Cell-Free Tumor DNA Detection-Based Liquid Biopsy of Plasma and Bile in Patients with Various Pancreatic Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Biomaterial Collection and Processing
2.3. DNA Analysis
2.4. Statistical Analysis
3. Results
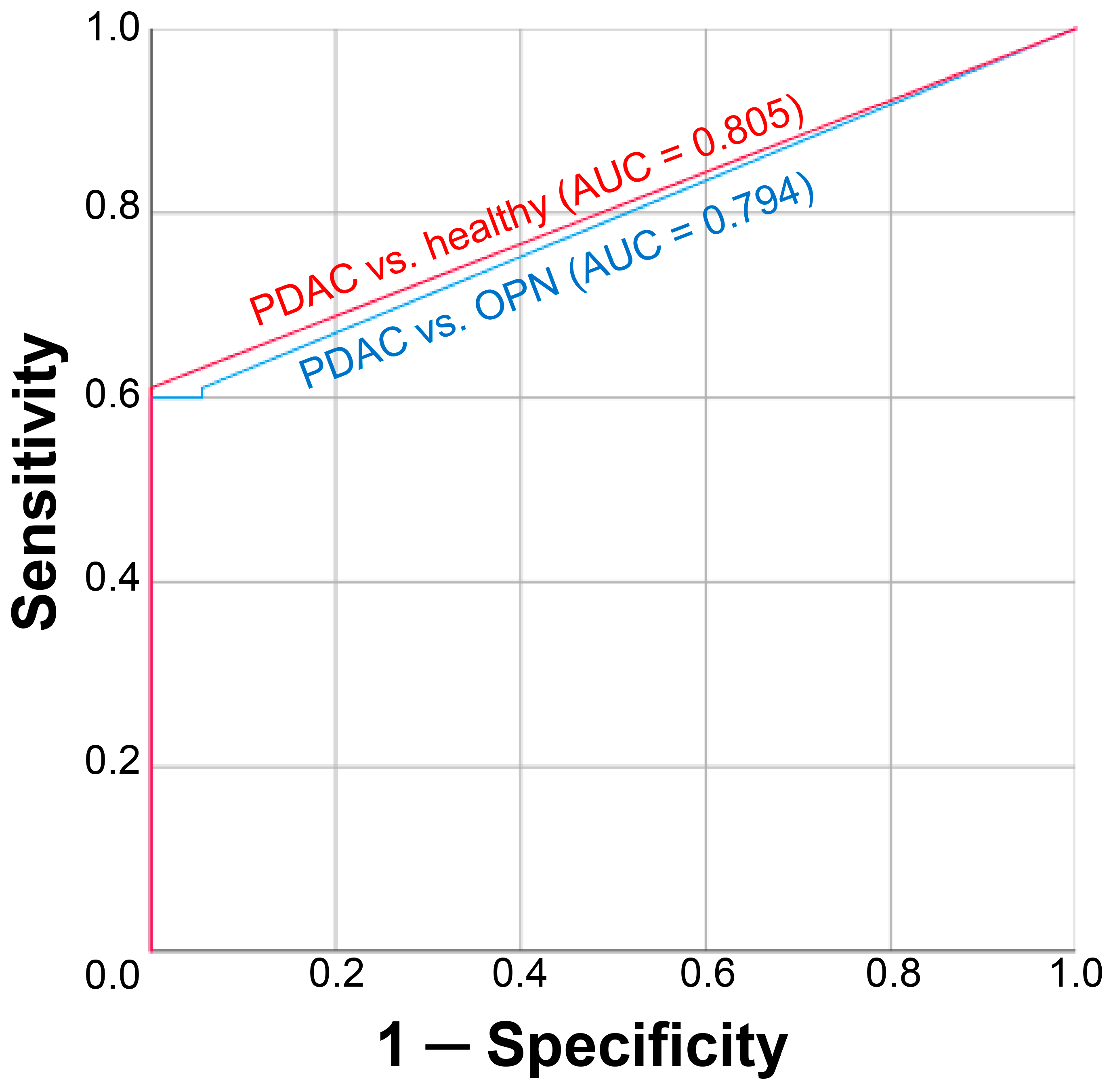
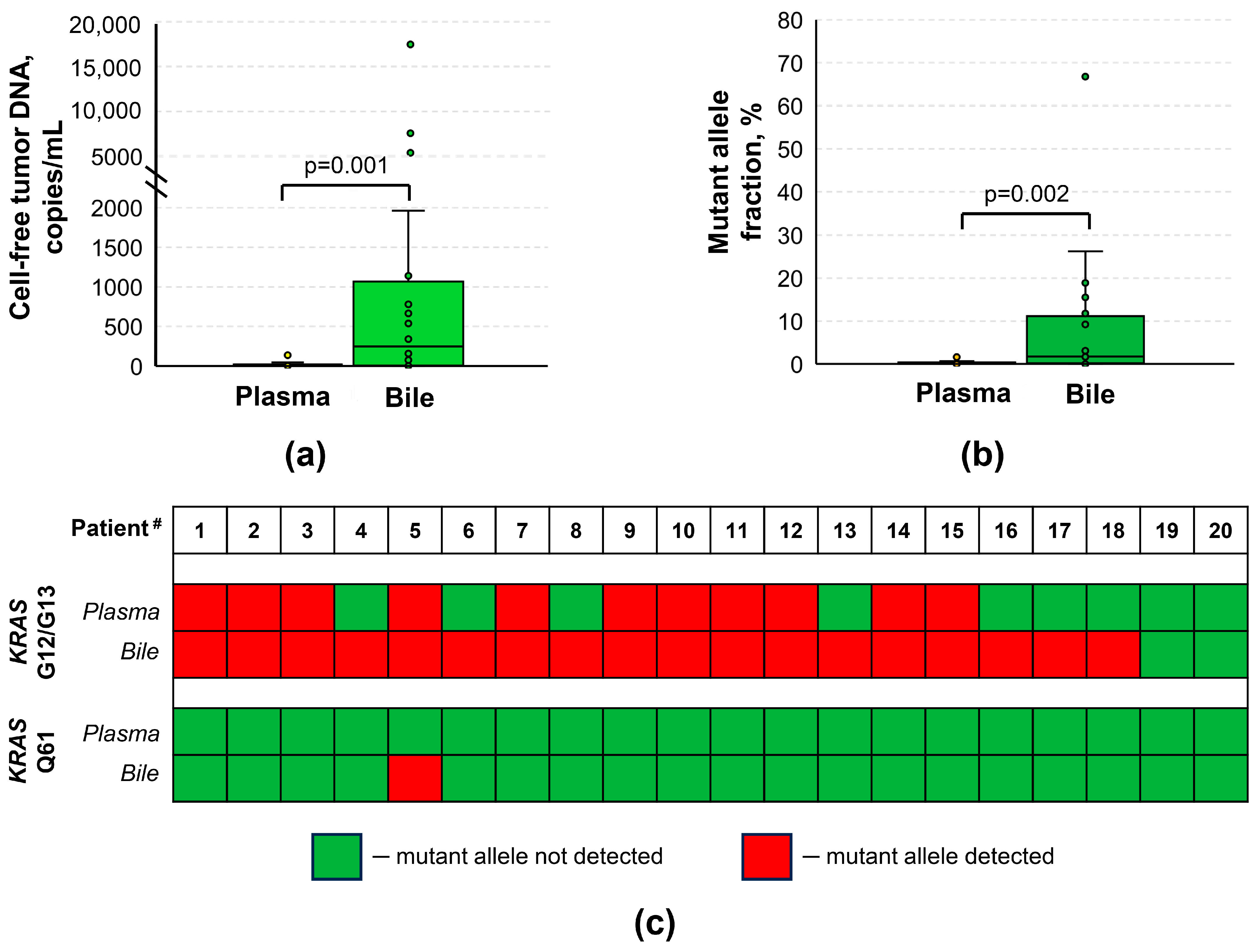
3.1. Diagnostic Performance of cftDNA Analysis
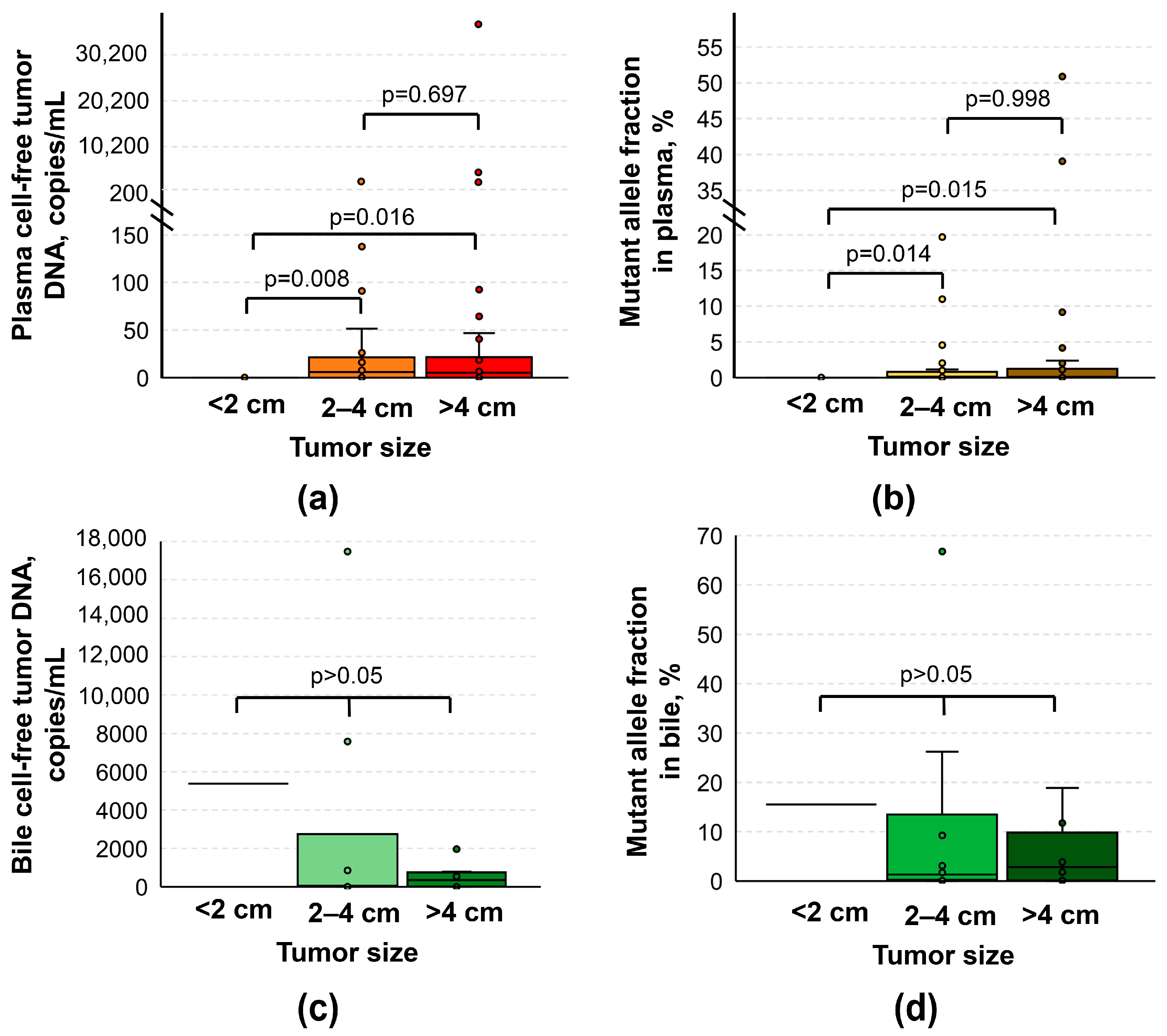
3.2. Relation of cftDNA to Clinical and Demographic Data
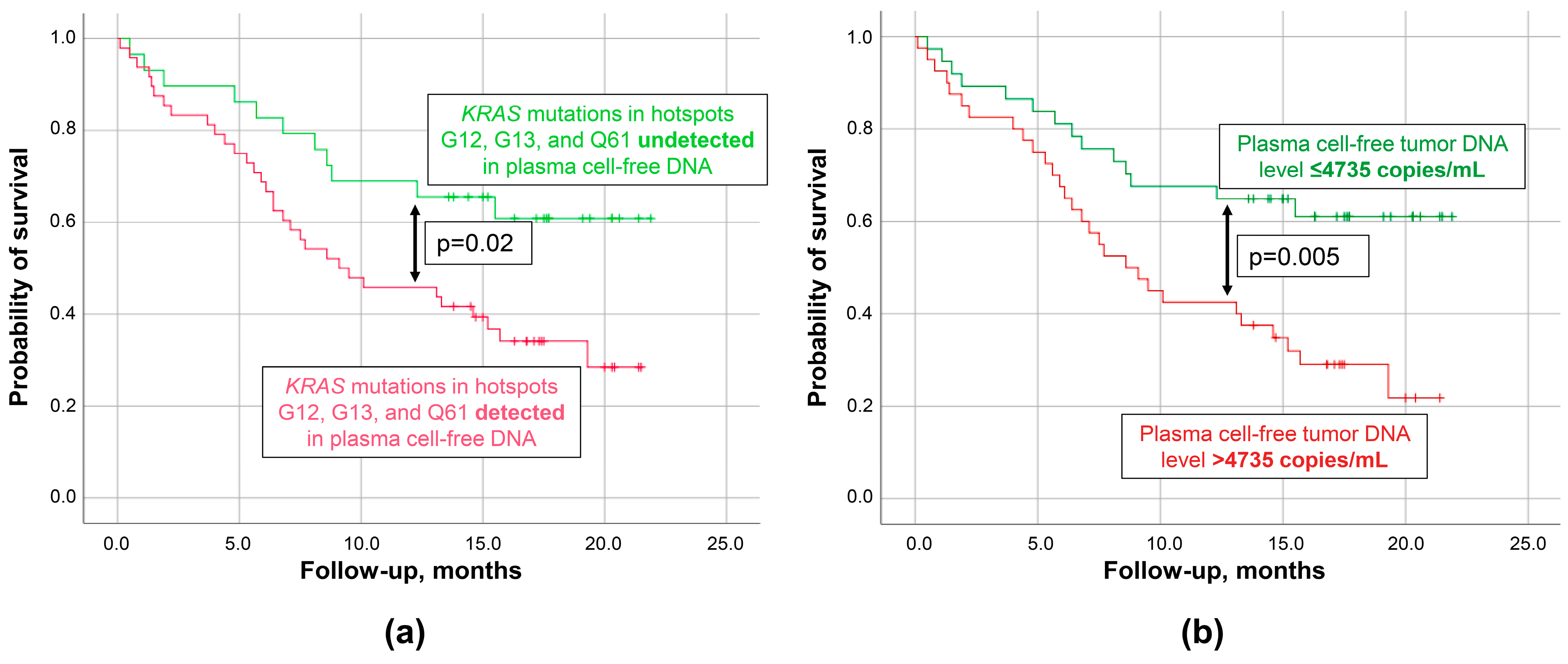
3.3. Prognostic Performance of cftDNA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P.; Lindström, S.; Mendelsohn, J.B.; Steplowski, E.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; Gallinger, S.; Gross, M.; Helzlsouer, K.; et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS ONE 2013, 8, e72311. [Google Scholar] [CrossRef]
- Nikšić, M.; Minicozzi, P.; Weir, H.K.; Zimmerman, H.; Schymura, M.J.; Rees, J.R.; Coleman, M.P.; Allemani, C. Pancreatic cancer survival trends in the US from 2001 to 2014: A CONCORD-3 study. Cancer Commun. 2023, 43, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014, 20, 11182–11198. [Google Scholar] [CrossRef] [PubMed]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef]
- Scholten, L.; van Huijgevoort, N.C.M.; van Hooft, J.E.; Besselink, M.G.; Del Chiaro, M. Pancreatic Cystic Neoplasms: Different Types, Different Management, New Guidelines. Visc. Med. 2018, 34, 173–177. [Google Scholar] [CrossRef]
- Kilgour, E.; Rothwell, D.G.; Brady, G.; Dive, C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020, 37, 485–495. [Google Scholar] [CrossRef]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating Tumor Cells: Moving Biological Insights into Detection. Theranostics 2017, 7, 2606–2619. [Google Scholar] [CrossRef]
- In ‘t Veld, S.G.J.G.; Wurdinger, T. Tumor-educated platelets. Blood 2019, 133, 2359–2364. [Google Scholar] [CrossRef]
