A Novel Classification of Endometriosis Based on Clusters of Comorbidities
Abstract
:1. Introduction
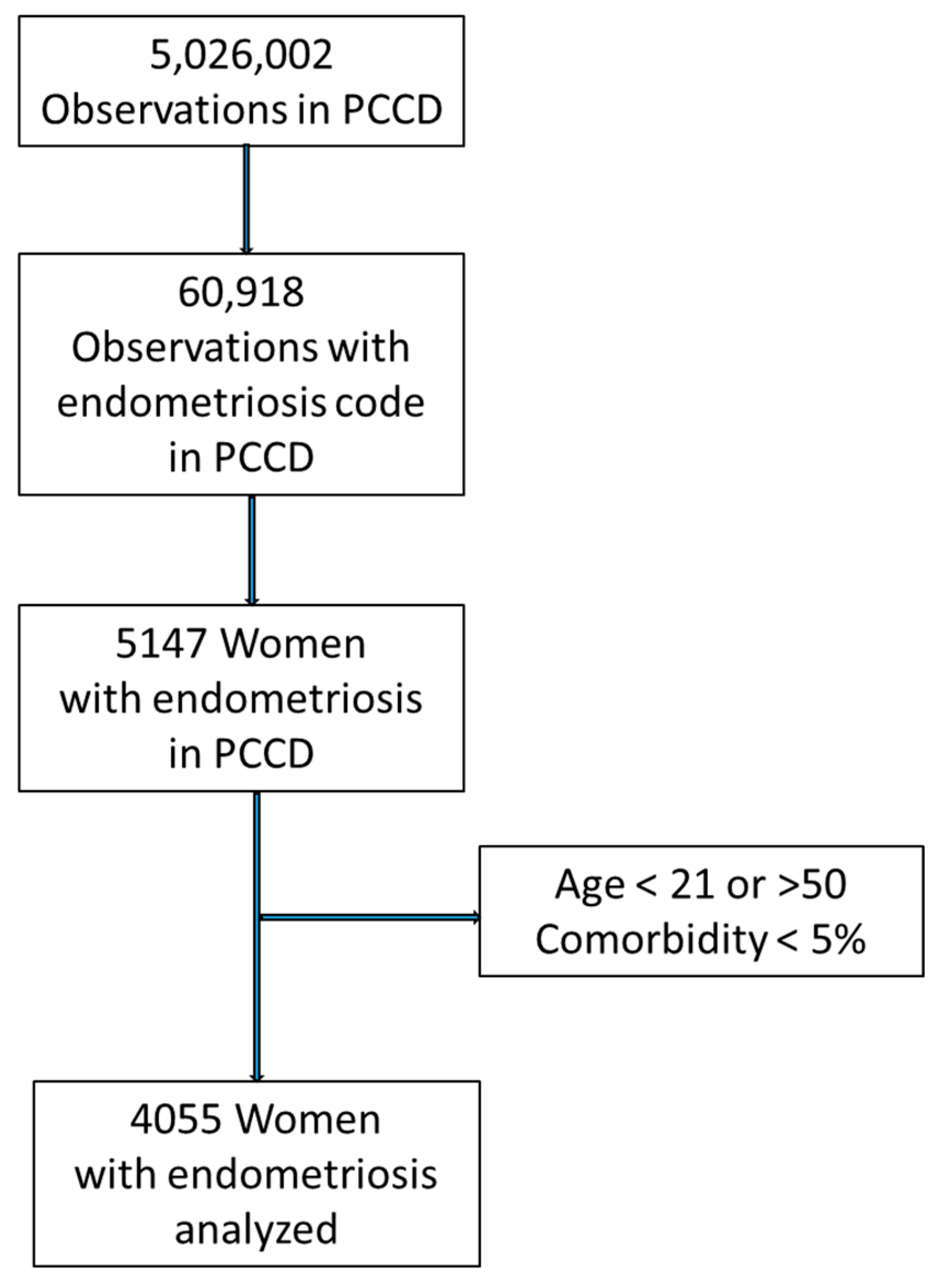
2. Materials and Methods
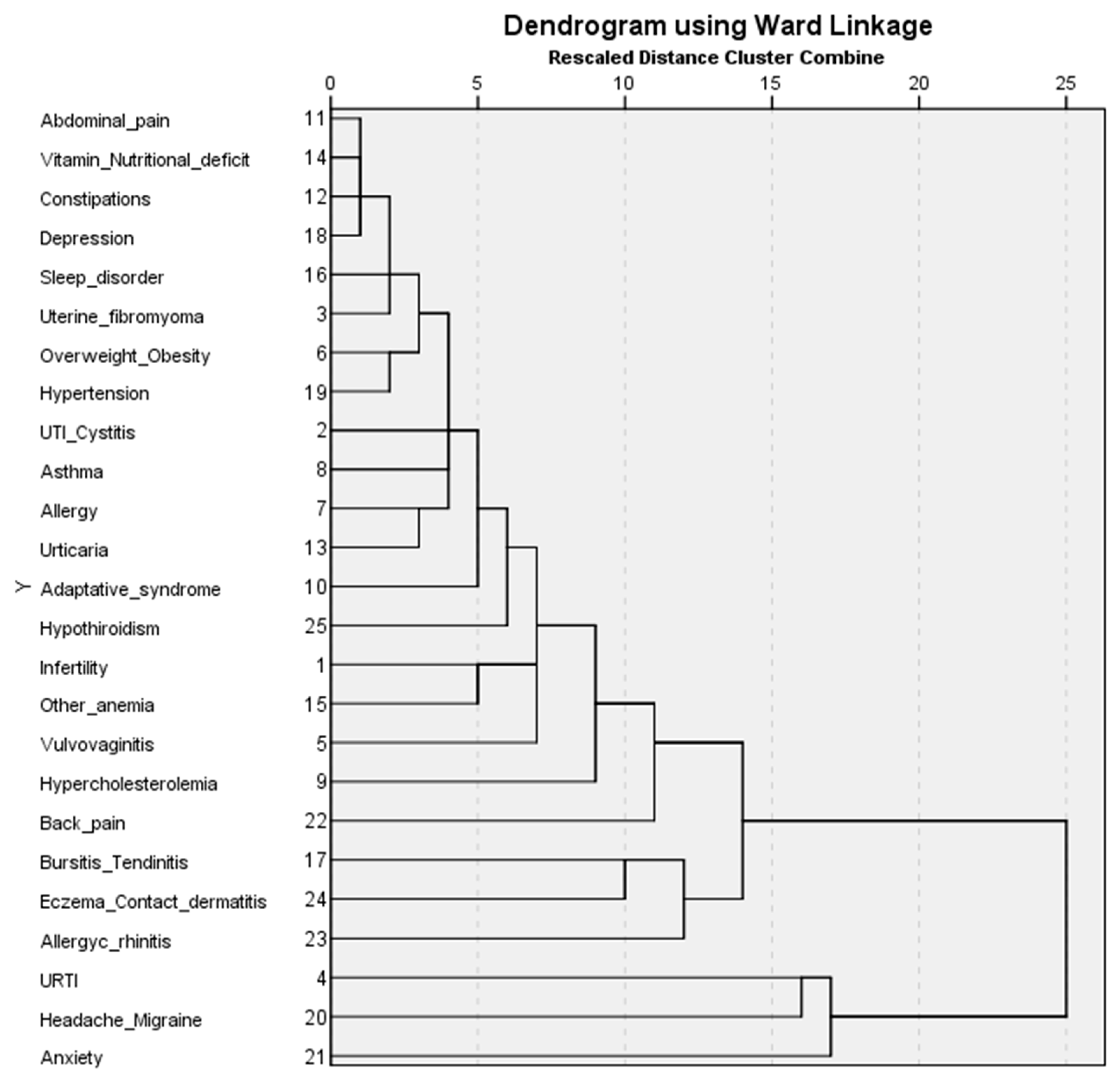
3. Results
- Cluster 1 (less comorbidity) has the second largest number of patients (n = 1212). It has the lowest GP visits (8.1%). All frequencies of the comorbidities are less than 17% and most comorbidities (n = 20) have frequencies between 0 and 4%.
- Cluster 2 (multiple comorbidities) has the lowest number of patients (n = 283) but shows the highest number of visits to the GP (28.3%) and to specialists (20.2%). Most patients are between 41 and 50 (61.48%). Patients in the cluster have a high frequency (>30%), of multiple comorbidities (n = 7) (anxiety (73.85%), headache/migraine (68.55%), URTI (51.59%), chronic/allergic rhinitis (48.6%), bursitis/tendinitis (44.88%), anemia (31.1%), and elevated cholesterol (31.8%)).
