Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology
Abstract
:1. Introduction
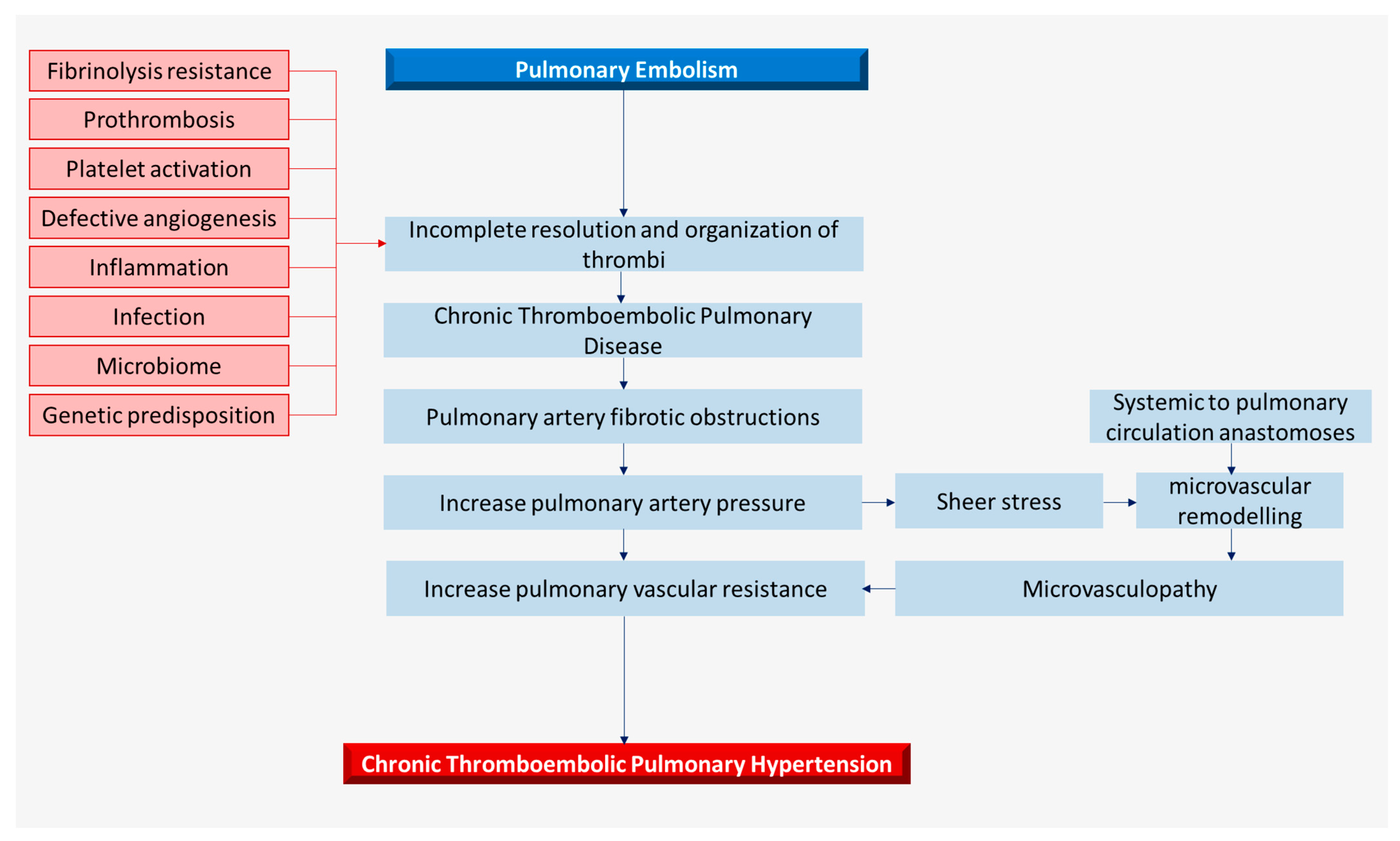
2. Pathophysiology and Histopathology
3. Role of Fibrinogen, Thrombosis, and Fibrinolysis in Thrombus Organization
4. Metabolomics- Angiogenesis and Inflammation
5. Microbiome and Infection
6. Microparticle and Plasma Metabolome
7. Genetic Background
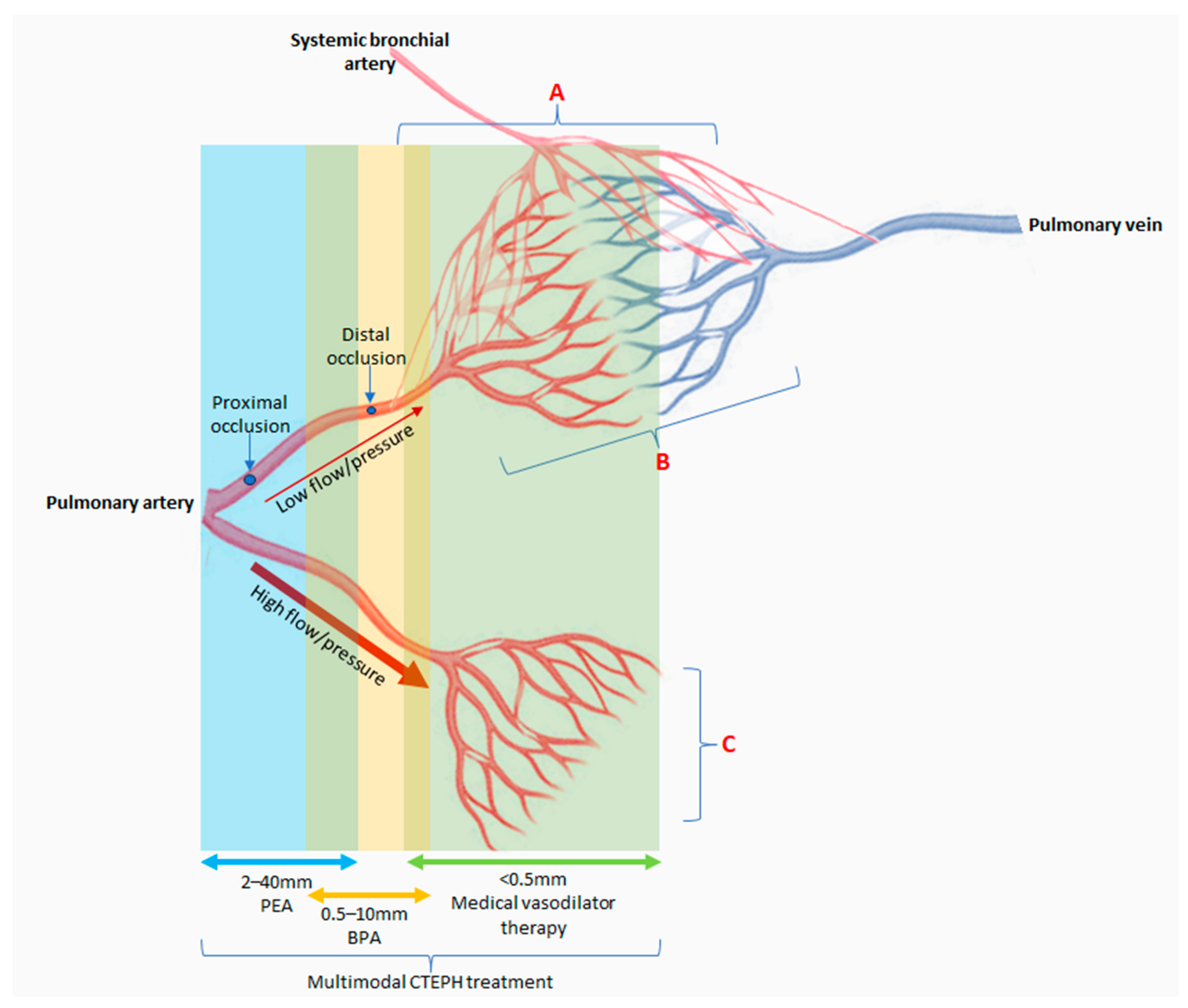
8. Does Current Understanding of Pathobiology Translate to CTEPH Management?
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.; Ghofrani, H.; Jenkins, D. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Dorfmüller, P.; Noordegraaf, A.V. The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S3), S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Dorfmüller, P.; Guignabert, C.; Mercier, O.; Humbert, M. Chronic thromboembolic pulmonary hypertension: The magic of pathophysiology. Ann. Cardiothorac. Surg. 2022, 11, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Dorfmüller, P.; Günther, S.; Ghigna, M.R.; Thomas de Montpréville, V.; Boulate, D.; Paul, J.F.; Jaïs, X.; Decante, B.; Simonneau, G.; Dartevelle, P.; et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: A role for pulmonary veins and systemic vasculature. Eur. Respir. J. 2014, 44, 1275–1288. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Mayer, E.; Simonneau, G.; Rubin, L.J. Chronic Thromboembolic Pulmonary Hypertension. Circulation 2006, 113, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef]
- Wartski, M.; Collignon, M.A. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou Heparin Standard: Evaluation dans l’Embolie Pulmonaire Study. J. Nucl. Med. 2000, 41, 1043–1048. [Google Scholar]
- Alonso-Martínez, J.L.; Anniccherico-Sánchez, F.J.; Urbieta-Echezarreta, M.A.; García-Sanchotena, J.L.; Herrero, H.G. Residual pulmonary thromboemboli after acute pulmonary embolism. Eur. J. Intern. Med. 2012, 23, 379–383. [Google Scholar] [CrossRef]
- Fernandes, T.; Planquette, B.; Sanchez, O.; Morris, T. From Acute to Chronic Thromboembolic Disease. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S3), S207–S214. [Google Scholar] [CrossRef]
- Gall, H.; Hoeper, M.M.; Richter, M.J.; Cacheris, W.; Hinzmann, B.; Mayer, E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur. Respir. Rev. 2017, 26, 160121. [Google Scholar] [CrossRef]
- Ruaro, B.; Baratella, E.; Caforio, G.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. Chronic Thromboembolic Pulmonary Hypertension: An Update. Diagnostics 2022, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Luijten, D.; Talerico, R.; Barco, S.; Cannegieter, S.C.; Delcroix, M.; Ende-Verhaar, Y.M.; Huisman, M.V.; Konstantinidis, S.; Mairuhu, A.T.A.; van Mens, T.E.; et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: An updated systematic review and meta-analysis. Eur. Respir. J. 2023, 62, 2300449. [Google Scholar] [CrossRef]
- Kim, N.H.; Lang, I.M. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2012, 21, 27–31. [Google Scholar] [CrossRef]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schafers, H.J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mercier, O.; Fadel, E. Chronic thromboembolic pulmonary hypertension: Animal models. Eur. Respir. J. 2013, 41, 1200–1206. [Google Scholar] [CrossRef]
- Karpov, A.A.; Vaulina, D.D.; Smirnov, S.S.; Moiseeva, O.M.; Galagudza, M.M. Rodent models of pulmonary embolism and chronic thromboembolic pulmonary hypertension. Heliyon 2022, 8, e09014. [Google Scholar] [CrossRef]