- Jung, A.; Kirchner, T. Liquid biopsy in tumor genetic diagnosis. Ärzteblatt Int. 2018, 115, 169–174. [Google Scholar]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Löhr, M.; Klöppel, G.; Maisonneuve, P.; Lowenfels, A.B.; Lüttges, J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-analysis. Neoplasia 2005, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef]
- Kinugasa, H.; Nouso, K.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Tsutsumi, K.; Kato, H.; Matsubara, T.; Okada, H.; Yamamoto, K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015, 121, 2271–2280. [Google Scholar] [CrossRef]
- Brychta, N.; Krahn, T.; von Ahsen, O. Detection of KRAS Mutations in Circulating Tumor DNA by Digital PCR in Early Stages of Pancreatic Cancer. Clin. Chem. 2016, 62, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, H.; Chen, N.; Hao, J.; Jin, H.; Ma, X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18581. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers 2019, 11, 497. [Google Scholar] [CrossRef]
- Distler, M.; Kersting, S.; Rückert, F.; Dobrowolski, F.; Miehlke, S.; Grützmann, R.; Saeger, H.-D. Palliative treatment of obstructive jaundice in patients with carcinoma of the pancreatic head or distal biliary tree. Endoscopic stent placement vs. hepaticojejunostomy. JOP 2010, 11, 568–574. [Google Scholar]
- Dong, L.; Wang, S.; Fu, B.; Wang, J. Evaluation of droplet digital PCR and next generation sequencing for characterizing DNA reference material for KRAS mutation detection. Sci. Rep. 2018, 8, 9650. [Google Scholar] [CrossRef]
- La Rocca, F.; Grieco, V.; Ruggieri, V.; Zifarone, E.; Villani, O.; Zoppoli, P.; Russi, S.; Laurino, S.; Falco, G.; Calice, G.; et al. Superiority of Droplet Digital PCR Over Real-Time Quantitative PCR for JAK2 V617F Allele Mutational Burden Assessment in Myeloproliferative Neoplasms: A Retrospective Study. Diagnostics 2020, 10, 143. [Google Scholar] [CrossRef]
