Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Mass Spectrometric Analysis
2.3. Data Processing and Label-Free Quantification
2.4. Data Analysis
3. Results
3.1. In an Inflammatory Uveitis Model, Granulocytes Show Distinct Protein Changes Dependent on Treatment with IL8, LPS, or PMA
3.2. Granulocytes Show Distinct Functional Heterogeneity in Different Inflammatory Environments
3.2.1. Biological Functions in Granulocytes with Predicted Activation
3.2.2. Canonical Pathways in Granulocytes with Predicted Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forrester, J.V.; Xu, H. Good news-bad news: The Yin and Yang of immune privilege in the eye. Front. Immunol. 2012, 3, 338. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W.; Takeuchi, M.; Taylor, A.W. Immune privilege, T-cell tolerance, and tissue-restricted autoimmunity. Hum. Immunol. 1997, 52, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef] [PubMed]
- Gery, I.; Caspi, R.R. Tolerance Induction in Relation to the Eye. Front. Immunol. 2018, 9, 2304. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.C.; Raveney, B.J.; Copland, D.A.; Dick, A.D.; Nicholson, L.B. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 2008, 31, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pepple, K.L.; Wilson, L.; Van Gelder, R.N. Comparison of Aqueous and Vitreous Lymphocyte Populations from Two Rat Models of Experimental Uveitis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R.; Chan, C.C.; Fujino, Y.; Najafian, F.; Grover, S.; Hansen, C.T.; Wilder, R.L. Recruitment of antigen-nonspecific cells plays a pivotal role in the pathogenesis of a T cell-mediated organ-specific autoimmune disease, experimental autoimmune uveoretinitis. J. Neuroimmunol. 1993, 47, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Michau, T.M. Equine recurrent uveitis: New methods of management. Vet. Clin. North. Am. Equine Pract. 2004, 20, 417–427. [Google Scholar] [CrossRef]
- McMullen, R.J., Jr.; Fischer, B.M. Medical and Surgical Management of Equine Recurrent Uveitis. Vet. Clin. North. Am. Equine Pract. 2017, 33, 465–481. [Google Scholar] [CrossRef]
- Gerding, J.C.; Gilger, B.C. Prognosis and impact of equine recurrent uveitis. Equine Vet. J. 2016, 48, 290–298. [Google Scholar] [CrossRef]
- Deeg, C.A.; Thurau, S.R.; Gerhards, H.; Ehrenhofer, M.; Wildner, G.; Kaspers, B. Uveitis in horses induced by interphotoreceptor retinoid-binding protein is similar to the spontaneous disease. Eur. J. Immunol. 2002, 32, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Reese, S.; Gerhards, H.; Wildner, G.; Kaspers, B. The uveitogenic potential of retinal S-antigen in horses. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Pompetzki, D.; Raith, A.J.; Hauck, S.M.; Amann, B.; Suppmann, S.; Goebel, T.W.; Olazabal, U.; Gerhards, H.; Reese, S.; et al. Identification and functional validation of novel autoantigens in equine uveitis. Mol. Cell Proteom. 2006, 5, 1462–1470. [Google Scholar] [CrossRef]
- Deeg, C.A.; Raith, A.J.; Amann, B.; Crabb, J.W.; Thurau, S.R.; Hauck, S.M.; Ueffing, M.; Wildner, G.; Stangassinger, M. CRALBP is a highly prevalent autoantigen for human autoimmune uveitis. Clin. Dev. Immunol. 2007, 2007, 39245. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Hauck, S.M.; Amann, B.; Pompetzki, D.; Altmann, F.; Raith, A.; Schmalzl, T.; Stangassinger, M.; Ueffing, M. Equine recurrent uveitis—A spontaneous horse model of uveitis. Ophthalmic Res. 2008, 40, 151–153. [Google Scholar] [CrossRef]
- Malalana, F.; Stylianides, A.; McGowan, C. Equine recurrent uveitis: Human and equine perspectives. Vet. J. 2015, 206, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Horohov, D.W. The equine immune responses to infectious and allergic disease: A model for humans? Mol. Immunol. 2015, 66, 89–96. [Google Scholar] [CrossRef]
- Weigand, M.; Hauck, S.M.; Deeg, C.A.; Degroote, R.L. Deviant proteome profile of equine granulocytes associates to latent activation status in organ specific autoimmune disease. J. Proteom. 2020, 230, 103989. [Google Scholar] [CrossRef]
