Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Primary Culture of TG Cells
2.2. Immunofluorescence Microscopy
2.3. Intracellular cAMP Level Assays in Living Cells
2.4. Measurement of Intracellular Free Ca2+ Concentration
2.5. Solutions and Reagents
2.6. Statistical and Offline Analysis
3. Results
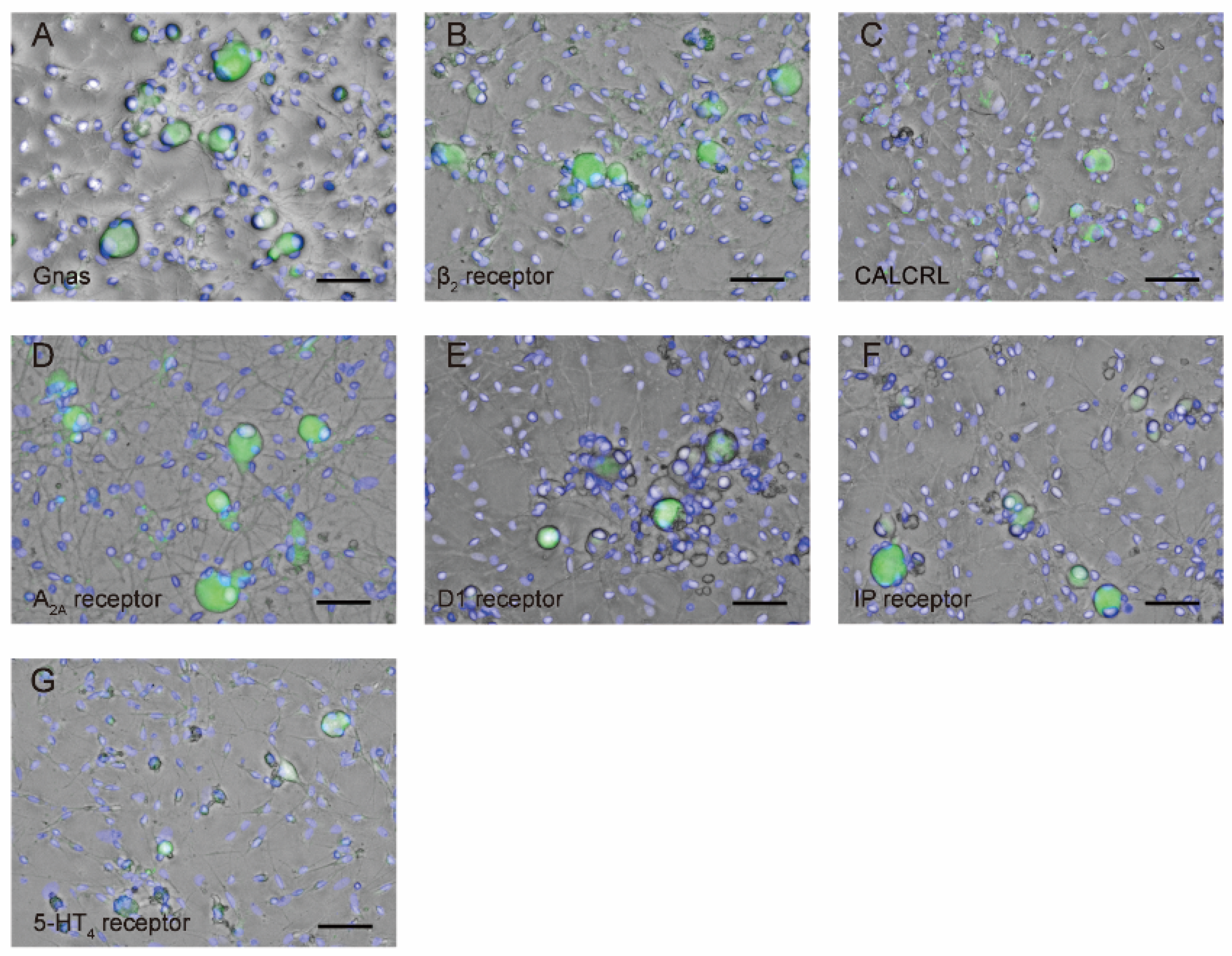
3.1. Immunolocalization of Gnas and NF-H in the TG Neurons
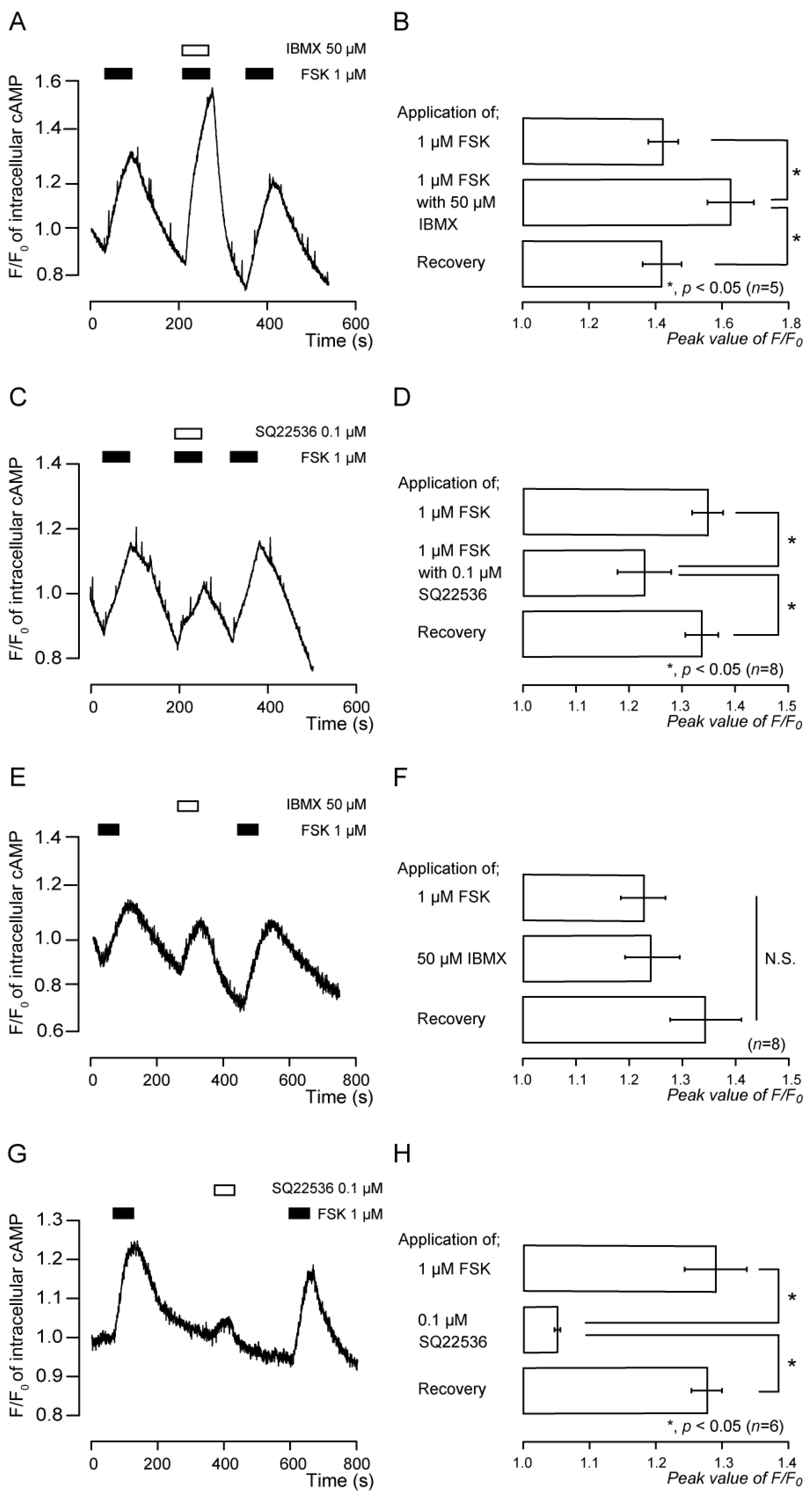
3.2. Forskolin-Induced Intracellular cAMP Increases Were Sensitive to Inhibitors of Adenylyl Cyclase and Phosphodiesterase
3.3. TG neurons Are Immunopositive for Gnas as well as Gαs Protein-Coupled Receptor Antibodies
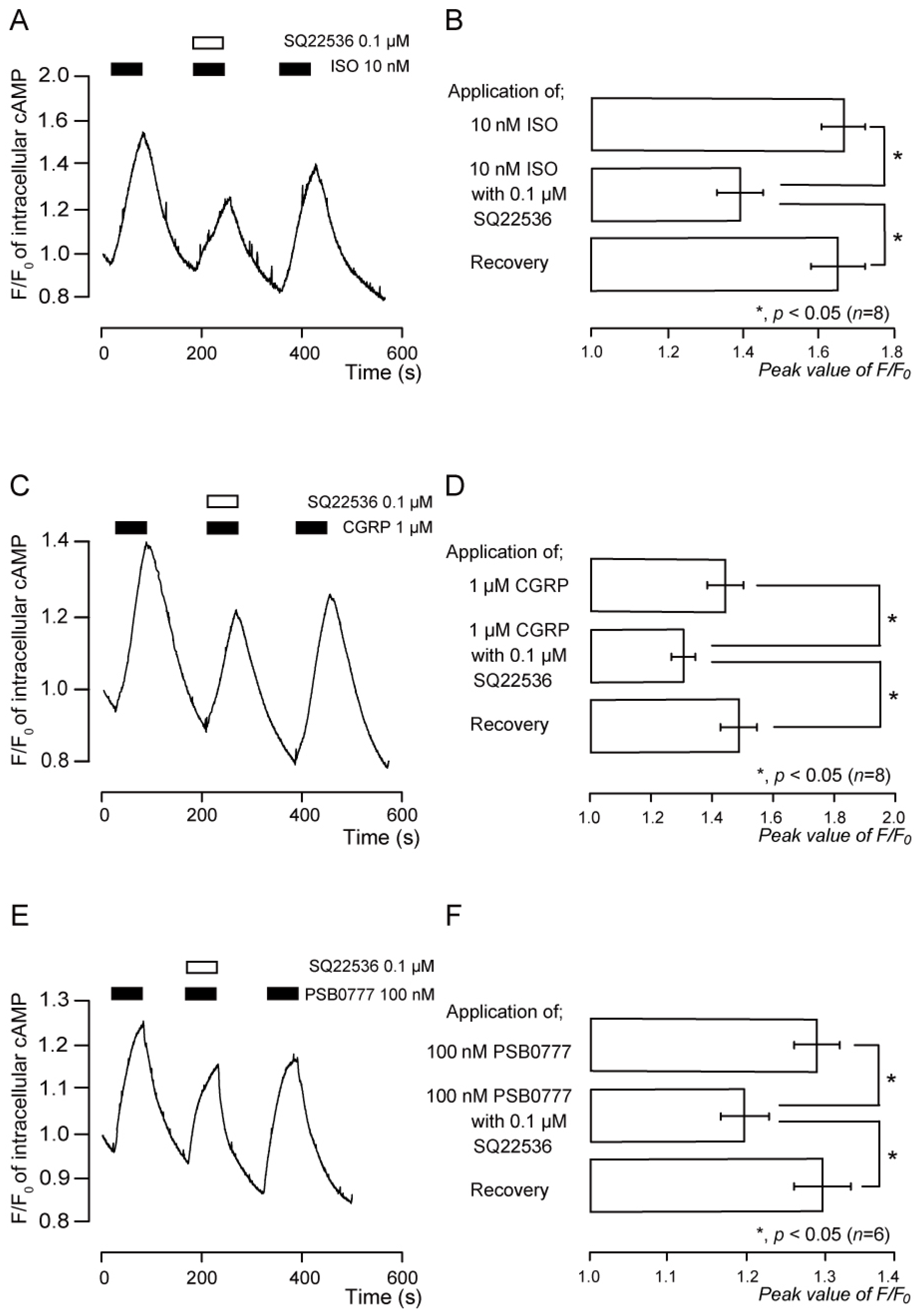
3.4. Functional Expression of β2 Receptors in TG Neurons
3.5. Functional Expression of CGRP Receptors in TG Neurons
3.6. Functional Expression of A2A Receptors in TG Neurons
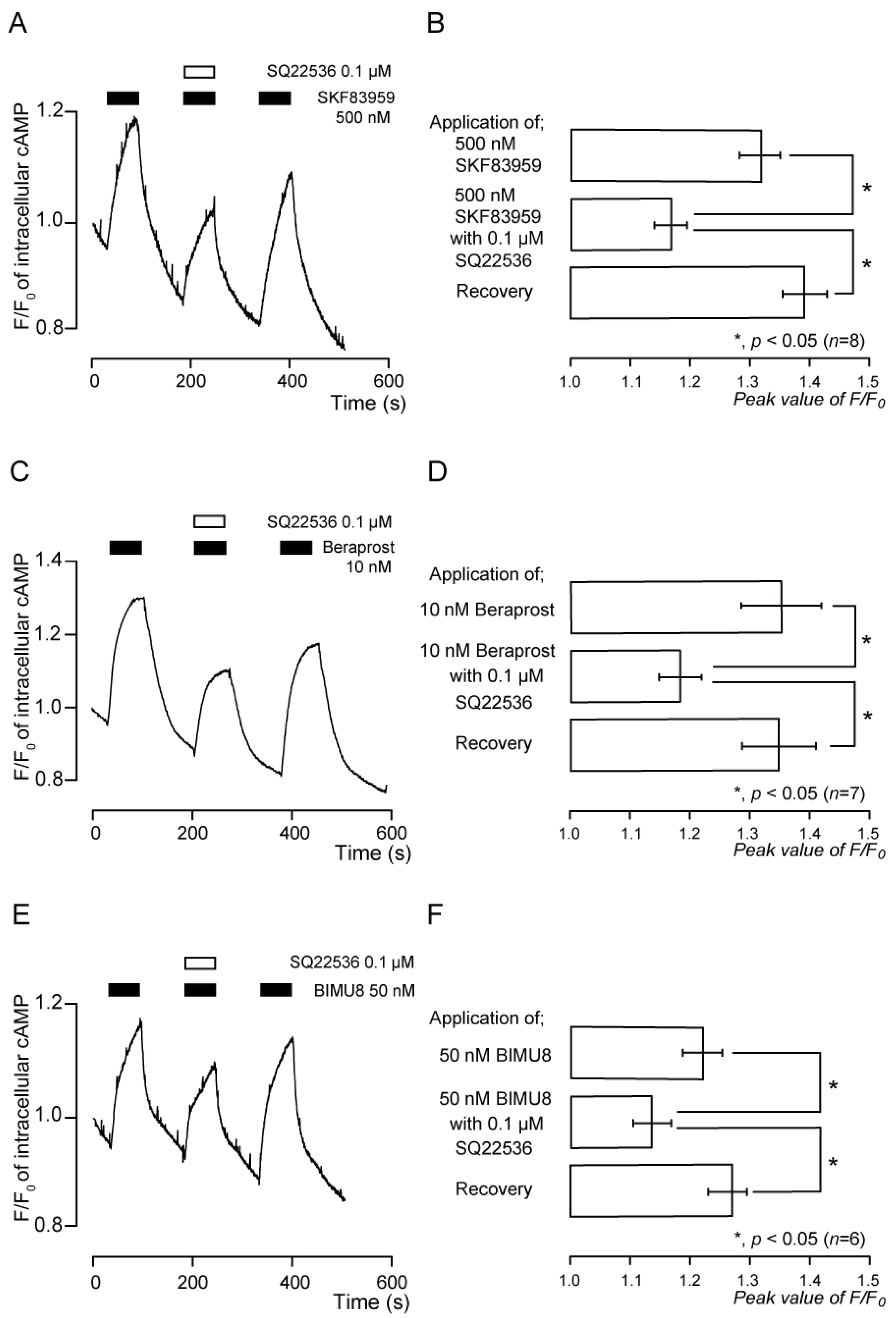
3.7. Functional Expression of D1-like Receptors in TG Neurons
3.8. Functional Expression of IP Receptors in TG Neurons
3.9. Functional Expression of 5-HT4 Receptors in TG Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kristiansen, K. Molecular Mechanisms of Ligand Binding, Signaling, and Regulation within the Superfamily of G-Protein-Coupled Receptors: Molecular Modeling and Mutagenesis Approaches to Receptor Structure and Function. Pharmacol. Ther. 2004, 103, 21–80. [Google Scholar] [CrossRef]
- Duc, N.M.; Du, Y.; Thorsen, T.S.; Lee, S.Y.; Zhang, C.; Kato, H.; Kobilka, B.K.; Chung, K.Y. Effective Application of Bicelles for Conformational Analysis of G Protein-Coupled Receptors by Hydrogen/Deuterium Exchange Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2015, 26, 808–817. [Google Scholar] [CrossRef]
