Cognitive Functions following Trigeminal Neuromodulation
Abstract
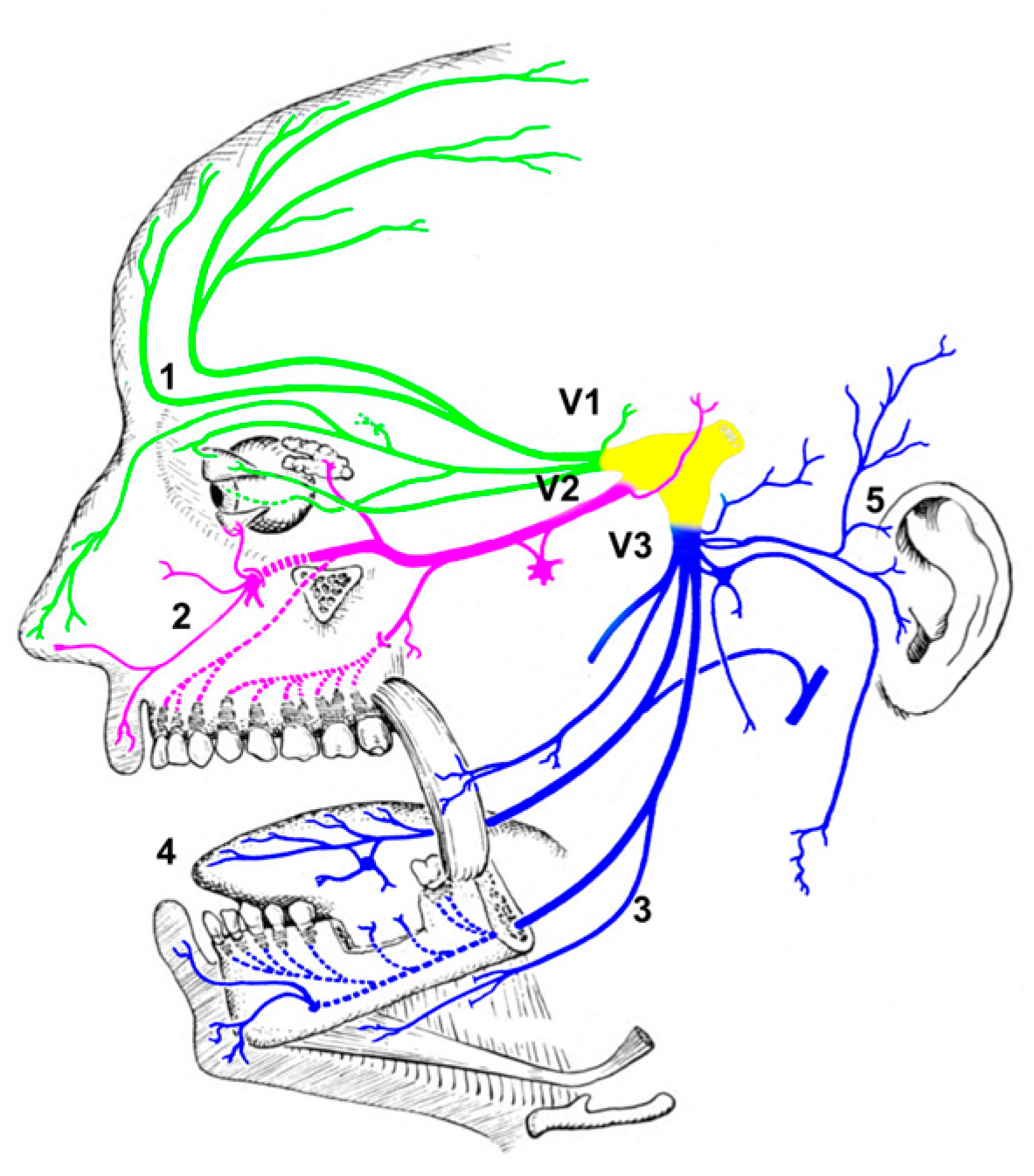
1. Introduction
2. Trigeminal Nerve Stimulation: Use and Safety
3. How Trigeminal Nerve Stimulation May Affect Cognitive Function
| Citation | Study Population | Stimulated Nerve | TNS Parameters | Time of Assessment | Assessment Methodology | Outcome | Side Effects |
|---|---|---|---|---|---|---|---|
| Piquet et al., 2011 [82] | HS | Supraorbital nerve, bilateral |
| After stimulation | Psychological test | Modulation of vigilance | No major side effects reported |
| Boasso et al., 2016 [48] | HS | Right supraorbital nerve, Cervical nerve |
| After one week of treatment | Psychological test Biochemical measurements | Improved sleep quality, stress reduction | No major side effects reported |
| Trevizol et al., 2016 [40] | NPD | Supraorbital nerve, bilateral |
| After 10 days of treatment | Psychological test | No significant difference in cognitive performances | No major side effects reported |
| Loo et al., 2020 [36] | ADHD | Supraorbital nerve, bilateral |
| After 4 weeks of treatment | Psychological test Resting state EEG | Improved/normalized executive functions, modulation of right frontal brain activity | Increase in fatigue, headache, appetite. Skin whitening/discoloration under electrode patch |
| Tramonti Fantozzi et al., 2020 [93] | HS | Mandibular motor branch |
| After stimulation | Psychological test Pupil size | Trigeminal sensory-motor imbalance may affect cognitive performance | No major side effects reported |
| Tramonti Fantozzi et al., 2021 [91] | HS | Mandibular motor branch |
| After stimulation | Pupil size EEG power change ERP (auditory oddball) | Reduced P300 amplitude, positive correlation between P300 and pupil size | No major side effects reported |
| Mercante et al., 2023 [92] | HS | Infraorbital nerve, bilateral |
| After stimulation | ERP (visual oddball, sensory gating) | No changes in ERP parameters measured | No major effects reported |
4. New Possibilities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Rooij, S.J.H.; Riva-Posse, P.; McDonald, W.M. The Efficacy and Safety of Neuromodulation Treatments in Late-Life Depression. Curr. Treat. Options Psychiatry 2020, 7, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Walsh, D. Transcutaneous Electrical Nerve Stimulation: Basic Science Mechanisms and Clinical Effectiveness. J. Pain 2003, 4, 109–121. [Google Scholar] [CrossRef]
- Adair, D.; Truong, D.; Esmaeilpour, Z.; Gebodh, N.; Borges, H.; Ho, L.; Bremner, J.D.; Badran, B.W.; Napadow, V.; Clark, V.P.; et al. Electrical Stimulation of Cranial Nerves in Cognition and Disease. Brain Stimul. 2020, 13, 717–750. [Google Scholar] [CrossRef] [PubMed]
- Pop, J.; Murray, D.; Markovic, D.; DeGiorgio, C.M. Acute and Long-Term Safety of External Trigeminal Nerve Stimulation for Drug-Resistant Epilepsy. Epilepsy Behav. 2011, 22, 574–576. [Google Scholar] [CrossRef]
- Darmani, G.; Bergmann, T.O.; Pauly, K.B.; Caskey, C.F.; de Lecea, L.; Fomenko, A.; Fouragnan, E.; Legon, W.; Murphy, K.R.; Nandi, T.; et al. Non-Invasive Transcranial Ultrasound Stimulation for Neuromodulation. Clin. Neurophysiol. 2022, 135, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Mercante, B.; Ginatempo, F.; Manca, A.; Melis, F.; Enrico, P.; Deriu, F. Anatomo-Physiologic Basis for Auricular Stimulation. Med. Acupunct. 2018, 30, 141–150. [Google Scholar] [CrossRef]
- Magis, D.; Sava, S.; d’Elia, T.S.; Baschi, R.; Schoenen, J. Safety and Patients’ Satisfaction of Transcutaneous Supraorbital NeuroStimulation (TSNS) with the Cefaly® Device in Headache Treatment: A Survey of 2313 Headache Sufferers in the General Population. J. Headache Pain 2013, 14, 95. [Google Scholar] [CrossRef]
- di Biase, L.; Falato, E.; Lazzaro, V.D. Transcranial Focused Ultrasound (TFUS) and Transcranial Unfocused Ultrasound (TUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front. Neurol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Marois, A.; Lafond, D. Augmenting Cognitive Work: A Review of Cognitive Enhancement Methods and Applications for Operational Domains. Cogn. Technol. Work 2022, 24, 589–608. [Google Scholar] [CrossRef]