- Saldinger, L.K.; Nelson, S.G.; Bellone, R.R.; Lassaline, M.; Mack, M.; Walker, N.J.; Borjesson, D.L. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet. Ophthalmol. 2020, 23, 160–170. [Google Scholar] [CrossRef]
- Hoffmann, A.L.C.; Hauck, S.M.; Deeg, C.A.; Degroote, R.L. Pre-Activated Granulocytes from an Autoimmune Uveitis Model Show Divergent Pathway Activation Profiles upon IL8 Stimulation In Vitro. Int. J. Mol. Sci. 2022, 23, 9555. [Google Scholar] [CrossRef]
- Degroote, R.L.; Weigand, M.; Hauck, S.M.; Deeg, C.A. IL8 and PMA Trigger the Regulation of Different Biological Processes in Granulocyte Activation. Front. Immunol. 2019, 10, 3064. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.S.; Rizzo, L.V.; Agarwal, R.K.; Tarrant, T.K.; Chan, C.C.; Wiggert, B.; Caspi, R.R. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J. Immunol. 1997, 158, 5997–6005. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Zhang, M.; Vistica, B.P.; Chan, C.C.; Shen, D.F.; Wawrousek, E.F.; Gery, I. Induction of ocular inflammation by T-helper lymphocytes type 2. Investig. Ophthalmol. Vis. Sci. 2002, 43, 758–765. [Google Scholar] [PubMed]
- Su, S.B.; Grajewski, R.S.; Luger, D.; Agarwal, R.K.; Silver, P.B.; Tang, J.; Tuo, J.; Chan, C.C.; Caspi, R.R. Altered chemokine profile associated with exacerbated autoimmune pathology under conditions of genetic interferon-gamma deficiency. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4616–4625. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Kaspers, B.; Gerhards, H.; Thurau, S.R.; Wollanke, B.; Wildner, G. Immune responses to retinal autoantigens and peptides in equine recurrent uveitis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 393–398. [Google Scholar] [PubMed]
- French, S.W.; Mendoza, A.S.; Afifiyan, N.; Tillman, B.; Vitocruz, E.; French, B.A. The role of the IL-8 signaling pathway in the infiltration of granulocytes into the livers of patients with alcoholic hepatitis. Exp. Mol. Pathol. 2017, 103, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kim, J.H.; Lima, R.; Zbytnuik, L.D.; Petri, B.; Swanlund, N.; Ho, M.; Szeto, V.G.; Tak, T.; Koenderman, L.; et al. The Lung is a Host Defense Niche for Immediate Neutrophil-Mediated Vascular Protection. Sci. Immunol. 2017, 2, eaam8929. [Google Scholar] [CrossRef]
- Saito, T.; Takahashi, H.; Doken, H.; Koyama, H.; Aratani, Y. Phorbol myristate acetate induces neutrophil death through activation of p38 mitogen-activated protein kinase that requires endogenous reactive oxygen species other than HOCl. Biosci. Biotechnol. Biochem. 2005, 69, 2207–2212. [Google Scholar] [CrossRef][Green Version]
- Werry, H.; Gerhards, H. The surgical therapy of equine recurrent uveitis. Tierarztl. Prax. 1992, 20, 178–186. [Google Scholar]
- Baien, S.H.; Langer, M.N.; Heppelmann, M.; von Kockritz-Blickwede, M.; de Buhr, N. Comparison Between K3EDTA and Lithium Heparin as Anticoagulant to Isolate Bovine Granulocytes from Blood. Front. Immunol. 2018, 9, 1570. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Grosche, A.; Hauser, A.; Lepper, M.F.; Mayo, R.; von Toerne, C.; Merl-Pham, J.; Hauck, S.M. The Proteome of Native Adult Muller Glial Cells From Murine Retina. Mol. Cell Proteom. 2016, 15, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Kall, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Trevisan-Herraz, M.; Bonzon-Kulichenko, E.; Nunez, E.; Martinez-Acedo, P.; Perez-Hernandez, D.; Jorge, I.; Mesa, R.; Calvo, E.; Carrascal, M.; et al. General statistical framework for quantitative proteomics by stable isotope labeling. J. Proteome Res. 2014, 13, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- RCoreTeam R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 22 November 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Slowikowski, K. ggrepel: Automatically Position Non-Overlapping Text Labels with ’ggplot2’. 2023. Available online: https://CRAN.R-project.org/package=ggrepel (accessed on 22 November 2023).
