Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation
Abstract
1. Introduction
2. Underlying Disease Mechanisms of AFib
2.1. Brief Overview of Normal Electrophysiology of Cardiomyocytes
2.2. Trigger and Substrate Cause AFib
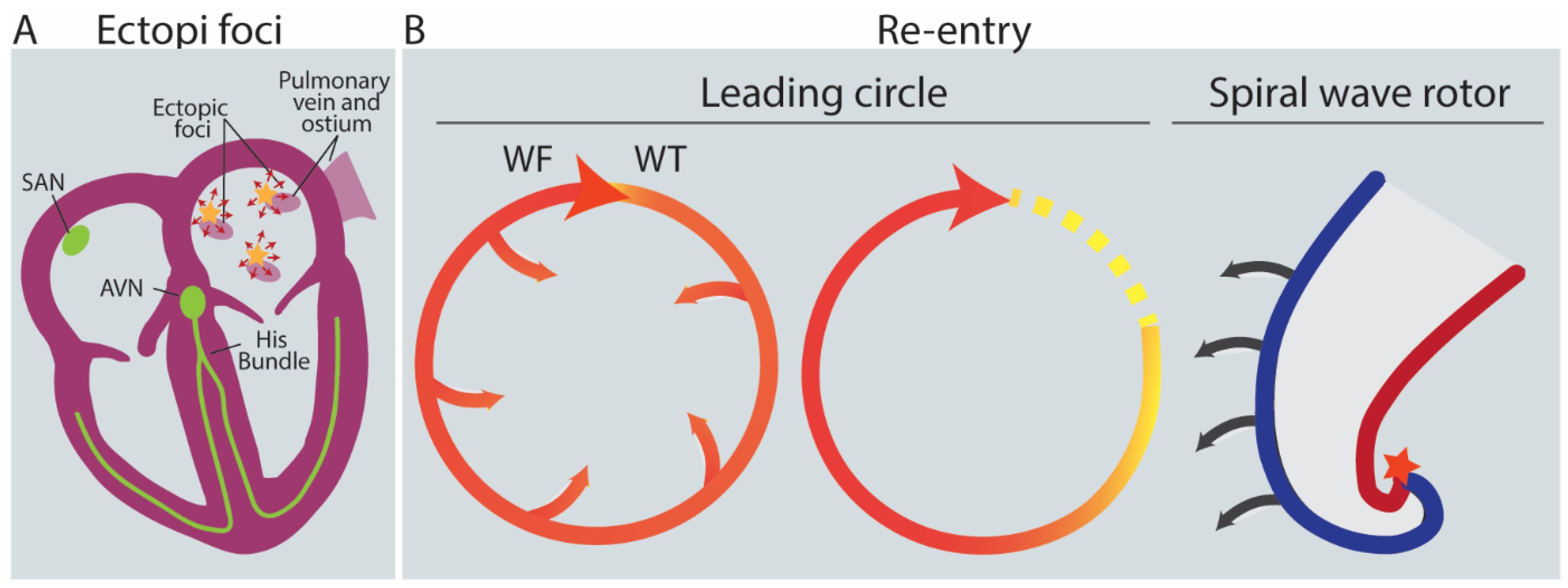
2.3. Triggers: AFib Is Induced by Ectopic Foci, Re-Entry and Rotors
2.4. Current-to-Load (Source-Sink) Mismatches Affect Conduction Velocity
2.5. Substrate: AFib Is Maintained via Electrical, Structural, or Autonomic Remodeling of Atrial Tissue
2.6. AFib Begets AFib
3. Treatment of AFib
4. Models of AFib
4.1. In Vivo Modeling: Animal Models
| Animal Model | AFib Model | AFib Promotion | Clinical Causes of AFib |
|---|---|---|---|
| Dog [50,53,87,88] | Paroxysmal and Persistent AFib models | Electrical, structural and autonomic remodeling | Sterile pericarditis, atrial tachycardia remodeling, CHF-related AFib, acute atrial ischemia, atrial volume overload, mitral regurgitation, cesium infusion |
| Goat [53,88,93,95] | Paroxysmal and Persistent AFib models | Electrical and structural remodeling | Atrial tachycardia remodeling |
| Pig [53,88,89] | Paroxysmal and Persistent AFib models | Structural remodeling | Atrial tachycardia remodeling |