- Jain, M.; Atayan, D.; Rakhmatullin, T.; Dakhtler, T.; Popov, P.; Kim, P.; Gontareva, J.; Samokhodskaya, L.; Egorov, V.; Kamalov, A. Tumor cell-free DNA detection-based liquid biopsy of plasma and bile in pancreatic ductal adenocarcinoma: A pilot study. Genes Cells 2023, 18, 41–51. [Google Scholar]
- Parra-Robert, M.; Santos, V.M.; Canis, S.M.; Pla, X.F.; Fradera, J.M.A.; Porto, R.M. Relationship Between CA 19.9 and the Lewis Phenotype: Options to Improve Diagnostic Efficiency. Anticancer Res. 2018, 38, 5883–5888. [Google Scholar] [CrossRef] [PubMed]
- Perales, S.; Torres, C.; Jimenez-Luna, C.; Prados, J.; Martinez-Galan, J.; Sanchez-Manas, J.M.; Caba, O. Liquid biopsy approach to pancreatic cancer. World J. Gastrointest. Oncol. 2021, 13, 1263–1287. [Google Scholar] [CrossRef]
- Vidal, L.; Pando, E.; Blanco, L.; Fabregat-Franco, C.; Castet, F.; Sierra, A.; Macarulla, T.; Balsells, J.; Charco, R.; Vivancos, A. Liquid biopsy after resection of pancreatic adenocarcinoma and its relation to oncological outcomes. Systematic review and meta-analysis. Cancer Treat. Rev. 2023, 120, 102604. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Soto, V.; Rodríguez-Salas, N.; Feliu, J. Liquid Biopsy in Pancreatic Cancer: Are We Ready to Apply It in the Clinical Practice? Cancers 2021, 13, 1986. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Chen, C.; Guo, J.; Qiao, M.; Lyu, J. Recent estimates and predictions of 5-year survival rate in patients with pancreatic cancer: A model-based period analysis. Front. Med. 2022, 9, 1049136. [Google Scholar]
- Hosen, M.I.; Sheikh, M.; Zvereva, M.; Scelo, G.; Forey, N.; Durand, G.; Voegele, C.; Poustchi, H.; Khoshnia, M.; Roshandel, G.; et al. Urinary TERT promoter mutations are detectable up to 10 years prior to clinical diagnosis of bladder cancer: Evidence from the Golestan Cohort Study. EBioMedicine 2020, 53, 102643. [Google Scholar] [CrossRef]