- Sunahara, R.K.; Dessauer, C.W.; Gilman, A.G. Complexity and Diversity of Mammalian Adenylyl Cyclases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 461–480. [Google Scholar] [CrossRef]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G Protein Pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef]
- Lefkowitz, R.J. G Protein–Coupled Receptors and Receptor Kinases: From Molecular Biology to Potential Therapeutic Applications. Nat. Biotechnol. 1996, 14, 283–286. [Google Scholar] [CrossRef]
- Klabunde, T.; Hessler, G. Drug Design Strategies for Targeting G-Protein-Coupled Receptors. Chembiochem 2002, 3, 928–944. [Google Scholar] [CrossRef]
- Pan, H.-L.; Wu, Z.-Z.; Zhou, H.-Y.; Chen, S.-R.; Zhang, H.-M.; Li, D.-P. Modulation of Pain Transmission by G Protein-Coupled Receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Sato, M.; Kimura, M.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Expression and Function of Purinergic P2Y12 Receptors in Rat Trigeminal Ganglion Neurons. Neurosci. Res. 2015, 98, 17–27. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Sato, M.; Kimura, M.; Yamazaki, T.; Yamamoto, H.; Tazaki, M.; Ichinohe, T.; Shibukawa, Y. Functional Expression of Bradykinin B1 and B2 Receptors in Neonatal Rat Trigeminal Ganglion Neurons. Front. Cell. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef]
- Terashima, R.; Kimura, M.; Higashikawa, A.; Kojima, Y.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Intracellular Ca2+ Mobilization Pathway via Bradykinin B1 Receptor Activation in Rat Trigeminal Ganglion Neurons. J. Physiol. Sci. 2019, 69, 199–209. [Google Scholar] [CrossRef]