| Sheep [88,96,97] | Paroxysmal and Persistent AFib models | Structural and autonomic remodeling | Atrial volume overload, aortopulmonary shunt, atrial tachycardia remodeling |
| Rabbit [50,88] | Paroxysmal AFib model | Electrical, structural and autonomic remodeling | Atrial volume overload |
| Transgenic mice [88,98,99] | Atrial conduction abnormalities models | - | Dilated cardiomyopathy, hypertrophic cardiomyopathy, atrial pathology in CHF, atrial tachycardia remodeling |
4.2. Alternatives to Animal Models
4.2.1. In Silico Models
4.2.2. In Vitro Modeling Using hPSC-Derived CMs
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFib | Atrial fibrillation |
| Hpsc | Human pluripotent stem cells |
| NCX | Rheogenic Na+/Ca2+-exchanger |
| SERCA2a | Sarco/endoplasmic reticulum Ca2+-ATPase |
| RyR2 | Ryanodine receptor 2 |
| SR | Sarcoplasmic reticulum |
| EAD | Early afterdepolarizations |
| DAD | Diastolic delayed afterdepolarizations |
| APs | Action potentials |
| AADs | Anti-arrhythmic drugs |
| EHTs | Engineered Heart Tissues |
| ECM | extracellular matrix |
| IKUR | ultrarapid outward current I. |
| Kv1.5 | Channel responsible for IKUR. |
| KCNA5 | Gene coding for Kv1.5 |