- Davis, S.E.; Smith, G.A. Transcranial Direct Current Stimulation Use in Warfighting: Benefits, Risks, and Future Prospects. Front. Hum. Neurosci. 2019, 13, 114. [Google Scholar] [CrossRef]
- Clark, V.P.; Coffman, B.A.; Mayer, A.R.; Weisend, M.P.; Lane, T.D.R.; Calhoun, V.D.; Raybourn, E.M.; Garcia, C.M.; Wassermann, E.M. TDCS Guided Using FMRI Significantly Accelerates Learning to Identify Concealed Objects. NeuroImage 2012, 59, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sanches, C.; Stengel, C.; Godard, J.; Mertz, J.; Teichmann, M.; Migliaccio, R.; Valero-Cabré, A. Past, Present, and Future of Non-Invasive Brain Stimulation Approaches to Treat Cognitive Impairment in Neurodegenerative Diseases: Time for a Comprehensive Critical Review. Front. Aging Neurosci. 2021, 12, 578339. [Google Scholar] [CrossRef]
- Siegert, A.; Diedrich, L.; Antal, A. New Methods, Old Brains—A Systematic Review on the Effects of TDCS on the Cognition of Elderly People. Front. Hum. Neurosci. 2021, 15, 730134. [Google Scholar] [CrossRef] [PubMed]
- Ghacibeh, G.A.; Shenker, J.I.; Shenal, B.; Uthman, B.M.; Heilman, K.M. Effect of Vagus Nerve Stimulation on Creativity and Cognitive Flexibility. Epilepsy Behav. 2006, 8, 720–725. [Google Scholar] [CrossRef]
- Ghacibeh, G.A.; Shenker, J.I.; Shenal, B.; Uthman, B.M.; Heilman, K.M. The Influence of Vagus Nerve Stimulation on Memory. Cogn. Behav. Neurol. 2006, 19, 119–122. [Google Scholar] [CrossRef]
- Goellner, E.; Rocha, C.E. Anatomy of Trigeminal Neuromodulation Targets: From Periphery to the Brain. Neuromodul. Facial Pain 2020, 35, 18–34. [Google Scholar] [CrossRef]
- Salles, A.D.; Gorgulho, A. Influence of the Brain Function through the Peripheral Nerves. Surg. Neurol. Int. 2012, 3, 245. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, E.E.; Reid, A.P.; Nicolelis, M.A.L. Reduction of Pentylenetetrazole-Induced Seizure Activity in Awake Rats by Seizure-Triggered Trigeminal Nerve Stimulation. J. Neurosci. 2000, 20, 8160–8168. [Google Scholar] [CrossRef]
- Liporace, J.; Hucko, D.; Morrow, R.; Barolat, G.; Nei, M.; Schnur, J.; Sperling, M. Vagal Nerve Stimulation: Adjustments to Reduce Painful Side Effects. Neurology 2001, 57, 885–886. [Google Scholar] [CrossRef]
- Redgrave, J.; Day, D.; Leung, H.; Laud, P.J.; Ali, A.; Lindert, R.; Majid, A. Safety and Tolerability of Transcutaneous Vagus Nerve Stimulation in Humans; a Systematic Review. Brain Stimul. 2018, 11, 1225–1238. [Google Scholar] [CrossRef]
- Giordano, F.; Zicca, A.; Barba, C.; Guerrini, R.; Genitori, L. Vagus Nerve Stimulation: Surgical Technique of Implantation and Revision and Related Morbidity. Epilepsia 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Spuck, S.; Tronnier, V.; Orosz, I.; Schönweiler, R.; Sepehrnia, A.; Nowak, G.; Sperner, J. Operative and Technical Complications of Vagus Nerve Stimulator Implantation. Oper. Neurosurg. 2010, 67, ons489–ons494. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.E.; Yugrakh, M.S.; Winegarner, D.; Rowe, V.; Kuruvilla, D.; Schoenen, J. Acute Migraine Therapy with External Trigeminal Neurostimulation (ACME): A Randomized Controlled Trial. Cephalalgia 2018, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, O.; Mahjoub, A.; Osman, N.; Surmava, A.-M.; Jan, S.; Lagman-Bartolome, A.M. Non-Invasive Neuromodulation in the Acute Treatment of Migraine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neurol. Sci. 2021, 43, 153–165. [Google Scholar] [CrossRef]
- Conlon, B.; Hamilton, C.; Hughes, S.; Meade, E.; Hall, D.A.; Vanneste, S.; Langguth, B.; Lim, H.H. Noninvasive Bimodal Neuromodulation for the Treatment of Tinnitus: Protocol for a Second Large-Scale Double-Blind Randomized Clinical Trial to Optimize Stimulation Parameters. Jmir. Res. Protoc. 2019, 8, e13176. [Google Scholar] [CrossRef]
- Cook, I.A.; Abrams, M.; Leuchter, A.F. Trigeminal Nerve Stimulation for Comorbid Posttraumatic Stress Disorder and Major Depressive Disorder. Neuromodul. Technol. Neural Interface 2016, 19, 299–305. [Google Scholar] [CrossRef]