- Xu, S.; Ono, K.; Inenaga, K. Electrophysiological and Chemical Properties in Subclassified Acutely Dissociated Cells of Rat Trigeminal Ganglion by Current Signatures. J. Neurophysiol. 2010, 104, 3451–3461. [Google Scholar] [CrossRef]
- Park, C.-K.; Kim, M.S.; Fang, Z.; Li, H.Y.; Jung, S.J.; Choi, S.-Y.; Lee, S.J.; Park, K.; Kim, J.S.; Oh, S.B. Functional Expression of Thermo-Transient Receptor Potential Channels in Dental Primary Afferent Neurons: Implication for tooth pain. J. Biol. Chem. 2006, 281, 17304–17311. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Muñoz, H.R.; Azuero-Holguín, M.M.; Ulate, E. Neuropeptides in Dental Pulp: The Silent Protagonists. J. Endod. 2008, 34, 773–788. [Google Scholar] [CrossRef]
- Liang, D.-Y.; Liao, G.; Wang, J.; Usuka, J.; Guo, Y.; Peltz, G.; Clark, J.D. A Genetic Analysis of Opioid-Induced Hyperalgesia in Mice. Anesthesiology 2006, 104, 1054–1062. [Google Scholar] [CrossRef]
- Liang, D.-Y.; Shi, X.; Li, X.; Li, J.; Clark, J.D. The Beta2 Adrenergic Receptor Regulates Morphine Tolerance and Physical Dependence. Behav. Brain Res. 2007, 181, 118–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samoshkin, A.; Convertino, M.; Viet, C.T.; Wieskopf, J.S.; Kambur, O.; Marcovitz, J.; Patel, P.; Stone, L.S.; Kalso, E.; Mogil, J.S.; et al. Structural and Functional Interactions between Six-Transmembrane μ-Opioid Receptors and β2-Adrenoreceptors Modulate Opioid Signaling. Sci. Rep. 2015, 5, 18198. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Edvinsson, L.; Grell, A.-S.; Warfvinge, K. Expression of the CGRP Family of Neuropeptides and Their Receptors in the Trigeminal Ganglion. J. Mol. Neurosci. 2020, 70, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Warfvinge, K. CGRP Receptor Antagonism and Migraine Therapy. Curr. Protein Pept. Sci. 2013, 14, 386–392. [Google Scholar] [CrossRef]
