Association of STAT4 Gene Polymorphisms (rs10181656, rs7574865, rs7601754, rs10168266) and Serum STAT4 Levels in Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Methods
2.1. DNA Extraction and Genotyping
2.2. ELISA
2.3. Statistical Analysis
3. Results
3.1. Frequencies of STAT4 (rs10181656, rs7574865, rs7601754, and rs10168266) Genotypes and Alleles in Patients with AMD and Control Group
3.2. STAT4 (rs10181656, rs7574865, rs7601754, and rs10168266) Genotypes and Allele Associations with Early and Exudative AMD
3.3. STAT4 (rs10181656, rs7574865, rs7601754, and rs10168266) Genotypes and Allele Associations with Early and Exudative AMD by Gender
3.4. Frequencies of STAT4 (rs10181656, rs7574865, rs7601754, and rs10168266) Genotypes and Alleles in Patients with AMD and in the Control Group by Age
3.5. STAT4 (rs10181656, rs7574865, rs7601754 and rs10168266) Genotype and Allele Associations with Early and Exudative AMD by Age
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Park, D.H.; Jeon, I.C. Medication Trends for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 11837. [Google Scholar] [CrossRef] [PubMed]
- Schnabolk, G. Systemic Inflammatory Disease and AMD Comorbidity. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1185, pp. 27–31. [Google Scholar] [CrossRef]
- Fan, Q.; Maranville, J.C.; Fritsche, L.; Sim, X.; Cheung, C.M.G.; Chen, L.J.; Gorski, M.; Yamashiro, K.; Ahn, J.; Laude, A.; et al. HDL-cholesterol levels and risk of age-related macular degeneration: A multiethnic genetic study using Mendelian randomization. Int. J. Epidemiol. 2017, 46, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Tie, L.J.; Wu, S.S.; Lv, P.L.; Huang, H.W.; Wang, W.Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, S.; Nakayama, H.; Woda, C.B.; Flynn, E.A.; Briscoe, D.M. DEPTOR regulates vascular endothelial cell activation and proinflammatory and angiogenic responses. Blood 2013, 122, 1833–1842. [Google Scholar] [CrossRef]
- Qing, M.; Schumacher, K.; Heise, R.; Wöltje, M.; Vazquez-Jimenez, J.F.; Richter, T.; Arranda-Carrero, M.; Hess, J.; von Bernuth, G.; Seghaye, M.-C. Intramyocardial synthesis of pro- and anti-inflammatory cytokines in infants with congenital cardiac defects. J. Am. Coll. Cardiol. 2003, 41, 2266–2274. [Google Scholar] [CrossRef]
- Torpey, N.; Maher, S.E.; Bothwell, A.L.; Pober, J.S. Interferon alpha but not interleukin 12 activates STAT4 signalling in human vascular endothelial cells. J. Biol. Chem. 2004, 279, 26789–26796. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, H.-F.; Ye, D.-Q. Therapeutic potential of STAT4 in autoimmunity. Expert Opin. Ther. Targets 2014, 18, 945–960. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. Signal Transducers and Activators of Transcription (STATs); Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Vidangos, N.; Maris, A.E.; Young, A.; Hong, E.; Pelton, J.G.; Batchelor, J.D.; Wemmer, D.E. Structure, function, and tethering of DNA-binding domains in σ54 transcriptional activators. Biopolymers 2013, 99, 1082. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, H.; Peng, J.; Zhou, B.; Hou, J.; Jiang, D. STAT4: An immunoregulator contributing to diverse human diseases. Int. J. Biol. Sci. 2020, 16, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya-Bahrami, P.; Moriarty, A.K.; de Melo, P.; Keeter, W.C.; Alakhras, N.S.; Nelson, A.S.; Hoover, M.; Barrios, M.S.; Nadler, J.L.; Serezani, C.H.; et al. STAT4 is expressed in neutrophils and promotes antimicrobial immunity. JCI Insight 2021, 6, e141326. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tong, R.; Xu, J.; Tian, Y.; Pan, J.; Wang, N.; Chen, H.; Peng, Y.; Fei, S.; Ling, W.; et al. Association between STAT4 gene polymorphism and type 2 diabetes risk in Chinese Han population. BMC Med. Genom. 2021, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tong, R.; Xu, J.; Tian, Y.; Pan, J.; Wang, N.; Chen, H.; Peng, Y.; Fei, S.; Ling, W.; et al. STAT4 sequence variant and elevated gene expression are associated with type 1 diabetes in Polish children. Cent. Eur. J. Immunol. 2020, 45, 22–28. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Q.; Chen, H.; Lian, Z.; Liu, J.; Feng, H.; Miao, X.; Du, Q.; Zhou, H. STAT4 Polymorphisms are Associated with Neuromyelitis Optica Spectrum Disorders. Neuromol. Med. 2017, 19, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yin, J.; Huang, R.; Petersen, F.; Yu, X. Meta-analysis reveals an association of STAT4 polymorphisms with systemic autoimmune disorders and anti-dsDNA antibody. Hum. Immunol. 2013, 74, 986–992. [Google Scholar] [CrossRef]
- Ghanavati, F.; Nezhad, S.R.K.; Hajjari, M.R.; Akhoond, M.R. Association of Signal Transducer and Activator of Transcription 4 rs10181656 Polymorphism with Rheumatoid Arthritis and Systemic Sclerosis in Khuzestan Province in Southwestern Iran. Arch. Rheumatol. 2019, 34, 434–442. [Google Scholar] [CrossRef]