- Kuvendjiska, J.; Müller, F.; Bronsert, P.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Kulemann, B. Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas. Life 2023, 13, 1570. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, J.; Deng, H.; Huang, S.-J.; Rao, J.; Xu, W.-B.; Huang, J.-S.; Sun, S.-Q.; Zhang, L. Cardiac-specific methylation patterns of circulating DNA for identification of cardiomyocyte death. BMC Cardiovasc. Disord. 2020, 20, 310. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Babaniamansour, S.; Shi, J. Updates in the Diagnosis of Intraductal Neoplasms of the Pancreas. Front. Physiol. 2022, 13, 856803. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.Y.; Wu, Y.; Li, Y.; Wang, J.; Chen, J. Intraductal papillary mucinous neoplasms of the pancreas: Clinical association with KRAS. Mol. Med. Rep. 2018, 17, 8061–8068. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Molin, M.D.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Collet, L.; Ghurburrun, E.; Meyers, N.; Assi, M.; Pirlot, B.; Leclercq, I.A.; Couvelard, A.; Komuta, M.; Cros, J.; Demetter, P.; et al. Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2020, 69, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Basturk, O.; Brannon, A.R.; Bhanot, U.; Scott, S.N.; Bouvier, N.; LaFemina, J.; Jarnagin, W.R.; Berger, M.F.; Klimstra, D.; et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J. Am. Coll. Surg. 2015, 220, 845–854.e1. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Costa, I.G.; Hackert, T.; Strobel, O.; Lam, S.; Barth, T.F.; Schröppel, B.; Meining, A.; Büchler, M.W.; et al. Detection of Hot-Spot Mutations in Circulating Cell-Free DNA From Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2016, 151, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Mizuma, M.; Motoi, F.; Omori, Y.; Ishida, M.; Nakagawa, K.; Hayashi, H.; Morikawa, T.; Kamei, T.; Furukawa, T.; et al. GNAS mutation detection in circulating cell-free DNA is a specific predictor for intraductal papillary mucinous neoplasms of the pancreas, especially for intestinal subtype. Sci. Rep. 2020, 10, 17761. [Google Scholar] [CrossRef]
