How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters
Abstract
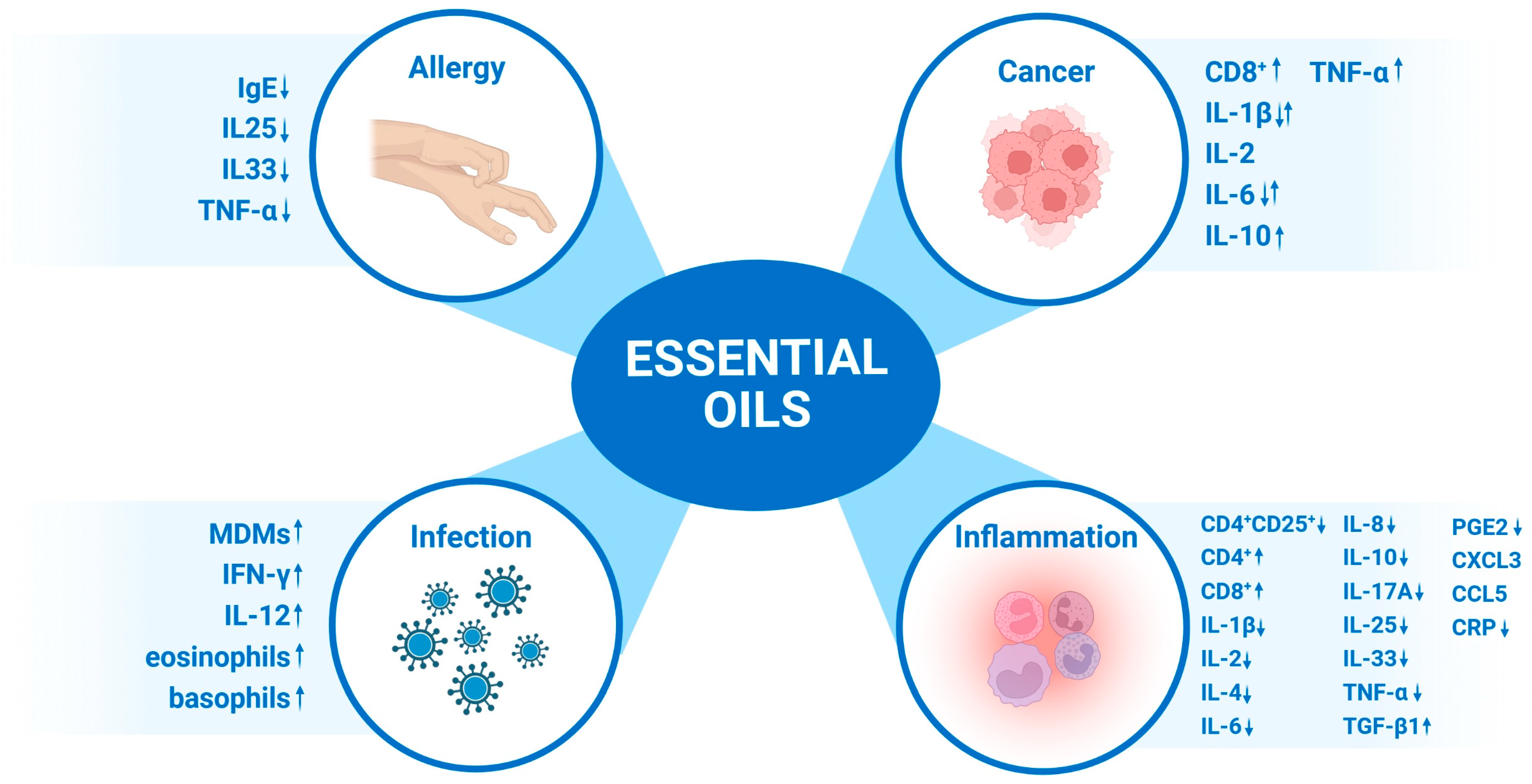
:1. Introduction
2. Action of the Human Immune System
3. Influence of the Immune System on Wound Healing
4. Influence of Selected Essential Oils on the Immune System
| Lp. | Plant Species | Activity | Main Constituents (>1%) (The Composition of Essential Oil May Differ Depending on e.g., a Part of the Plant, Geographical Area, Season When It Was Harvested) (–Means Lack of the Peesence of the Component in Tested Material) | Literature Source |
|---|---|---|---|---|
| 1. | Myrtus communis (M. communis, myrtle) |
| The composition of essential oil isolated from leafs of the plant grown in Jericho/Jenin/Tunisia:
| [50,51,52,143,144] |
| 2. | Chamaecyparis obtusa (C. obtuse, Cupressaceae) | It is able to inhibit inflammatory processes by:
| The composition of essential oils isolated from leafs of the tree grown in South Korea/from fruit of the tree grown in Sudan:
| [48,57,58,145,146] |
| 3. | Nigella sativa (N. sativa, Ranunculaceae) | Inhibits inflammation by:
May increase proinflamatory processess in the Alzheimer’s disease model by:
Plays a role in combating infections by:
Plays a role in anticancer treatment because:
| The composition of essential oil of Iranian/Brazilian black cumin seed:
| [71,74,75,76,77,78,79,80,81,147,148] |
| 4. | Houttuynia cordata Thunb. (H. cordata; Saururaceae) | May reduce side effects of bleomycine in cancer treatment as:
| The composition of essential oil isolated from dried aerial part of leafs/fresh whole grass of the plant grown in China:
| [92,93,94,149] |
| 5. | Thymus vulgaris L. (T. vulgaris) | It is involved in antiinflammatory processes by:
| The composition of essential oil isolated from aerial parts of the plant grown in Nyons, France/Jablanicki, Serbia/Pomoravje District, Serbia/Richerenches, France:
| [8,97,98,99,100,101,150] |
| 6. | Artemisia judaica L. (A. judaica; Asteraceae; Compositae) | It exhibits antiinflamamtory activity by:
| The composition of essential oil isolated from leafs of the plant grown in Saudi Arabia/Algeria/Egypt/Sinai, Egypt/Jordan/Libya:
| [9,10,107,108,109,110] |
| 7. | Patchouli (Pogostemon cablin, P. cablin) | Plays a role in antiinflammatory procesess and wound healing by:
| The composition of essential oil isolated from leafs of the plant grown in Aceh Indinesia/India:
| [111,151] |
| 8. | Echinacea purpurea (E. purpurea) L. Moench (Asteraceae) |
| The composition of essential oil isolated from the leaf/root of plant grown in South Africa:
| [113,114,152] |
| 9. | Cardamom (Elettaria cardamomum Maton, Zingiberaceae) | It is involved in antiinflammatory response by:
| The composition of essential oil isolated from the grains/fruit of the plant grown in Jordan/Mersin Turkey:
| [125,153] |
| 10. | Citrus sudachi (sudachi oil, Rutaceae) |
| The composition of essential oil from fruit of the plant grown in Kagoshima, Japan/Yamaguchi, Japan:
| [127,154,155] |
| 11. | Euodia ruticarpa (E. ruticarpia) (Rutaceae) | It exhibits anticancer propeties by:
| The composition of essential oil from leaf /fruit:
| [32,130,131,132,133,134,135,156] |
| 12. | Eucalyptus plant (Myrtaceae) |
| The composition of essential oil from leaf of the plant Eucalyptus maculate/Eucalyptus globulus grown in Tanzania:
| [2,40,137,138,141,142,157] |
| Lp. | Constituent | Activity | Chemical Classification | Literature |
|---|---|---|---|---|
| 1 | α-pinene |
| Monoterpene hydrocarbons | [158,159,160,161,162,163] |
| 2 | p-cymen-8-ol |
| Monoterpene alcohol | [158,160] |
| 3 | limonene, |
| Cyclic monoterpene | [158,164,165,166,167] |
| 4 | β-pinene |
| Monoterpene hydrocarbons | [158,168] |
| 5 | camphor |
| Monoterpenoid | [110,169,170] |
| 6 | myrcene |
| Monoterpene hydrocarbons | [158,165,171] |
| 7 | β- and α-phellandrene |
| Monoterpene hydrocarbons | [167,172,173,174,175,176] |
| 8 | spathulenol |
| Oxygenated sesquiterpene | [172,177,178,179,180,181] |
| 9 | myristicin |
| Phenylpropene | [158] |
| 10 | myrtenol |
| Monoterpene alcohol | [158,182] |
| 11 | (+/−)-sabinene |
| Monoterpene hydrocarbons | [158,183,184] |
| 12 | γ-terpinene |
| Monoterpene hydrocarbons | [158,163] |
| 13 | terpinen-4-ol |
| Oxygenated monoterpene | [185,186,187] |
| 14 | terpinolene |
| Monoterpene hydrocarbons | [158,160,188] |
| 15 | p-cymene |
| Monoterpene | [98,163,168,185,189] |
| 16 | nerolidol |
| Oxygenated sesquiterpenes | [185,190,191] |
| 17 | myrtenol |
| Monoterpene alcohol | [158,182,192] |
| 18 | (±)-bornyl acetate |
| Bicyclic monoterpene | [185,193,194,195,196] |
| 19 | borneol |
| Oxygenated monoterpenes | [110,185,197,198]. |
| 20 | geraniol |
| Monoterpenoid | [169,199,200] |
| 21 | o-cymene |
| Monoterpene | [201,202] |
| 22 | 1.8-cineole |
| Monoterpene | [110,126,160,161,198,202,203,204,205,206,207] |
| 23 | linalool |
| Cyclic monoterpene | [185,201,208] |
| 24 | Cis thujanol |
| Monoterpenoid | [10,163,200] |
| 25 | b-caryophyllene |
| Sesquiterpene hydrocarbons | [168,177] |
| 26 | citronellal |
| Monoterpene | [167,209,210] |
| 27 | Alpha terpineol |
| Oxygenated monoterpenes | [174,176,187,197,211,212,213] |
| 28 | carvacrol |
| Monoterpene | [214] |
| 29 | Thymoquinone |
| Monoterpene | [164,215,216,217,218,219,220,221,222] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ataur-Rahman, F.R.S.; Iqbal Choudhary, M. (Eds.) Medicinal plants as immune response enhancers to prevent infectious diseases of veterinary interest. In Frontiers in Anti-Infective Drug Discovery; Bentham Science Publishers: Sharja, United Arab Emirates, 2017; Volume 5, pp. 132–144. [Google Scholar]