- Hellquist, A.; Sandling, J.K.; Zucchelli, M.; Koskenmies, S.; Julkunen, H.; D'Amato, M.; Garnier, S.; Syvänen, A.-C.; Kere, J. Variation in STAT4 is associated with systemic lupus erythematosus in a Finnish family cohort. Ann. Rheum. Dis. 2010, 69, 883–886. [Google Scholar] [CrossRef]
- Beltrán Ramírez, O.; Mendoza Rincón, J.F.; Barbosa Cobos, R.E.; Alemán Ávila, I.; Ramírez Bello, J. STAT4 confers risk for rheumatoid arthritis and systemic lupus erythematosus in Mexican patients. Immunol. Lett. 2016, 175, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Myrthianou, E.; Zervou, M.; Budu-Aggrey, A.; Eliopoulos, E.; Kardassis, D.; Boumpas, D.; Kougkas, N.; Barton, A.; Sidiropoulos, P.; Goulielmos, G. Investigation of the genetic overlap between rheumatoid arthritis and psoriatic arthritis in a Greek population. Scand. J. Rheumatol. 2016, 46, 180–186. [Google Scholar] [CrossRef]
- Yan, N.; Meng, S.; Zhou, J.; Xu, J.; Muhali, F.S.; Jiang, W.; Shi, L.; Shi, X.; Zhang, J. Association between STAT4 Gene Polymorphisms and Autoimmune Thyroid Diseases in a Chinese Population. Int. J. Mol. Sci. 2014, 15, 12280–12293. [Google Scholar] [CrossRef] [PubMed]
- Gu, E.; Lu, J.; Xing, D.; Chen, X.; Xie, H.; Liang, J.; Li, L. Rs7574865 polymorphism in signal transducers and activators of transcription 4 gene and rheumatoid arthritis: An updated meta-analysis of 28 case-control comparisons. Int. J. Rheum. Dis. 2015, 18, 3–16. [Google Scholar] [CrossRef]
- Liang, Y.L.; Wu, H.; Shen, X.; Li, P.Q.; Yang, X.Q.; Liang, L.; Tian, W.H.; Zhang, L.F.; Xie, X.D. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: A meta-analysis. Mol. Biol. Rep. 2012, 39, 8873–8882. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Feng, J.-B.; Pan, H.-F.; Qiu, L.-X.; Li, L.-H.; Zhang, N.; Ye, D.-Q. A meta-analysis of the association of STAT4 polymorphism with systemic lupus erythematosus. Mod. Rheumatol. 2014, 20, 257–262. [Google Scholar] [CrossRef]
- Chai, H.C.; Chua, K.H.; Lim, S.K.; Phipps, M.E. Insight into gene polymorphisms involved in toll-like receptor/interferon signalling pathways for systemic lupus erythematosus in South East Asia. J. Immunol. Res. 2014, 2014, 529167. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Zhou, B.; Yin, J.; Cao, G.; Hou, J.; Jiang, D. The effects of the interactions of STAT4 rs7574865 with HBV mutations on the risk of hepatocellular carcinoma. Mol. Carcinog. 2022, 61, 933–940. [Google Scholar] [CrossRef]
- Shi, H.; He, H.; Ojha, S.C.; Sun, C.; Fu, J.; Yan, M.; Deng, C.; Sheng, Y.J. Association of STAT3 and STAT4 polymorphisms with susceptibility to chronic hepatitis B virus infection and risk of hepatocellular carcinoma: A meta-analysis. Biosci. Rep. 2019, 39, 20190783. [Google Scholar] [CrossRef]
- Gao, W.; Dong, X.; Yang, Z.; Mao, G.; Xing, W. Association between rs7574865 polymorphism in STAT4 gene and rheumatoid arthritis: An updated meta-analysis. Eur. J. Intern. Med. 2020, 71, 101–103. [Google Scholar] [CrossRef]
- Wang, J.M.; Xu, W.D.; Huang, A.F. Association of STAT4 Gene Rs7574865, Rs10168266 Polymorphisms and Systemic Lupus Erythematosus Susceptibility: A Meta-analysis. Immunol. Investig. 2020, 50, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Abdelmajed, S.S.; El-Dessouky, M.A.; SalahElDin, D.S.; Hassan, N.A.M.; Zaki, M.E.; Ismail, S. Assessing the association of rs7574865 STAT4 gene variant and type 1 diabetes mellitus among Egyptian patients. J. Genet. Eng. Biotechnol. 2021, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimiyan, H.; Rezaei, R.; Mostafaei, S.; Aslani, S.; Goulielmos, G.N.; Jamshidi, A.; Mahmoudi, M. Association study between STAT4 polymorphisms and susceptibility to systemic lupus erythematosus disease: A systematic review and meta-analysis. Meta Gene 2018, 16, 241–247. [Google Scholar] [CrossRef]
- Kawasaki, A.; Ito, I.; Hikami, K.; Ohashi, J.; Hayashi, T.; Goto, D.; Matsumoto, I.; Ito, S.; Tsutsumi, A.; Koga, M.; et al. Role of STAT4 polymorphisms in systemic lupus erythematosus in a Japanese population: A case-control association study of the STAT1-STAT4 region. Arthritis Res. Ther. 2008, 10, R113. [Google Scholar] [CrossRef]
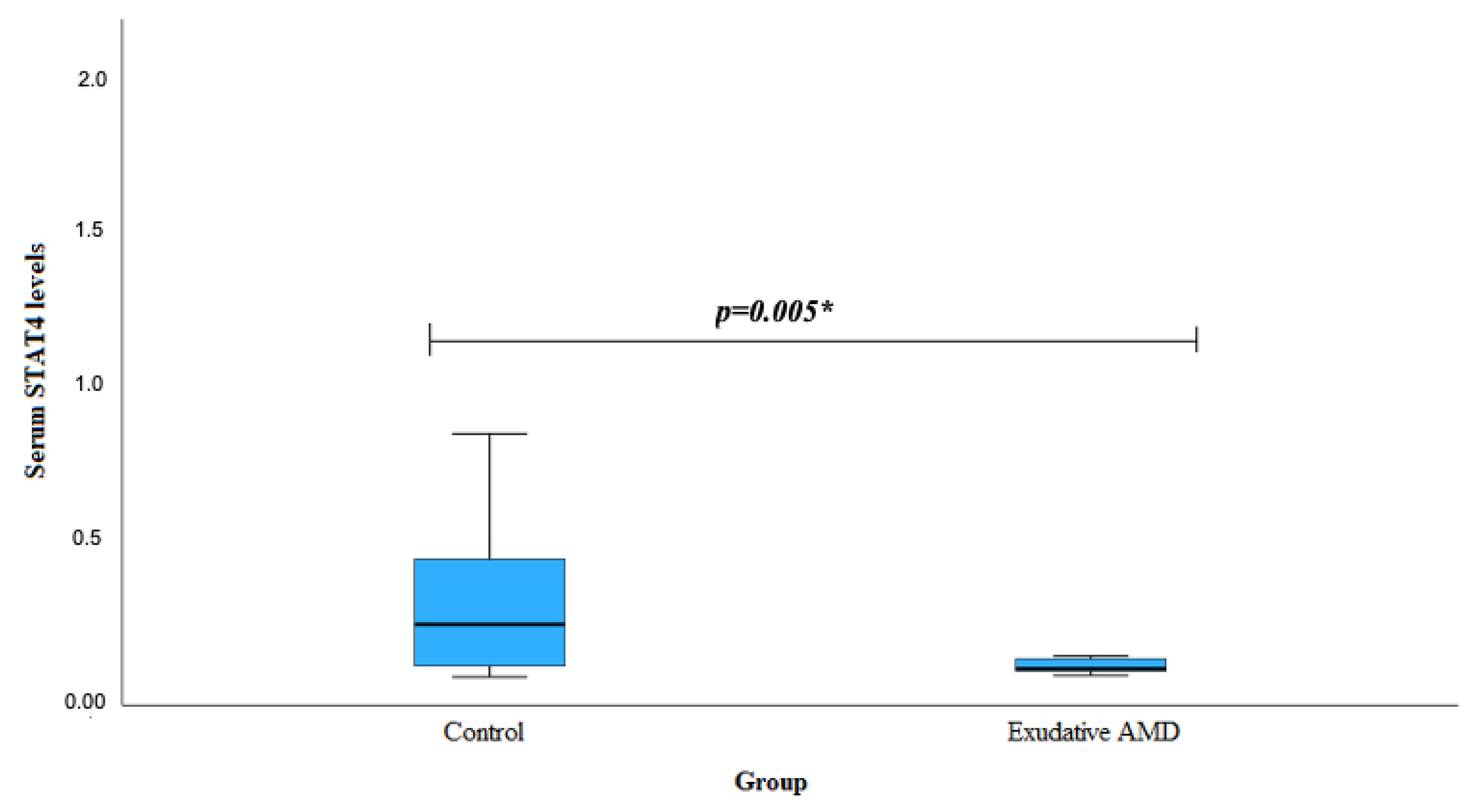
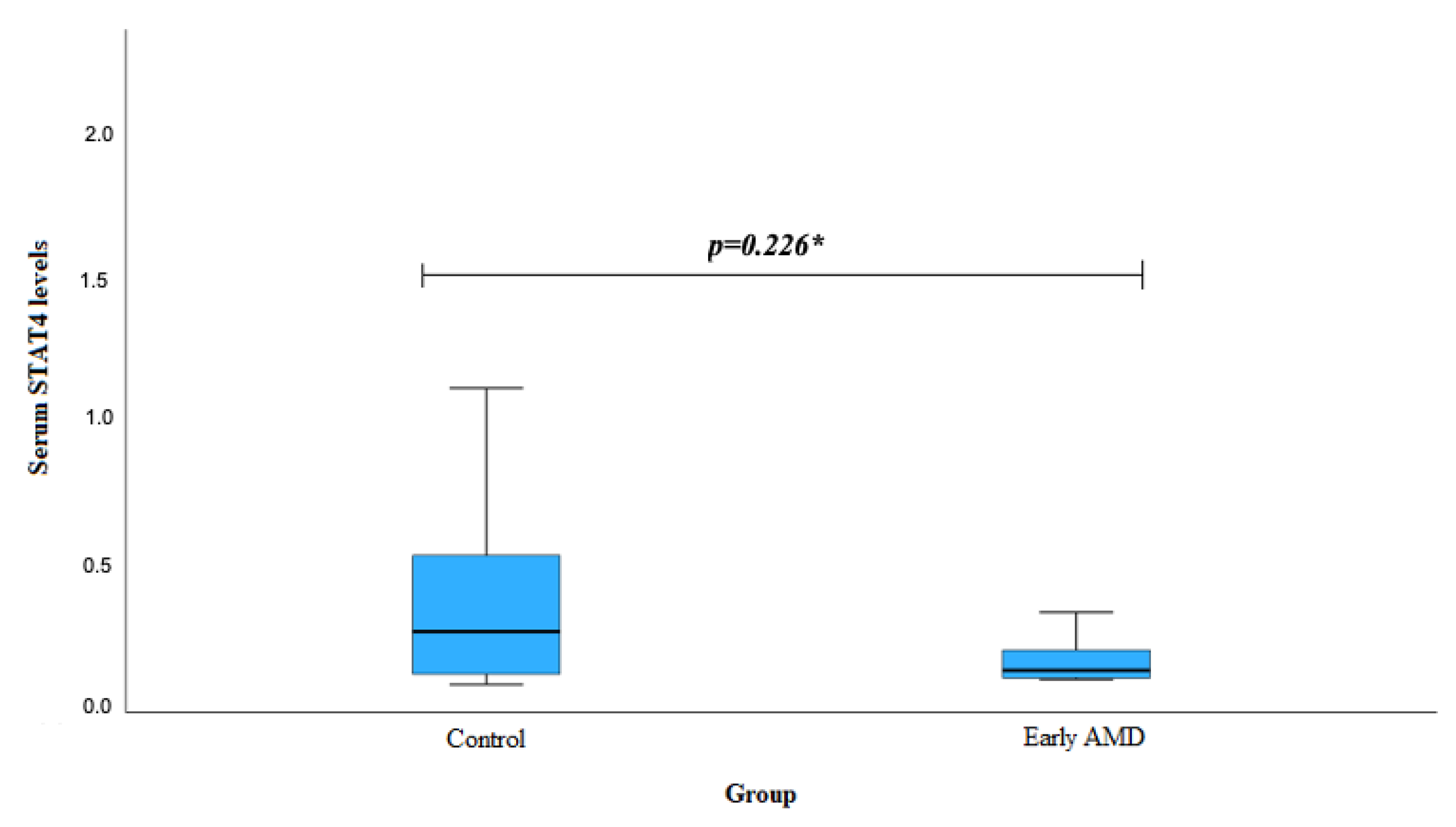
| Characteristics | Group | p-Value | |||
|---|---|---|---|---|---|
| Early AMD n = 150 | Exudative AMD n = 150 | Control n = 200 | |||
| Gender | Women, N (%) | 75 (50) | 75 (50) | 100 (50) | 1 * 1 ** |
| Men, N (%) | 75 (50) | 75 (50) | 100 (50) | ||
| Interquartile range (IQR) | 71 (11) | 72.5 (11) | 71 (4) | 0.726 * 0.152 ** | |
| SNP | Genotype/Allele | Group | p-Value | |
|---|---|---|---|---|
| Control, N (%) | Early AMD, N (%) | |||
| STAT4 rs10181656 | CC | 118 (59.0) | 90 (60.0) | 0.672 |
| CG | 65 (32.5) | 51 (34.0) | ||