- Kim, S.G.; Wu, T.-T.; Lee, J.H.; Yun, Y.K.; Issa, J.-P.; Hamilton, S.R.; Rashid, A. Comparison of epigenetic and genetic alterations in mucinous cystic neoplasm and serous microcystic adenoma of pancreas. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2003, 16, 1086–1094. [Google Scholar] [CrossRef][Green Version]
- Zhu, C.; Guan, X.; Zhang, X.; Luan, X.; Song, Z.; Cheng, X.; Zhang, W.; Qin, J.-J. Targeting KRAS mutant cancers: From druggable therapy to drug resistance. Mol. Cancer 2022, 21, 159. [Google Scholar] [CrossRef]
- Mohan, S.; Ayub, M.; Rothwell, D.G.; Gulati, S.; Kilerci, B.; Hollebecque, A.; Sun Leong, H.; Smith, N.K.; Sahoo, S.; Descamps, T.; et al. Analysis of circulating cell-free DNA identifies KRAS copy number gain and mutation as a novel prognostic marker in Pancreatic cancer. Sci. Rep. 2019, 9, 11610. [Google Scholar] [CrossRef]
- Botrus, G.; Uson Junior, P.L.S.; Raman, P.; Kaufman, A.E.; Kosiorek, H.; Yin, J.; Fu, Y.; Majeed, U.; Sonbol, M.B.; Ahn, D.H.; et al. Circulating Cell-Free Tumor DNA in Advanced Pancreatic Adenocarcinoma Identifies Patients with Worse Overall Survival. Front. Oncol. 2021, 11, 794009. [Google Scholar] [CrossRef] [PubMed]
- Milin-Lazovic, J.; Madzarevic, P.; Rajovic, N.; Djordjevic, V.; Milic, N.; Pavlovic, S.; Veljkovic, N.; Milic, N.M.; Radenkovic, D. Meta-Analysis of Circulating Cell-Free DNA’s Role in the Prognosis of Pancreatic Cancer. Cancers 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Combs, S.E.; Springfeld, C.; Hartwig, W.; Hackert, T.; Büchler, M.W. Advanced-stage pancreatic cancer: Therapy options. Nat. Rev. Clin. Oncol. 