- Zonfrillo, M.; Andreola, F.; Krasnowska, E.K.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Essential Oil from Eucalyptus globulus (Labill.) Activates Complement Receptor-Mediated Phagocytosis and Stimulates Podosome Formation in Human Monocyte-Derived Macrophages. Molecules 2022, 27, 3488. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338. [Google Scholar] [CrossRef]
- Sampson, A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy 2000, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Akira, S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006, 117, 979. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.M. Alterations in blood components. Compr. Toxicol. 2018, 2018, 249–293. [Google Scholar] [CrossRef]
- Naseem, S.; Iqbal, R.; Munir, T. Role of interleukin-6 in immunity: A Review. Int. J. Life Sci. Res. 2016, 4, 268–274. [Google Scholar]
- Ismail, H.T.H. The ameliorative efficacy of Thymus vulgaris essential oil against Escherichia coli O157:H7-induced hematological alterations, hepatorenal dysfunction and immune-inflammatory disturbances in experimentally infected rats. Environ. Sci. Pollut. Res. 2022, 29, 41476–41491. [Google Scholar] [CrossRef] [PubMed]
- Pereira Beserra, F.; Sérgio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Alberto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants 2022, 11, 332. [Google Scholar] [CrossRef]
- Guermonprez, P.; Amigorena, S. Pathways for antigen cross presentation. Springer Semin. Immunopathol. 2005, 26, 257–271. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 2003, 3, 373. [Google Scholar] [CrossRef] [PubMed]
- Badea, D.; Genunche-Dumitrescu, A.; Badea, M.; Marinaș, C.; Cojan, T.T. Immunoglobulins: Functions, biosynthesis and biological properties. Res. Sci. Today J. 2020, 8, 67–74. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777. [Google Scholar] [CrossRef]
- Maschalidi, S.; Mehrotra, P.; Keçeli, B.N.; De Cleene, H.K.L.; Lecomte, K.; Van der Cruyssen, R.; Janssen, P.; Pinney, J.; van Loo, G.; Elewaut, D.; et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes. Nature 2022, 606, 776–784. [Google Scholar] [CrossRef]
- Moulik, P.K.; Mtonga, R.; Gill, G.V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003, 26, 491–494. [Google Scholar] [CrossRef]
- Sayama, K.; Komatsuzawa, H.; Yamasaki, K.; Shirakata, Y.; Hanakawa, Y.; Ouhara, K.; Tokumaru, S.; Dai, X.; Tohyama, M.; Ten Dijke, P.; et al. New mechanisms of skin innate immunity: ASK1-mediated keratinocyte differentiation regulates the expression of beta-defensins, LL37, and TLR2. Eur. J. Immunol. 2005, 35, 1886. [Google Scholar] [CrossRef]
- Sorensen, O.E.; Cowland, J.B.; Theilgaard-Monch, K.; Liu, L.; Ganz, T.; Borregaard, N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 2003, 170, 5583. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Koehler, T.; Jacobsen, F.; Daigeler, A.; Goertz, O.; Langer, S.; Kesting, M.; Steinau, H.; Eriksson, E.; Hirsch, T. Host defense peptides in wound healing. Mol. Med. 2008, 14, 528. [Google Scholar] [CrossRef]
- Chen, L.; Guo, S.; Ranzer, M.J.; Dipietro, L.A. Toll-like receptor 4 has an essential role in early skin wound healing. J. Investig. Dermatol. 2013, 133, 258. [Google Scholar] [CrossRef]
- Kluwe, J.; Mencin, A.; Schwabe, R.F. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. 2009, 87, 125. [Google Scholar] [CrossRef] [PubMed]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513. [Google Scholar] [CrossRef] [PubMed]
- Strbo, N.; Yin, N.; Stojadinovic, O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv. Wound Care 2014, 3, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Gurjala, A.N.; Geringer, M.R.; Seth, A.K.; Hong, S.J.; Smeltzer, M.S.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair. Regen. 2011, 19, 400–410. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P. Biofilms and host response: Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine; World Health Organization: Genèva, Switzerland, 2019. [Google Scholar]
- Anand, U.; Jacobo-Herrera, N.J.; Altemimi, A.B.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Sarto, M.P.M.; da Silva, H.F.L.; de Souza Fernandes, N.; de Abreu, A.P.; Junior, G.Z.; de Ornelas Toledo, M.J. Essential oils from Syzygium aromaticum and Zingiber officinale, administered alone or in combination with benznidazole, reduce the parasite load in mice orally inoculated with Trypanosoma cruzi, I.I. BMC Complement. Med. Ther. 2021, 21, 77. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Lin, J.-Y. Active Ingredients from Euodia ruticarpa Steam Distilled Essential Oil Inhibit PC-3 Prostate Cancer Cell Growth via Direct Action and Indirect Immune Cells Conditioned Media In Vitro. Curr. Issues Mol. Biol. 2021, 43, 996–1018. [Google Scholar] [CrossRef]
- Shiina, Y.; Funabashi, N.; Lee, K.; Toyoda, T.; Sekine, T.; Honjo, S.; Hasegawa, R.; Kawata, T.; Wakatsuki, Y.; Hayashi, S. Relaxation effects of lavender aromatherapy improve coronary flow velocity reserve in healthy men evaluated by transthoracic doppler echocardiography. Int. J. Cardiol. Res. 2008, 129, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: A review. J. Ethnopharmacol. 2020, 263, 113231. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Mesaik, M.A.; Jabeen, A.; Kan, Y. Immunomodulatory properties of various natural compounds and essential oils through modulation of human cellular immune response. Ind. Crop Prod. 2016, 81, 117–122. [Google Scholar] [CrossRef]