| GG | 17 (8.5) | 9 (6.0) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| C | 301 (75.25) | 231 (77.0) | ||
| G | 99 (24.75) | 69 (23.0) | 0.592 | |
| STAT4 rs7574865 | GG | 118 (59.0) | 92 (61.3) | 0.670 |
| GT | 65 (32.5) | 49 32.7) | ||
| TT | 17 (8.5) | 9 (6.0) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| G | 301 (75.25) | 233 (77.67) | ||
| T | 99 (24.75) | 67 (22.33) | 0.457 | |
| STAT4 rs7601754 | AA | 150 (75.0) | 113 (75.3) | 0.902 |
| GA | 46 (23.0) | 33 (22.0) | ||
| GG | 4 (2.0) | 4 (2.7) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| A | 346 (86.5) | 259 (86.34) | ||
| G | 54 (13.5) | 41 (13.66) | 0.949 | |
| STAT4 rs10168266 | CC | 133 (66.5) | 100 (66.7) | 0.852 |
| CT | 58 (29.0) | 45 (30.0) | ||
| TT | 9 (4.5) | 5 (3.3) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| C | 324 (81.0) | 245 (81.67) | ||
| T | 76 (19.0) | 55 (18.33) | 0.823 | |
| SNP | Genotype/Allele | Group | p-Value | |
|---|---|---|---|---|
| Control, N (%) | Exudative AMD, N (%) | |||
| STAT4 rs10181656 | CC | 118 (59.0) | 80 (53.3) | 0.568 |
| CG | 65 (32.5) | 56 (37.3) | ||
| GG | 17 (8.5) | 14 (9.3) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| C | 301 (75.25) | 216 (72.0) | ||
| G | 99 (24.75) | 84 (28.0) | 0.333 | |
| STAT4 rs7574865 | GG | 118 (59.0) | 80 (53.3) | 0.568 |
| GT | 65 (32.5) | 56 (37.3) | ||
| TT | 17 (8.5) | 14 (9.3) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| G | 301 (75.25) | 216 (72.0) | ||
| T | 99 (24.75) | 84 (28.0) | 0.333 | |
| STAT4 rs7601754 | AA | 150 (75.0) | 111 (74.0) | 0.529 |
| GA | 46 (23.0) | 38 (25.3) | ||
| GG | 4 (2.0) | 1 (0.7) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| A | 346 (86.5) | 260 (86.67) | ||
| G | 54 (13.5) | 40 (13.33) | 0.949 | |
| STAT4 rs10168266 | CC | 133 (66.5) | 98 (65.3) | 0.674 |
| CT | 58 (29.0) | 42 (28.0) | ||
| TT | 9 (4.5) | 10 (6.7) | ||
| Total: | 200 | 150 | ||
| Allele: | ||||
| C | 324 (81.0) | 238 (79.34) | ||
| T | 76 (19.0) | 62 (20.96) | 0.583 | |
| Model | Genotype/Allele | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 1.029 (0.651–1.626) 0.694 (0.296–1.629) | 0.904 0.402 | 481.227 |
| Dominant | CG+GG vs. CC | 0.959 (0.623–1.477) | 0.850 | 480.000 |
| Recessive | GG vs. CC+CG | 0.687 (0.297–1.587) | 0.380 | 479.241 |
| Overdominant | CG vs. CC+GG | 1.070 (0.683–1.676) | 0.768 | 479.949 |
| Additive | C | 0.915 (0.653–1.283) | 0.608 | 479.771 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 0.967 (0.610–1.532) 0.679 (0.289–1.593) | 0.886 0.374 | 481.221 |
| Dominant | GT+TT vs. GG | 0.907 (0.588–1.399) | 0.659 | 479.841 |
| Recessive | TT vs. GG+GT | 0.687 (0.297–1.587) | 0.380 | 479.241 |
| Overdominant | GT vs. GG+TT | 1.008 (0.641–1.583) | 0.974 | 480.035 |
| Additive | G | 0.885 (0.631–1.241) | 0.478 | 479.530 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 0.952 (0.572–1.585) 1.327 (0.325–5.422) | 0.851 0.693 | 481.831 |
| Dominant | GA+GG vs. AA | 0.982 (0.602–1.604) | 0.943 | 480.031 |
| Recessive | GG vs. AA+GA | 1.342 (0.330–5.457) | 0.681 | 479.867 |
| Overdominant | GA vs. AA+GG | 0.944 (0.568–1.569) | 0.825 | 479.987 |
| Additive | A | 1.014 (0.660–1.556) | 0.950 | 480.032 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 1.032 (0.646–1.648) 0.739 (0.240–2.273) | 0.895 0.598 | 481.709 |
| Dominant | CT+TT vs. CC | 0.993 (0.634–1.555) | 0.974 | 480.035 |
| Recessive | TT vs. CC+CT | 0.732 (0.240–2.230) | 0.583 | 479.727 |
| Overdominant | CT vs. CC+TT | 1.049 (0.660–1.669) | 0.839 | 479.994 |
| Additive | C | 0.958 (0.656–1.399) | 0.826 | 479.987 |
| Model | Genotype/Allele | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 1.271 (0.805–2.006) 1.215 (0.567–2.603) | 0.303 0.617 | 480.904 |
| Dominant | CG+GG vs. CC | 1.259 (0.822–1.930) | 0.290 | 478.916 |
| Recessive | GG vs. CC+CG | 1.108 (0.528–2.326) | 0.786 | 479.962 |
| Overdominant | CG vs. CC+GG | 1.237 (0.794–1.929) | 0.347 | 479.153 |
| Additive | C | 1.164 (0.842–1.608) | 0.357 | 479.189 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 1.271 (0.805–2.006) 1.215 (0.567–2.603) | 0.303 0.617 | 480.904 |
| Dominant | GT+TT vs. GG | 1.259 (0.822–1.930) | 0.290 | 478.916 |
| Recessive | TT vs. GG+GT | 1.108 (0.528–2.326) | 0.786 | 479.962 |
| Overdominant | GT vs. GG+TT | 1.237 (0.794–1.929) | 0.347 | 479.153 |
| Additive | G | 1.164 (0.842–1.608) | 0.357 | 479.189 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 1.116 (0.681–1.831) 0.338 (0.037–3.064) | 0.663 0.335 | 480.663 |
| Dominant | GA+GG vs. AA | 1.054 (0.649–1.713) | 0.832 | 479.991 |
| Recessive | GG vs. AA+GA | 0.329 (0.036–2.973) | 0.322 | 478.853 |
| Overdominant | GA vs. AA+GG | 1.136 (0.693–1.861) | 0.613 | 479.781 |
| Additive | A | 0.985 (0.630–1.540) | 0.948 | 480.031 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 0.983 (0.611–1.581) 1.508 (0.590–3.851) | 0.943 0.391 | 481.256 |
