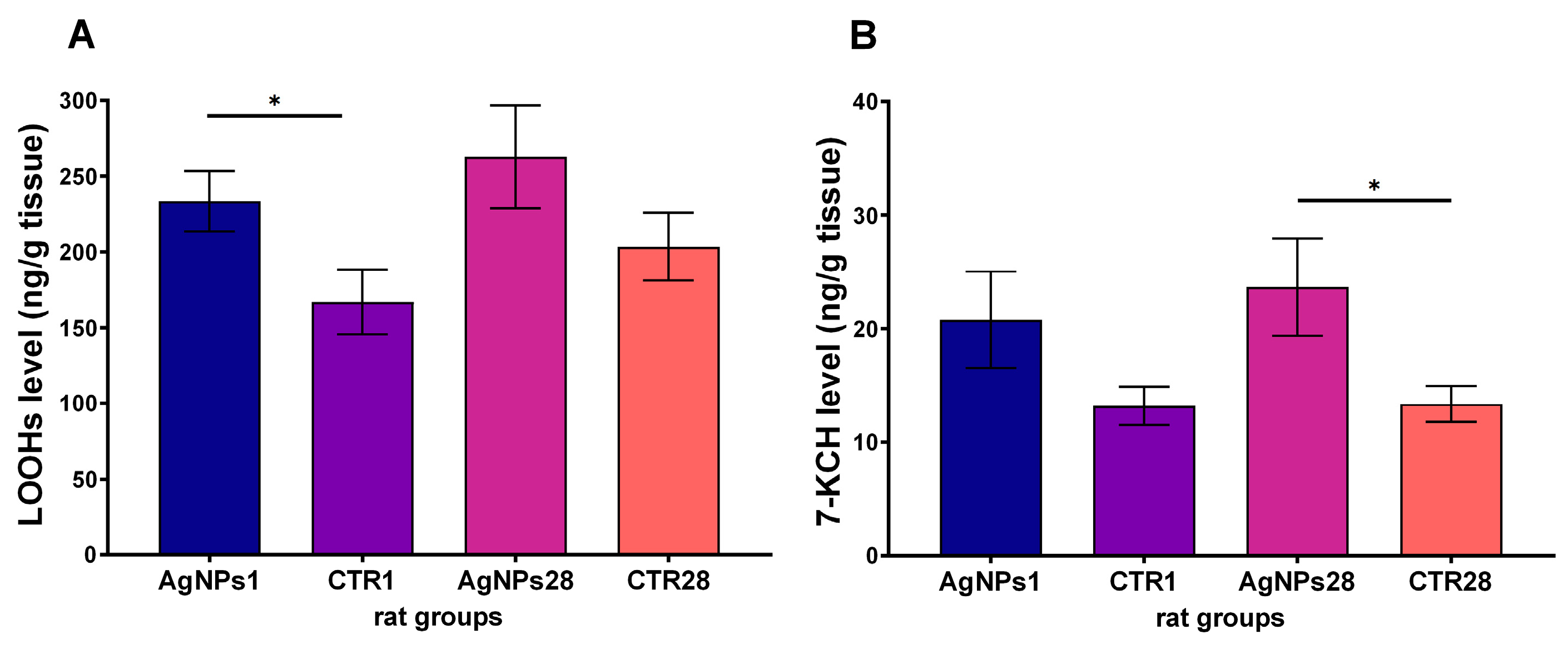
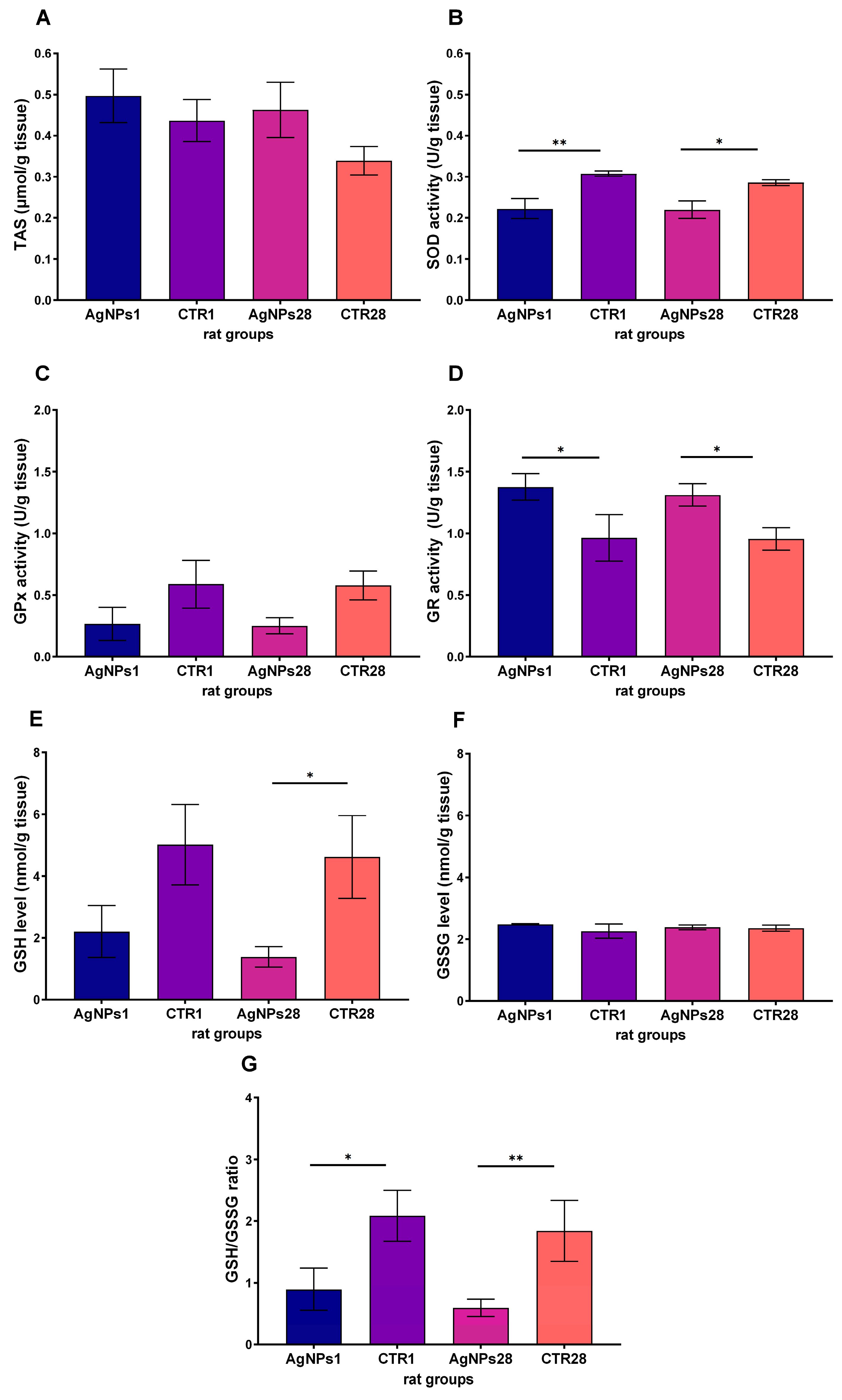
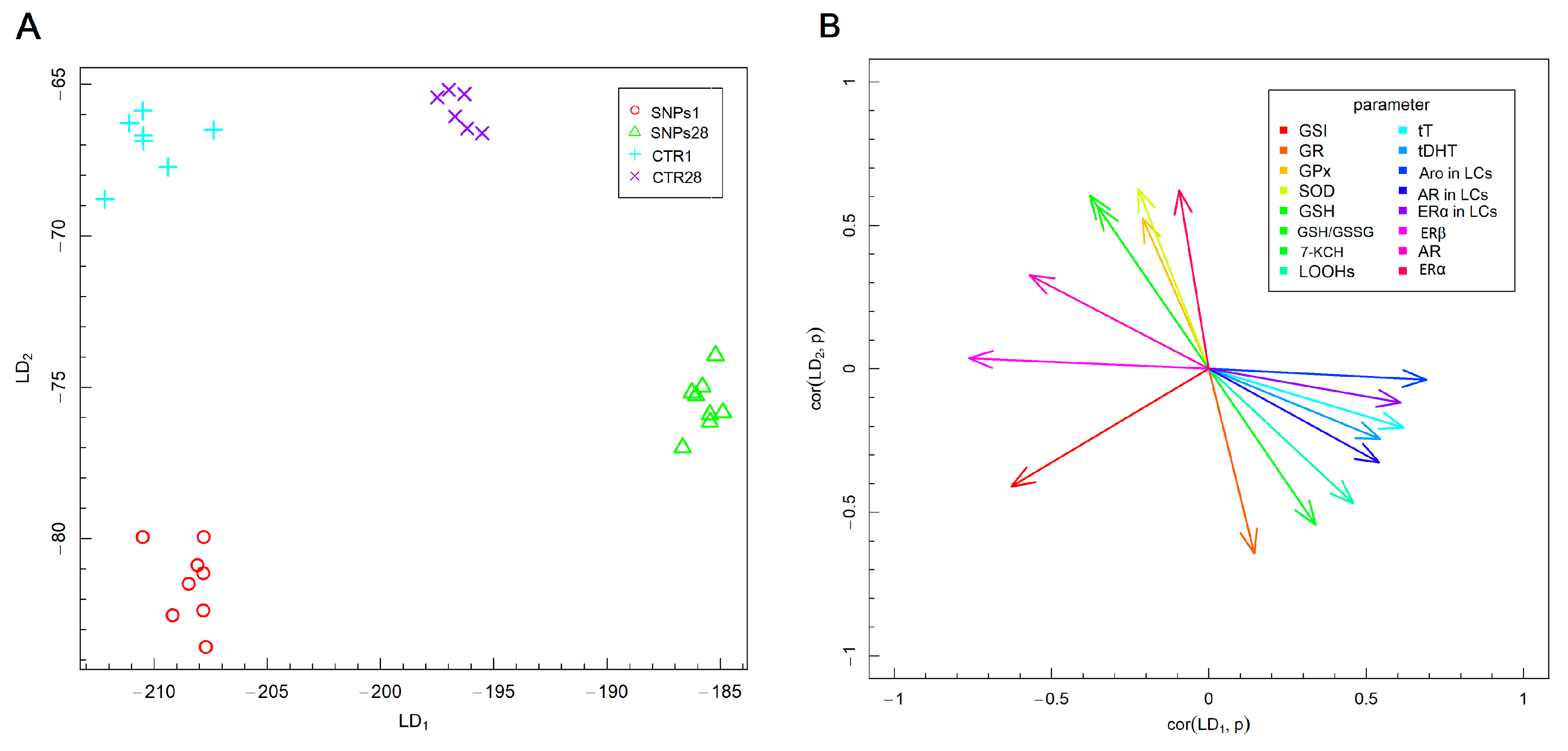
3.2. Male Gonads Redox Parameters
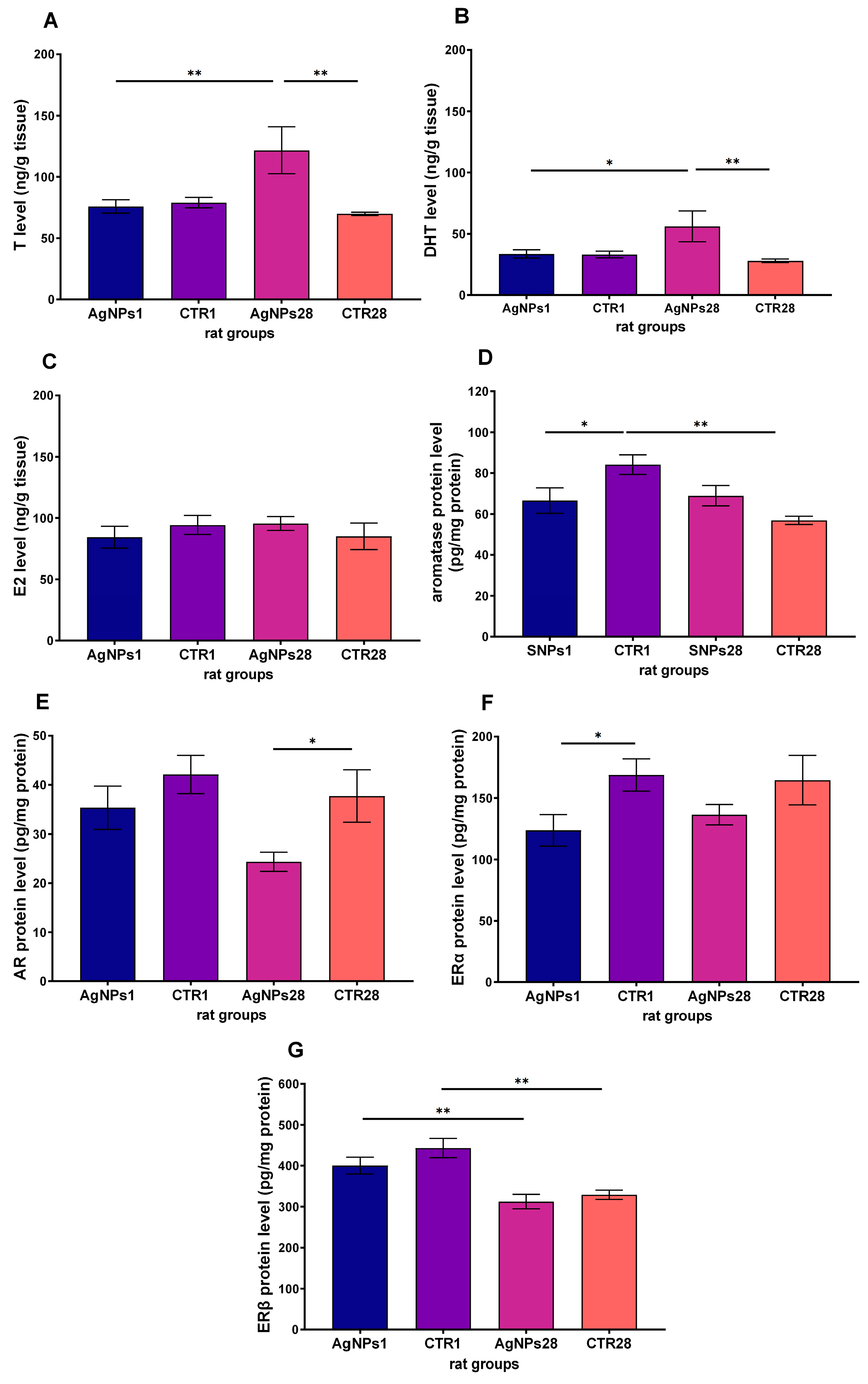
3.3. Steroid Hormones Concentration in Testes Homogenate Supernatant
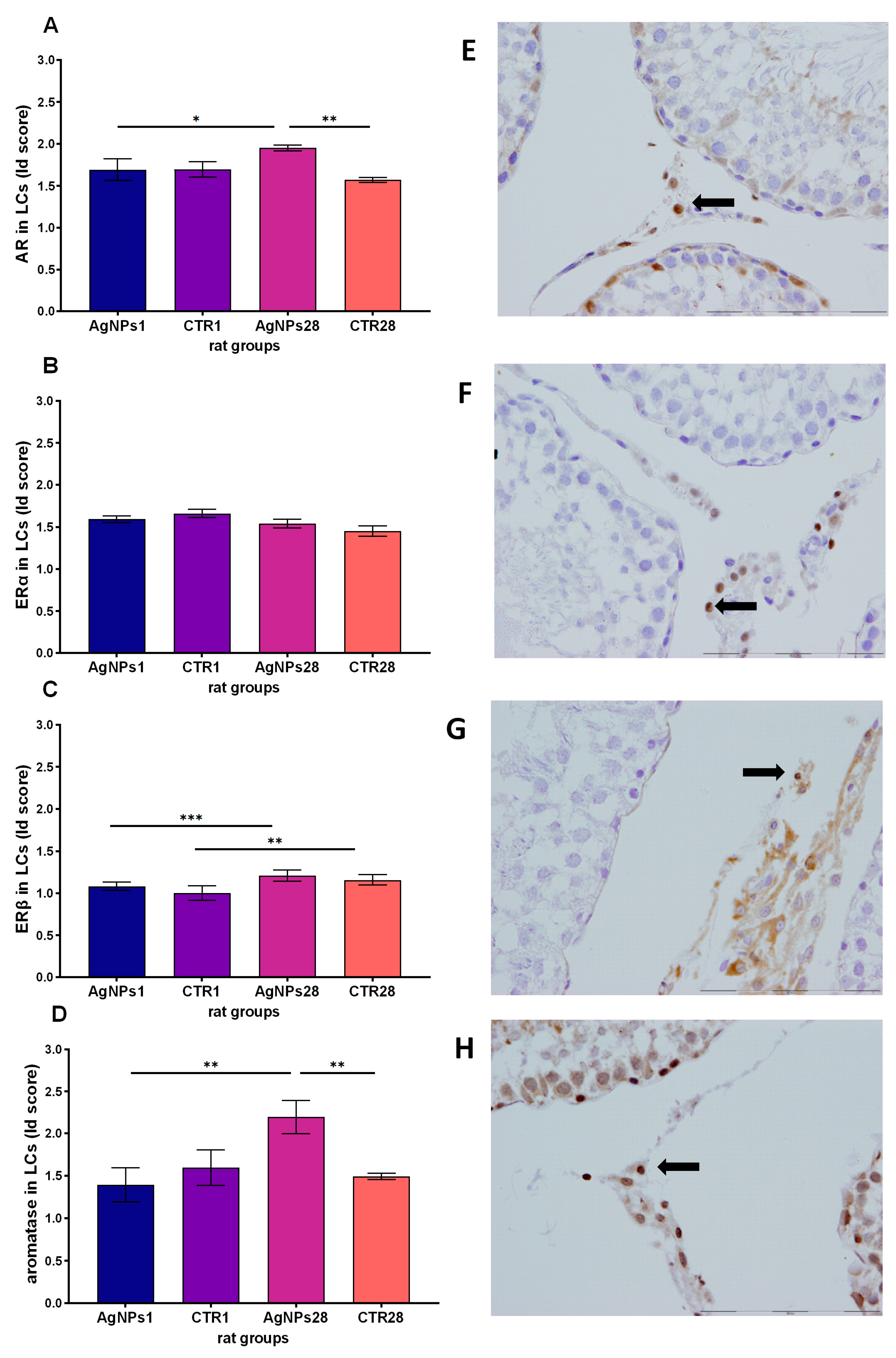
3.4. Steroid Hormones Receptors and Aromatase Protein in Leydig Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temizel-Sekeryan, S.; Hicks, A.L. Global environmental impacts of silver nanoparticle production methods supported by life cycle assessment. Resour. Conserv. Recycl. 2020, 156, 104676. [Google Scholar] [CrossRef]
- Dziendzikowska, K.; Wilczak, J.; Grodzicki, W.; Gromadzka-Ostrowska, J.; Węsierska, M.; Kruszewski, M. Coating-Dependent Neurotoxicity of Silver Nanoparticles—An In Vivo Study on Hippocampal Oxidative Stress and Neurosteroids. Int. J. Mol. Sci. 2022, 23, 1365. [Google Scholar] [CrossRef] [PubMed]