- Degroote, R.L.; Hauck, S.M.; Kremmer, E.; Amann, B.; Ueffing, M.; Deeg, C.A. Altered expression of talin 1 in peripheral immune cells points to a significant role of the innate immune system in spontaneous autoimmune uveitis. J. Proteom. 2012, 75, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Degroote, R.L.; Hauck, S.M.; Treutlein, G.; Amann, B.; Frohlich, K.J.; Kremmer, E.; Merl, J.; Stangassinger, M.; Ueffing, M.; Deeg, C.A. Expression Changes and Novel Interaction Partners of Talin 1 in Effector Cells of Autoimmune Uveitis. J. Proteome Res. 2013, 12, 5812–5819. [Google Scholar] [CrossRef]
- Fingerhut, L.; Ohnesorge, B.; von Borstel, M.; Schumski, A.; Strutzberg-Minder, K.; Morgelin, M.; Deeg, C.A.; Haagsman, H.P.; Beineke, A.; von Kockritz-Blickwede, M.; et al. Neutrophil Extracellular Traps in the Pathogenesis of Equine Recurrent Uveitis (ERU). Cells 2019, 8, 1528. [Google Scholar] [CrossRef]
- Fingerhut, L.; Yucel, L.; Strutzberg-Minder, K.; von Kockritz-Blickwede, M.; Ohnesorge, B.; de Buhr, N. Ex Vivo and In Vitro Analysis Identify a Detrimental Impact of Neutrophil Extracellular Traps on Eye Structures in Equine Recurrent Uveitis. Front. Immunol. 2022, 13, 830871. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Coffer, P.J. Regulation of granulocyte apoptosis by phosphatidylinositol 3-kinase. Biochem. Soc. Trans. 2004, 32, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, C.; Masinovsky, B.; Dick, K.; Sowell, C.G.; Staunton, D.E. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J. Immunol. 2003, 170, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Blees, A.; Januliene, D.; Hofmann, T.; Koller, N.; Schmidt, C.; Trowitzsch, S.; Moeller, A.; Tampe, R. Structure of the human MHC-I peptide-loading complex. Nature 2017, 551, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khan, H.; Singh, T.G.; Grewal, A.K.; Najda, A.; Kawecka-Radomska, M.; Kamel, M.; Altyar, A.E.; Abdel-Daim, M.M. Pharmacological Modulation of Ubiquitin-Proteasome Pathways in Oncogenic Signaling. Int. J. Mol. Sci. 2021, 22, 11971. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Luan, L.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Boykin, O.A.; Sivanesam, K.; Schaedel, J.F.; Patil, T.K.; Wang, J.; et al. MyD88-dependent signaling drives toll-like receptor-induced trained immunity in macrophages. Front. Immunol. 2022, 13, 1044662. [Google Scholar] [CrossRef] [PubMed]
- Blunsom, N.J.; Cockcroft, S. CDP-Diacylglycerol Synthases (CDS): Gateway to Phosphatidylinositol and Cardiolipin Synthesis. Front. Cell Dev. Biol. 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef]