- Cook, I.A.; Schrader, L.M.; DeGiorgio, C.M.; Miller, P.R.; Maremont, E.R.; Leuchter, A.F. Trigeminal Nerve Stimulation in Major Depressive Disorder: Acute Outcomes in an Open Pilot Study. Epilepsy Behav. 2013, 28, 221–226. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Fanselow, E.E.; Schrader, L.M.; Cook, I.A. Trigeminal Nerve Stimulation: Seminal Animal and Human Studies for Epilepsy and Depression. Neurosurg. Clin. N. Am. 2011, 22, 449–456. [Google Scholar] [CrossRef]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411.e3. [Google Scholar] [CrossRef]
- Generoso, M.B.; Taiar, I.T.; Garrocini, L.P.; Bernardon, R.; Cordeiro, Q.; Uchida, R.R.; Shiozawa, P. Effect of a 10-Day Transcutaneous Trigeminal Nerve Stimulation (TNS) Protocol for Depression Amelioration: A Randomized, Double Blind, and Sham-Controlled Phase II Clinical Trial. Epilepsy Behav. 2019, 95, 39–42. [Google Scholar] [CrossRef]
- Gil-López, F.; Boget, T.; Manzanares, I.; Donaire, A.; Conde-Blanco, E.; Baillés, E.; Pintor, L.; Setoaín, X.; Bargalló, N.; Navarro, J.; et al. External Trigeminal Nerve Stimulation for Drug Resistant Epilepsy: A Randomized Controlled Trial. Brain Stimul. 2020, 13, 1245–1253. [Google Scholar] [CrossRef]
- Ginatempo, F.; Fois, C.; Carli, F.D.; Todesco, S.; Mercante, B.; Sechi, G.; Deriu, F. Effect of Short-Term Transcutaneous Trigeminal Nerve Stimulation on EEG Activity in Drug-Resistant Epilepsy. J. Neurol. Sci. 2019, 400, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Olivié-García, L.; Giraldez, B.G.; Sierra-Marcos, A.; Díaz-Gómez, E.; Serratosa, J.M. External Trigeminal Nerve Stimulation: A Long Term Follow up Study. Seizure 2019, 69, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, P.; da Silva, M.E.; de Carvalho, T.C.; Cordeiro, Q.; Brunoni, A.R.; Fregni, F. Transcutaneous Vagus and Trigeminal Nerve Stimulation for Neuropsychiatric Disorders: A Systematic Review. Arq. Neuro-Psiquiat. 2014, 72, 542–547. [Google Scholar] [CrossRef]
- Koek, R.J.; Roach, J.; Athanasiou, N.; van’t Wout-Frank, M.; Philip, N.S. Neuromodulatory Treatments for Post-Traumatic Stress Disorder (PTSD). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Salgari, G.C.; Ellis, A.; Cowen, J.; Dillon, A.; McGough, J.J. Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder: Cognitive and Electrophysiological Predictors of Treatment Response. J. Am. Acad. Child Adolesc. Psychiatry 2020, 60, 856–864.e1. [Google Scholar] [CrossRef]
- Slaght, S.J.; Nashef, L. An Audit of External Trigeminal Nerve Stimulation (ETNS) in Epilepsy. Seizure 2017, 52, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Pieton, D.; Pezeshkian, P.; Miller, P.; Gorgulho, A.A.; Pouratian, N.; Salles, A.A.F.D. Surgical Approaches to Tinnitus Treatment: A Review and Novel Approaches. Surg. Neurol. Int. 2011, 2, 154. [Google Scholar] [CrossRef]
- Soss, J.; Heck, C.; Murray, D.; Markovic, D.; Oviedo, S.; Corrale-Leyva, G.; Gordon, S.; Kealey, C.; DeGiorgio, C. A Prospective Long-Term Study of External Trigeminal Nerve Stimulation for Drug-Resistant Epilepsy. Epilepsy Behav. 2015, 42, 44–47. [Google Scholar] [CrossRef]
- Trevizol, A.; Bonadia, B.; Gomes, J.S.; Cordeiro, Q.; Shiozawa, P. Integrity of Cognitive Functions in Trigeminal Nerve Stimulation Trials in Neuropsychiatry. Trends Psychiatry Psychother. 2016, 38, 60–61. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Sato, I.A.; Cook, I.A.; Shiozawa, P.; Lowenthal, R.; Cordeiro, Q. Trigeminal Nerve Stimulation (TNS) for Posttraumatic Stress Disorder and Major Depressive Disorder: An Open-Label Proof-of-Concept Trial. Epilepsy Behav. 2016, 60, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Canady, V.A. FDA Approves Marketing of First Nondrug Treatment for ADHD. Ment. Health Wkly. 2019, 29, 3–4. [Google Scholar] [CrossRef]