- Dziendzikowska, K.; Krawczyńska, A.; Oczkowski, M.; Królikowski, T.; Brzóska, K.; Lankoff, A.; Dziendzikowski, M.; Stępkowski, T.; Kruszewski, M.; Gromadzka-Ostrowska, J. Progressive effects of silver nanoparticles on hormonal regulation of reproduction in male rats. Toxicol. Appl. Pharmacol. 2016, 313, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cummins, E. Hazard characterization of silver nanoparticles for human exposure routes. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 704–725. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring silver nanoparticles for cancer therapy and diagnosis. Colloids Surf. B Biointerfaces 2022, 210, 112254. [Google Scholar] [CrossRef]
- Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Oczkowski, M.; Krawczyńska, A.; Chwastowska, J.; Sadowska-Bratek, M.; Chajduk, E.; Wojewódzka, M.; Dušinská, M.; et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012, 32, 920–928. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Masterson, C.M.; Jang, W.; Xiao, Z.; Bohloul, A.; Garcia-Rojas, D.; Puppala, H.L.; Bennett, G.; Colvin, V.L. When function is biological: Discerning how silver nanoparticle structure dictates antimicrobial activity. iScience 2022, 25, 104475. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques to Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef]
- Krawczyńska, A.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Herman, A.P.; Oczkowski, M.; Królikowski, T.; Wilczak, J.; Wojewódzka, M.; Kruszewski, M. Silver and titanium dioxide nanoparticles alter oxidative/inflammatory response and renin-angiotensin system in brain. Food Chem. Toxicol. 