- Chen, R.; Wu, W.; Chen, S.Y.; Liu, Z.Z.; Wen, Z.P.; Yu, J.; Zhang, L.B.; Liu, Z.; Zhang, J.; Luo, P.; et al. A Pan-Cancer Analysis Reveals CLEC5A as a Biomarker for Cancer Immunity and Prognosis. Front. Immunol. 2022, 13, 831542. [Google Scholar] [CrossRef]
- Epps, S.J.; Coplin, N.; Luthert, P.J.; Dick, A.D.; Coupland, S.E.; Nicholson, L.B. Features of ectopic lymphoid-like structures in human uveitis. Exp. Eye Res. 2020, 191, 107901. [Google Scholar] [CrossRef]
- Martin-Martin, B.; Nabokina, S.M.; Blasi, J.; Lazo, P.A.; Mollinedo, F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 2000, 96, 2574–2583. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.C.; Copland, D.A.; Dick, A.D.; Nicholson, L.B. The dynamics of leukocyte infiltration in experimental autoimmune uveoretinitis. Prog. Retin. Eye Res. 2008, 27, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, C.L.; Pemberton, A.; Watson, E.D.; Miller, H.R. Neutrophil chemotaxis in the horse is not mediated by a complex of equine neutrophil elastase and equine alpha-1-proteinase inhibitor. Br. Vet. J. 1993, 149, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Brazil, T.J.; Dixon, P.M.; Haslett, C.; Murray, J.; McGorum, B.C. Constitutive apoptosis in equine peripheral blood neutrophils in vitro. Vet. J. 2014, 202, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir. Res. 2017, 18, 207. [Google Scholar] [CrossRef]
- Bright, L.A.; Dittmar, W.; Nanduri, B.; McCarthy, F.M.; Mujahid, N.; Costa, L.R.; Burgess, S.C.; Swiderski, C.E. Modeling the pasture-associated severe equine asthma bronchoalveolar lavage fluid proteome identifies molecular events mediating neutrophilic airway inflammation. Vet. Med. 2019, 10, 43–63. [Google Scholar] [CrossRef]
- Saraogi, I.; Akopian, D.; Shan, S.O. Regulation of cargo recognition, commitment, and unloading drives cotranslational protein targeting. J. Cell Biol. 2014, 205, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, M.K.; Miller, S.C.; Tikhonova, E.B.; Karamyshev, A.L. SRPassing Co-translational Targeting: The Role of the Signal Recognition Particle in Protein Targeting and mRNA Protection. Int. J. Mol. Sci. 2021, 22, 6284. [Google Scholar] [CrossRef] [PubMed]
- Derhaag, P.J.; de Waal, L.P.; Linssen, A.; Feltkamp, T.E. Acute anterior uveitis and HLA-B27 subtypes. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1137–1140. [Google Scholar] [PubMed]