| Dominant | CT+TT vs. CC | 1.053 (0.674–1.646) | 0.820 | 479.984 |
| Recessive | TT vs. CC+CT | 1.516 (0.600–3.829) | 0.379 | 479.261 |
| Overdominant | CT vs. CC+TT | 0.952 (0.595–1.522) | 0.838 | 479.994 |
| Additive | C | 1.100 (0.769–1.574) | 0.601 | 479.762 |
| SNP | Genotype/Allele | Women | p-Value | Men | p-Value | ||
|---|---|---|---|---|---|---|---|
| Control, N (%) | Early AMD, N (%) | Control, N (%) | Early AMD, N (%) | ||||
| STAT4 rs10181656 | CC CG GG Allele: C G | 58 (58.0) 33 (33.0) 9 (9.0) 149 (74.5) 51 (25.5) | 48 (64.0) 22 (29.3) 5 (6.7) 118 (78.67) 32 (21.33) | 0.694 0.364 | 60 (60.0) 32 (32.0) 8 (8.0) 152 (76.0) 48 (24.0) | 42 (56.0) 29 (38.7) 4 (5.3) 113 (75.34) 37 (24.66) | 0.574 0.886 |
| STAT4 rs7574865 | GG GT TT Allele: G T | 58 (58.0) 33 (33.0) 9 (9.0) 149 (74.5) 51 (25.5) | 48 (64.0) 21 (28.0) 6 (8.0) 117 (78.0) 33 (22.0) | 0.722 0.448 | 60 (60.0) 32 (32.0) 8 (8.0) 152 (76.0) 48 (24.0) | 44 (58.7) 28 (37.3) 3 (4.0) 116 (77.34) 34 (22.66) | 0.482 0.771 |
| STAT4 rs7601754 | AA GAGG Allele: A G | 73 (73.0) 25 (25.0) 2 (2.0) 171 (85.5) 29 (14.5) | 50 (66.7) 21 (28.0) 4 (5.3) 121 (80.67) 29 (19.33) | 0.411 0.229 | 77 (77.0) 21 (21.0) 2 (2.0) 175 (87.5) 25 (12.5) | 63 (84.0) 12 (16.0) 0 (0.0) 138 (92.0) 12 (8.0) | 0.312 0.175 |
| STAT4 rs10168266 | CC CT TT Allele: C T | 62 (62.0) 32 (32.0) 6 (6.0) 156 (78.0) 44 (22.0) | 50 (66.7) 22 (29.3) 3 (4.0) 122 (81.33) 28 (18.67) | 0.749 0.445 | 71 (71.0) 26 (26.0) 3 (3.0) 168 (84.0) 32 (16.0) | 50 (66.7) 23 (30.7) 2 (2.7) 123 (82.0) 27 (18.0) | 0.792 0.621 |
| SNP | Genotype/Allele | Women | p-Value | Men | p-Value | ||
|---|---|---|---|---|---|---|---|
| Control, N (%) | Exudative AMD, N (%) | Control, N (%) | Exudative AMD, N (%) | ||||
| STAT4 rs10181656 | CC CG GG Allele: C G | 58 (58.0) 33 (33.0) 9 (9.0) 149 (74.5) 51 (25.5) | 37 (49.3) 31 (41.3) 7 (9.3) 105 (70.0) 45 (30.0) | 0.494 0.350 | 60 (60.0) 32 (32.0) 8 (8.0) 152 (76.0) 48 (24.0) | 43 (57.3) 25 (33.3) 7 (9.3) 111 (74.0) 39 (26.0) | 0.921 0.668 |
| STAT4 rs7574865 | GG GT TT Allele: G T | 58 (58.0) 33 (33.0) 9 (9.0) 149 (74.5) 51 (25.5) | 37 (49.3) 31 (41.3) 7 (9.3) 105 (70.0) 45 (30.0) | 0.494 0.350 | 60 (60.0) 32 (32.0) 8 (8.0) 152 (76.0) 48 (24.0) | 43 (57.3) 25 (33.3) 7 (9.3) 111 (74.0) 39 (26.0) | 0.921 0.668 |
| STAT4 rs7601754 | AA GA GG Allele: A G | 73 (73.0) 25 (25.0) 2 (2.0) 171 (85.5) 29 (14.5) | 54 (72.0) 21 (28.0) 0 (0) 129 (86.0) 21 (14.0) | 0.438 0.895 | 77 (77.0) 21 (21.0) 2 (2.0) 175 (87.5) 25 (12.5) | 57 (76.0) 17 (22.7) 1 (1.3) 131 (87.34) 19 (12.66) | 0.918 0.963 |
| STAT4 rs10168266 | CC CT TT Allele: C T | 62 (62.0) 32 (32.0) 6 (6.0) 156 (78.0) 44 (22.0) | 47 (62.7) 23 (30.7) 5 (6.7) 117 (78.0) 33 (22.0) | 0.972 1 | 71 (71.0) 26 (26.0) 3 (3.0) 168 (84.0) 32 (16.0) | 51 (68.0) 19 (25.3) 5 (6.7) 121 (80.67) 29 (19.33) | 0.516 0.416 |
| Model | Genotype/Allele | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|
| Women | ||||
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 0.806 (0.416–1.561) 0.671 (0.211–2.137) | 0.522 0.500 | 242.283 |
| Dominant | CG+GG vs. CC | 0.777 (0.419–1.439) | 0.422 | 240.370 |
| Recessive | GG vs. CC+CG | 0.722 (0.232–2.251) | 0.575 | 240.696 |
| Overdominant | CG vs. CC+GG | 0.843 (0.441–1.612) | 0.605 | 240.750 |
| Additive | C | 0.814 (0.506–1.309) | 0.395 | 240.285 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 0.769 (0.394–1.499) 0.806 (0.268–2.424) | 0.440 0.700 | 242.364 |
| Dominant | GT+TT vs. GG | 0.777 (0.419–1.439) | 0.422 | 240.370 |
| Recessive | TT vs. GG+GT | 0.879 (0.299–2.587) | 0.815 | 240.963 |
| Overdominant | GT vs. GG+TT | 0.790 (0.411–1.519) | 0.479 | 240.513 |
| Additive | G | 0.845 (0.530–1.348) | 0.481 | 240.516 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 1.226 (0.620–2.427) 2.920 (0.515–16.555) | 0.558 0.226 | 239.247 |
| Dominant | GA+GG vs. AA | 1.352 (0.704–2.595) | 0.365 | 240.198 |
| Recessive | GG vs. AA+GA | 2.761 (0.492–15.488) | 0.249 | 239.590 |
| Overdominant | GA vs. AA+GG | 1.167 (0.593–2.297) | 0.656 | 240.819 |
| Additive | A | 1.392 (0.799–2.424) | 0.242 | 239.646 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 0.853 (0.441–1.647) 0.620 (0.148–2.604) | 0.635 0.514 | 242.431 |
| Dominant | CT+TT vs. CC | 0.816 (0.436–1.528) | 0.525 | 240.611 |
| Recessive | TT vs. CC+CT | 0.653 (0.158–2.699) | 0.556 | 240.658 |
| Overdominant | CT vs. CC+TT | 0.882 (0.460–1.691) | 0.706 | 240.875 |
| Additive | C | 0.822 (0.490–1.379) | 0.458 | 240.461 |
| Men | ||||
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 1.295 (0.684–2.452) 0.714 (0.202–2.527) | 0.428 0.602 | 241.902 |
| Dominant | CG+GG vs. CC | 1.179 (0.643–2.162) | 0.595 | 240.736 |
| Recessive | GG vs. CC+CG | 0.648 (0.188–2.238) | 0.493 | 240.529 |
| Overdominant | CG vs. CC+GG | 1.340 (0.716–2.507) | 0.360 | 240.182 |