- Cluster 3 (anxiety and musculoskeletal disorders) is the largest in terms of patients (n = 1334). The cluster has a significantly higher prevalence of anxiety (37.48%), back pain (22.56%), and bursitis/tendinitis (22.26%).
- Cluster 4 (type 1 allergies or immediate hypersensitivity) has the highest prevalence of chronic/allergic rhinitis (49.06%), asthma (35.58%), and urticaria (30.71%).
- Cluster 5 (anemia and infertility) has the highest prevalence of anemia (54.68%) and infertility (58.91%).
- Cluster 6 (headache and migraine) has the highest frequency of patients aged between 21 and 30 (14.96%). The cluster has the highest prevalence of headache/migraine (96.4%).
4. Discussion
4.1. Multiple Comorbidities and Advanced Age Cluster
4.2. Anxiety and Musculoskeletal Disorders Cluster
4.3. Type 1 Allergies/Immediate-Type Hypersensitivity Cluster
4.4. Infertility and Anemia Cluster
4.5. Headache/Migraine Cluster
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S.; Laganà, A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelley, C.; Winkel, C. The direct and indirect costs associated with endometriosis: A systematic literature review. Hum. Reprod. 2016, 31, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Garzon, S.; Bapayeva, G.; Ukybassova, T.; Terzic, S.; Norton, M.; Laganà, A.S. Ovulation induction in infertile women with endometriotic ovarian cysts: Current evidence and potential pitfalls. Minerva Medica 2020, 111, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Malvezzi, H.; Marengo, E.B.; Podgaec, S.; Piccinato, C.d.A. Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology. J. Transl. Med. 2020, 18, 311. [Google Scholar] [CrossRef]
- Vetvicka, V.; Fiala, L.; Garzon, S.; Buzzaccarini, G.; Terzic, M.; Laganà, A.S. Endometriosis and gynaecological cancers: Molecular insights behind a complex machinery. Menopausal Rev. 2021, 20, 201–206. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef]
- Mechsner, S. Endometriosis, an Ongoing Pain—Step-by-Step Treatment. J. Clin. Med. 2022, 11, 467. [Google Scholar] [CrossRef]
- Masciullo, L.; Viscardi, M.F.; Piacenti, I.; Scaramuzzino, S.; Cavalli, A.; Piccioni, M.G.; Porpora, M.G. A deep insight into pelvic pain and endometriosis: A review of the literature from pathophysiology to clinical expressions. Minerva Obstet. Gynecol. 2021, 73, 511–522. [Google Scholar] [CrossRef]
- Vitale, S.G.; Capriglione, S.; Zito, G.; Lopez, S.; Gulino, F.A.; Di Guardo, F.; Vitagliano, A.; Noventa, M.; La Rosa, V.L.; Sapia, F.; et al. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch. Gynecol. Obstet. 2018, 299, 299–315. [Google Scholar] [CrossRef]
- Namazi, M.; Moghadam, Z.B.; Zareiyan, A.; Jafarabadi, M. Exploring the impact of endometriosis on women’s lives: A qualitative study in Iran. Nurs. Open 2020, 8, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, X.; Chen, Y.; Wang, J.; Zheng, W.; Cao, L. Miscarriage on Endometriosis and Adenomyosis in Women by Assisted Reproductive Technology or with Spontaneous Conception: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Filip, L.; Duică, F.; Prădatu, A.; Crețoiu, D.; Suciu, N.; Crețoiu, S.M.; Predescu, D.-V.; Varlas, V.N.; Voinea, S.-C. Endometriosis Associated Infertility: A Critical Review and Analysis on Etiopathogenesis and Therapeutic Approaches. Medicina 2020, 56, 460. [Google Scholar] [CrossRef]