- Chandan, G.; Kumar, C.; Verma, M.K.; Satti, N.K.; Saini, A.K.; Saini, R.V. Datura stramonium essential oil composition and it’s immunostimulatory potential against colon cancer cells. 3 Biotech 2020, 10, 451. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Roana, J.; Cavallo, L.; Mandras, N. Immune Defences: A View from the Side of the Essential Oils. Molecules 2023, 28, 435. [Google Scholar] [CrossRef]
- Serafino, A.; Sinibaldi Vallebona, P.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of Eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Ciesielska-Figlon, K.; Daca, A.; Kokotkiewicz, A.; Łuczkiewicz, M.; Zabiegała, B.; Witkowski, J.M.; Lisowska, K.A. The influence of Nigella sativa essential oil on proliferation, activation, and apoptosis of human T lymphocytes in vitro. Biomed. Pharmacother. 2022, 153, 113349. [Google Scholar] [CrossRef]
- Kutlu, Z.; Gulaboglu, M.; Halıcı, Z.; Cınar, I.; Dıyarbakır, B. Biochemical Research of the Effects of Essential Oil Obtained from the Fruit of Myrtus communis L. on Cell Damage Associated with Lipopolysaccharide-Induced Endotoxemia in a Human Umbilical Cord Vein Endothelial Cells. Biochem. Genet. 2020, 59, 315–334. [Google Scholar] [CrossRef]
- Nikoliĉ, M.; Glamoĉlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Markoviĉ, T.; Markoviĉ, D.; Giweli, A.; Sokoviĉ, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop. Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The effects of lavender essential oil on wound healing: A review of the current evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 11, 4530. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Gashaw, T. Ethnobotanical, Ethnopharmacological, and Phytochemical Studies of Myrtus communis Linn: A Popular Herb in Unani System of Medicine. J. Evid. Based Integr. Med. 2017, 22, 1035–1043. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of Pharmacological Effects of Myrtus communis L. and its Active Constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef]
- Elfadaly, H.A.; Hassanain, N.A.; Shaapan, R.M.; Hassanain, M.A.; Barakat, A.M.A.; AbdelRahma, K.A. Molecular Detection and Genotyping of Toxoplasma gondii from Egyptian Isolates. Asian, J. Epidemiol. 2016, 10, 37–44. [Google Scholar] [CrossRef]
- Oliveira, C.B.S.; Meurer, Y.S.R.; Medeiros, T.L.; Pohlit, A.M.; Silva, M.V.; Mineo, T.W.P.; Andrade-Neto, V.F. Anti-ToxoplasmaActivity of Estragole and Thymol in Murine Models of Congenital and Noncongenital Toxoplasmosis. J. Parasitol. 2016, 102, 369–376. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Fallahi, S.; Mahmoudvand, H.; Shakibaie, M.; Harandi, M.F.; Dezaki, E.S. Efficacy of Myrtus communis L. to Inactivate the Hydatid Cyst Protoscoleces. J. Investig. Surg. 2015, 29, 137–143. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Ezzatkhah, F.; Sharififar, F.; Sharifi, I.; Dezaki, E.S. Antileishmanial and Cytotoxic Effects of Essential Oil and Methanolic Extract of Myrtus communis L. Korean J. Parasitol. 2015, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shaapan, R.M.; Al-Abodi, H.R.; Alanazi, A.D.; Abdel-Shafy, S.; Rashidipour, M.; Shater, A.F.; Mahmoudvand, H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules 2021, 26, 819. [Google Scholar] [CrossRef] [PubMed]
- Delfani, S.; Mohammadrezaei-Khorramabadi, R.; Ghamari, S.; Boroujeni, R.K.; Khodabandeloo, N.; Khorzoughi, M.G.; Shahsavari, S. Systematic review for phytotherapy in Streptococcus Mutans. J. Pharm. Sci. Res. 2017, 9, 552. [Google Scholar]
- Mose, J.M.; Kagira, J.M.; Kamau, D.M.; Maina, N.; Ngotho, M.; Karanja, S.M. A Review on the Present Advances on Studies of Toxoplasmosis in Eastern Africa. Biomed. Res. Int. 2020, 2020, 7135268. [Google Scholar] [CrossRef] [PubMed]
- Delfani, S.; Mohammadrezaei-Khorramabadi, R.; Abbaszadeh, S.; Naghdi, N.; Shahsavari, S. Phytotherapy for Streptococcus pyogenes. J. Pharm. Sci. Res. 2017, 9, 513. [Google Scholar]
- Shin, S.H.; Ye, M.K.; Chae, M.H.; Lee, D.W. Chamaecyparis obtusa Essential Oil Inhibits House Dust Mite Induced Nasal Epithelial Cell Activation and Immune Responses. J. Oleo Sci. 2021, 70, 431–438. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Asker, M.M.S.; Tadros, M. Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. Eur. Food Res. Technol. 2012, 234, 833–844. [Google Scholar] [CrossRef]
- Ahmad, I.; Tripathi, J.; Manik, S.; Umar, L.; Rabia, J. Preliminary Phytochemical Studies of the Miracle Herb of the Century, Nigella sativa L. (Black Seed) Indo Am. J. Pharm. Res. 2013, 3, 3000–3007. [Google Scholar]
- Ali, S.A.; Parveen, N.; Ali, A.S. Links between the Prophet Muhammad (PBUH) recommended foods and disease management: A review in the light of modern superfoods. Int. J. Health Sci. 2018, 12, 61–69. [Google Scholar]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Chapter 13—Cumin. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178. [Google Scholar]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Haq, A.; Lobo, P.I.; Al-Tufail, M.; Rama, N.R.; Al-Sedairy, S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int. J. Immunopharmacol. 1999, 21, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Halawani, E. Antibacterial activity of thymo-quinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Adv. Biol. Res. 2009, 3, 148–152. [Google Scholar]
- Gali-Muhtasib, H.; Ocker, M.; Kuester, D.; Krueger, S.; El-Hajj, Z.; Diestel, A.; Evert, M.; El-Najjar, N.; Peters, B.; Jurjus, A.; et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell. Mol. Med. 2008, 12, 330–342. [Google Scholar] [CrossRef]
- Carroll, J.F.; Babish, J.G.; Pacioretty, L.M.; Kramer, M. Repellency to ticks (Acari: Ixodidae) of extracts of Nigella sativa (Ranunculaceae) and the anti-inflammatory DogsBestFriend™. Exp. Appl. Acarol. 2016, 70, 89–97. [Google Scholar] [CrossRef]