| Additive | C | 1.035 (0.640–1.674) | 0.888 | 240.998 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 1.193 (0.630–2.261) 0.511 (0.128–2.038) | 0.588 0.342 | 241.505 |
| Dominant | GT+TT vs. GG | 1.057 (0.575–1.944) | 0.859 | 240.986 |
| Recessive | TT vs. GG+GT | 0.479 (0.123–1.871) | 0.290 | 239.798 |
| Overdominant | GT vs. GG+TT | 1.266 (0.675–2.374) | 0.462 | 240.478 |
| Additive | G | 0.931 (0.570–1.521) | 0.776 | 240.936 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 0.698 (0.319–1.529) - | 0.369 - | 239.941 |
| Dominant | GA+GG vs. AA | 0.638 (0.294–1.382) | 0.254 | 239.683 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 0.717 (0.328–1.567) | 0.404 | 240.309 |
| Additive | A | 0.608 (0.294–1.259) | 0.180 | 239.135 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 1.256 (0.644–2.449) 0.947 (0.153–5.874) | 0.503 0.953 | 242.553 |
| Dominant | CT+TT vs. CC | 1.224 (0.642–2.335) | 0.539 | 240.642 |
| Recessive | TT vs. CC+CT | 0.886 (0.144–5.439) | 0.896 | 241.001 |
| Overdominant | CT vs. CC+TT | 1.259 (0.648–2.445) | 0.497 | 240.557 |
| Additive | C | 1.152 (0.656–2.023) | 0.621 | 240.774 |
| Model | Genotype/Allele | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|
| Women | ||||
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 1.473 (0.776–2.794) 1.219 (0.418–3.556) | 0.236 0.717 | 241.608 |
| Dominant | CG+GG vs. CC | 1.418 (0.777–2.590) | 0.255 | 239.721 |
| Recessive | GG vs. CC+CG | 1.041 (0.369–2.934) | 0.940 | 241.012 |
| Overdominant | CG vs. CC+GG | 1.430 (0.769–2.660) | 0.258 | 239.739 |
| Additive | C | 1.232 (0.781–1.942) | 0.370 | 240.214 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 1.473 (0.776–2.794) 1.219 (0.418–3.556) | 0.236 0.717 | 241.608 |
| Dominant | GT+TT vs. GG | 1.418 (0.777–2.590) | 0.255 | 239.721 |
| Recessive | TT vs. GG+GT | 1.041 (0.369–2.934) | 0.940 | 241.012 |
| Overdominant | GT vs. GG+TT | 1.430 (0.769–2.660) | 0.258 | 239.739 |
| Additive | G | 1.232 (0.781–1.942) | 0.370 | 240.214 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 1.136 (0.576–2.238) - | 0.713 - | 240.627 |
| Dominant | GA+GG vs. AA | 1.051 (0.538–2.055) | 0.883 | 240.996 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 1.167 (0.593–2.297) | 0.656 | 240.819 |
| Additive | A | 0.957 (0.510–1.796) | 0.891 | 240.999 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 0.948 (0.492–1.828) 1.099 (0.316–3.821) | 0.874 0.882 | 242.960 |
| Dominant | CT+TT vs. CC | 0.972 (0.524–1.803) | 0.928 | 241.010 |
| Recessive | TT vs. CC+CT | 1.119 (0.328–3.815) | 0.857 | 240.986 |
| Overdominant | CT vs. CC+TT | 0.940 (0.493–1.793) | 0.851 | 240.982 |
| Additive | C | 1.000 (0.612–1.634) | 1.000 | 241.018 |
| Men | ||||
| STAT4 rs10181656 | ||||
| Codominant | CG vs. CC GG vs. CC | 1.090 (0.567–2.095) 1.221 (0.412–3.622) | 0.796 0.719 | 242.854 |
| Dominant | CG+GG vs. CC | 1.116 (0.608–2.050) | 0.723 | 240.892 |
| Recessive | GG vs. CC+CG | 1.184 (0.409–3.423) | 0.755 | 240.921 |
| Overdominant | CG vs. CC+GG | 1.062 (0.561–2.011) | 0.852 | 240.983 |
| Additive | C | 1.099 (0.694–1.741) | 0.687 | 240.856 |
| STAT4 rs7574865 | ||||
| Codominant | GT vs. GG TT vs. GG | 1.090 (0.567–2.095) 1.221 (0.412–3.622) | 0.796 0.719 | 242.854 |
| Dominant | GT+TT vs. GG | 1.116 (0.608–2.050) | 0.723 | 240.892 |
| Recessive | TT vs. GG+GT | 1.184 (0.409–3.423) | 0.755 | 240.921 |
| Overdominant | GT vs. GG+TT | 1.062 (0.561–2.011) | 0.852 | 240.983 |
| Additive | G | 1.099 (0.694–1.741) | 0.687 | 240.856 |
| STAT4 rs7601754 | ||||
| Codominant | GA vs. AA GG vs. AA | 1.094 (0.529–2.259) 0.675 (0.060–7.632) | 0.809 0.751 | 242.844 |
| Dominant | GA+GG vs. AA | 1.057 (0.522–2.141) | 0.877 | 240.994 |
| Recessive | GG vs. AA+GA | 0.662 (0.059–7.442) | 0.738 | 240.902 |
| Overdominant | GA vs. AA+GG | 1.103 (0.535–2.274) | 0.791 | 240.948 |
| Additive | A | 1.015 (0.538–1.914) | 0.963 | 241.016 |
| STAT4 rs10168266 | ||||
| Codominant | CT vs. CC TT vs. CC | 1.017 (0.509–2.033) 2.320 (0.530–10.151) | 0.961 0.264 | 241.709 |
| Dominant | CT+TT vs. CC | 1.152 (0.602–2.206) | 0.669 | 240.836 |
| Recessive | TT vs. CC+CT | 2.310 (0.534–9.984) | 0.262 | 239.712 |
| Overdominant | CT vs. CC+TT | 0.966 (0.486–1.917) | 0.920 | 241.008 |
| Additive | C | 1.231 (0.726–2.087) | 0.440 | 240.423 |
| SNP | Genotype/Allele | ≤65 y/o | p-Value | >65 y/o–≤75 y/o | p-Value | >75 y/o | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group, | Early AMD | Exudative AMD | Control Group, | Early AMD | Exudative AMD | Control Group, | Early AMD | Exudative AMD | |||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| rs10181656 | CC | 13 (52.0) | 15 (50.0) | 17 (54.8) | 0.965 (1) 0.754 (2) | 89 (59.7) | 48 (62.3) | 32 (50.0) | 0.493 (1) 0.411 (2) | 16 (61.5) | 27 (62.8) | 31 (56.4) | 0.797 (1) 0.447 (2) |
| CG | 10 (40.0) | 12 (40.0) | 10 (32.3) | 48 (32.2) | 26 (33.8) | 25 (39.1) | 7 (26.9) | 13 (30.2) | 21 (38.2) | ||||