- Eftekhari, S.; Warfvinge, K.; Blixt, F.W.; Edvinsson, L. Differentiation of Nerve Fibers Storing CGRP and CGRP Receptors in the Peripheral Trigeminovascular System. J. Pain 2013, 14, 1289–1303. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Strassman, A.M.; Nir, R.-R.; Schain, A.J.; Noseda, R.; Stratton, J.; Burstein, R. Fremanezumab—A Humanized Monoclonal Anti-CGRP Antibody—Inhibits Thinly Myelinated (Aδ) But Not Unmyelinated (C) Meningeal Nociceptors. J. Neurosci. 2017, 37, 10587–10596. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kimura, M.; Ouchi, T.; Ichinohe, T.; Shibukawa, Y. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. Biomolecules 2022, 12, 1747. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, I.; Ohkubo, S.; Kimura, J.; Uezono, Y. Adenine Nucleotide-Induced Activation of Adenosine A2B Receptors Expressed in Xenopus LaevisOocytes: Involvement of a Rapid and Localized Adenosine Formation by Ectonucleotidases. Mol. Pharmacol. 2002, 61, 606–613. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Diao, L.; Proctor, W.R. Adenine Nucleotides Undergo Rapid, Quantitative Conversion to Adenosine in the Extracellular Space in Rat Hippocampus. J. Neurosci. 1997, 17, 7673–7682. [Google Scholar] [CrossRef]
- Chen, J.-F.; Sonsalla, P.K.; Pedata, F.; Melani, A.; Domenici, M.R.; Popoli, P.; Geiger, J.; Lopes, L.V.; de Mendonça, A. Adenosine A2A Receptors and Brain Injury: Broad Spectrum of Neuroprotection, Multifaceted Actions and “Fine Tuning” Modulation. Prog. Neurobiol. 2007, 83, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine Receptors and the Central Nervous System. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 471–534. ISBN 978-3-540-89615-9. [Google Scholar]