2013, 10, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Herrera, R.; Ucar, A.; Aparo, S.; De Zarraga, F.; Asbun, H.; Jimenez, R.; Asbun, D.; Narayanan, G.; Joseph, S.; et al. Causes of Death Among Patients with Initially Inoperable Pancreas Cancer after Induction Chemotherapy and Ablative 5-fraction Stereotactic Magnetic Resonance Image Guided Adaptive Radiation Therapy. Adv. Radiat. Oncol. 2023, 8, 101084. [Google Scholar] [CrossRef]
- Huang, C.-J.; Huang, W.-Y.; Chen, C.-Y.; Chao, Y.-J.; Chiang, N.-J.; Shan, Y.-S. Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: A prospective cohort study. Ther. Adv. Med. Oncol. 2022, 14, 17588359221106558. [Google Scholar] [CrossRef] [PubMed]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef]
- Earl, J.; Garcia-Nieto, S.; Martinez-Avila, J.C.; Montans, J.; Sanjuanbenito, A.; Rodríguez-Garrote, M.; Lisa, E.; Mendía, E.; Lobo, E.; Malats, N.; et al. Circulating tumor cells (CTC) and KRAS mutant circulating free DNA (cfDNA) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer 2015, 15, 797. [Google Scholar] [CrossRef]
- Guo, S.; Shi, X.; Shen, J.; Gao, S.; Wang, H.; Shen, S.; Pan, Y.; Li, B.; Xu, X.; Shao, Z.; et al. Preoperative detection of KRAS G12D mutation in ctDNA is a powerful predictor for early recurrence of resectable PDAC patients. Br. J. Cancer 2020, 122, 857–867. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Q.; Li, X.; Su, W.; Li, G.; Ma, T.; Gao, S.; Lou, J.; Que, R.; Zheng, L.; et al. Monitoring Tumor Burden in Response to FOLFIRINOX Chemotherapy via Profiling Circulating Cell-Free DNA in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 196–203. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef]