| GG | 2 (8.0) | 3 (10.0) | 4 (12.9) | 12 (8.1) | 3 (3.9) | 7 (10.9) | 3 (11.5) | 3 (7.0) | 3 (5.5) | ||||
| C | 36 (72) | 42 (70) | 44 (71) | 0.818 (1) 0.904 (2) | 226 (75.8) | 122 (79.2) | 89 (69.5) | 0.418 (1) 0.174 (2) | 39 (75) | 67 (77.9) | 83 (75.5) | 0.695 (1) 0.950 (2) | |
| G | 14 (28) | 18 (30) | 18 (29) | 72 (24.2) | 32 (20.8) | 39 (30.5) | 13 (25) | 19 (22.1) | 27 (24.5) | ||||
| rs7574865 | GG | 13 (52.0) | 17 (56.7) | 17 (54.8) | 0.938 (1) 0.754 (2) | 89 (59.7) | 48 (62.3) | 31 (48.4) | 0.725 (1) 0.277 (2) | 16 (61.5) | 27 (62.8) | 32 (58.2) | 0.797 (1) 0.291 (2) |
| GT | 10 (40.0) | 11 (36.7) | 10 (32.3) | 48 (32.2) | 25 (32.5) | 25 (39.1) | 7 (26.0) | 13 (30.2) | 21 (38.2) | ||||
| TT | 2 (8.0) | 2 (7.7) | 4 (12.9) | 12 (8.1) | 4 (5.2) | 8 (12.5) | 3 (11.5) | 3 (7.0) | 2 (3.6) | ||||
| G | 36 (72) | 45 (75) | 44 (71) | 0.722 (1) 0.904 (2) | 226 (75.8) | 121 (78.6) | 87 (68) | 0.514 (1) 0.092 (2) | 39 (75) | 67 (77.9) | 85 (77.3) | 0.695 (1) 0.749 (2) | |
| T | 14 (28) | 15 (25) | 18 (29) | 72 (24.2) | 33 (21.4) | 41 (32) | 13 (25) | 19 (22.1) | 25 (22.7) | ||||
| rs7601754 | AA | 19 (76.0) | 18 (60.0) | 21 (67.7) | 0.453 (1) 0.342 (2) | 113 (75.8) | 57 (74.0) | 51 (79.7) | 0.857 (1) 0.486 (2) | 18 (69.2) | 38 (88.4) | 39 (70.9) | 0.060 (1) 0.757 (2) |
| GA | 5 (20.0) | 10 (33.3) | 10 (32.3) | 33 (22.1) | 19 (24.7) | 13 (20.3) | 8 (30.8) | 4 (9.3) | 15 (27.3) | ||||
| GG | 1 (4.0) | 2 (6.7) | 0 (0) | 3 (2.0) | 1 (1.3) | 0 (0) | 0 (0) | 1 (2.3) | 1 (1.8) | ||||
| A | 43 (86) | 46 (76.7) | 52 (83.9) | 0.215 (1) 0.755 (2) | 259 (86.9) | 133 (86.4) | 115 (89.8) | 0.870 (1) 0.397 (2) | 44 (84.6) | 80 (93) | 93 (84.6) | 0.113 (1) 0.991 (2) | |
| G | 7 (14) | 14 (23.3) | 10 (16.1) | 39 (13.1) | 21 (13.6) | 13 (10.2) | 8 (15.4) | 6 (7) | 17 (15.4) | ||||
| rs10168266 | CC | 14 (56.0) | 17 (56.7) | 18 (58.1) | 0.956 (1) 0.972 (2) | 101 (67.8) | 53 (68.8) | 38 (59.4) | 0.528 (1) 0.234 (2) | 18 (69.2) | 30 (69.8) | 42 (76.1) | 0.934 (1) 0.777 (2) |
| CT | 9 (36.0) | 10 (33.3) | 11 (35.5) | 42 (28.2) | 23 (29.9) | 20 (31.3) | 7 (26.9) | 12 (27.9) | 11 (20.0) | ||||
| TT | 2 (8.0) | 3 (10.0) | 2 (6.5) | 6 (4.0) | 1 (1.3) | 6 (9.4) | 1 (3.8) | 1 (2.3) | 2 (3.6) | ||||
| C | 37 (74) | 44 (73.3) | 47 (75.8) | 0.937 (1) 0.826 (2) | 244 (81.9) | 129 (83.8) | 96 (75) | 0.617 (1) 0.105 (2) | 43 (82.7) | 72 (83.7) | 95 (86.4) | 0.875 (1) 0.539 (2) | |
| T | 13 (26) | 16 (26.7) | 15 (24.2) | 54 (18.1) | 25 (16.2) | 32 (25) | 9 (17.3) | 14 (16.3) | 15 (13.6) | ||||
| ≤65 y/o | ||||
|---|---|---|---|---|
| Model | Genotype/Allele | OR (95% CI) * | p-Value | AIC |
| Early AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 1.040 (0.339–3.190) | 0.945 | 79.720 |
| GG vs. CC | 1.300 (0.187–9.021) | 0.791 | ||
| Dominant | CG+GG vs. CC | 1.083 (0.375–3.133) | 0.883 | 77.769 |
| Recessive | GG vs. CG+CC | 1.278 (0.196–8.321) | 0.798 | 77.724 |
| Overdominant | CG vs. CC+GG | 1.000 (0.338–2.955) | 1.000 | 77.791 |
| Additive | C | 1.099 (0.486–2.486) | 0.821 | 77.740 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 0.841 (0.274–2.579) | 0.762 | 79.664 |
| TT vs. GG | 0.765 (0.095–6.175) | 0.801 | ||
| Dominant | GT+TT vs. GG | 0.828 (0.285–2.406) | 0.729 | 77.671 |
| Recessive | TT vs. GG+GT | 0.821 (0.107–6.293) | 0.850 | 77.755 |
| Overdominant | GT vs. GG+TT | 0.868 (0.292–2.587) | 0.800 | 77.727 |
| Additive | G | 0.859 (0.369–1.999) | 0.725 | 77.667 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 2.111 (0.604–7.385) | 0.242 | 78.180 |
| GG vs. AA | 2.111 (0.176–25.349) | 0.556 | ||
| Dominant | GA+GG vs. AA | 2.111 (0.653–6.823) | 0.212 | 76.180 |
| Recessive | GG vs. AA+GA | 1.714 (0.146–20.097) | 0.668 | 77.598 |
| Overdominant | GA vs. AA+GG | 2.000 (0.579–6.908) | 0.273 | 76.547 |
| Additive | A | 1.766 (0.673–4.634) | 0.248 | 76.375 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 0.915 (0.291–2.876) | 0.879 | 79.701 |
| TT vs. CC | 1.235 (0.180–8.459) | 0.830 | ||
| Dominant | CT+TT vs. CC | 0.973 (0.334–2.838) | 0.960 | 77.789 |
| Recessive | TT vs. CC+CT | 1.278 (0.196–8.321) | 0.798 | 77.724 |
| Overdominant | CT vs. CC+TT | 0.889 (0.291–2.711) | 0.836 | 77.748 |
| Additive | C | 1.031 (0.459–2.317) | 0.940 | 77.785 |
| Exudative AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 0.765 (0.246–2.381) | 0.643 | 80.418 |
| GG vs. CC | 1.529 (0.242–9.674) | 0.652 | ||
| Dominant | CG+GG vs. CC | 0.892 (0.310–2.566) | 0.832 | 78.944 |
| Recessive | GG vs. CG+CC | 1.704 (0.286–10.165) | 0.559 | 78.633 |
| Overdominant | CG vs. CC+GG | 0.714 (0.238–2.143) | 0.548 | 78.628 |
| Additive | C | 1.046 (0.480–2.279) | 0.910 | 78.976 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 0.765 (0.246–2.381) | 0.643 | 80.418 |
| TT vs. GG | 1.529 (0.242–9.674) | 0.652 | ||
| Dominant | GT+TT vs. GG | 0.892 (0.310–2.566) | 0.832 | 78.944 |