- Oskouei, Z.; Akaberi, M.; Hosseinzadeh, H. A glance at black cumin (Nigella sativa) and its active constituent, thymoquinone, in ischemia: A review. Iran. J. Basic Med. Sci. 2018, 21, 1200–1209. [Google Scholar] [CrossRef]
- Haseena, S.; Aithal, M.; Das, K.K.; Saheb, S.H. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J. Pharm. Sci. Res. 2015, 7, 514–517. [Google Scholar]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Taiari, S.; Nassiri-Asl, M. Effect of thymoquinone, a constituent of Nigella sativa L., on ischemia-reperfusion in rat skeletal muscle. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 503–508. [Google Scholar] [CrossRef]
- Dwita, L.P.; Yati, K.; Gantini, S.N. The anti-inflammatory activity of nigella sativa balm sticks. Sci. Pharm. 2019, 87, 3. [Google Scholar] [CrossRef]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Fallahi, M.; Khamaneh, A.M.; Ebrahimi Saadatlou, M.A.; Saadat, S.; Keyhanmanesh, R. Effect of alpha-Hederin on IL-2 and IL-17 mRNA and miRNA-133a levels in lungs of ovalbumin-sensitized male rats. Drug Dev. Res. 2016, 77, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef]
- Attia, H.N.; Ibrahim, F.M.; Maklad, Y.A.; Ahmed, K.A.; Ramadan, M.F. Characterization of antiradical and anti-inflammatory activities of some cold pressed oils in carrageenan-induced rat model of acute inflammation. Der Pharma. Chem. 2016, 8, 148–158. [Google Scholar]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol. Cell. Biochem. 2017, 435, 149–162. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Sheik, N.M.M.; Jaikumar, K.; Marimuthu, S.; Wyson, W.J.; Anand, D.; Saravanan, P. In vitro immunostimulation activity of Nigella sativa Linn. and psoralea Corylifolia Linn. seeds using a murine macrophage cell line. Asian J. Pharm. Clin. Res. 2017, 10, 329–332. [Google Scholar]
- Hakim, A.S.; Abouelhag, H.A.; Abdou, A.M.; Fouad, E.A.; Khalaf, D.D. Assessment of immunomodulatory effects of black cumin seed (Nigella Sativa) extract on macrophage activity in vitro. Int. J. Vet. Sci. 2019, 8, 385–389. [Google Scholar]
- Koshak, A.E.; Yousif, N.M.; Fiebich, B.L.; Koshak, E.A.; Heinrich, M. Comparative immunomodulatory activity of Nigella sativa L. preparations on proinflammatory mediators: A focus on asthma. Front. Pharm. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Meles, D.K.; Safitri, E.; Mustofa, I.; Susilowati, S.; Putri, D.K.S.C. Immunomodulatory Activity of Black Jinten Oil (Nigella sativa) as Macrophage Activator for Salmonella typimurium Infected Rat. Indian Vet. J. 2020, 97, 12–14. [Google Scholar]
- Kensara, O.A.; El-Shemi, A.G.; Mohamed, A.M.; Refaat, B.; Idris, S.; Ahmad, J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des. Dev. Ther. 2016, 10, 2239–2253. [Google Scholar]
- Talib, W.H. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A Current Update on the Phytopharmacological Aspects of Houttuynia Cordata Thunb. Pharmacogn. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in Chemical Composition and Antibacterial Activities of Essential Oils from Two Species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical Composition and Determination of the Antibacterial Activity of Essential Oils in Liquid and Vapor Phases Extracted from Two Different Southeast Asian Herbs-Houttuynia Cordata (Saururaceae) and Persicaria Odorata (Polygonaceae). Molecules 2020, 25, 2432. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A.; et al. Chemical Composition and Allelopathic, Antibacterial, Antifungal, and Antiacetylcholinesterase Activity of Fish-Mint (Houttuynia cordataThunb.) from India. Chem. Biodivers. 2017, 14, e1700189. [Google Scholar] [CrossRef]
- Ng, A.W.T.; Poon, S.L.; Ni Huang, M.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic Acids and Their Derivatives Are Widely Implicated in Liver Cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Du, S.; Li, H.; Cui, Y.; Yang, L.; Wu, J.; Huang, H.; Chen, Y.; Huang, W.; Zhang, R.; Yang, J.; et al. Houttuynia Cordata Inhibits Lipopolysaccharide-Induced Rapid Pulmonary Fibrosis by Up-Regulating IFN-γ and Inhibiting the TGF-β1/Smad Pathway. Int. Immunopharmacol. 2012, 13, 331–340. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An Ethnopharmacological Review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef]
- Bukovská, A.; Cikos, S.; Juhás, S.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediators Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef]
- Ocaña, A.; Reglero, G. Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevo-Silva, C.F.; de Carvalho, M.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Zhong, W.; Chi, G.; Jiang, L.; Soromou, L.W.; Chen, N.; Huo, M.; Guo, W.; Deng, X.; Feng, H. p-Cymene modulates in vitro and in vivo cytokine production by inhibiting MAPK and NF-κB activation. Inflammation 2012, 36, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Gholijani, N.; Gharagozloo, M.; Kalantar, F.; Ramezani, A.; Amirghofran, Z. Modulation of cytokine production and transcription factors activities in human jurkat T cells by thymol and carvacrol. Adv. Pharm. Bull. 2015, 5, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, W.; Jirovetz, L. Chemical composition and anti-inflammatory activity of Algerian, T. vulgaris essential oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Lin, C.-C.; Lin, W.-C.; Yang, C.-H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011, 75, 1977–1983. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Benmansour, N.; Benmansour, A.; El Hanbali, F.; González-Mas, M.C.; Blázquez, M.A.; El Hakmaoui, A.; Akssira, M. Antimicrobial activity of essential oil of Artemisia judaica L. from Algeria against multi-drug resistant bacteria from clinical origin. Flavour Fragr. J. 2016, 31, 137–142. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Janacković, P.; Novaković, J.; Soković, M.; Vujisić, L.; Giweli, A.A.; Dajić-Stevanović, Z.; Marin, P.D. Composition and antimicrobial activity of essential oils of Artemisia judaica, A. herba-alba and A. arborescens from Libya. Arch. Biol. Sci. 2015, 67, 455–466. [Google Scholar] [CrossRef]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 96. [Google Scholar] [CrossRef]