- Lauritsen, C.G.; Silberstein, S.D. Rationale for Electrical Parameter Determination in External Trigeminal Nerve Stimulation (ETNS) for Migraine: A Narrative Review. Cephalalgia 2018, 39, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J.; Coppola, G. Efficacy and Mode of Action of External Trigeminal Neurostimulation in Migraine. Expert Rev. Neurother. 2018, 18, 545–555. [Google Scholar] [CrossRef]
- Konstantinos, S.; Vikelis, M.; Rapoport, A. Acute Care and Treatment of Migraine. J. Neuro-Ophthalmol. 2020, 40, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Nune, G.; DeGiorgio, C.; Heck, C. Neuromodulation in the Treatment of Epilepsy. Curr. Treat. Option Neurol. 2015, 17, 43. [Google Scholar] [CrossRef]
- Colzato, L.S.; Vonck, K. Theory-Driven Approaches to Cognitive Enhancement; Springer International Publishing: Cham, Switzerland, 2017; pp. 115–126. [Google Scholar] [CrossRef]
- Boasso, A.M.; Mortimore, H.; Silva, R.; Aven, L.; Tyler, W.J. Transdermal Electrical Neuromodulation of the Trigeminal Sensory Nuclear Complex Improves Sleep Quality and Mood. Biorxiv 2016, 043901. [Google Scholar] [CrossRef]
- Neisser. Cognition and Reality: Principles and Implications of Cognitive Psychology; W.H. Freeman and Co.: San Francisco, CA, USA, 1976. [Google Scholar]
- Bayne, T.; Brainard, D.; Byrne, R.W.; Chittka, L.; Clayton, N.; Heyes, C.; Mather, J.; Ölveczky, B.; Shadlen, M.; Suddendorf, T.; et al. What Is Cognition? Curr. Biol. 2019, 29, R608–R615. [Google Scholar] [CrossRef]
- Beaty, R.E.; Benedek, M.; Silvia, P.J.; Schacter, D.L. Creative Cognition and Brain Network Dynamics. Trends Cogn. Sci. 2016, 20, 87–95. [Google Scholar] [CrossRef]
- Park, H.-J.; Friston, K. Structural and Functional Brain Networks: From Connections to Cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef]
- Petersen, S.E.; Sporns, O. Brain Networks and Cognitive Architectures. Neuron 2015, 88, 207–219. [Google Scholar] [CrossRef]
- Cohen, J.R.; D’Esposito, M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef]
- Hearne, L.J.; Cocchi, L.; Zalesky, A.; Mattingley, J.B. Reconfiguration of Brain Network Architectures between Resting-State and Complexity-Dependent Cognitive Reasoning. J. Neurosci. 2017, 37, 8399–8411. [Google Scholar] [CrossRef]
- Luckey, A.M.; McLeod, L.S.; Huang, Y.; Mohan, A.; Vanneste, S. Making Memories Last Using the Peripheral Effect of Direct Current Stimulation. eLife 2023, 12, e75586. [Google Scholar] [CrossRef]
- Luckey, A.M.; Adcock, K.; Vanneste, S. Peripheral Nerve Stimulation: A Neuromodulation-Based Approach. Neurosci. Biobehav. Rev. 2023, 149, 105180. [Google Scholar] [CrossRef]
- Joo, W.; Yoshioka, F.; Funaki, T.; Mizokami, K.; Rhoton, A.L. Microsurgical Anatomy of the Trigeminal Nerve. Clin. Anat. 2014, 27, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Mihailoff, G.A. Chapter 14—A Synopsis of Cranial Nerves of the Brainstem. In Fundamental Neuroscience for Basic and Clinical Applications, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–211. [Google Scholar] [CrossRef]
- Ricardo, J.A.; Koh, E.T. Anatomical Evidence of Direct Projections from the Nucleus of the Solitary Tract to the Hypothalamus, Amygdala, and Other Forebrain Structures in the Rat. Brain Res. 1978, 153, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mercante, B.; Enrico, P.; Floris, G.; Quartu, M.; Boi, M.; Serra, M.P.; Follesa, P.; Deriu, F. Trigeminal Nerve Stimulation Induces Fos Immunoreactivity in Selected Brain Regions, Increases Hippocampal Cell Proliferation and Reduces Seizure Severity in Rats. Neuroscience 2017, 361, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, D.J.; Mackay, E.W.; Hamlin, A.S.; Marathe, S.V.; Nandam, L.S.; Vaidya, V.A.; Bartlett, P.F. Norepinephrine Directly Activates Adult Hippocampal Precursors via 3-Adrenergic Receptors. J. Neurosci. 2010, 30, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased Extracellular Concentrations of Norepinephrine in Cortex and Hippocampus Following Vagus Nerve Stimulation in the Rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef]