- Masino, S.A.; Kawamura, M.; Ruskin, D.N. Adenosine Receptors and Epilepsy: Current Evidence and Future Potential. Int. Rev. Neurobiol. 2014, 119, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration. J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Sawynok, J. Adenosine Receptor Activation and Nociception. Eur. J. Pharmacol. 1998, 347, 1–11. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine Receptor Signaling. J. Recept. Signal Transduct. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Charbit, A.R.; Akerman, S.; Holland, P.R.; Goadsby, P.J. Neurons of the Dopaminergic/Calcitonin Gene-Related Peptide A11 Cell Group Modulate Neuronal Firing in the Trigeminocervical Complex: An Electrophysiological and Immunohistochemical Study. J. Neurosci. 2009, 29, 12532–12541. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, N.; Forssell, H.; Rinne, J.O.; Scheinin, H.; Taiminen, T.; Aalto, S.; Luutonen, S.; Någren, K.; Jääskeläinen, S. Striatal Dopamine D1 and D2 Receptors in Burning Mouth Syndrome. Pain 2003, 101, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shamsizadeh, A.; Pahlevani, P.; Haghparast, A.; Moslehi, M.; Zarepour, L.; Haghparast, A. Involvement of Dopamine Receptors within the Dorsal Hippocampus in Suppression of the Formalin-Induced Orofacial Pain. Pharmacol. Biochem. Behav. 2013, 114–115, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Soleimannejad, E.; Semnanian, S.; Fathollahi, Y.; Naghdi, N. Microinjection of Ritanserin into the Dorsal Hippocampal CA1 and Dentate Gyrus Decrease Nociceptive Behavior in Adult Male Rat. Behav. Brain Res. 2006, 168, 221–225. [Google Scholar] [CrossRef]