- Patzkowsky, K. Rethinking endometriosis and pelvic pain. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Urteaga, I.; McKillop, M.; Elhadad, N. Learning endometriosis phenotypes from patient-generated data. NPJ Digit. Med. 2020, 3, 88. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, C.W.; Yun, J.; Lee, J.H.; Yun, B.H.; Park, J.H.; Seo, S.K.; Cho, S.; Choi, Y.S.; Lee, B.S. Use of the Endometriosis Fertility Index to Predict Natural Pregnancy after Endometriosis Surgery: A Single-Center Study. Gynecol. Obstet. Investig. 2018, 84, 86–93. [Google Scholar] [CrossRef]
- Pascoal, E.; Wessels, J.M.; Aas-Eng, M.K.; Abrao, M.S.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S.; et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 2022, 60, 309–327. [Google Scholar] [CrossRef] [PubMed]
- International working group of AAGL, ESGE, ESHRE and WES; Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.; Missmer, S.; Petrozza, J.; Tomassetti, C. Endometriosis classification, staging and reporting systems: A review on the road to a universally accepted endometriosis classification. Hum. Reprod. Open 2021, 2021, hoab025. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Sarria-Santamera, A.; Orazumbekova, B.; Terzic, M.; Issanov, A.; Chaowen, C.; Asúnsolo-Del-Barco, A. Systematic Review and Meta-Analysis of Incidence and Prevalence of Endometriosis. Healthcare 2020, 9, 29. [Google Scholar] [CrossRef]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J. Women's Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.-W.; Horng, H.-C.; Ho, C.-H.; Yen, M.-S.; Chao, H.-T.; Wang, P.-H.; Chang, Y.-H.; Chang, Y.; Chao, K.-C.; Chen, Y.-J.; et al. Women with endometriosis have higher comorbidities: Analysis of domestic data in Taiwan. J. Chin. Med. Assoc. 2016, 79, 577–582. [Google Scholar] [CrossRef]
- Gandini, S.; Lazzeroni, M.; Peccatori, F.; Bendinelli, B.; Saieva, C.; Palli, D.; Masala, G.; Caini, S. The risk of extra-ovarian malignancies among women with endometriosis: A systematic literature review and meta-analysis. Crit. Rev. Oncol. 2019, 134, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Saruarov, Y.; Nuskabayeva, G.; Gencer, M.Z.; Sadykova, K.; Zhunissova, M.; Tatykayeva, U.; Iskandirova, E.; Sarsenova, G.; Durmanova, A.; Gaipov, A.; et al. Associations of Clusters of Cardiovascular Risk Factors with Insulin Resistance and Β-Cell Functioning in a Working-Age Diabetic-Free Population in Kazakhstan. Int. J. Environ. Res. Public Health 2023, 20, 3918. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Primary Care, 2nd ed.; (ICPC-2). Available online: https://www.who.int/standards/classifications/other-classifications/international-classification-of-primary-care (accessed on 30 June 2022).
- Larsson, B.; Søgaard, K.; Rosendal, L. Work related neck–shoulder pain: A review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract. Res. Clin. Rheumatol. 2007, 21, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef]
- Vargas, E.; Aghajanova, L.; Gemzell-Danielsson, K.; Altmäe, S.; Esteban, F.J. Cross-disorder analysis of endometriosis and its comorbid diseases reveals shared genes and molecular pathways and proposes putative biomarkers of endometriosis. Reprod. Biomed. Online 2019, 40, 305–318. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Frangez, H.B.; Bokal, E.V.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Women's Health 2017, 9, 323–330. [Google Scholar] [CrossRef]