- Perros, F.; Ghigna, M.R.; Loisel, F.; Chemla, D.; Decante, B.; de Montpreville, V.; Montani, D.; Humbert, M.; Fadel, E.; Mercier, O.; et al. Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension. Biomedicines 2020, 8, 493. [Google Scholar] [CrossRef]
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef]
- Toshner, M.; Pepke-Zaba, J. Chronic thromboembolic pulmonary hypertension: Time for research in pathophysiology to catch up with developments in treatment. F1000Prime Rep. 2014, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Plácido, R.; Guimarães, T.; Jenkins, D.; Cortez-Dias, N.; Pereira, S.C.; Campos, P.; Mineiro, A.; Lousada, N.; Martins, S.R.; Moreira, S.; et al. Chronic thromboembolic pulmonary hypertension: Initial experience of patients undergoing pulmonary thromboendarterectomy. Rev. Port. Cardiol. 2021, 40, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Quarck, R.; Wynants, M.; Verbeken, E.; Meyns, B.; Delcroix, M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2015, 46, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Magaña, M.M.; Liang, N.C.; Soler, X.; Desantis, D.J.; Ngo, D.; Woods, V.L., Jr. High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood 2009, 114, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.J.; Chiles, P.G.; Liang, N.C.; Morris, T.A. Chronic thromboembolic pulmonary hypertension-associated dysfibrinogenemias exhibit disorganized fibrin structure. Thromb. Res. 2013, 132, 729–734. [Google Scholar] [CrossRef]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Auger, W.R.; Fedullo, P.F.; Woods, V.L. Fibrin Derived from Patients with Chronic Thromboembolic Pulmonary Hypertension Is Resistant to Lysis. Am. J. Respir. Crit. Care Med. 2006, 173, 1270–1275. [Google Scholar] [CrossRef]
- Firth, A.L.; Yau, J.; White, A.; Chiles, P.G.; Marsh, J.J.; Morris, T.A.; Yuan, J.X. Chronic exposure to fibrin and fibrinogen differentially regulates intracellular Ca2+ in human pulmonary arterial smooth muscle and endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L979–L986. [Google Scholar] [CrossRef]
- Liang, N.C.; Firth, A.L.; Marsh, J.J.; Yuan, J.X.; Morris, T.A. Human Pulmonary Artery Endothelial Cell Exposure to Fibrin(ogen) Augments Intracellular Calcium Responses to Thrombin. In A106 Chronic Thromboembolic Pulmonary Hypertension: Bench to Bedside; American Thoracic Society: New York, NY, USA, 2011; p. A2422. [Google Scholar]
- Wolf, M.; Boyer-Neumann, C.; Parent, F.; Eschwege, V.; Jaillet, H.; Meyer, D.; Simonneau, G. Thrombotic risk factors in pulmonary hypertension. Eur. Respir. J. 2000, 15, 395. [Google Scholar] [CrossRef]
- Bonderman, D.; Turecek, P.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; Kneussl, M.; Rubin, L.J.; Kyrle, P.A.; Klepetko, W.; et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef]
- Remková, A.; Šimková, I.; Valkovičová, T. Platelet abnormalities in chronic thromboembolic pulmonary hypertension. Int. J. Clin. Exp. Med. 2015, 8, 9700–9707. [Google Scholar]
- Newnham, M.; South, K.; Bleda, M.; Auger, W.R.; Barberà, J.A.; Bogaard, H.; Bunclark, K.; Cannon, J.E.; Delcroix, M.; Hadinnapola, C.; et al. The ADAMTS13–VWF axis is dysregulated in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801805. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Szulcek, R.; Pan, X.; Symersky, P.; Dickhoff, C.; Majolée, J.; Kremer, V.; Michielon, E.; Jordanova, E.S.; Radonic, T.; et al. Epigenetic Modification of the von Willebrand Factor Promoter Drives Platelet Aggregation on the Pulmonary Endothelium in Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Vrigkou, E.; Tsantes, A.; Konstantonis, D.; Rapti, E.; Maratou, E.; Pappas, A.; Halvatsiotis, P.; Tsangaris, I. Platelet, Fibrinolytic and Other Coagulation Abnormalities in Newly-Diagnosed Patients with Chronic Thromboembolic Pulmonary Hypertension. Diagnostics 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, N.; Shirakawa, R.; Fukumoto, Y.; Sugimura, K.; Miyata, S.; Miura, Y.; Nochioka, K.; Miura, M.; Tatebe, S.; Aoki, T.; et al. Platelets Are Highly Activated in Patients of Chronic Thromboembolic Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Olman, M.A.; Marsh, J.J.; Lang, I.M.; Moser, K.M.; Binder, B.R.; Schleef, R.R. Endogenous fibrinolytic system in chronic large-vessel thromboembolic pulmonary hypertension. Circulation 1992, 86, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Marsh, J.J.; Olman, M.A.; Moser, K.M.; Schleef, R.R. Parallel analysis of tissue-type plasminogen activator and type 1 plasminogen activator inhibitor in plasma and endothelial cells derived from patients with chronic pulmonary thromboemboli. Circulation 1994, 90, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Marsh, J.J.; Olman, M.A.; Moser, K.M.; Loskutoff, D.J.; Schleef, R.R. Expression of type 1 plasminogen activator inhibitor in chronic pulmonary thromboemboli. Circulation 1994, 89, 2715–2721. [Google Scholar] [CrossRef]
- Yaoita, N.; Satoh, K.; Satoh, T.; Sugimura, K.; Tatebe, S.; Yamamoto, S.; Aoki, T.; Miura, M.; Miyata, S.; Kawamura, T.; et al. Thrombin-Activatable Fibrinolysis Inhibitor in Chronic Thromboembolic Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1293–1301. [Google Scholar] [CrossRef]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-Term Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Hadinnapola, C.M.; Southwood, M.; Hernández-Sánchez, J.; Bunclark, K.; Newnham, M.; Swietlik, E.M.; Cannon, J.; Preston, S.D.; Sheares, K.; Taboada, D.; et al. Angiopoietin 2 and hsCRP are associated with pulmonary hemodynamics and long-term mortality respectively in CTEPH—Results from a prospective discovery and validation biomarker study. J. Heart Lung Transplant. 2023, 42, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lang, I.M. More Evidence for Inflammatory Thrombosis in Chronic Thromboembolic Pulmonary Hypertension: Is the Embolic Hypothesis Losing Ground? Am. J. Respir. Crit. Care Med. 2022, 205, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Smolders, V.F.E.D.; Lodder, K.; Rodríguez, C.; Tura-Ceide, O.; Barberà, J.A.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J.; Kurakula, K. The Inflammatory Profile of CTEPH-Derived Endothelial Cells Is a Possible Driver of Disease Progression. Cells 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Hadinnapola, C.; Southwood, M.; Jenkins, D.; Sheares, K.; Toshner, M.; Pepke-Zaba, J. P16 A Systematic Characterisation Of Inflammation in Chronic Thromboembolic Pulmonary Hypertension. Thorax 2014, 69 (Suppl. S2), A84. [Google Scholar] [CrossRef]
- Alias, S.; Redwan, B.; Panzenböck, A.; Winter, M.P.; Schubert, U.; Voswinckel, R.; Frey, M.K.; Jakowitsch, J.; Alimohammadi, A.; Hobohm, L.; et al. Defective Angiogenesis Delays Thrombus Resolution. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 810–819. [Google Scholar] [CrossRef]
- Kellermair, J.; Redwan, B.; Alias, S.; Jabkowski, J.; Panzenboeck, A.; Kellermair, L.; Winter, M.P.; Weltermann, A.; Lang, I.M. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood 2013, 122, 3376–3384. [Google Scholar] [CrossRef]
- Woodfin, A.; Voisin, M.B.; Nourshargh, S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef]