- Wakefield, D.; Clarke, D.; McCluskey, P. Recent Developments in HLA B27 Anterior Uveitis. Front. Immunol. 2020, 11, 608134. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Colbert, J.D.; Merino, E.; Kriegsman, B.A.; Rock, K.L. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol. 2017, 35, 149–176. [Google Scholar] [CrossRef]
- Colbert, J.D.; Cruz, F.M.; Rock, K.L. Cross-presentation of exogenous antigens on MHC I molecules. Curr. Opin. Immunol. 2020, 64, 1–8. [Google Scholar] [CrossRef]
- Meaney, M.P.; Nieman, D.C.; Henson, D.A.; Jiang, Q.; Wang, F.Z. Measuring Granulocyte and Monocyte Phagocytosis and Oxidative Burst Activity in Human Blood. J. Vis. Exp. 2016, 115, e54264. [Google Scholar] [CrossRef]
- Czaja, A.J. Incorporating the Molecular Mimicry of Environmental Antigens into the Causality of Autoimmune Hepatitis. Dig. Dis. Sci. 2023, 68, 2824–2842. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Schwimmbeck, P.L.; Oldstone, M.B. Molecular mimicry between human leukocyte antigen B27 and Klebsiella. Consequences for spondyloarthropathies. Am. J. Med. 1988, 85, 51–53. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Lin, P.; Asquith, M. Does the Microbiome Cause B27-related Acute Anterior Uveitis? Ocul. Immunol. Inflamm. 2016, 24, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; Asquith, M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat. Rev. Rheumatol. 2018, 14, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Lin, P.; Bach, M.; Asquith, M.; Lee, A.Y.; Akileswaran, L.; Stauffer, P.; Davin, S.; Pan, Y.; Cambronne, E.D.; Dorris, M.; et al. HLA-B27 and human beta2-microglobulin affect the gut microbiota of transgenic rats. PLoS ONE 2014, 9, e105684. [Google Scholar] [CrossRef]
- Martin de Bustamante, M.; Gomez, D.; MacNicol, J.; Hamor, R.; Plummer, C. The Fecal Bacterial Microbiota in Horses with Equine Recurrent Uveitis. Animals 2021, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Barros-Becker, F.; Lam, P.Y.; Fisher, R.; Huttenlocher, A. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J. Cell Sci. 2017, 130, 3801–3808. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, S.; Muroi, Y.; Chiba, T.; Ohmura, R.; Yoneyama, M.; Magarisawa, M.; Dodo, K.; Terayama, N.; Sodeoka, M.; Aoyagi, R.; et al. Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation. Sci. Rep. 2017, 7, 16026. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef]