- Laganà, A.S.; Condemi, I.; Retto, G.; Muscatello, M.R.A.; Bruno, A.; Zoccali, R.A.; Triolo, O.; Cedro, C. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mamillapalli, R.; Ding, S.; Chang, H.; Liu, Z.-W.; Gao, X.-B.; Taylor, H.S. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice†. Biol. Reprod. 2018, 99, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Schlicke, C.P. Ectopic Endometrial Tissue in the Thigh. J. Am. Med Assoc. 1946, 132, 445. [Google Scholar] [CrossRef]
- Pacchiarotti, A.; Milazzo, G.N.; Biasiotta, A.; Truini, A.; Antonini, G.; Frati, P.; Gentile, V.; Caserta, D.; Moscarini, M. Pain in the upper anterior-lateral part of the thigh in women affected by endometriosis: Study of sensitive neuropathy. Fertil. Steril. 2013, 100, 122–126. [Google Scholar] [CrossRef]
- Linton, S.J. A Review of Psychological Risk Factors in Back and Neck Pain. Spine 2000, 25, 1148–1156. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Matalliotakis, I.; Cakmak, H.; Matalliotakis, M.; Kappou, D.; Arici, A. High rate of allergies among women with endometriosis. J. Obstet. Gynaecol. 2012, 32, 291–293. [Google Scholar] [CrossRef]
- Yuk, J.-S.; Kim, Y.J.; Yi, K.-W.; Tak, K.; Hur, J.-Y.; Shin, J.-H. High rate of nickel allergy in women with endometriosis: A 3-year population-based study. J. Obstet. Gynaecol. Res. 2015, 41, 1255–1259. [Google Scholar] [CrossRef]
- Bungum, H.F.; Vestergaard, C.; Knudsen, U.B. Endometriosis and type 1 allergies/immediate type hypersensitivity: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 209–215. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [CrossRef] [PubMed]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to Know Endometriosis-Related Infertility Better: A Review on How Endometriosis Affects Oocyte Quality and Embryo Development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Wender-Ozegowska, E.; Kedzia, M. Epigenetic Factors in Eutopic Endometrium in Women with Endometriosis and Infertility. Int. J. Mol. Sci. 2022, 23, 3804. [Google Scholar] [CrossRef]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- De Graaff, A.; Van Lankveld, J.; Smits, L.; Van Beek, J.; Dunselman, G. Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum. Reprod. 2016, 31, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, C.; D'Hooghe, T. Endometriosis and infertility: Insights into the causal link and management strategies. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 51, 25–33. [Google Scholar] [CrossRef]
- Atkins, H.M.; Appt, S.; Taylor, R.N.; Torres-Mendoza, Y.; Lenk, E.; Rosenthal, N.S.; Caudell, D.L. Systemic Iron Deficiency in a Nonhuman Primate Model of Endometriosis. Comp. Med. 2018, 68, 298–307. [Google Scholar] [CrossRef]
- Petraglia, F.; Dolmans, M.M. Iron deficiency anemia: Impact on women’s reproductive health. Fertil. Steril. 2022, 118, 605–606. [Google Scholar] [CrossRef]
- Gemmill, J.A.L.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil. Steril. 2010, 94, 1627–1631. [Google Scholar] [CrossRef]
- Miller, J.A.; Missmer, S.A.; Vitonis, A.F.; Sarda, V.; Laufer, M.R.; DiVasta, A.D. Prevalence of migraines in adolescents with endometriosis. Fertil. Steril. 2018, 109, 685–690. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Chen, S.; Lin, Y.; Xie, X.; Zhong, G.; Zhang, Q. Migraine Is More Prevalent in Advanced-Stage Endometriosis, Especially When Co-Occuring with Adenomoysis. Front. Endocrinol. 2022, 12, 814474. [Google Scholar] [CrossRef]