- Bushiken, L.F.S.; Hussni, C.A.; Bastos, J.K.; Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Padovani, C.R.; Lemos, M.; Polizello Junior, M.; da Silva, J.J.M. Skin wound healing potential and mechanisms of the hydroalcoholic extract of leaves and oleoresin of Copaifera langsdorffii Desf. Kuntze in rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 6589270. [Google Scholar] [CrossRef]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-inflammatory effects of 1, 8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1, 8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef]
- Leong, W.; Huang, G.; Liao, W.; Xia, W.; Li, X.; Su, Z.; Liu, L.; Wu, Q.; Kam Wai Wong, V.; Yuen Kwan Law, B.; et al. Traditional Patchouli essential oil modulates the host’s immune responses and gut microbiota and exhibits potent anti-cancer effects in ApcMin/+ mice. Pharmacol. Res. 2022, 176, 106082. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Contreras, M.d.M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M.; Lasseigne, T. Variability in the composition of anti-oxidant compounds in Echinacea species by HPLC. Phytochem. Anal. 2005, 16, 77–85. [Google Scholar] [CrossRef]
- Mistrikova, I.; Vaverkova, S. Echinacea—Chemical composition, immunostimulatory activities and uses. Thaiszia J. Bot. 2006, 16, 11–26. [Google Scholar]
- Tan, B.K.; Vanitha, J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: A review. Curr. Med. Chem. 2004, 11, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 2020, 61, 102089. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Chatterjee, J. Identification of proapoptopic, anti-inflammatory, anti-proliferative, anti-invasive and anti-angiogenic targets of essential oils in cardamom by dual reverse virtual screening and binding pose analysis. Asian Pac. J. Cancer Prev. 2013, 14, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kiran, S.; Marimuthu, P.; Isidorov, V.; Vinogorova, V. Antioxidant and antimicrobial activities of essential oil and various oleoresins of Elettaria cardamomum (seeds and pods). J. Sci. Food Agric. 2008, 88, 280–289. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.-D.; Saptami, K.; Caputo, L.; De Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E. Chemical and biological evaluation of essential oils from cardamom species. Molecules 2018, 23, 2818. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Human campylobacteriosis. In Campylobacter; Klein, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–25. [Google Scholar]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, Cumin, and Dill Weed Essential Oils: Chemical Compositions, Antimicrobial Activities, and Mechanisms of Action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Weschka, D.; Bereswill, S. Anti-Pathogenic and Immune-Modulatory Effects of Peroral Treatment with Cardamom Essential Oil in Acute Murine Campylobacteriosis. Microorganisms 2021, 9, 169. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Carr, R.I. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J. Med. Food 2010, 13, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1, 8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, A.; Goto, M.; Hasegawa, H.; Anzaki, C.; Nakamoto, M.; Shuto, E.; Sakai, T. Essential Oil of Citrus sudachi Suppresses T Cell Activation both in Vitro and in Vivo. J. Nutr. Sci. Vitaminol. 2022, 68, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.S.; Tsai, C.H.; Li, T.C.; Yu, C.J.; Chou, J.W.; Feng, C.L.; Wang, K.T.; Lai, H.C.; Hsieh, C.L. Effect of wu chu yu tang on gastroesophagel reflux disease: Randomized, double-blind, placebo-controlled trial. Phytomedicine 2019, 56, 118–125. [Google Scholar] [CrossRef]
- Yeh, T.H.; Lin, J.Y. Acorus gramineusand and Euodia ruticarpa steam distilled essential oils exert anti-inflammatory effects through decreasing Th1/Th2 and pro-/anti-inflammatory cytokine secretion ratios in vitro. Biomolecules 2020, 10, 338. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.E.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- Santin, A.D.; Hermonat, P.L.; Ravaggi, A.; Bellone, S.; Pecorelli, S.; Roman, J.J.; Parham, G.P.; Cannon, M.J. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8+ cytotoxic T lymphocytes. J. Virol. 2000, 74, 4729–4737. [Google Scholar] [CrossRef]
- Li, X.; Lu, P.; Li, B.; Zhang, W.; Yang, R.; Chu, Y.; Luo, K. Interleukin 2 and interleukin 10 function synergistically to promote CD8+ T cell cytotoxicity, which is suppressed by regulatory T cells in breast cancer. Int. J. Biochem. Cell Biol. 2017, 87, 1–7. [Google Scholar] [CrossRef]
- Trueblood, E.S.; Brown, W.C.; Palmer, G.H.; Davis, W.C.; Stone, D.M.; McElwain, T.F. B-lymphocyte proliferation during bovine leukemia virus-induced persistent lymphocytosis is enhanced by T-lymphocyte-derived interleukin-2. J. Virol. 1998, 72, 3169–3177. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335. [Google Scholar] [CrossRef]