- Jakus, Z.; Simon, E.; Frommhold, D.; Sperandio, M.; Mocsai, A. Critical role of phospholipase Cgamma2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J. Exp. Med. 2009, 206, 577–593. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Hu, J. Study of the shared gene signatures of polyarticular juvenile idiopathic arthritis and autoimmune uveitis. Front. Immunol. 2023, 14, 1048598. [Google Scholar] [CrossRef] [PubMed]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Luger, D.; Silver, P.B.; Tang, J.; Cua, D.; Chen, Z.; Iwakura, Y.; Bowman, E.P.; Sgambellone, N.M.; Chan, C.C.; Caspi, R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 2008, 205, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.P.; Aarnio, M.C.; Davis, W.S.; Carmichael, K.P.; Vandenplas, M.L.; Lauderdale, J.D.; Moore, P.A. Characterization of cytokines associated with Th17 cells in the eyes of horses with recurrent uveitis. Vet. Ophthalmol. 2012, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, L.; Liu, L.; Yang, Y.; Huang, J.; Ji, D.; Zhou, Y.; Chen, Y.; Zhu, Z.; Sun, B. CD44/ERM/F-actin complex mediates targeted nuclear degranulation and excessive neutrophil extracellular trap formation during sepsis. J. Cell Mol. Med. 2022, 26, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.J.; Krautter, F.; Blacksell, I.A.; Wright, R.D.; Austin-Williams, S.N.; Voisin, M.B.; Hussain, M.T.; Law, H.L.; Niki, T.; Hirashima, M.; et al. Galectin-9 mediates neutrophil capture and adhesion in a CD44 and beta2 integrin-dependent manner. FASEB J. 2022, 36, e22065. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.W.; Zhu, Q.; Wang, S.; Ortiz, G.; Huckfeldt, R.M.; Chen, Y. Long-lived autoreactive memory CD4(+) T cells mediate the sustained retinopathy in chronic autoimmune uveitis. FASEB J. 2023, 37, e22855. [Google Scholar] [CrossRef]
- Fan, N.W.; Li, J.; Mittal, S.K.; Foulsham, W.; Elbasiony, E.; Huckfeldt, R.M.; Chauhan, S.K.; Chen, Y. Characterization of Clinical and Immune Responses in an Experimental Chronic Autoimmune Uveitis Model. Am. J. Pathol. 2021, 191, 425–437. [Google Scholar] [CrossRef]
- Xu, H.; Manivannan, A.; Liversidge, J.; Sharp, P.F.; Forrester, J.V.; Crane, I.J. Involvement of CD44 in leukocyte trafficking at the blood-retinal barrier. J. Leukoc. Biol. 2002, 72, 1133–1141. [Google Scholar] [CrossRef]
- Kuppner, M.C.; Liversidge, J.; McKillop-Smith, S.; Lumsden, L.; Forrester, J.V. Adhesion molecule expression in acute and fibrotic sympathetic ophthalmia. Curr. Eye Res. 1993, 12, 923–934. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
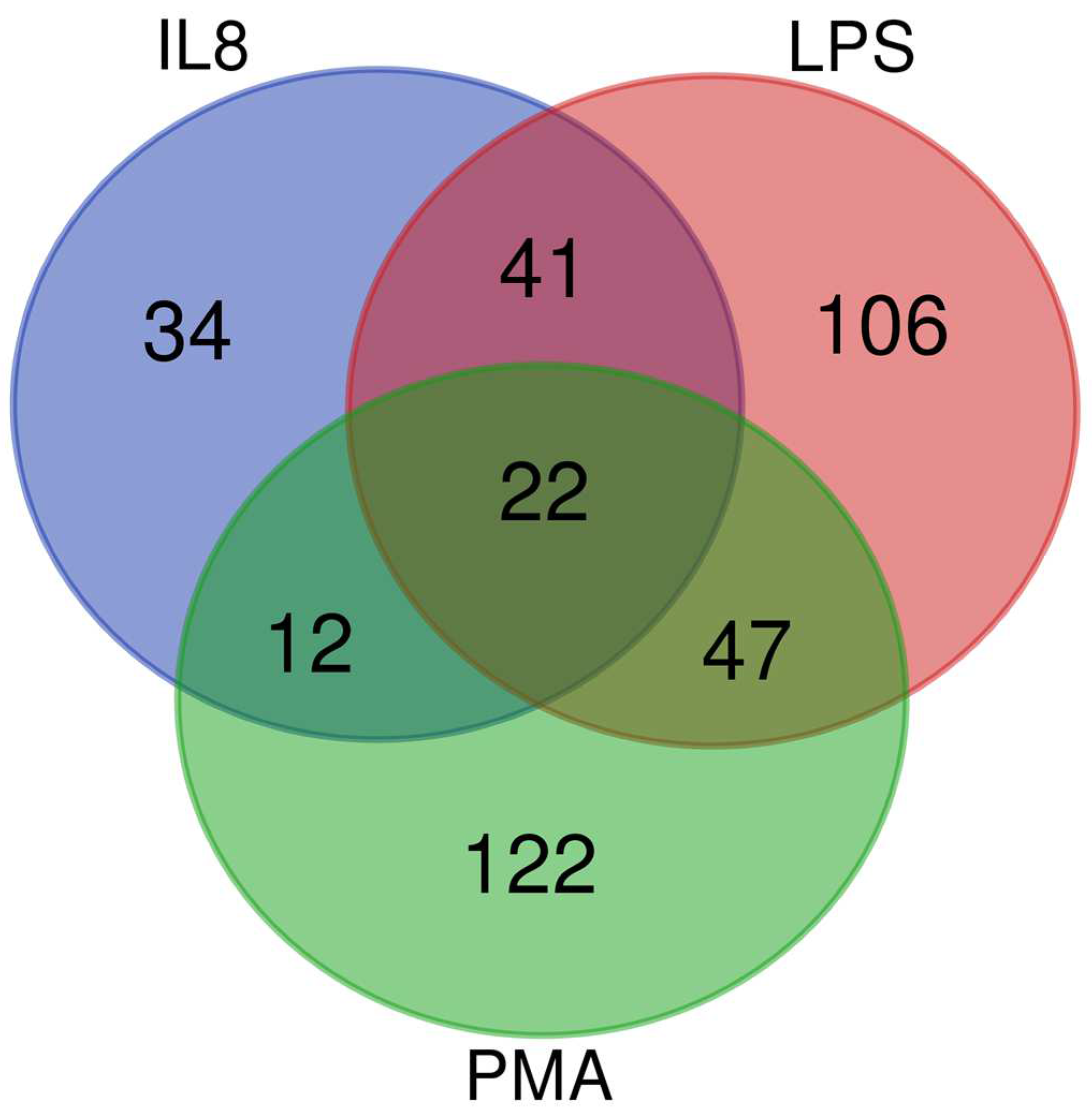
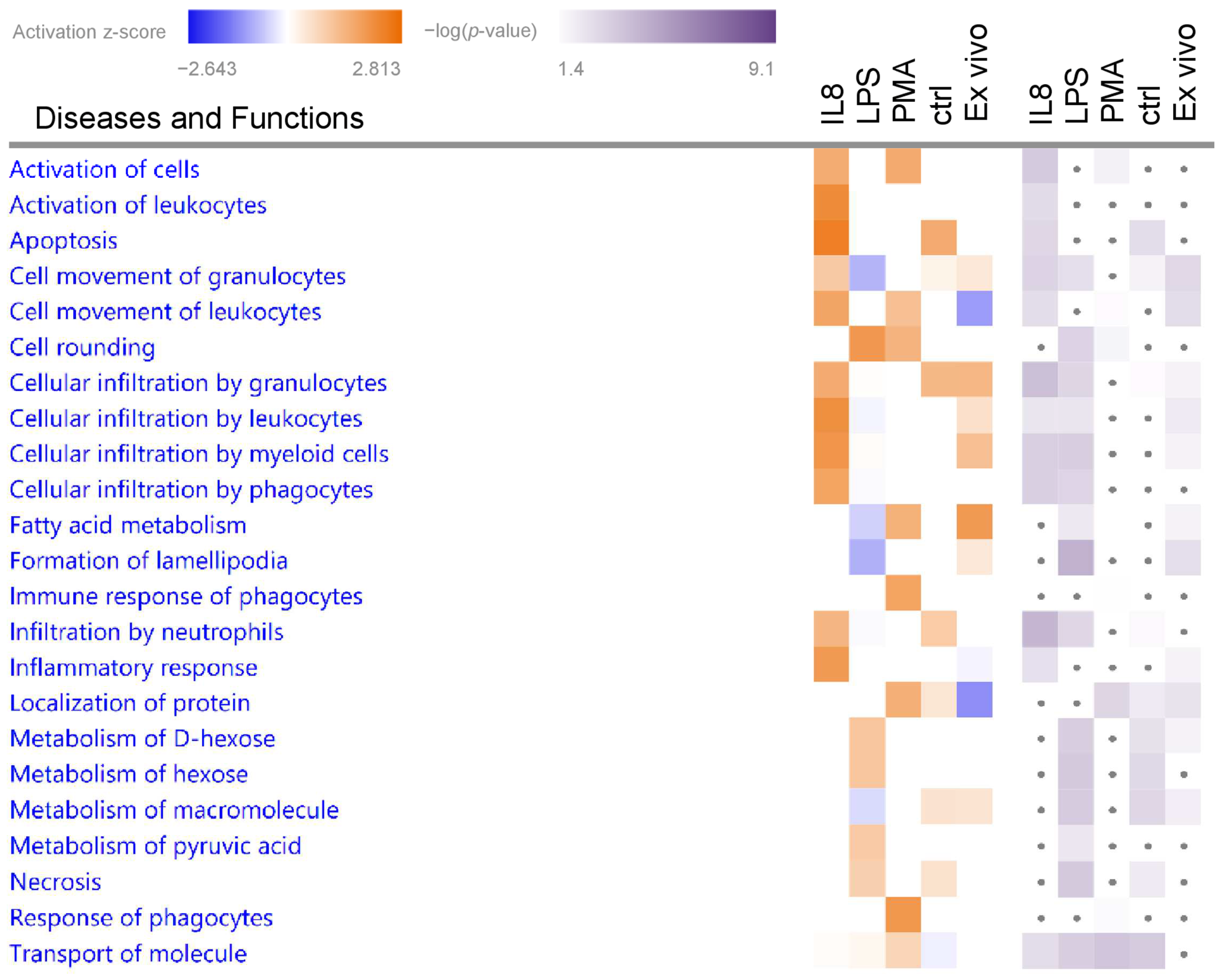

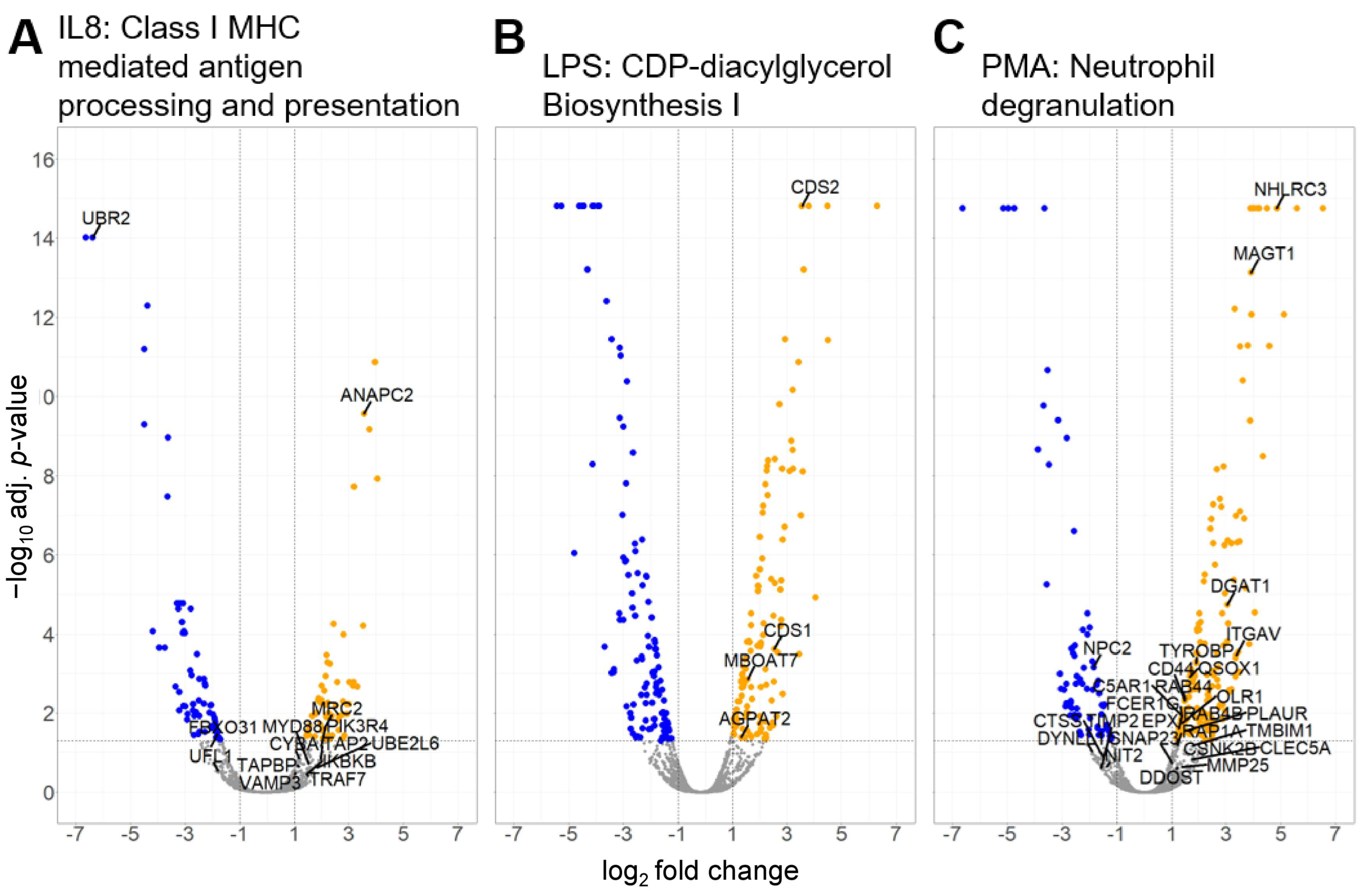

| Category 1 | Diseases and Biological Functions 2 | z-Score 3 | p-Value 4 | Molecules 5 |
|---|---|---|---|---|