- Bandara, S.M.R.; Samita, S.; Kiridana, A.M.; Herath, H.M.M.T.B. Elevated nitric oxide and carbon monoxide concentration in nasal-paranasal sinus air as a diagnostic tool of migraine: A case—Control study. BMC Neurol. 2021, 21, 407. [Google Scholar] [CrossRef]
- Maitrot-Mantelet, L.; Hugon-Rodin, J.; Vatel, M.; Marcellin, L.; Santulli, P.; Chapron, C.; Plu-Bureau, G. Migraine in relation with endometriosis phenotypes: Results from a French case-control study. Cephalalgia 2019, 40, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R.; Gillespie, N.G.; Merikangas, K.R.; Treloar, S.A.; Martin, N.G.; Montgomery, G.W. Common genetic influences underlie comorbidity of migraine and endometriosis. Genet. Epidemiol. 2008, 33, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Overeem, L.H.; Mecklenburg, J.; Hofacker, M.D.; Knoth, H.; Nowak, C.P.; Neeb, L.; Ebert, A.D.; Sehouli, J.; Mechsner, S.; et al. Plasma calcitonin gene-related peptide (CGRP) in migraine and endometriosis during the menstrual cycle. Ann. Clin. Transl. Neurol. 2021, 8, 1251–1259. [Google Scholar] [CrossRef]
- Bora, G.; Yaba, A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1610–1623. [Google Scholar] [CrossRef]
- Signorile, P.G.; Cassano, M.; Viceconte, R.; Marcattilj, V.; Baldi, A. Endometriosis: A Retrospective Analysis of Clinical Data from a Cohort of 4,083 Patients, With Focus on Symptoms. In Vivo 2022, 36, 874–883. [Google Scholar] [CrossRef]
- Mormile, R.; Vittori, G. Endometriosis and migraine: What is there behind the scenes? J. Pediatr. Endocrinol. Metab. 2014, 27, 389–390. [Google Scholar] [CrossRef]
- Webster, A.J.; Gaitskell, K.; Turnbull, I.; Cairns, B.J.; Clarke, R. Characterisation, identification, clustering, and classification of disease. Sci. Rep. 2021, 11, 5405. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. New Insights in Pathogenesis of Endometriosis. Front. Med. 2022, 9, 879015. [Google Scholar] [CrossRef]
- Sarría-Santamera, A.; Khamitova, Z.; Gusmanov, A.; Terzic, M.; Polo-Santos, M.; Ortega, M.A.; Asúnsolo, A. History of Endometriosis Is Independently Associated with an Increased Risk of Ovarian Cancer. J. Pers. Med. 2022, 12, 1337. [Google Scholar] [CrossRef] [PubMed]
- Secosan, C.; Balulescu, L.; Brasoveanu, S.; Balint, O.; Pirtea, P.; Dorin, G.; Pirtea, L. Endometriosis in Menopause—Renewed Attention on a Controversial Disease. Diagnostics 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]

| Cases | % | |
| Age | ||
| Mean | ||
| 21–30 | 368 | 9.08 |
| 31–40 | 1555 | 38.35 |
| 41–50 | 2132 | 52.58 |
| Comorbidities | Cases | % |
| Infertility | 404 | 9.96 |
| UTI/Cystitis | 313 | 7.72 |
| Uterine fibromyoma | 275 | 6.78 |
| Upper respiratory tract infection | 836 | 20.62 |
| Vulvovaginitis | 449 | 11.07 |
| Obesity | 284 | 7.00 |
| Allergy | 321 | 7.92 |
| Asthma | 335 | 8.26 |
| Chronic/Allergic rhinitis | 695 | 17.14 |
| Thyroid disorders | 412 | 10.16 |
| High cholesterol | 527 | 13.00 |
| Smoking | 434 | 10.70 |
| Adaptation syndrome | 370 | 9.12 |
| Abdominal pain | 207 | 5.10 |
| Constipation | 244 | 6.02 |
| Urticaria | 353 | 8.71 |
| Contact dermatitis/Eczema | 625 | 15.41 |
| Vitamin/Nutrient deficiency | 203 | 5.01 |
| Anemia | 415 | 10.23 |
| Sleep disorder | 285 | 7.03 |
| Back pain | 595 | 14.67 |
| Bursitis/Tendinitis | 632 | 15.59 |
| Depression | 255 | 6.29 |
| Hypertension | 298 | 7.35 |
| Headache/Migraine | 1023 | 25.23 |