- Tahvildari, M.; Dana, R. Low-dose IL-2 therapy in transplantation, autoimmunity, and inflammatory diseases. J. Immunol. 2019, 203, 2749–2755. [Google Scholar] [CrossRef]
- Byard, R. Traditional medicine of aboriginal Australia. CMAJ 1988, 139, 792–794. [Google Scholar] [PubMed]
- Akhtar, M.A.; Raju, R.; Beattie, K.D.; Bodkin, F.; Munch, G. Medicinal plants of the Australian Aboriginal Dharawal people exhibiting anti-inflammatory activity. Evid.-Based Complement. Altern. Med. 2016, 2016, 2935403. [Google Scholar] [CrossRef] [PubMed]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral activities of eucalyptus essential oils: Their effectiveness as therapeutic targets against human viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Goncalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yoshida, N.; Yamanoi, Y.; Honryo, A.; Tomita, H.; Kuwabara, H.; Kojima, Y. Eucalyptus oil reduces allergic reactions and suppresses mast cell degranulation by downregulating IgE-FcepsilonRI signalling. Sci. Rep. 2020, 10, 20940. [Google Scholar] [CrossRef]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis Essential Oil: Chemical Composition and Antimicrobial Activities against Food Spoilage Pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Al-Hajj, N.; Jaber, S. Myrtus communis L.: Essential oil chemical composition, total phenols and flavonoids contents, antimicrobial, antioxidant, anticancer, and α-amylase inhibitory activity. Chem. Biol. Technol. Agric. 2023, 10, 41. [Google Scholar] [CrossRef]
- Yang, J.-K.; Choi, M.-S.; Seo, W.-T.; Rinker, D.L.; Han, S.W.; Cheong, G.-W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, L.M.H.; Yagi, S.; Mohamed, H.M.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential Oils Composition and Biological Activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia Grown Wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Jafarabadi, H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother. Res. 2004, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Gerige, S.J.; Gerige, M.K.Y.; Rao, M. Ramanjaneyulu GC-MS analysis of Nigella sativa seeds and antimicrobial activity of its volatile oil. Braz. Arch. Biol. Technol. 2009, 52, 1189–1192. [Google Scholar] [CrossRef]
- Pan, X.; Li, H.; Chen, D.; Zheng, J.; Yin, L.; Zou, J.; Zhang, Y.; Deng, K.; Xiao, M.; Meng, L.; et al. Comparison of Essential Oils of Houttuynia cordata Thunb from Different Processing Methods and Harvest. Seasons Based on GC-MS and Chemometric Analysis. Int. J. Anal. Chem. 2021, 2021, 8324169. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Ermaya, D.; Sari, S.P.; Patria, A.; Hidayat, F.; Razi, F. Identification of patchouli oil chemical components as the results on distillation using GC-MS. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012039. [Google Scholar] [CrossRef]
- Nyalambisa, M.; Oyemitan, I.A.; Matewu, R.; Oyedeji, O.O.; Oluwafemi, O.S.; Songca, S.P.; Nkeh-Chungag, B.N.; Oyedeji, A.O. Volatile constituents and biological activities of the leaf and root of Echinacea species from South Africa. Saudi Pharm. J. 2017, 25, 381–386. [Google Scholar] [CrossRef]
- Al-Zereini, W.A.; Al-Trawneh, I.N.; Al-Qudah, M.A.; TumAllah, H.M.; Al Rawashdeh, H.A.; Abudayeh, Z.H. Essential oils from Elettaria cardamomum (L.) Maton grains and Cinnamomum verum J. Presl barks: Chemical examination and bioactivity studies. J. Pharm. Pharmacogn. Res. 2022, 10, 173–185. [Google Scholar] [CrossRef]
- Akakabe, Y.; Sakamoto, M.; Ikeda, Y.; Tanaka, M. Identification and Characterization of Volatile Components of the Japanese Sour Citrus Fruit Citrus nagato-yuzukichi Tanaka. Biosci. Biotechnol. Biochem. 2008, 72, 1965–1968. [Google Scholar] [CrossRef]
- Hamada, T.; Harano, K.; Niihara, R.; Kitahara, H.; Yamamoto, M.; Vairrapan, C.S.; Tani, F.; Onitsuka, S.; Okamura, H. Composition and Monthly Changes of the Volatile Constituents in the Sour Hetsuka-daidai Citrus Peel. J. Oleo Sci. 2000, 69, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Q.; Li, L.; Li, P.; Yin, M.; Xu, S.; Chen, Y.; Feng, X.; Wang, B. Chemical Composition and Antifungal Activity of Zanthoxylum armatum Fruit Essential Oil against Phytophthora capsici. Molecules 2022, 27, 8636. [Google Scholar] [CrossRef] [PubMed]
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Chemical composition of essential oils from Eucalyptus globulus and Eucalyptus maculata grown in Tanzania. Sci. Afr. 2021, 12, e00758. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.; Kovrizhina, A.R.; et al. Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents. J. Agric. Food Chem. 2016, 38, 7156–7170. [Google Scholar] [CrossRef]
- Nam, S.Y.; Chung, C.K.; Seo, J.H.; Rah, S.Y.; Kim, H.M.; Jeong, H.J. The therapeutic efficacy of alpha-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food. Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 4, 731–742. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. alpha-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Kazemi, M. Phytochemical Composition, Antioxidant, Anti-inflammatory and Antimicrobial Activity of Nigella sativa L. J. Essent. Oil Bear. Plants 2014, 17, 1002–1011. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Sens. Actuators A Phys. 2000, 83, 47–53. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Lee, N.; Hyun, C. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2 and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef]