2015, 85, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, L.; Bai, R.; Liu, Y.; Han, L.; Xu, Z.; Chen, F.; Autrup, H.; Long, D.; Chen, C. Silver nanoparticles induced oxidative and endoplasmic reticulum stresses in mouse tissues: Implications for the development of acute toxicity after intravenous administration. Toxicol. Res. 2016, 5, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka-Ostrowska, J.; Dziendzikowska, K.; Lankoff, A.; Dobrzyńska, M.; Instanes, C.; Brunborg, G.; Gajowik, A.; Radzikowska, J.; Wojewódzka, M.; Kruszewski, M. Silver nanoparticles effects on epididymal sperm in rats. Toxicol. Lett. 2012, 214, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Meczyńska-Wielgosz, S.; Wojewódzka, M.; Matysiak-Kucharek, M.; Czajka, M.; Jodlowska-Jedrych, B.; Kruszewski, M.; Kapka-Skrzypczak, L. Susceptibility of HepG2 cells to silver nanoparticles in combination with other metal/metal oxide nanoparticles. Materials 2020, 13, 2221. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Simple Procedure for Specific Assay of Lipid Hydroperoxides in Serum or Plasma. In Free Radical and Antioxidant Protocols; Humana Press: Clifton, NJ, USA, 1998; pp. 107–110. [Google Scholar]
- Suchecka, D.; Harasym, J.P.; Wilczak, J.; Gajewska, M.; Oczkowski, M.; Gudej, S.; Błaszczyk, K.; Kamola, D.; Filip, R.; Gromadzka-Ostrowska, J. Antioxidative and anti-inflammatory effects of high beta-glucan concentration purified aqueous extract from oat in experimental model of LPS-induced chronic enteritis. J. Funct. Foods 2015, 14, 244–254. [Google Scholar] [CrossRef]
- Rebrin, I.; Forster, M.J.; Sohal, R.S. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007, 1127, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Habert, R.; Picon, R. Control of testicular steroidogenesis in foetal rat: Effect of decapitation on testosterone and plasma luteinizing hormone-like activity. Eur. J. Endocrinol. 1982, 99, 466–473. [Google Scholar] [CrossRef]
- Oczkowski, M.; Rembiszewska, A.; Dziendzikowska, K.; Wolińska-Witort, E.; Kołota, A.; Malik, A.; Stachoń, M.; Lachowicz, K.; Gromadzka-Ostrowska, J. Beer consumption negatively regulates hormonal reproductive status and reduces apoptosis in Leydig cells in peripubertal rats. Alcohol 2019, 78, 21–31. [Google Scholar] [CrossRef]
- Ball, E.; Bond, J.; Franc, B.; DeMicco, C.; Wynford-Thomas, D. An immunohistochemical study of p16INK4a expression in multistep thyroid tumourigenesis. Eur. J. Cancer 2007, 43, 194–201. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002; Available online: https://www.stats.ox.ac.uk/pub/MASS4/ (accessed on 30 March 2023).