- Del Rey, A.; Yau, H.-J.; Randolf, A.; Centeno, M.V.; Wildmann, J.; Martina, M.; Besedovsky, H.O.; Apkarian, A.V. Chronic Neuropathic Pain-like Behavior Correlates with IL-1β Expression and Disrupts Cytokine Interactions in the Hippocampus. Pain 2011, 152, 2827–2835. [Google Scholar] [CrossRef]
- Gao, Y.-H.; Chen, S.-P.; Wang, J.-Y.; Qiao, L.-N.; Meng, F.-Y.; Xu, Q.-L.; Liu, J.-L. Differential Proteomics Analysis of the Analgesic Effect of Electroacupuncture Intervention in the Hippocampus Following Neuropathic Pain in Rats. BMC Complement. Altern. Med. 2012, 12, 241. [Google Scholar] [CrossRef]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed Messages: Modulation of Inflammation and Immune Responses by Prostaglandins and Thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef]
- Davies, P.; Bailey, P.J.; Goldenberg, M.M.; Ford-Hutchinson, A.W. The Role of Arachidonic Acid Oxygenation Products in Pain and Inflammation. Annu. Rev. Immunol. 1984, 2, 335–357. [Google Scholar] [CrossRef]
- Kumazawa, T.; Mizumura, K.; Koda, H.; Fukusako, H. EP Receptor Subtypes Implicated in the PGE2-Induced Sensitization of Polymodal Receptors in Response to Bradykinin and Heat. J. Neurophysiol. 1996, 75, 2361–2368. [Google Scholar] [CrossRef]
- Campos, M.M.; Calixto, J.B. Involvement of B1 and B2 Receptors in Bradykinin-induced Rat Paw Oedema. Br. J. Pharmacol. 1995, 114, 1005–1013. [Google Scholar] [CrossRef]