- Follesa, P.; Biggio, F.; Gorini, G.; Caria, S.; Talani, G.; Dazzi, L.; Puligheddu, M.; Marrosu, F.; Biggio, G. Vagus Nerve Stimulation Increases Norepinephrine Concentration and the Gene Expression of BDNF and BFGF in the Rat Brain. Brain Res. 2007, 1179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Zhu, L.-J.; Wang, X.-H.; Zuo, J.; He, H.-Y.; Tian, M.-M.; Wang, L.; Liang, G.-L.; Wang, Y. Chronic Trigeminal Nerve Stimulation Protects Against Seizures, Cognitive Impairments, Hippocampal Apoptosis, and Inflammatory Responses in Epileptic Rats. J. Mol. Neurosci. 2016, 59, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, S.; Huo, L.; Zhang, Q.; Liu, L.; Ye, Z.; Cao, J.; Ma, H.; Shang, C.; Ma, C. Trigeminal Nerve Stimulation Restores Hippocampal Dopamine Deficiency to Promote Cognitive Recovery in Traumatic Brain Injury. Prog. Neurobiol. 2023, 227, 102477. [Google Scholar] [CrossRef]
- Axelson, H.W.; Isberg, M.; Flink, R.; Amandusson, Å. Trigeminal Nerve Stimulation Does Not Acutely Affect Cortical Excitability in Healthy Subjects. Brain Stimul. 2014, 7, 613–617. [Google Scholar] [CrossRef]
- Mercante, B.; Pilurzi, G.; Ginatempo, F.; Manca, A.; Follesa, P.; Tolu, E.; Deriu, F. Trigeminal Nerve Stimulation Modulates Brainstem More than Cortical Excitability in Healthy Humans. Exp. Brain Res. 2015, 233, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Pilurzi, G.; Mercante, B.; Ginatempo, F.; Follesa, P.; Tolu, E.; Deriu, F. Transcutaneous Trigeminal Nerve Stimulation Induces a Long-Term Depression-like Plasticity of the Human Blink Reflex. Exp. Brain Res. 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Mercante, B.; Loi, N.; Ginatempo, F.; Biggio, M.; Manca, A.; Bisio, A.; Enrico, P.; Bove, M.; Deriu, F. Transcutaneous Trigeminal Nerve Stimulation Modulates the Hand Blink Reflex. Sci. Rep. 2020, 10, 21116. [Google Scholar] [CrossRef] [PubMed]
- Sambo, C.F.; Forster, B.; Williams, S.C.; Iannetti, G.D. To Blink or Not to Blink: Fine Cognitive Tuning of the Defensive Peripersonal Space. J. Neurosci. 2012, 32, 12921–12927. [Google Scholar] [CrossRef]
- Ginatempo, F.; Carli, F.D.; Todesco, S.; Mercante, B.; Sechi, G.P.; Deriu, F. Effects of Acute Trigeminal Nerve Stimulation on Rest EEG Activity in Healthy Adults. Exp. Brain Res. 2018, 236, 2839–2845. [Google Scholar] [CrossRef]
- Ritland, B.M.; Neumeier, W.H.; Jiang, S.H.; Smith, C.D.; Heaton, K.J.; Hildebrandt, A.M.; Jabbar, M.A.; Liao, H.J.; Coello, E.; Zhao, W.; et al. Short-term Neurochemical Effects of Transcutaneous Trigeminal Nerve Stimulation Using 7T Magnetic Resonance Spectroscopy. J. Neuroimaging 2022, 33, 279–288. [Google Scholar] [CrossRef]
- Ferguson, K.J.; MacLullich, A.M.J.; Marshall, I.; Deary, I.J.; Starr, J.M.; Seckl, J.R.; Wardlaw, J.M. Magnetic Resonance Spectroscopy and Cognitive Function in Healthy Elderly Men. Brain 2002, 125, 2743–2749. [Google Scholar] [CrossRef]
- GOADSBY, P.J.; HOSKIN, K.L. The Distribution of Trigeminovascular Afferents in the Nonhuman Primate Brain Macaca Nemestrina: A C-fos Immunocytochemical Study. J. Anat. 1997, 190, 367–375. [Google Scholar] [CrossRef]
- Kumada, M.; Dampney, R.A.L.; Reis, D.J. The Trigeminal Depressor Response: A Novel Vasodepressor Response Originating from the Trigeminal System. Brain Res. 1977, 119, 305–326. [Google Scholar] [CrossRef]
- Chiluwal, A.; Narayan, R.K.; Chaung, W.; Mehan, N.; Wang, P.; Bouton, C.E.; Golanov, E.V.; Li, C. Neuroprotective Effects of Trigeminal Nerve Stimulation in Severe Traumatic Brain Injury. Sci. Rep. 2017, 7, 6792. [Google Scholar] [CrossRef]
- Golanov, E.V. Forehead Stimulation Decreases Volume of the Infarction Triggered by Permanent Occlusion of Middle Cerebral Artery in Rats. J. Neurol. Stroke 2015, 2, 15406. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Chiluwal, A.; Afridi, A.; Chaung, W.; Powell, K.; Yang, W.-L.; Wang, P.; Narayan, R.K. Trigeminal Nerve Stimulation: A Novel Method of Resuscitation for Hemorrhagic Shock. Crit. Care Med. 2019, 47, e478–e484. [Google Scholar] [CrossRef]
- Mercante, B.; Nuvoli, S.; Sotgiu, M.A.; Manca, A.; Todesco, S.; Melis, F.; Spanu, A.; Deriu, F. SPECT Imaging of Cerebral Blood Flow Changes Induced by Acute Trigeminal Nerve Stimulation in Drug-Resistant Epilepsy. A Pilot Study. Clin. Neurophysiol. 2021, 132, 1274–1282. [Google Scholar] [CrossRef]