- Driescher, C.; Fuchs, K.; Haeberle, L.; Goering, W.; Frohn, L.; Opitz, F.V.; Haeussinger, D.; Knoefel, W.T.; Keitel, V.; Esposito, I. Bile-Based Cell-Free DNA Analysis Is a Reliable Diagnostic Tool in Pancreatobiliary Cancer. Cancers 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Zhang, C.Z.; Sun, Z.H.; Wu, L.G.; Chen, Y.; Mo, Z.Q.; Mai, Q.C.; He, J.; Zhou, Z.X.; Shi, F.; et al. Cell-free DNA from bile outperformed plasma as a potential alternative to tissue biopsy in biliary tract cancer. ESMO Open 2021, 6, 100275. [Google Scholar] [CrossRef] [PubMed]
- Toll, J.; Li, Q.; Osipov, A.; Zhou, J.; Patel, R.; Pan, D.; Khanuja, J.; Bryant, M.; Tuli, R.; Hendifar, A.E. Identifying novel genetic alterations for risk stratification and prognostication in pancreatic cancer. J. Clin. Oncol. 2016, 34, e15754. [Google Scholar] [CrossRef]
- Molina, H.; Chan, M.M.; Lewandowski, R.J.; Gabr, A.; Riaz, A. Complications of Percutaneous Biliary Procedures. Semin. Intervent. Radiol. 2021, 38, 364–372. [Google Scholar] [CrossRef]
- Mizuide, M.; Ryozawa, S.; Fujita, A.; Ogawa, T.; Katsuda, H.; Suzuki, M.; Noguchi, T.; Tanisaka, Y. Complications of Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagnostics 2020, 10, 964. [Google Scholar] [CrossRef]
- Hart, P.A.; Topazian, M.; Raimondo, M.; Cruz-Monserrate, Z.; Fisher, W.E.; Lesinski, G.B.; Steen, H.; Conwell, D.L. Endoscopic Pancreas Fluid Collection: Methods and Relevance for Clinical Care and Translational Science. Am. J. Gastroenterol. 2016, 111, 1258–1266. [Google Scholar] [CrossRef]

| Parameters | PDAC Group | OPN Group | Control Group |
|---|---|---|---|
| (n = 95) | (n = 18) | (n = 38) | |
| Age, years 1 | 65 (41–88) | 58 (43–66) | 20 (19–22) |
| Sex, n (%): | |||
| male | 46/95 | 5/18 | 17/38 |
| female | 49/95 | 13/18 | 21/38 |
| OPN types: | |||
| mdIPMN, n | N/A | 6/18 | N/A |
| multIPMN, n | N/A | 1/18 | N/A |
| bdIPMN, n | N/A | 7/18 | N/A |
| adenoma, n | N/A | 1/18 | N/A |
| serous cystadenoma, n | N/A | 1/18 | N/A |
| SPPN, n | N/A | 1/18 | N/A |
| NET, n | N/A | 1/18 | N/A |
| Tumor localization: | |||
| head, n | 44/87 2 | N/A | N/A |
| body, n | 6/87 2 | N/A | N/A |