| Anxiety | 1060 | 26.14 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
|---|---|---|---|---|---|---|
| Number of cases | 1212 | 283 | 1334 | 534 | 331 | 361 |
| % | 29.89 | 6.98 | 32.90 | 13.17 | 8.16 | 8.90 |
| Visits to general practitioners (mean) | 8.1 | 28.3 | 13.1 | 14.1 | 14.4 | 14.6 |
| Visits to specialists (mean) | 10.2 | 20.2 | 11.8 | 12.4 | 8.5 | 14.0 |
| Comorbidities (mean) | 1.40 | 9.72 | 3.96 | 4.14 | 3.82 | 4.06 |
| Age (mean) | 39.9 | 41.6 | 41.1 | 39.5 | 39.1 | 39 |
| Age groups (%) | ||||||
| 21–30 | 10.07 | 6.36 | 6.30 | 12.36 | 7.25 | 14.96 |
| 31–40 | 38.94 | 32.16 | 34.63 | 42.13 | 49.55 | 39.06 |
| 41–50 | 50.99 | 61.48 | 59.07 | 45.51 | 43.20 | 45.98 |
| Comorbidity (%) | ||||||
| Infertility | 2.72 | 11.66 | 5.70 | 7.87 | 58.91 | 6.93 |
| Urinary tract infection/Cystitis | 13.37 | 20.85 | 3.67 | 4.31 | 4.53 | 1.39 |
| Uterine fibromyoma | 7.92 | 15.90 | 5.85 | 4.68 | 7.55 | 1.66 |
| Upper respiratory tract infection | 16.50 | 51.59 | 18.44 | 16.85 | 21.15 | 23.27 |
| Vulvovaginitis | 3.71 | 22.97 | 13.79 | 9.93 | 10.57 | 18.56 |
| Obesity | 0.66 | 26.15 | 11.62 | 3.56 | 5.14 | 3.05 |
| Allergy | 0.99 | 24.73 | 4.12 | 27.34 | 5.74 | 5.26 |
| Asthma | 0.99 | 14.84 | 5.47 | 35.58 | 3.63 | 1.66 |
| Thyroid disorders | 3.63 | 6.71 | 18.89 | 6.93 | 11.78 | 5.82 |
| High cholesterol | 3.71 | 31.80 | 17.47 | 14.04 | 5.74 | 18.01 |
| Smoking | 3.22 | 20.85 | 17.39 | 8.61 | 6.34 | 10.25 |
| Adaptation syndrome | 10.15 | 16.25 | 7.57 | 9.55 | 4.83 | 9.14 |
| Abdominal pain | 5.86 | 10.25 | 3.37 | 5.81 | 1.81 | 6.93 |
| Constipation | 1.49 | 18.37 | 9.30 | 5.24 | 2.72 | 3.60 |
| Urticaria | 3.05 | 24.38 | 4.12 | 30.71 | 3.32 | 4.71 |
| Vitamin/Nutrient deficiency | 4.54 | 9.54 | 4.65 | 6.18 | 3.93 | 3.60 |
| Anemia | 2.15 | 31.10 | 4.65 | 7.30 | 54.68 | 5.26 |
| Sleep disorder | 1.49 | 32.51 | 9.97 | 3.93 | 3.93 | 2.22 |
| Bursitis/Tendinitis | 1.90 | 44.88 | 22.26 | 15.54 | 9.67 | 19.39 |
| Depression | 0.91 | 26.86 | 8.17 | 4.12 | 6.04 | 4.71 |
| Hypertension | 1.90 | 26.15 | 12.52 | 2.62 | 3.63 | 2.22 |
| Headache/Migraine | 7.26 | 68.55 | 14.92 | 23.22 | 21.15 | 96.40 |
| Anxiety | 4.70 | 73.85 | 37.48 | 23.97 | 20.24 | 27.42 |
| Back pain | 2.97 | 43.82 | 22.56 | 12.55 | 13.29 | 6.37 |
| Chronic/Allergic rhinitis | 2.72 | 48.06 | 10.42 | 49.06 | 16.62 | 19.39 |
| Contact dermatitis/Eczema | 11.39 | 34.28 | 12.44 | 13.30 | 19.03 | 24.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarria-Santamera, A.; Yemenkhan, Y.; Terzic, M.; Ortega, M.A.; Asunsolo del Barco, A. A Novel Classification of Endometriosis Based on Clusters of Comorbidities. Biomedicines 2023, 11, 2448. https://doi.org/10.3390/biomedicines11092448
Sarria-Santamera A, Yemenkhan Y, Terzic M, Ortega MA, Asunsolo del Barco A. A Novel Classification of Endometriosis Based on Clusters of Comorbidities. Biomedicines. 2023; 11(9):2448. https://doi.org/10.3390/biomedicines11092448
Chicago/Turabian StyleSarria-Santamera, Antonio, Yerden Yemenkhan, Milan Terzic, Miguel A. Ortega, and Angel Asunsolo del Barco. 2023. "A Novel Classification of Endometriosis Based on Clusters of Comorbidities" Biomedicines 11, no. 9: 2448. https://doi.org/10.3390/biomedicines11092448
APA StyleSarria-Santamera, A., Yemenkhan, Y., Terzic, M., Ortega, M. A., & Asunsolo del Barco, A. (2023). A Novel Classification of Endometriosis Based on Clusters of Comorbidities. Biomedicines, 11(9), 2448. https://doi.org/10.3390/biomedicines11092448