- White, T.G.; Powell, K.; Shah, K.A.; Woo, H.H.; Narayan, R.K.; Li, C. Trigeminal Nerve Control of Cerebral Blood Flow: A Brief Review. Front. Neurosci. 2021, 15, 649910. [Google Scholar] [CrossRef]
- Piquet, M.; Balestra, C.; Sava, S.L.; Schoenen, J.E. Supraorbital Transcutaneous Neurostimulation Has Sedative Effects in Healthy Subjects. BMC Neurol. 2011, 11, 135. [Google Scholar] [CrossRef]
- Ginatempo, F.; Pirina, P.; Melis, F.; Deriu, F. Short-Term Trigeminal Neuromodulation Does Not Alter Sleep Latency in Healthy Subjects: A Pilot Study. Neurol. Sci. 2018, 39, 145–147. [Google Scholar] [CrossRef]
- Cicco, V.D.; Fantozzi, M.P.T.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Boasso, A.M.; Mortimore, H.M.; Silva, R.S.; Charlesworth, J.D.; Marlin, M.A.; Aebersold, K.; Aven, L.; Wetmore, D.Z.; Pal, S.K. Transdermal Neuromodulation of Noradrenergic Activity Suppresses Psychophysiological and Biochemical Stress Responses in Humans. Sci. Rep. 2015, 5, 13865. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, R.F.; Knight, R.T. Chapter 36 Cognitive Neurophysiology: Event-Related Potentials. Handb. Clin. Neurol. 2019, 160, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J. An Introduction to the Event-Related Potential Technique, 2nd ed.; The MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Wongupparaj, P.; Sumich, A.; Wickens, M.; Kumari, V.; Morris, R.G. Individual Differences in Working Memory and General Intelligence Indexed by P200 and P300: A Latent Variable Model. Biol. Psychol. 2018, 139, 96–105. [Google Scholar] [CrossRef]
- Verleger, R. Effects of Relevance and Response Frequency on P3b Amplitudes: Review of Findings and Comparison of Hypotheses about the Process Reflected by P3b. Psychophysiology 2020, 57, e13542. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Knight, R.T. Mechanisms of Human Attention: Event-Related Potentials and Oscillations. Neurosci. Biobehav. Rev. 2001, 25, 465–476. [Google Scholar] [CrossRef]
- Fantozzi, M.P.T.; Artoni, F.; Galante, M.D.; Briscese, L.; Cicco, V.D.; Bruschini, L.; d’Ascanio, P.; Manzoni, D.; Faraguna, U.; Carboncini, M.C. Effect of the Trigeminal Nerve Stimulation on Auditory Event-Related Potentials. Cereb. Cortex Commun. 2021, 2, tgab012. [Google Scholar] [CrossRef]
- Mercante, B.; Enrico, P.; Ginatempo, F.; Loi, N.; Deriu, F. Short-Term Transcutaneous Trigeminal Nerve Stimulation Does Not Affect Visual Oddball Task and Paired-Click Paradigm ERP Responses in Healthy Volunteers. Exp. Brain Res. 2022, 241, 327–339. [Google Scholar] [CrossRef]
- Fantozzi, M.P.T.; Cicco, V.D.; Argento, S.; Cicco, D.D.; Barresi, M.; Cataldo, E.; Bruschini, L.; d’Ascanio, P.; Faraguna, U.; Manzoni, D. Trigeminal Input, Pupil Size and Cognitive Performance: From Oral to Brain Matter. Brain Res. 2020, 1751, 147194. [Google Scholar] [CrossRef]
- Dunn, B.R.; Dunn, D.A.; Languis, M.; Andrews, D. The Relation of ERP Components to Complex Memory Processing. Brain Cogn. 1998, 36, 355–376. [Google Scholar] [CrossRef]
- Sara, S.J. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M. The Ascending Reticular Activating System. Neurocrit. Care 2019, 31, 419–422. [Google Scholar] [CrossRef]
- Monaco, A.; Cattaneo, R.; Smurra, P.; Nicolantonio, S.D.; Cipriano, F.; Pietropaoli, D.; Ortu, E. Trigeminal Electrical Stimulation with ULFTENS of the Dorsal Anterior Mucosal Surface of the Tongue: Effects on Heart Rate Variability (HRV). PLoS ONE 2023, 18, e0285464. [Google Scholar] [CrossRef]
- Danilov, Y.P.; Tyler, M.E.; Kaczmarek, K.A.; Skinner, K.L. New Approach to Neurorehabilitation: Cranial Nerve Noninvasive Neuromodulation (CN-NINM) Technology. In Sensing Technologies for Global Health, Military Medicine, and Environmental Monitoring IV; SPIE: Bellingham, UK, 2014; pp. 91120L–91120L-10. [Google Scholar] [CrossRef]