- Zabini, D.; Nagaraj, C.; Stacher, E.; Lang, I.M.; Nierlich, P.; Klepetko, W.; Heinemann, A.; Olschewski, H.; Bálint, Z.; Olschewski, A. Angiostatic Factors in the Pulmonary Endarterectomy Material from Chronic Thromboembolic Pulmonary Hypertension Patients Cause Endothelial Dysfunction. PLoS ONE 2012, 7, e43793. [Google Scholar] [CrossRef]
- Hobohm, L.; Kölmel, S.; Niemann, C.; Kümpers, P.; Krieg, V.J.; Bochenek, M.L.; Lukasz, A.H.; Reiss, Y.; Plate, K.H.; Liebetrau, C.; et al. Role of angiopoietin-2 in venous thrombus resolution and chronic thromboembolic disease. Eur. Respir. J. 2021, 58, 2004196. [Google Scholar] [CrossRef]
- Fiedler, U.; Scharpfenecker, M.; Koidl, S.; Hegen, A.; Grunow, V.; Schmidt, J.M.; Kriz, W.; Thurston, G.; Augustin, H.G. The Tie-2 ligand Angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004, 103, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, U.; Reiss, Y.; Scharpfenecker, M.; Grunow, V.; Koidl, S.; Thurston, G.; Gale, N.W.; Witzenrath, M.; Rosseau, S.; Suttorp, N.; et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat. Med. 2006, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Schramm, R.; Bauer, M.; Tscholl, D.; Kunihara, T.; Schäfers, H.J. Cytokine Response to Pulmonary Thromboendarterectomy. Chest 2004, 126, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hofbauer, T.M.; Ondracek, A.S.; Chausheva, S.; Alimohammadi, A.; Artner, T.; Panzenboeck, A.; Rinderer, J.; Shafran, I.; Mangold, A.; et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood 2021, 137, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Li, X.; Dong, G.; Zhang, M.; Xu, Z.; Yang, J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediat. Inflamm. 2020, 2020, 9254087. [Google Scholar] [CrossRef]
- Savchenko, A.S.; Martinod, K.; Seidman, M.A.; Wong, S.L.; Borissoff, J.I.; Piazza, G.; Libby, P.; Goldhaber, S.Z.; Mitchell, R.N.; Wagner, D.D. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 2014, 12, 860–870. [Google Scholar] [CrossRef]
- Wynants, M.; Quarck, R.; Ronisz, A.; Alfaro-Moreno, E.; Van Raemdonck, D.; Meyns, B.; Delcroix, M. Effects of C-reactive protein on human pulmonary vascular cells in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2012, 40, 886–894. [Google Scholar] [CrossRef]
- Shih, H.H.; Zhang, S.; Cao, W.; Hahn, A.; Wang, J.; Paulsen, J.E.; Harnish, D.C. CRP is a novel ligand for the oxidized LDL receptor LOX-1. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1643–H1650. [Google Scholar] [CrossRef]
- Kanaji, N.; Sato, T.; Nelson, A.; Wang, X.; Li, Y.; Kim, M.; Nakanishi, M.; Basma, H.; Michalski, J.; Farid, M.; et al. Inflammatory cytokines regulate endothelial cell survival and tissue repair functions via NF-κB signaling. J. Inflamm. Res. 2011, 4, 127–138. [Google Scholar] [PubMed]
- Farkas, D.; Alhussaini, A.A.; Kraskauskas, D.; Kraskauskiene, V.; Cool, C.D.; Nicolls, M.R.; Natarajan, R.; Farkas, L. Nuclear Factor κB Inhibition Reduces Lung Vascular Lumen Obliteration in Severe Pulmonary Hypertension in Rats. Am. J. Respir. Cell Mol. Biol. 2014, 51, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Wynants, M.; Vengethasamy, L.; Ronisz, A.; Meyns, B.; Delcroix, M.; Quarck, R. NF-κB pathway is involved in CRP-induced effects on pulmonary arterial endothelial cells in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L934–L942. [Google Scholar] [CrossRef] [PubMed]