- Lee, J.H.; Chang, K.M.; Kim, G.H. Composition and anti-inflammatory activities of Zanthoxylum schinifolium essential oil: Suppression of inducible nitric oxide synthase, cyclooxygenase-2, cytokines and cellular adhesion. J. Sci. Food Agric. 2009, 89, 1762–1769. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Vonapart, A.; Karioti, A.; Recio, M.C.; Máñez, S.; Ríos, J.L.; Skaltsa, E.; Giner, R.M. Effects of Terpenoids from Salvia willeana in Delayed-type Hypersensitivity, Human Lymphocyte Proliferation and Cytokine Production. Nat. Prod. Commun. 2008, 3, 1953–1958. [Google Scholar] [CrossRef]
- Islam, A.U.S.; Hellman, B.; Nyberg, F.; Amir, N.; Jayaraj, R.L.; Petroianu, G.; Adem, A. Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat. Model. Molecules 2020, 25, 4492. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical Composition and Immunomodulatory Activity of Essential Oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef]
- Kawata, J.; Kameda, M.; Miyazawa, M. Cyclooxygenase-2 inhibitory effects of monoterpenoids with a p-methane skeleton. Int. J. Essent. Oil Ther. 2008, 2, 145–148. [Google Scholar]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.G.; Marques, R.B.; Santana, M.F.; Santos, A.B.; Brito, F.A.; Barreto, E.O.; Sousa, D.P.; Almeida, F.R.; Badauê-Passos, D.; Antoniolli, A.R.; et al. α-Terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin. Pharmacol. Toxicol. 2012, 2, 120–125. [Google Scholar] [CrossRef]
- Apel, M.; Lima, M.E.L.; Sobral, M.; Young, M.C.C.; Cordeiro, I.; Schapoval, E.E.S.; Henriques, A.T.; Moreno, P.R.H. Anti-inflammatory activity of essential oil from leaves of Myciaria tenella and Calycorectes sellowianus. Pharm. Biol. 2010, 4, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Kurkcuoglu, M.; Yildirim, A.; Senkardes, I.; Bitis, L.; Baser, K.H.C. Chemical composition, antiradical, and enzyme inhibitory potential of essential oil obtained from aerial part of Centaureapterocaula Trautv. J. Essent. Oil Res. 2020, 33, 44–52. [Google Scholar] [CrossRef]
- Ziaei, A.; Ramezani, M.; Wright, L.; Paetz, C.; Schneider, B.; Amirghofran, Z. Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytother. Res. 2011, 25, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Liu, J.Y.; Chang, S.T. Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J. Agric. Food Chem. 2005, 53, 7274–7278. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kim, K.D.; Nam, D.; Chung, W.S.; Jang, H.J.; Lee, S.G.; Shim, B.S.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NFκB-regulated gene products. Apoptosis 2014, 19, 708–718. [Google Scholar] [CrossRef]
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Freitas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant-derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192. [Google Scholar] [CrossRef]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Cryptomeria japonica essential oil ninhibits the growth of drug-resistant skin pathogens and LPS-induced NO and pro-inflammatory cytokine production. Pol. J. Microbiol. 2009, 58, 61–68. [Google Scholar]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Kokorina, P.I.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil Immunomodulatory activity of nerolidol, a major component of essential oils from Populus balsamifera buds and propolis. Plants 2022, 11, 3399. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.; Brand, C.; Carson, C.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.N.M.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef]
- De Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 2, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Martínez, C.A.; Rosas-López, R.; Rodríguez-Monroy, M.A.; Canales-Martínez, M.M.; Román-Guerrero, A.; Jiménez-Alvarado, R. Chemical composition and in vivo anti-inflammatory activity of Bursera morelensis Ramírez essential oil. J. Essent. Oil Bear. Plants 2014, 17, 758–768. [Google Scholar]
- Fonsêca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.; Penha, A.R.; Leite, F.C.; Borges, C.J.; Piuvezam, M.R.; Pordeus, L.C.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 1, 14–22. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef]
- Gomes, B.S.; Neto, B.P.; Lopes, E.M.; Cunha, F.V.; Araújo, A.R.; Wanderley, C.W.; Wong, D.V.; Júnior, R.C.P.; Ribeiro, R.A.; Sousa, D.P.; et al. Anti-inflammatory effect of the monoterpene myrtenol is dependent on the direct modulation of neutrophil migration and oxidative stress. Chem.-Biol. Interact. 2017, 273, 73–81. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, R.; Chen, H.; Jia, P.; Bao, L.; Tang, H. Bornyl acetate has an anti-inflammatory effect in human chondrocytes via induction of IL-11. IUBMB Life 2014, 66, 854–859. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Li, Y.; Qi, G. Bornyl acetate suppresses ox-LDL-induced attachment of THP-1 monocytes to endothelial cells. Biomed. Pharmacother. 2018, 103, 234–239. [Google Scholar] [CrossRef]
- Chen, N.; Sun, G.; Yuan, X.; Hou, J.; Wu, Q.; Soromou, L.W.; Feng, H. Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J. Surg. Res. 2014, 1, 436–445. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Sun, Y.I.; Ruan, X. Bornyl Acetate: A Promising Agent In Phytomedicine for Inflammation and Immune Modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.G.; Mahajan, K.; Dhiman, S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J. Pharm. Pharmacol. 2020, 72, 1306–1327. [Google Scholar] [CrossRef]
- Juergens, U.R.; Engelen, T.; Racke, K.; Stober, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes Pulm. Pharmacol. Ther. 2004, 17, 281. [Google Scholar]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Kaviyani, N.; Tavako, S. Monoterpenes modulating autophagy: A review study. Basic Clin. Pharmacol. Toxicol. 2020, 126, 9–20. [Google Scholar] [CrossRef]