- Daniel, O.; Sharon, R.; Tepper, S.J. A Device Review of Relivion®: An External Combined Occipital and Trigeminal Neurostimulation (ECOT-NS) System for Self-Administered Treatment of Migraine and Major Depressive Disorder. Expert Rev. Med. Devic. 2021, 18, 333–342. [Google Scholar] [CrossRef]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The Anatomical Basis for Transcutaneous Auricular Vagus Nerve Stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Peuker, E.T.; Filler, T.J. The Nerve Supply of the Human Auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Mercante, B.; Deriu, F.; Rangon, C.-M. Auricular Neuromodulation: The Emerging Concept beyond the Stimulation of Vagus and Trigeminal Nerves. Medicines 2018, 5, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Hu, L. Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness. Int. J. Environ. Res. Public Health 2023, 20, 1402. [Google Scholar] [CrossRef]
- Gurtubay, I.G.; Perez-Rodriguez, D.R.; Fernandez, E.; Librero-Lopez, J.; Calvo, D.; Bermejo, P.; Pinin-Osorio, C.; Lopez, M. Immediate Effects and Duration of a Short and Single Application of Transcutaneous Auricular Vagus Nerve Stimulation on P300 Event Related Potential. Front. Neurosci. 2023, 17, 1096865. [Google Scholar] [CrossRef]
- Konjusha, A.; Yu, S.; Mückschel, M.; Colzato, L.; Ziemssen, T.; Beste, C. Auricular Transcutaneous Vagus Nerve Stimulation Specifically Enhances Working Memory Gate Closing Mechanism: A System Neurophysiological Study. J. Neurosci. 2023, 43, 4709–4724. [Google Scholar] [CrossRef]
- Smet, S.D.; Ottaviani, C.; Verkuil, B.; Kappen, M.; Baeken, C.; Vanderhasselt, M. Effects of Non-invasive Vagus Nerve Stimulation on Cognitive and Autonomic Correlates of Perseverative Cognition. Psychophysiology 2023, 60, e14250. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Fischer, R.; Borges, U.; Laborde, S.; Achtzehn, S.; Liepelt, R. The Effect of Transcutaneous Auricular Vagus Nerve Stimulation (TaVNS) on Cognitive Control in Multitasking. Neuropsychologia 2023, 187, 108614. [Google Scholar] [CrossRef] [PubMed]
- Hassert, D.L.; Miyashita, T.; Williams, C.L. The Effects of Peripheral Vagal Nerve Stimulation at a Memory-Modulating Intensity on Norepinephrine Output in the Basolateral Amygdala. Behav. Neurosci. 2004, 118, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Giraudier, M.; Ventura-Bort, C.; Weymar, M. Transcutaneous Vagus Nerve Stimulation (TVNS) Improves High-Confidence Recognition Memory but Not Emotional Word Processing. Front. Psychol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Giraudier, M.; Ventura-Bort, C.; Burger, A.M.; Claes, N.; D’Agostini, M.; Fischer, R.; Franssen, M.; Kaess, M.; Koenig, J.; Liepelt, R.; et al. Evidence for a Modulating Effect of Transcutaneous Auricular Vagus Nerve Stimulation (TaVNS) on Salivary Alpha-Amylase as Indirect Noradrenergic Marker: A Pooled Mega-Analysis. Brain Stimul. 2022, 15, 1378–1388. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 2020, 41, 320–330. [Google Scholar] [CrossRef]
- Ventura-Bort, C.; Wirkner, J.; Genheimer, H.; Wendt, J.; Hamm, A.O.; Weymar, M. Effects of Transcutaneous Vagus Nerve Stimulation (TVNS) on the P300 and Alpha-Amylase Level: A Pilot Study. Front. Hum. Neurosci. 2018, 12, 202. [Google Scholar] [CrossRef]
- Diep, D.; Lam, A.C.L.; Ko, G. A Review of the Evidence and Current Applications of Portable Translingual Neurostimulation Technology. Neuromodul. Technol. Neural Interface 2021, 24, 1377–1387. [Google Scholar] [CrossRef]
- Wildenberg, J.C.; Tyler, M.E.; Danilov, Y.P.; Kaczmarek, K.A.; Meyerand, M.E. Sustained Cortical and Subcortical Neuromodulation Induced by Electrical Tongue Stimulation. Brain Imaging Behav. 2010, 4, 199–211. [Google Scholar] [CrossRef]
- Danilov, Y.P. Translingual Neurostimulation (TLNS): A Novel Approach to Neurorehabilitation. J. Neurol. Neurophysiol. 2017, 8, 1117. [Google Scholar] [CrossRef]
- Spencer, S.; Mielczarek, M.; Olszewski, J.; Sereda, M.; Joossen, I.; Vermeersch, H.; Gilles, A.; Michiels, S. Effectiveness of Bimodal Auditory and Electrical Stimulation in Patients with Tinnitus: A Feasibility Study. Front. Neurosci. 2022, 16, 971633. [Google Scholar] [CrossRef]