- Zabini, D.; Heinemann, A.; Foris, V.; Nagaraj, C.; Nierlich, P.; Bálint, Z.; Kwapiszewska, G.; Lang, I.M.; Klepetko, W.; Olschewski, H.; et al. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 2014, 44, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Mercier, O.; Arthur Ataam, J.; Langer, N.B.; Dorfmüller, P.; Lamrani, L.; Lecerf, F.; Decante, B.; Dartevelle, P.; Eddahibi, S.; Fadel, E. Abnormal pulmonary endothelial cells may underlie the enigmatic pathogenesis of chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2017, 36, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Johns, R.A.; Gao, W.D. Right heart in pulmonary hypertension: From adaptation to failure. Pulm. Circ. 2019, 9, 2045894019845611. [Google Scholar] [CrossRef]
- Sun, X.Q.; Abbate, A.; Bogaard, H.J. Role of cardiac inflammation in right ventricular failure. Cardiovasc. Res. 2017, 113, 1441–1452. [Google Scholar] [CrossRef]
- Samson, N.; Paulin, R. Epigenetics, inflammation and metabolism in right heart failure associated with pulmonary hypertension. Pulm. Circ. 2017, 7, 572–587. [Google Scholar] [CrossRef]
- Yang, B.Q.; Park, A.C.; Liu, J.; Byrnes, K.; Javaheri, A.; Mann, D.L.; Schilling, J.D. Distinct Inflammatory Milieu in Patients with Right Heart Failure. Circ. Heart Fail. 2023, 16, 657. [Google Scholar] [CrossRef]
- Bonderman, D.; Jakowitsch, J.; Adlbrecht, C.; Schemper, M.; Kyrle, P.; Schönauer, V.; Exner, M.; Klepetko, W.; Kneussl, M.P.; Maurer, G.; et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2005, 93, 512–516. [Google Scholar] [CrossRef]
- Bonderman, D.; Jakowitsch, J.; Redwan, B.; Bergmeister, H.; Renner, M.K.; Panzenböck, H.; Adlbrecht, C.; Georgopoulos, A.; Klepetko, W.; Kneussl, M.; et al. Role for Staphylococci in Misguided Thrombus Resolution of Chronic Thromboembolic Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.K.; Zhang, G.; Zhang, B.T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Aasen, A.O.; Wang, J.E. Mediator Responses in Surgical Infections. Surg. Infect. 2006, 7 (Suppl. S2),, s-3–s-4. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.I.; O’Seaghdha, M.; Magargee, M.; Foster, T.J.; Prince, A.S. Staphylococcus aureus Protein A Activates TNFR1 Signaling through Conserved IgG Binding Domains. J. Biol. Chem. 2006, 281, 20190–20196. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Kim, S.; Rigatto, K.; Gazzana, M.B.; Knorst, M.M.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Altered Gut Microbiome Profile in Patients with Pulmonary Arterial Hypertension. Hypertension 2020, 75, 1063–1071. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, T.; Tan, Z.; Chen, S.; Fang, Z. Role of Gut Microbiota in Pulmonary Arterial Hypertension. Front. Cell Infect. Microbiol. 2022, 12, 493. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Tang, W.H.W. Intersections between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef]
- Ikubo, Y.; Sanada, T.J.; Hosomi, K.; Park, J.; Naito, A.; Shoji, H.; Misawa, T.; Suda, R.; Sekine, A.; Sugiura, T.; et al. Altered gut microbiota and its association with inflammation in patients with chronic thromboembolic pulmonary hypertension: A single-center observational study in Japan. BMC Pulm. Med. 2022, 22, 138. [Google Scholar] [CrossRef]
- Boxer, M.A.; Braun, J.; Ellman, L. Thromboembolic risk of postsplenectomy thrombocytosis. Arch. Surg. 1978, 113, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, X.; Ioos, V.; Jardim, C.; Sitbon, O.; Parent, F.; Hamid, A.; Fadel, E.; Dartevelle, P.; Simonneau, G.; Humbert, M. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax 2005, 60, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Barmparas, G.; Fierro, N.; Sun, B.J.; Ashrafian, S.; Li, T.; Ley, E.J. Splenectomy is associated with a higher risk for venous thromboembolism: A prospective cohort study. Int. J. Surg. 2015, 24, 27–32. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Schoonen, W.M.; Farkas, D.K.; Riis, A.; Fryzek, J.P.; Sørensen, H.T. Risk of venous thromboembolism in splenectomized patients compared with the general population and appendectomized patients: A 10-year nationwide cohort study. J. Thromb. Haemost. 2010, 8, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Atichartakarn, V.; Angchaisuksiri, P.; Aryurachai, K.; Onpun, S.; Chuncharunee, S.; Thakkinstian, A.; Atamasirikul, K. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/β-thalassaemic patients. Br. J. Haematol. 2002, 118, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.K.; Alias, S.; Winter, M.P.; Redwan, B.; Stübiger, G.; Panzenboeck, A.; Alimohammadi, A.; Bonderman, D.; Jakowitsch, J.; Bergmeister, H.; et al. Splenectomy Is Modifying the Vascular Remodeling of Thrombosis. J. Am. Heart Assoc. 2014, 3, e000772. [Google Scholar] [CrossRef] [PubMed]
- Heresi, G.A.; Mey, J.T.; Bartholomew, J.R.; Haddadin, I.S.; Tonelli, A.R.; Dweik, R.A.; Kirwan, J.P.; Kalhan, S.C. Plasma metabolomic profile in chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2020, 10, 2045894019890553. [Google Scholar] [CrossRef]
- Swietlik, E.M.; Ghataorhe, P.; Zalewska, K.I.; Wharton, J.; Howard, L.S.; Taboada, D.; Cannon, J.E.; UK National Cohort Study of PAH; Morrell, N.W.; Wilkins, M.R.; et al. Plasma metabolomics exhibit response to therapy in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2003201. [Google Scholar] [CrossRef]
- Vardon Bounes, F.; Mujalli, A.; Cenac, C.; Severin, S.; Le Faouder, P.; Chicanne, G.; Gaits-Iacovoni, F.; Minville, V.; Gratacap, M.P.; Payrastre, B. The importance of blood platelet lipid signaling in thrombosis and in sepsis. Adv. Biol. Regul. 2018, 67, 66–73. [Google Scholar] [CrossRef]
- Doehner, W.; Frenneaux, M.; Anker, S.D. Metabolic Impairment in Heart Failure. J. Am. Coll. Cardiol. 2014, 64, 1388–1400. [Google Scholar] [CrossRef]
- Suntharalingam, J.; Goldsmith, K.; van Marion, V.; Long, L.; Treacy, C.M.; Dudbridge, F.; Toshner, M.R.; Pepke-Zaba, J.; Eikenboom, J.C.; Morrell, N.W. Fibrinogen A Thr312Ala polymorphism is associated with chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2008, 31, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Lin, Y.; Yang, Y.H.; Gan, H.L.; Liang, Y.; Liu, J.; Yang, S.Q.; Zhang, W.J.; Cui, N.; Zhao, L.; et al. Fibrinogen Aα Thr312Ala Polymorphism Specifically Contributes to Chronic Thromboembolic Pulmonary Hypertension by Increasing Fibrin Resistance. PLoS ONE 2013, 8, e69635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L. Structure–function and regulation of ADAMTS-13 protease. J. Thromb. Haemost. 2013, 11, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.G.; Nichols, W.C.; Lian, E.C.; Foroud, T.; McClintick, J.N.; McGee, B.M.; Yang, A.Y.; Siemieniak, D.R.; Stark, K.R.; Gruppo, R.; et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001, 413, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, M.A.H.; de Maat, M.P.M.; Leebeek, F.W.G. Von Willebrand factor and ADAMTS13 in arterial thrombosis: A systematic review and meta-analysis. Blood Rev. 2014, 28, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Deng, C.; Wu, D.; Zhong, Z.; Lv, X.; Huang, Z.; Lian, N.; Liu, K.; Zhang, Q. The role of mononuclear cell tissue factor and inflammatory cytokines in patients with chronic thromboembolic pulmonary hypertension. J. Thromb. Thrombolysis 2016, 42, 38–45. [Google Scholar] [CrossRef]