| tail, n | 11/87 2 | N/A | N/A |
| head + body, n | 10/87 2 | N/A | N/A |
| body + tail, n | 15/87 2 | N/A | N/A |
| head + body + tail, n | 1/87 2 | N/A | N/A |
| Tumor size: | |||
| >4 cm, n | 39/87 2 | N/A | N/A |
| 2–4 cm, n | 41/87 2 | N/A | N/A |
| <2 cm, n | 7/87 2 | N/A | N/A |
| Contact with | |||
| arteries/veins, n | 78/87 2 | N/A | N/A |
| Bile ducts invasion, n | 39/87 2 | N/A | N/A |
| Distant metastases, n | 43/87 2 | N/A | N/A |
| Serum CA 19-9, U/mL 3 | 36.5 [0.9; 883.3] | 3.9 [0.8; 7.9] | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, M.; Atayan, D.; Rakhmatullin, T.; Dakhtler, T.; Popov, P.; Kim, P.; Viborniy, M.; Gontareva, I.; Samokhodskaya, L.; Egorov, V. Cell-Free Tumor DNA Detection-Based Liquid Biopsy of Plasma and Bile in Patients with Various Pancreatic Neoplasms. Biomedicines 2024, 12, 220. https://doi.org/10.3390/biomedicines12010220
Jain M, Atayan D, Rakhmatullin T, Dakhtler T, Popov P, Kim P, Viborniy M, Gontareva I, Samokhodskaya L, Egorov V. Cell-Free Tumor DNA Detection-Based Liquid Biopsy of Plasma and Bile in Patients with Various Pancreatic Neoplasms. Biomedicines. 2024; 12(1):220. https://doi.org/10.3390/biomedicines12010220
Chicago/Turabian StyleJain, Mark, David Atayan, Tagir Rakhmatullin, Tatyana Dakhtler, Pavel Popov, Pavel Kim, Mikhail Viborniy, Iuliia Gontareva, Larisa Samokhodskaya, and Vyacheslav Egorov. 2024. "Cell-Free Tumor DNA Detection-Based Liquid Biopsy of Plasma and Bile in Patients with Various Pancreatic Neoplasms" Biomedicines 12, no. 1: 220. https://doi.org/10.3390/biomedicines12010220
APA StyleJain, M., Atayan, D., Rakhmatullin, T., Dakhtler, T., Popov, P., Kim, P., Viborniy, M., Gontareva, I., Samokhodskaya, L., & Egorov, V. (2024). Cell-Free Tumor DNA Detection-Based Liquid Biopsy of Plasma and Bile in Patients with Various Pancreatic Neoplasms. Biomedicines, 12(1), 220. https://doi.org/10.3390/biomedicines12010220