- Conlon, B.; Langguth, B.; Hamilton, C.; Hughes, S.; Meade, E.; Connor, C.O.; Schecklmann, M.; Hall, D.A.; Vanneste, S.; Leong, S.L.; et al. Bimodal Neuromodulation Combining Sound and Tongue Stimulation Reduces Tinnitus Symptoms in a Large Randomized Clinical Study. Sci. Transl. Med. 2020, 12, eabb2830. [Google Scholar] [CrossRef]
- Frehlick, Z.; Lakhani, B.; Fickling, S.D.; Livingstone, A.C.; Danilov, Y.; Sackier, J.M.; D’Arcy, R.C.N. Human Translingual Neurostimulation Alters Resting Brain Activity in High-Density EEG. J. Neuroeng. Rehabil. 2019, 16, 60. [Google Scholar] [CrossRef]
- Hou, J.; Mohanty, R.; Chu, D.; Nair, V.A.; Danilov, Y.; Kaczmarek, K.A.; Meyerand, B.; Tyler, M.; Prabhakaran, V. Translingual Neural Stimulation Affects Resting-state Functional Connectivity in Mild-moderate Traumatic Brain Injury. J. Neuroimaging 2022, 32, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.D. The Trigeminal (V) and Facial (VII) Cranial Nerves: Head and Face Sensation and Movement. Psychiatry (Edgmont) 2010, 7, 13–16. [Google Scholar] [PubMed]
- Alhelal, M.A.; Palaska, I.; Panagiotidou, S.; Letourneau, R.; Theoharides, T.C. Trigeminal Nerve Stimulation Triggers Oral Mast Cell Activation and Vascular Permeability. Ann. Allergy Asthma Immunol. 2014, 112, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dingle, A.; Zeng, W.; Ness, J.P.; Albano, N.; Minor, R.L.; Feldman, C.; Austin, M.; Brodnick, S.K.; Shulzhenko, N.; Sanchez, R.; et al. Strategies for Interfacing with the Trigeminal Nerves in Rodents for Bioelectric Medicine. J. Neurosci. Meth. 2019, 324, 108321. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; You, Z.; Shen, S.; Yang, J.; Lim, G.; Doheny, J.T.; Chen, L.; Zhu, S.; Mao, J. An Improved Rodent Model of Trigeminal Neuropathic Pain by Unilateral Chronic Constriction Injury of Distal Infraorbital Nerve. J. Pain 2017, 18, 899–907. [Google Scholar] [CrossRef]
- Pitcher, G.M.; Ritchie, J.; Henry, J.L. Nerve Constriction in the Rat: Model of Neuropathic, Surgical and Central Pain. PAIN 1999, 83, 37–46. [Google Scholar] [CrossRef]
- Vos, B.; Strassman, A.; Maciewicz, R. Behavioral Evidence of Trigeminal Neuropathic Pain Following Chronic Constriction Injury to the Rat’s Infraorbital Nerve. J. Neurosci. 1994, 14, 2708–2723. [Google Scholar] [CrossRef]
- Araya, E.I.; Carvalho, E.C.; Andreatini, R.; Zamponi, G.W.; Chichorro, J.G. Trigeminal Neuropathic Pain Causes Changes in Affective Processing of Pain in Rats. Mol. Pain 2022, 18, 17448069211057750. [Google Scholar] [CrossRef] [PubMed]
- Benoist, J.-M.; Gautron, M.; Guilbaud, G. Experimental Model of Trigeminal Pain in the Rat by Constriction of One Infraorbital Nerve: Changes in Neuronal Activities in the Somatosensory Cortices Corresponding to the Infraorbital Nerve. Exp. Brain Res. 1999, 126, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Finn, E.S.; Scheinost, D.; Rosenberg, M.D.; Chun, M.M.; Papademetris, X.; Constable, R.T. Using Connectome-Based Predictive Modeling to Predict Individual Behavior from Brain Connectivity. Nat. Protoc. 2017, 12, 506–518. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Jin, Z.; Bashivan, P.; Li, L. Using Modular Connectome-Based Predictive Modeling to Reveal Brain-Behavior Relationships of Individual Differences in Working Memory. Brain Struct. Funct. 2023, 228, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercante, B.; Enrico, P.; Deriu, F. Cognitive Functions following Trigeminal Neuromodulation. Biomedicines 2023, 11, 2392. https://doi.org/10.3390/biomedicines11092392
Mercante B, Enrico P, Deriu F. Cognitive Functions following Trigeminal Neuromodulation. Biomedicines. 2023; 11(9):2392. https://doi.org/10.3390/biomedicines11092392
Chicago/Turabian StyleMercante, Beniamina, Paolo Enrico, and Franca Deriu. 2023. "Cognitive Functions following Trigeminal Neuromodulation" Biomedicines 11, no. 9: 2392. https://doi.org/10.3390/biomedicines11092392
APA StyleMercante, B., Enrico, P., & Deriu, F. (2023). Cognitive Functions following Trigeminal Neuromodulation. Biomedicines, 11(9), 2392. https://doi.org/10.3390/biomedicines11092392