- Horváth, G.; Horváth, A.; Reichert, G.; Böszörményi, A.; Sipos, K.; Pandur, E. Three chemotypes of thyme (Thymus vulgaris L.) essential oil and their main compounds affect differently the IL-6 and TNFα cytokine secretions of BV-2 microglia by modulating the NF-κB and C/EBPβ signalling pathways. BMC Complement. Med. Ther. 2021, 1, 148. [Google Scholar] [CrossRef]
- De Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; Silva, J.C.; Bonjardim, L.R.; de Lucca Júnior, W.; Ferro, J.N.; Barreto Ede, O.; dos Santos, F.E.; Soares, M.B.; et al. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 2015, 11, 1583–1590. [Google Scholar] [CrossRef]
- Xie, G.; Chen, N.; Soromou, L.W.; Liu, F.; Xiong, Y.; Wu, Q.; Li, H.; Feng, H.; Liu, G. p-Cymene protects mice against lipopolysaccharide-induced acute lung injury by inhibiting inflammatory cell activation. Molecules 2012, 7, 8159–8173. [Google Scholar] [CrossRef]
- Pries, R.; Jeschke, S.; Leichtle, A.; Bruchhage, K.L. Modes of Action of 1,8-Cineol in Infections and Inflammation. Metabolites 2023, 6, 751. [Google Scholar] [CrossRef]
- Juergens, U.R.; Stober, M.; Vetter, H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1,8- cineole) in human monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Greiner, J.F.; Müller, J.; Zeuner, M.T.; Hauser, S.; Seidel, T.; Klenke, C.; Grunwald, L.M.; Schomann, T.; Widera, D.; Sudhoff, H.; et al. 1,8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. Biophys. Acta 2013, 12, 2866–2878. [Google Scholar] [CrossRef]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1, 8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation 2016, 4, 1582–1593. [Google Scholar] [CrossRef]
- Rui, Y.; Han, X.; Han, A.; Hu, J.; Li, M.; Liu, B.; Qian, F.; Huang, L. Eucalyptol prevents bleomycin-induced pulmonary fibrosis and M2 macrophage polarization. Eur. J. Pharmacol. 2022, 931, 175184. [Google Scholar] [CrossRef]
- Chang, M.Y.; Shen, Y.L. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules 2014, 5, 6694–6706. [Google Scholar] [CrossRef]
- Su, Y.W.; Chao, S.H.; Lee, M.H.; Ou, T.Y.; Tsai, Y.C. Inhibitory effects of citronellol and geraniol on nitric oxide and prostaglandin E2 production in macrophages. Planta Med. 2010, 76, 1666–1671. [Google Scholar] [CrossRef]
- Mikami, T.; Miyasaka, K. Effects of several anti-inflammatory drugs on the various parameters involved in the inflammatory response in rat carrageenin-induced pleurisy. Eur. J. Pharmacol. 1983, 95, 1–12. [Google Scholar] [CrossRef]
- Sousa, G.M.; Cazarin, C.B.B.; Marostica Junior, M.R.; Lamas, C.A.; Quitete, V.H.A.C.; Pastore, G.M.; Bicas, J.L. The effect of α-terpineol enantiomers on biomarkers of rats fed a high fat-diet. Heliyon Food Sci. 2020, 4, e03752. [Google Scholar] [CrossRef]
- Khaleel, C.; Nurhayat, T.; Gerhard, B. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Trinh, H.T.; Lee, I.A.; Hyun, Y.J.; Kim, D.H. Artemisia princepsPamp. Essential Oil and Its Constituents Eucalyptol andα-terpineol Ameliorate Bacterial Vaginosis and Vulvovaginal Candidiasis in Mice by Inhibiting Bacterial Growth and NF-κB Activation. Planta Medica 2011, 18, 1996–2002. [Google Scholar] [CrossRef]
- da Silva Lima, M.; Quintans-Junior, L.J.; de Santana, W.A.; Martins Kaneto, C.; Botelho Pereira Soares, M.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Zia, S.; Marzouqi, F.; Al-Dhaheri, A.; Subramaniyan, D.; Dhanasekaran, S.; Yasin, J.; Ali, B.H.; Kazzam, E.E. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br. J. Pharmacol. 2011, 164, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.S.M.; Gameil, N.M.; Shawky, N.M.; Nader, M.A. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int. Immunopharmacol. 2011, 11, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Zargan, J.; Umar, K.; SAhmad, S.; Katiyar, C.K.; Khan, H.A. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem. Biol. Interact. 2012, 197, 40–46. [Google Scholar] [CrossRef]
- El Gazzar, M.; El Mezayen, R.; Nicolls, M.R.; Marecki, J.C.; Dreskin, S.C. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Anti-inflammatory effects of thymoquinone and its protective effects against several diseases. Biomed. Pharmacother. 2021, 138, 111492. [Google Scholar] [CrossRef] [PubMed]
- El Mezayen, R.; El Gazzar, M.; Nicolls, M.R.; Marecki, J.C.; Dreskin, S.C.; Nomiyama, H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol. Lett. 2006, 1, 72–81. [Google Scholar] [CrossRef]
- Al Wafai, R.J. Nigella Sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas 2013, 42, 841–849. [Google Scholar] [CrossRef]
- Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem. Biophys. Res. Commun. 2013, 438, 721–727. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grazul, M.; Kwiatkowski, P.; Hartman, K.; Kilanowicz, A.; Sienkiewicz, M. How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters. Biomedicines 2023, 11, 2381. https://doi.org/10.3390/biomedicines11092381
Grazul M, Kwiatkowski P, Hartman K, Kilanowicz A, Sienkiewicz M. How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters. Biomedicines. 2023; 11(9):2381. https://doi.org/10.3390/biomedicines11092381
Chicago/Turabian StyleGrazul, Magdalena, Paweł Kwiatkowski, Kacper Hartman, Anna Kilanowicz, and Monika Sienkiewicz. 2023. "How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters" Biomedicines 11, no. 9: 2381. https://doi.org/10.3390/biomedicines11092381
APA StyleGrazul, M., Kwiatkowski, P., Hartman, K., Kilanowicz, A., & Sienkiewicz, M. (2023). How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters. Biomedicines, 11(9), 2381. https://doi.org/10.3390/biomedicines11092381