- Wu, O.; Bayoumi, N.; Vickers, M.A.; Clark, P. ABO(H) blood groups and vascular disease: A systematic review and meta-analysis. J. Thromb. Haemost. 2008, 6, 62–69. [Google Scholar] [CrossRef]
- Ward, S.E.; O’Sullivan, J.M.; O’Donnell, J.S. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood 2020, 136, 2864–2874. [Google Scholar] [CrossRef]
- Koster, T.; Vandenbroucke, J.P.; Rosendaal, F.R.; Briët, E.; Rosendaal, F.R.; Blann, A.D. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995, 345, 152–155. [Google Scholar] [CrossRef]
- McCord, R.P.; Kaplan, N.; Giorgetti, L. Chromosome Conformation Capture and Beyond: Toward an Integrative View of Chromosome Structure and Function. Mol. Cell. 2020, 77, 688–708. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, J.; Machado, R.D.; Sharples, L.D.; Toshner, M.R.; Sheares, K.K.; Hughes, R.J.; Jenkins, D.P.; Trembath, R.C.; Morrell, N.W.; Pepke-Zaba, J. Demographic features, BMPR2 status and outcomes in distal chronic thromboembolic pulmonary hypertension. Thorax 2007, 62, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.X.; Liu, D.; Sun, M.L.; Jiang, X.; Sun, N.; Mao, Y.M.; Jing, Z.C. BMPR2 germline mutation in chronic thromboembolic pulmonary hypertension. Lung 2014, 192, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Szamalek-Hoegel, J.; Hersberger, M.; Fischler, M.; Garcia, J.S.; Huber, L.C.; Grünig, E.; Janssen, B.; Speich, R. Sequence variants in BMPR2 and genes involved in the serotonin and nitric oxide pathways in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Relation to clinical parameters and comparison with left heart disease. Respiration 2010, 79, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Heeneman, S.; Sluimer, J.C.; Daemen, M.J.A.P. Angiotensin-Converting Enzyme and Vascular Remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Amano, S.; Tatsumi, K.; Kominami, S.; Igarashi, N.; Shimura, R.; Matsubara, H.; Kasahara., Y.; Takiguchi, Y.; Kuriyama, T. Angiotensin-Converting Enzyme Gene Polymorphisms and Prognosis in Chronic Thromboembolic Pulmonary Hypertension. Circ. J. 2006, 70, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, L.; Liu, J.; Wang, W.; Yuan, J.X.-J.; Zhao, L.; Wang, J.; Wang, C. MicroRNA Expression Profile of Pulmonary Artery Smooth Muscle Cells and the Effect of Let-7d in Chronic Thromboembolic Pulmonary Hypertension. Pulm. Circ. 2013, 3, 654–664. [Google Scholar] [CrossRef]
- Miao, R.; Dong, X.; Gong, J.; Li, Y.; Guo, X.; Wang, J.; Huang, Q.; Wang, Y.; Li, J.; Yang, S.; et al. Single-cell RNA-sequencing and microarray analyses to explore the pathological mechanisms of chronic thromboembolic pulmonary hypertension. Front. Cardiovasc. Med. 2022, 9, 900353. [Google Scholar] [CrossRef]
- Gu, S.; Su, P.; Yan, J.; Zhang, X.; An, X.; Gao, J.; Xin, R.; Liu, Y. Comparison of gene expression profiles and related pathways in chronic thromboembolic pulmonary hypertension. Int. J. Mol. Med. 2014, 33, 277–300. [Google Scholar] [CrossRef]
- Liley, J.; Newnham, M.; Bleda, M.; Auger, W.; Barbera, J.A.; Bogaard, H.; Delcroix, M.; Fernandes, T.M.; Howard, L.; Jenkins, D.; et al. CTEPH has shared and distinct genetic associations with pulmonary embolism in a genome-wide association study. medRxiv 2023. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindström, S.; Weng, L.C.; de Andrade, M.; de Visser, M.C.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 Individuals Identifies TSPAN15 and SLC44A2 as Two Susceptibility Loci for Venous Thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef] [PubMed]
- TSPAN15. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000099282-TSPAN15 (accessed on 6 September 2023).