- Yazdani, S.; Jaldin-Fincati, J.R.; Pereira, R.V.S.; Klip, A. Endothelial cell barriers: Transport of molecules between blood and tissues. Traffic 2019, 20, 390–403. [Google Scholar] [CrossRef]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the blood–brain barrier: Beyond the endothelial cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The mammalian blood-testis barrier: Its biology and regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Z.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef]
- Garcia, T.X.; Costa, G.M.J.; França, L.R.; Hofmann, M.-C. Sub-acute intravenous administration of silver nanoparticles in male mice alters Leydig cell function and testosterone levels. Reprod. Toxicol. 2014, 45, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, J.; Wang, J.; Hu, Y.; Meng, P.; Xiong, Y.; Huang, P. Genome-wide transcriptional analysis of oxidative stress-related genes and pathways induced by cdte aqqds in mice. Nanotheranostics 2018, 2, 271–279. [Google Scholar] [CrossRef]
- Assar, D.H.; Mokhbatly, A.A.A.; ELazab, M.F.A.; Ghazy, E.W.; Gaber, A.A.; Elbialy, Z.I.; Hassan, A.A.; Nabil, A.; Asa, S.A. Silver nanoparticles induced testicular damage targeting NQO1 and APE1 dysregulation, apoptosis via Bax/Bcl-2 pathway, fibrosis via TGF-β/α-SMA upregulation in rats. Environ. Sci. Pollut. Res. 2023, 30, 26308–26326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Choi, Y.-J.; Han, J.W.; Kim, E.; Park, J.H.; Gurunathan, S.; Kim, J.-H. Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells. Int. J. Nanomed. 2015, 10, 1335–1357. [Google Scholar] [CrossRef]
- Han, J.W.; Jeong, J.K.; Gurunathan, S.; Choi, Y.J.; Das, J.; Kwon, D.N.; Cho, S.G.; Park, C.; Seo, H.G.; Park, J.K.; et al. Male- and female-derived somatic and germ cell-specific toxicity of silver nanoparticles in mouse. Nanotoxicology 2016, 10, 361–373. [Google Scholar] [CrossRef]
- Russell, L.D.; Ettlin, R.A.; Hikim, A.P.S.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis. Int. J. Androl. 1993, 16, 83. [Google Scholar] [CrossRef]
- Bois, C.; Delalande, C.; Nurmio, M.; Parvinen, M.; Zanatta, L.; Toppari, J.; Carreau, S. Age- and cell-related gene expression of aromatase and estrogen receptors in the rat testis. J. Mol. Endocrinol. 2010, 45, 147–159. [Google Scholar] [CrossRef]
- Schulster, M.; Bernie, A.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435. [Google Scholar] [CrossRef]
- Marchioni, M.; Jouneau, P.H.; Chevallet, M.; Michaud-Soret, I.; Deniaud, A. Silver nanoparticle fate in mammals: Bridging in vitro and in vivo studies. Coord. Chem. Rev. 2018, 364, 118–136. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Albetran, H.M.; Al-Mutary, M.G.; Ahmad, M.; Low, I.M. Alleviation of silver nanoparticle-induced sexual behavior and testicular parameters dysfunction in male mice by yttrium oxide nanoparticles. Toxicol. Rep. 2021, 8, 1121–1130. [Google Scholar] [CrossRef]
- Kose, O.; Mantecca, P.; Costa, A.; Carrière, M. Putative adverse outcome pathways for silver nanoparticle toxicity on mammalian male reproductive system: A literature review. Part. Fibre Toxicol. 2023, 20, 1. [Google Scholar] [CrossRef]