| IL8 | ||||
| Cell-To-Cell Signaling and Interaction | Activation of cells | 1.56 | 3.56 | AIP AMFR APOE BPI BSG CCN2 CD300LF CD37 CD81 CD84 CXCR2 ENTPD1 EPX FCER1G GBA1 GP5 HSPH1 HVCN1 IKBKB ITGB3 JAK3 LTB4R MGST1 MYD88 NPC2 NUCB2 PLAT PLSCR1 PPIF RIGI SEMA4A SLC11A1 SPTB SPTLC2 THBS1 TNFSF14 VWF |
| Cell-To-Cell Signaling and Interaction, Hematological System Development and Function, Immune Cell Trafficking, Inflammatory Response | Activation of leukocytes | 2.17 | 2.86 | AIP APOE BPI BSG CD300LF CD37 CD81 CD84 CXCR2 EPX FCER1G GBA1 HSPH1 HVCN1 IKBKB JAK3 LTB4R MGST1 MYD88 PLAT PLSCR1 RIGI SEMA4A SLC11A1 SPTLC2 THBS1 TNFSF14 |
| Inflammatory Response | Inflammatory response | 1.90 | 2.65 | ALOX5AP APOE BPI BSG CD37 CD84 CXCR2 CYBA DDT EPX FCER1G HSPB1 IKBKB IRF3 ITGB3 LTB4R MSRA MYD88 NLRC4 PLAT SBDS SLC11A1 TBXAS1 THBS1 TNFSF14 TUBA4A TUBB1 TUBB2A VWF |
| Cell Death and Survival | Apoptosis | 2.41 | 3.00 | ACTC1 AIP ALDH3A1 AMFR APOE ARHGAP18 ASS1 BPI BSG CCDC12 CCDC47 CCN2 CIAPIN1 CKAP5 CKB COX6B1 CSTA CXCR2 CYBA DDX5 DENR EIF2B5 EIF4E ENTPD1 FBXO31 FCER1G FHL1 GBA1 GCLC GPX7 HK2 HNRNPH1 HSPB1 HSPH1 IKBKB ILKAP IRF3 ITGB3 ITM2B JAK3 KDELR1 LIMS1 MBOAT7 MRC2 MYD88 NLRC4 OAS1 PARVB PLAT PLSCR1 PPIF PPP1R2 PRDX4 PRKAR2B RABL6 RBM3 RIGI SAP18 SBDS SEMA4A SEPTIN2 SEPTIN9 SH3GLB1 SHROOM2 SNRPG SPTLC2 THBS1 TMBIM6 TNFSF14 TRAF7 TSPO TTC39C UBR2 UFL1 VPS41 VTI1A VWF |
| Cellular Movement | Cellular infiltration by myeloid cells | 1.99 | 3.34 | ALOX5AP AMFR APOE BSG CCN2 CD300LF CXCR2 FCER1G GBA1 HSPB1 IKBKB IRF3 ITGB3 JAK3 LTB4R MYD88 PLAT VWF |
| Cellular Movement, Hematological System Development and Function, Immune Cell Trafficking | Cellular infiltration by leukocytes | 2.09 | 2.43 | ALOX5AP AMFR APOE BSG CCN2 CD300LF CXCR2 FCER1G GBA1 HSPB1 IKBKB IRF3 ITGB3 JAK3 LTB4R MYD88 PLAT SPTLC2 THBS1 VWF |
| Cellular infiltration by phagocytes | 1.74 | 3.34 | ALOX5AP APOE BSG CCN2 CD300LF CXCR2 FCER1G GBA1 HSPB1 IKBKB IRF3 ITGB3 JAK3 LTB4R MYD88 PLAT VWF | |
| Cell movement of leukocytes | 1.70 | 2.74 | ALOX5AP AMFR APOE ARHGAP18 BSG CCN2 CD300LF CD37 CD81 COMMD8 CXCR2 DDT DPYSL2 EIF4E FCER1G GBA1 HSPB1 IKBKB IRF3 ITGB3 JAK3 LTB4R MYD88 NLRC4 PLAT RIGI SBDS SEMA4A SPTLC2 THBS1 TNFSF14 VWF | |
| Cellular infiltration by granulocytes | 1.57 | 3.94 | ALOX5AP AMFR APOE CCN2 CD300LF CXCR2 FCER1G HSPB1 IKBKB IRF3 JAK3 LTB4R MYD88 PLAT VWF | |
| LPS | ||||
| Cell morphology | Cell rounding | 1.95 | 3.23 | ITGAV ITGB8 NEDD9 PARVB PIP5K1A RAF1 SOD2 |
| PMA | ||||