- Taiwo, Y.O.; Levine, J.D. Further Confirmation of the Role of Adenyl Cyclase and of CAMP-Dependent Protein Kinase in Primary Afferent Hyperalgesia. Neuroscience 1991, 44, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, Y.O.; Bjerknes, L.K.; Goetzl, E.J.; Levine, J.D. Mediation of Primary Afferent Peripheral Hyperalgesia by the CAMP Second Messenger System. Neuroscience 1989, 32, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, Pharmacological and Functional Diversity of 5-HT Receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Sharp, T. A Review of Central 5-HT Receptors and Their Function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Kumamoto, E. Cellular Mechanisms for Antinociception Produced by Oxytocin and Orexins in the Rat Spinal Lamina II—Comparison with Those of Other Endogenous Pain Modulators. Pharmaceuticals 2019, 12, 136. [Google Scholar] [CrossRef]
- Cardenas, L.M.; Cardenas, C.G.; Scroggs, R.S. 5HT Increases Excitability of Nociceptor-Like Rat Dorsal Root Ganglion Neurons Via CAMP-Coupled TTX-Resistant Na+Channels. J. Neurophysiol. 2001, 86, 241–248. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunioku, Y.; Kimura, M.; Ouchi, T.; Fukuda, K.; Shibukawa, Y. Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines 2023, 11, 2347. https://doi.org/10.3390/biomedicines11092347
Kunioku Y, Kimura M, Ouchi T, Fukuda K, Shibukawa Y. Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines. 2023; 11(9):2347. https://doi.org/10.3390/biomedicines11092347
Chicago/Turabian StyleKunioku, Yuki, Maki Kimura, Takehito Ouchi, Kenichi Fukuda, and Yoshiyuki Shibukawa. 2023. "Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells" Biomedicines 11, no. 9: 2347. https://doi.org/10.3390/biomedicines11092347
APA StyleKunioku, Y., Kimura, M., Ouchi, T., Fukuda, K., & Shibukawa, Y. (2023). Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines, 11(9), 2347. https://doi.org/10.3390/biomedicines11092347