| Recessive | TT vs. GG+GT | 1.704 (0.286–10.165) | 0.559 | 78.633 |
| Overdominant | GT vs. GG+TT | 0.714 (0.238–2.143) | 0.548 | 78.628 |
| Additive | G | 1.046 (0.480–2.279) | 0.910 | 78.976 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 1.810 (0.524–6.253) | 0.349 | 78.447 |
| GG vs. AA | - | - | ||
| Dominant | GA+GG vs. AA | 1.508 (0.460–4.943) | 0.498 | 78.522 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 1.905 (0.553–6.555) | 0.307 | 77.909 |
| Additive | A | 1.190 (0.408–3.467) | 0.750 | 78.886 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 0.951 (0.309–2.926) | 0.930 | 80.931 |
| TT vs. CC | 0.778 (0.097–6.230) | 0.813 | ||
| Dominant | CT+TT vs. CC | 0.919 (0.317–2.664) | 0.877 | 78.964 |
| Recessive | TT vs. CC+CT | 0.793 (0.104–6.069) | 0.823 | 78.939 |
| Overdominant | CT vs. CC+TT | 0.978 (0.326–2.935) | 0.968 | 78.987 |
| Additive | C | 0.912 (0.394–2.112) | 0.830 | 78.942 |
| >65 y/o–≤75 y/o | ||||
|---|---|---|---|---|
| Model | Genotype/Allele | OR (95% CI) * | p-Value | AIC |
| Early AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 1.004 (0.555–1.816) | 0.989 | 292.420 |
| GG vs. CC | 0.464 (0.125–1.723) | 0.251 | ||
| Dominant | CG+GG vs. CC | 0.896 (0.509–1.577) | 0.704 | 291.815 |
| Recessive | GG vs. CG+CC | 0.463 (0.127–1.692 | 0.244 | 290.420 |
| Overdominant | CG vs. CC+GG | 1.073 (0.598–1.924) | 0.814 | 291.904 |
| Additive | C | 0.834 (0.529–1.316) | 0.436 | 291.342 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 0.966 (0.531–1.755) | 0.909 | 293.285 |
| TT vs. GG | 0.618 (0.189–2.021) | 0.426 | ||
| Dominant | GT+TT vs. GG | 0.896 (0.509–1.577) | 0.704 | 291.815 |
| Recessive | TT vs. GG+GT | 0.626 (0.195–2.009) | 0.431 | 291.298 |
| Overdominant | GT vs. GG+TT | 1.012 (0.562–1.821) | 0.969 | 291.958 |
| Additive | G | 0.867 (0.554–1.357) | 0.534 | 291.567 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 1.141 (0.597–2.182) | 0.689 | 293.644 |
| GG vs. AA | 0.661 (0.067–6.496) | 0.722 | ||
| Dominant | GA+GG vs. AA | 1.101 (0.585–2.073) | 0.765 | 291.871 |
| Recessive | GG vs. AA+GA | 0.640 (0.065–6.261) | 0.702 | 291.803 |
| Overdominant | GA vs. AA+GG | 1.152 (0.603–2.198) | 0.669 | 291.778 |
| Additive | A | 1.049 (0.593–1.854) | 0.871 | 291.933 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 1.044 (0.568–1.916) | 0.891 | 292.491 |
| TT vs. CC | 0.318 (0.037–2.707) | 0.294 | ||
| Dominant | CT+TT vs. CC | 0.953 (0.527–1.723) | 0.873 | 291.934 |
| Recessive | TT vs. CC+CT | 0.314 (0.037–2.653) | 0.287 | 290.510 |
| Overdominant | CT vs. CC+TT | 1.085 (0.593–1.986) | 0.791 | 291.890 |
| Additive | C | 0.876 (0.521–1.473) | 0.617 | 291.707 |
| Exudative AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 1.449 (0.771–2.720) | 0.249 | 262.633 |
| GG vs. CC | 1.622 (0.587–4.481) | 0.351 | ||
| Dominant | CG+GG vs. CC | 1.483 (0.823–2.674) | 0.190 | 260.678 |
| Recessive | GG vs. CG+CC | 1.402 (0.525–3.743) | 0.500 | 261.955 |
| Overdominant | CG vs. CC+GG | 1.349 (0.734–2.479) | 0.335 | 261.476 |
| Additive | C | 1.333 (0.860–2.067) | 0.199 | 260.763 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 1.495 (0.794–2.816) | 0.213 | 261.862 |
| TT vs. GG | 1.914 (0.716–5.118) | 0.196 | ||
| Dominant | GT+TT vs. GG | 1.579 (0.876–2.847) | 0.129 | 260.086 |
| Recessive | TT vs. GG+GT | 1.631 (0.633–4.205) | 0.311 | 261.405 |
| Overdominant | GT vs. GG+TT | 1.349 (0.734–2.479) | 0.335 | 261.476 |
| Additive | G | 1.419 (0.920–2.190) | 0.113 | 259.911 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 0.873 (0.424–1.797) | 0.712 | 262.097 |
| GG vs. AA | - | - | ||
| Dominant | GA+GG vs. AA | 0.800 (0.391–1.636) | 0.541 | 262.017 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 0.896 (0.436–1.843) | 0.765 | 262.308 |
| Additive | A | 0.749 (0.384–1.462) | 0.397 | 261.651 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 1.266 (0.661–2.425) | 0.478 | 261.680 |
| TT vs. CC | 2.658 (0.807–8.750) | 0.108 | ||
| Dominant | CT+TT vs. CC | 1.440 (0.786–2.638) | 0.238 | 261.018 |
| Recessive | TT vs. CC+CT | 2.466 (0.764–7.960) | 0.131 | 260.179 |
| Overdominant | CT vs. CC+TT | 1.158 (0.612–2.191) | 0.652 | 262.196 |
| Additive | C | 1.454 (0.902–2.344) | 0.124 | 260.067 |
| >75 y/o | ||||
|---|---|---|---|---|
| Model | Genotype/Allele | OR (95% CI) * | p-Value | AIC |
| Early AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 1.101 (0.364–3.331) | 0.865 | 94.981 |
| GG vs. CC | 0.593 (0.107–3.295) | 0.550 | ||
| Dominant | CG+GG vs. CC | 0.948 (0.348–2.586) | 0.917 | 93.412 |
| Recessive | GG vs. CG+CC | 0.575 (0.107–3.087) | 0.519 | 93.010 |
| Overdominant | CG vs. CC+GG | 1.176 (0.398–3.477) | 0.769 | 93.336 |
| Additive | C | 0.873 (0.415–1.833) | 0.719 | 93.294 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 1.101 (0.364–3.331) | 0.865 | 94.981 |