| Cell-To-Cell Signaling and Interaction, Inflammatory Response | Response of phagocytes | 1.96 | 1.60 | C5AR1 CD44 CLEC5A FCER1G G6PC3 ITGB3 MAP4K1 PLAUR PRKAA1 RIGI TAPBP TYROBP |
| Immune response of phagocytes | 1.74 | 1.52 | CD44 CLEC5A FCER1G G6PC3 ITGB3 MAP4K1 PLAUR PRKAA1 RIGI TAPBP TYROBP | |
| Cell-To-Cell Signaling and Interaction | Activation of cells | 1.59 | 2.04 | AMFR ANK3 APOE C1GALT1C1 C5AR1 CCN2 CD300LF CD44 CD81 CD84 CLCN7 CLEC5A CTSS DDOST DGAT1 EPX FCER1G FERMT2 FGG IKBKG ITGAV ITGB3 JAK3 KRT8 LSP1 MAGT1 MAP4K1 NAMPT NPC2 NUCB2 PLAUR PRKAA1 RIGI SLC11A1 SNAP23 SPTB TIMP2 TYROBP VWF |
| Canonical Pathways 1 | z-Score 2 | p-Value 3 | Molecules 4 |
|---|---|---|---|
| IL8 | |||
| SRP-dependent cotranslational protein targeting to membrane | 2.24 | 1.57 | RPL18 RPL19 SEC11A SEC61B SPCS2 |
| Class I MHC-mediated antigen processing and presentation | 2.14 | 3.07 | ANAPC2 CYBA FBXO31 IKBKB MRC2 MYD88 PIK3R4 TAP2 TAPBP TRAF7 UBE2L6 UBR2 UFL1 VAMP3 |
| TEC Kinase Signaling | 2.00 | 1.66 | ACTC1 FCER1G GNG10 ITGB3 ITGB8 JAK3 PIK3R4 |
| LPS | |||
| SRP-dependent cotranslational protein targeting to membrane | 2.31 | 5.58 | RPL13 RPL15 RPL26 RPL27A RPL35 RPL7A RPLP1 RPS13 RPS26 SEC11A SEC61A1 SPCS2 |
| CDP-diacylglycerol Biosynthesis I | 2.00 | 2.76 | AGPAT2 CDS1 CDS2 MBOAT7 |
| Phosphatidylglycerol Biosynthesis II (Non-plastidic) | 2.00 | 2.63 | AGPAT2 CDS1 CDS2 MBOAT7 |
| PMA | |||
| Neutrophil degranulation | 3.53 | 7.61 | ALDH3B1 C5AR1 CD44 CHIT1 CLEC5A CSNK2B CTSS DDOST DGAT1 DYNLL1 EPX FCER1G ITGAV MAGT1 MMP25 NHLRC3 NIT2 NPC2 OLR1 PLAUR QSOX1 RAB44 RAB4B RAP1A SLC11A1 SNAP23 TIMP2 TMBIM1 TYROBP |
| SRP-dependent cotranslational protein targeting to membrane | 2.12 | 2.80 | DDOST RPL19 RPL22 RPL9 RPLP0 RPS10 RPS9 SEC11A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degroote, R.L.; Schmalen, A.; Hauck, S.M.; Deeg, C.A. Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis. Biomedicines 2024, 12, 19. https://doi.org/10.3390/biomedicines12010019
Degroote RL, Schmalen A, Hauck SM, Deeg CA. Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis. Biomedicines. 2024; 12(1):19. https://doi.org/10.3390/biomedicines12010019
Chicago/Turabian StyleDegroote, Roxane L., Adrian Schmalen, Stefanie M. Hauck, and Cornelia A. Deeg. 2024. "Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis" Biomedicines 12, no. 1: 19. https://doi.org/10.3390/biomedicines12010019
APA StyleDegroote, R. L., Schmalen, A., Hauck, S. M., & Deeg, C. A. (2024). Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis. Biomedicines, 12(1), 19. https://doi.org/10.3390/biomedicines12010019