- Nicy, V.; Das, M.; Gurusubramanian, G.; Mondal, P.; Roy, V.K. Treatment of copper nanoparticles (CuNPs) for two spermatogenic cycles impairs testicular activity via down-regulating steroid receptors and inhibition of germ cell proliferation in a mice model. Nanotoxicology 2022, 16, 658–678. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological Role of ROS in Sperm Function. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 337–345. ISBN 9783030323004. [Google Scholar]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Paciorek, P.; Zuberek, M.; Grzelak, A. Products of lipid peroxidation as a factor in the toxic effect of silver nanoparticles. Materials 2020, 13, 2460. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Verratti, V.; Mrakic-Sposta, S.; Fusi, J.; Sabovic, I.; Franzoni, F.; Pietrangelo, T.; Bondi, D.; Dall’Acqua, S.; Daniele, S.; Scarfò, G.; et al. Fertility Impairment after Trekking at High Altitude: A Proof of Mechanisms on Redox and Metabolic Seminal Changes. Int. J. Mol. Sci. 2022, 23, 9066. [Google Scholar] [CrossRef]
- Coimbra, J.L.P.; Dantas, G.d.P.F.; de Andrade, L.M.; Brener, M.R.G.; Viana, P.I.M.; Lopes, R.A.; Gontijo, D.O.G.; Ervilha, L.O.G.; Assis, M.Q.; Barcelos, L.S.; et al. Gold nanoparticle intratesticular injections as a potential animal sterilization tool: Long-term reproductive and toxicological implications. Toxicology 2023, 492, 153543. [Google Scholar] [CrossRef]
- Fang, W.; Chi, Z.; Li, W.; Zhang, X.; Zhang, Q. Comparative Study on the Toxic Mechanisms of Medical Nanosilver and Silver Ions on the Antioxidant System of Erythrocytes: From the Aspects of Antioxidant Enzyme Activities and Molecular Interaction Mechanisms. J. Nanobiotechnol. 2019, 17, 66. [Google Scholar] [CrossRef]

| Parameter | BSA-Coated AgNPs |
|---|---|
| nominal size of Ag particles [nm] | 20 ± 5 |
| dynamic light scattering [nm] | 84.4 ± 3.7 |
| polydispersity index | 0.295 |
| zeta potential [mV] | −33.6 |
| Parameters | After 1 Day | After 28 Days | |
|---|---|---|---|
| Following Administration of AgNPs (Compared to Control Groups): | |||
| oxidative stress parameters | LOOHs concentration | ↑ | = |
| 7-KCH concentration | = | ↑ | |
| anti-oxidative defense parameters | SOD activity | ↓ | ↓ |
| GR activity | ↑ | ↑ | |
| GSH concentration | = | ↓ | |
| GSH/GSSG ratio | ↓ | ↓ | |
| steroid hormones level and steroid receptors abundance | T | = | ↑ |
| DHT | = | ↑ | |
| Aro protein level | ↓ | = | |
| AR protein level | = | ↓ | |
| ERα protein level | ↓ | = | |
| AR in Leydig cells | = | ↑ | |
| Aro in Leydig cells | = | ↑ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oczkowski, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Rakowski, M.; Kruszewski, M. Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption. Biomedicines 2024, 12, 73. https://doi.org/10.3390/biomedicines12010073
Oczkowski M, Dziendzikowska K, Gromadzka-Ostrowska J, Rakowski M, Kruszewski M. Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption. Biomedicines. 2024; 12(1):73. https://doi.org/10.3390/biomedicines12010073
Chicago/Turabian StyleOczkowski, Michał, Katarzyna Dziendzikowska, Joanna Gromadzka-Ostrowska, Michał Rakowski, and Marcin Kruszewski. 2024. "Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption" Biomedicines 12, no. 1: 73. https://doi.org/10.3390/biomedicines12010073
APA StyleOczkowski, M., Dziendzikowska, K., Gromadzka-Ostrowska, J., Rakowski, M., & Kruszewski, M. (2024). Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption. Biomedicines, 12(1), 73. https://doi.org/10.3390/biomedicines12010073