| TT vs. GG | 0.593 (0.107–3.295) | 0.550 | ||
| Dominant | GT+TT vs. GG | 0.948 (0.348–2.586) | 0.917 | 93.412 |
| Recessive | TT vs. GG+GT | 0.575 (0.107–3.087) | 0.519 | 93.010 |
| Overdominant | GT vs. GG+TT | 1.176 (0.398–3.477) | 0.769 | 93.336 |
| Additive | G | 0.873 (0.415–1.833) | 0.719 | 93.294 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 0.237 (0.063–0.891) | 0.033 | 89.606 |
| GG vs. AA | - | - | ||
| Dominant | GA+GG vs. AA | 0.296 (0.085–1.034) | 0.056 | 89.653 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 0.231 (0.061–0.867) | 0.030 | 88.373 |
| Additive | A | 0.422 (0.137–1.303) | 0.134 | 91.082 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 1.029 (0.342–3.091) | 0.960 | 95.291 |
| TT vs. CC | 0.600 (0.035–10.195) | 0.724 | ||
| Dominant | CT+TT vs. CC | 0.975 (0.339–2.806) | 0.963 | 93.420 |
| Recessive | TT vs. CC+CT | 0.595 (0.036–9.943) | 0.718 | 93.293 |
| Overdominant | CT vs. CC+TT | 1.051 (0.352–3.135) | 0.929 | 93.415 |
| Additive | C | 0.930 (0.372–2.321) | 0.876 | 93.398 |
| Exudative AMD | ||||
| rs10181656 | ||||
| Codominant | CG vs. CC | 1.548 (0.544–4.410) | 0.413 | 104.092 |
| GG vs. CC | 0.516 (0.093–2.854) | 0.448 | ||
| Dominant | CG+GG vs. CC | 1.239 (0.478–3.213) | 0.660 | 103.478 |
| Recessive | GG vs. CG+CC | 0.442 (0.083–2.359) | 0.339 | 102.779 |
| Overdominant | CG vs. CC+GG | 1.676 (0.603–4.664) | 0.322 | 102.661 |
| Additive | C | 0.977 (0.467–2.044) | 0.952 | 103.669 |
| rs7574865 | ||||
| Codominant | GT vs. GG | 1.500 (0.528–4.265) | 0.447 | 103.326 |
| TT vs. GG | 0.333 (0.051–2.200) | 0.254 | ||
| Dominant | GT+TT vs. GG | 1.150 (0.443–2.987) | 0.774 | 103.590 |
| Recessive | TT vs. GG+GT | 0.289 (0.045–1.849) | 0.190 | 101.918 |
| Overdominant | GT vs. GG+TT | 1.676 (0.603–4.664) | 0.322 | 102.661 |
| Additive | G | 0.886 (0.416–1.888) | 0.754 | 103.576 |
| rs7601754 | ||||
| Codominant | GA vs. AA | 0.865 (0.311–2.409) | 0.782 | 104.817 |
| GG vs. AA | - | - | ||
| Dominant | GA+GG vs. AA | 0.923 (0.334–2.550) | 0.877 | 103.649 |
| Recessive | GG vs. AA+GA | - | - | - |
| Overdominant | GA vs. AA+GG | 0.844 (0.303–2.346) | 0.745 | 103.568 |
| Additive | A | 1.006 (0.386–2.618) | 0.990 | 103.673 |
| rs10168266 | ||||
| Codominant | CT vs. CC | 0.673 (0.225–2.017) | 0.480 | 105.180 |
| TT vs. CC | 0.857 (0.073–10.064) | 0.902 | ||
| Dominant | CT+TT vs. CC | 0.696 (0.246–1.969) | 0.495 | 103.214 |
| Recessive | TT vs. CC+CT | 0.943 (0.082–10.901) | 0.963 | 103.671 |
| Overdominant | CT vs. CC+TT | 0.679 (0.228–2.018) | 0.486 | 103.195 |
| Additive | C | 0.778 (0.331–1.824) | 0.563 | 103.344 |
| Genotype | Serum STAT4 Levels | p-Value | ||
|---|---|---|---|---|
| Early AMD Mean (Std. Deviation) | Exudative AMD Median (IQR) | Control Median (IQR) | ||
| STAT4 rs10181656 | ||||
| CC | 0.431 (-) | 0.109 (0.038) | 0.202 (0.560) | 0.555 1 0.405 2 |
| CG+GG | 0.256 (0.198) | 0.178 (0.823) | 0.292 (0.209) | 0.826 1 0.011 2 |
| STAT4 rs7574865 | ||||
| GG | 0.182 (0.198) | 0.194 (0.123) | 0.324 (0.318) | 0.728 1 0.054 2 |
| GT+TT | 0.356 (0.188) | 0.198 (0.353) | 0.292 (0.209) | 0.756 1 0.062 2 |
| STAT4 rs7601754 | ||||
| AA | 0.305 (0.187) | 0.221 (0.198) | 0.458 (0.268) | 0.631 1 0.858 2 |
| GA+GG | 0.176 (0.124) | 0.174 (0.155) | 0.258 (0.144) | 0.972 1 0.889 2 |
| STAT4 rs10168266 | ||||
| CC | 0.256 (0.266) | 0.152 (0.190) | 0.268 (0.268) | 0.821 1 0.658 2 |
| CT+TT | 0.255 (0.178) | 0.185 (0.562) | 0.268 (0.154) | 0.956 1 0.039 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blekeris, T.; Gedvilaite, G.; Kaikaryte, K.; Kriauciuniene, L.; Zaliuniene, D.; Liutkevciene, R. Association of STAT4 Gene Polymorphisms (rs10181656, rs7574865, rs7601754, rs10168266) and Serum STAT4 Levels in Age-Related Macular Degeneration. Biomedicines 2024, 12, 18. https://doi.org/10.3390/biomedicines12010018
Blekeris T, Gedvilaite G, Kaikaryte K, Kriauciuniene L, Zaliuniene D, Liutkevciene R. Association of STAT4 Gene Polymorphisms (rs10181656, rs7574865, rs7601754, rs10168266) and Serum STAT4 Levels in Age-Related Macular Degeneration. Biomedicines. 2024; 12(1):18. https://doi.org/10.3390/biomedicines12010018
Chicago/Turabian StyleBlekeris, Tomas, Greta Gedvilaite, Kriste Kaikaryte, Loresa Kriauciuniene, Dalia Zaliuniene, and Rasa Liutkevciene. 2024. "Association of STAT4 Gene Polymorphisms (rs10181656, rs7574865, rs7601754, rs10168266) and Serum STAT4 Levels in Age-Related Macular Degeneration" Biomedicines 12, no. 1: 18. https://doi.org/10.3390/biomedicines12010018
APA StyleBlekeris, T., Gedvilaite, G., Kaikaryte, K., Kriauciuniene, L., Zaliuniene, D., & Liutkevciene, R. (2024). Association of STAT4 Gene Polymorphisms (rs10181656, rs7574865, rs7601754, rs10168266) and Serum STAT4 Levels in Age-Related Macular Degeneration. Biomedicines, 12(1), 18. https://doi.org/10.3390/biomedicines12010018