- TAP2. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000204267-TAP2 (accessed on 6 September 2023).
- Tang, W.; Schwienbacher, C.; Lopez, L.M.; Ben-Shlomo, Y.; Oudot-Mellakh, T.; Johnson, A.D.; Samani, N.J.; Basu, S.; Gögele, M.; Davies, G.; et al. Genetic Associations for Activated Partial Thromboplastin Time and Prothrombin Time, their Gene Expression Profiles, and Risk of Coronary Artery Disease. Am. J. Hum. Genet. 2012, 91, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Mastrangelo, M.A.; Ture, S.K.; Smith, C.O.; Loelius, S.G.; Berg, R.A.; Shi, X.; Burke, R.M.; Spinelli, S.L.; Cameron, S.J.; et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat. Commun. 2020, 11, 3479. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Auger, W.R.; Benza, R.L.; Channick, R.N.; Davis, R.D.; Elliott, C.G.; He, F.; Jain, S.; Madani, M.M.; McLaughlin, V.V.; et al. Long-term Survival and Quality of Life: Results from the United States Chronic Thromboembolic Pulmonary Hypertension Registry. CHEST Pulm. 2023, 1, 100008. [Google Scholar] [CrossRef]
- Wiedenroth, C.B.; Rolf, A.; Steinhaus, K.; Adameit, M.S.D.; Kriechbaum, S.D.; Haas, M.; Roller, F.; Hamm, C.W.; Ghofrani, H.A.; Mayer, E.; et al. Riociguat and balloon pulmonary angioplasty improve prognosis in patients with inoperable chronic thromboembolic pulmonary Hypertension. J. Heart Lung Transplant. 2023, 42, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Matsuoka, Y.; Onishi, H.; Nakai, H.; Okada, K.; Emoto, N.; Hirata, K. Survival in patients with chronic thromboembolic pulmonary hypertension in the modern management era. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946-2292. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, P.; Caforio, G.; Baratella, E.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lerda, S.; Geri, P.; Biolo, M.; et al. Chronic Thromboembolic Pulmonary Hypertension: An Observational Study. Medicina 2022, 58, 1094. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Ghofrani, H.A.; Hoeper, M.M. Medical management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160107. [Google Scholar] [CrossRef]
- Yang, J.; Madani, M.M.; Mahmud, E.; Kim, N.H. Evaluation and Management of Chronic Thromboembolic Pulmonary Hypertension. Chest 2023, 164, 490–502. [Google Scholar] [CrossRef]
- Jeong, I.; Alotaibi, M.; Fernandes, T.M.; Kim, S.; Kerr, K.M.; Yang, J.; Pretorius, V.; Madani, M.; Kim, N.H. Direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension and the presence of recent thrombus during pulmonary endarterectomy. Pulm. Circ. 2022, 12, e12110. [Google Scholar] [CrossRef] [PubMed]
- Bunclark, K.; Newnham, M.; Chiu, Y.; Ruggiero, A.; Villar, S.S.; Cannon, J.E.; Coghlan, G.; Corris, P.A.; Howard, L.; Jenkins, D.; et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J. Thromb. Haemost. 2020, 18, 114–122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghani, H.; Pepke-Zaba, J. Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology. Biomedicines 2024, 12, 46. https://doi.org/10.3390/biomedicines12010046
Ghani H, Pepke-Zaba J. Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology. Biomedicines. 2024; 12(1):46. https://doi.org/10.3390/biomedicines12010046
Chicago/Turabian StyleGhani, Hakim, and Joanna Pepke-Zaba. 2024. "Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology" Biomedicines 12, no. 1: 46. https://doi.org/10.3390/biomedicines12010046
APA StyleGhani, H., & Pepke-Zaba, J. (2024). Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology. Biomedicines, 12(1), 46. https://doi.org/10.3390/biomedicines12010046