| IKACh | Acetylcholine-activated inward rectifier potassium current |
| Kir3.1 | Inward-rectifier potassium ion channel mediating IKACh. Also called GIRK1. |
| Kir3.4 | Inward-rectifier potassium ion channel mediating IKACh. Also called GIRK4. |
| KCNJ3 | Gene coding for Kir3.1 |
| KCNJ5 | Gene coding for Kir3.4 |
| IKr | Delayed rectifier K+ current |
| Ito | Transient outward K+ current |
| INa | Late sodium current |
| Cav1.2 | subunit of L-type Ca2+ channels, mediating L-type calcium current (ICa,L) |
| IK1 | inward rectifying K+ current |
| KIR2 | channelprotein of IK1 current |
References
- WHO. Cardiovascular Diseases WHO, 2017. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 11 June 2020).
- Lip, G.Y.H.; Fauchier, L.; Freedman, S.B.; Van Gelder, I.; Natale, A.; Gianni, C.; Nattel, S.; Potpara, T.; Rienstra, M.; Tse, H.-F.; et al. Atrial fibrillation. Nat. Rev. Dis. Prim. 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef] [PubMed]
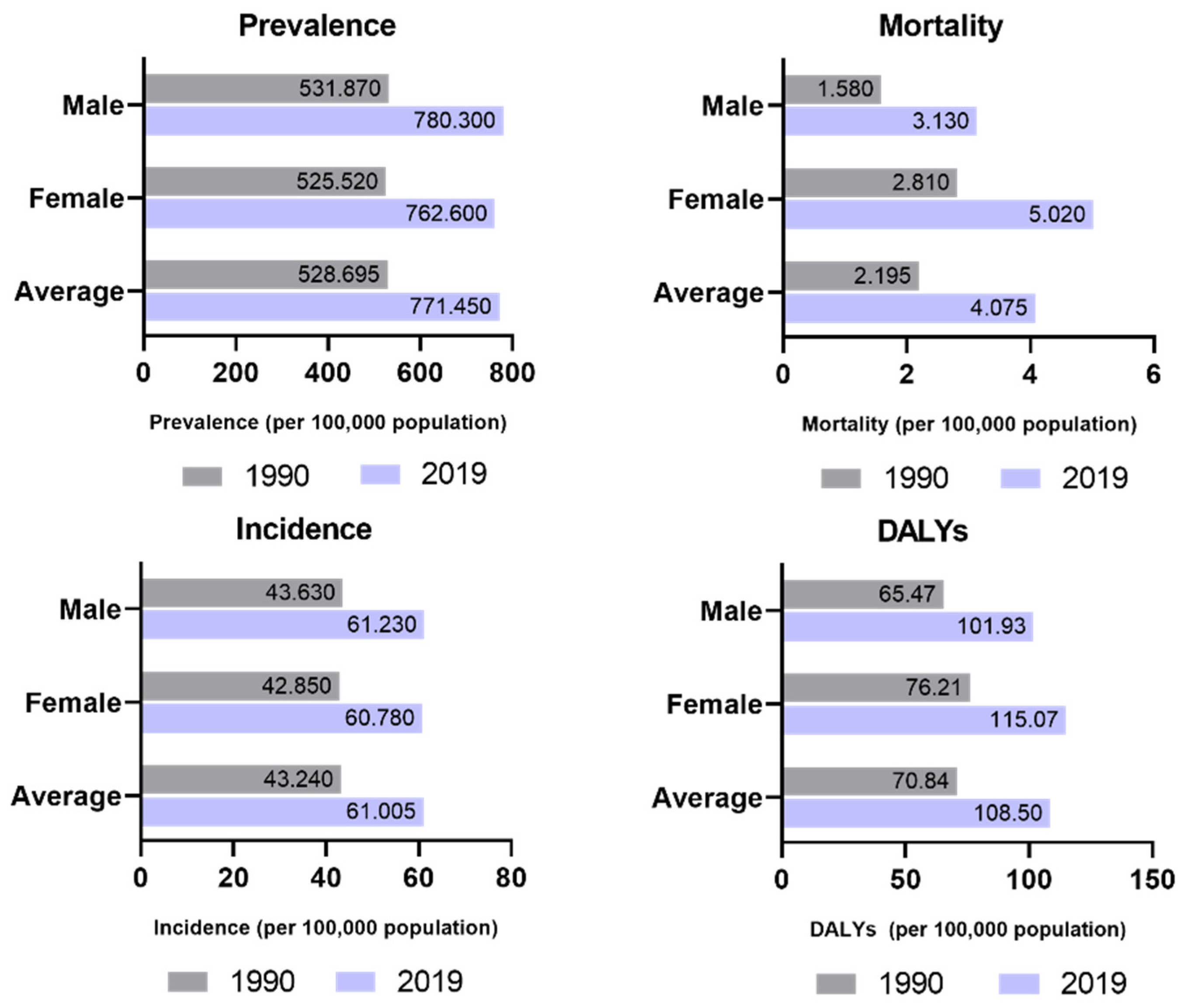
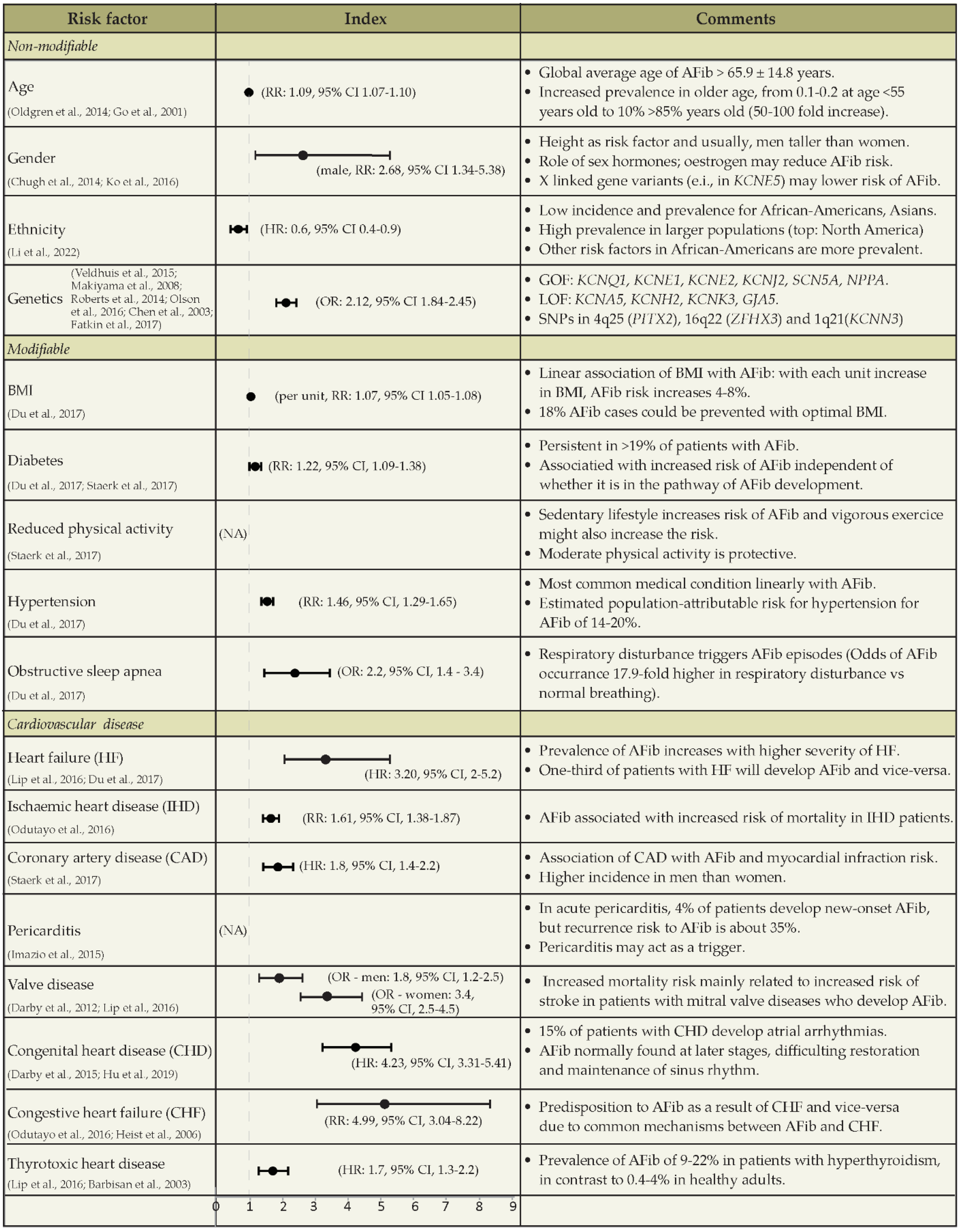
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.J.; et al. Worldwide Epidemiology of Atrial Fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Padanilam, B.J.; Prystowsky, E.N. Epidemiology of Atrial Fibrillation The Rising Prevalence. In Atrial Fibrillation; Humana: Totowa, NJ, USA, 2008; pp. 3–11. [Google Scholar] [CrossRef]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global epidemiology of atrial fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 4 July 2023).
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.S.; Tang, C.; Chen, W.; Zhao, L. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990–2019: Results from a global burden of disease study, 2019. BMC Public Health 2022, 22, 2015. [Google Scholar] [CrossRef]
- Christophersen, I.E.; Ellinor, P.T. Genetics of atrial fibrillation: From families to genomes. J. Hum. Genet. 2016, 61, 61–70. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, S.-J.; Bendahhou, S.; Wang, X.-L.; Wang, Y.; Xu, W.-Y.; Jin, H.; Sun, H.; Su, X.; Zhuang, Q.; et al. KCNQ1 Gain-of-Function Mutation in Familial Atrial Fibrillation. Science 2003, 299, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M.; Alekseev, A.E.; Liu, X.K.; Park, S.; Zingman, L.V.; Bienengraeber, M.; Sattiraju, S.; Ballew, J.D.; Jahangir, A.; Terzic, A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006, 15, 2185–2191. [Google Scholar] [CrossRef]
- Oldgren, J.; Healey, J.S.; Ezekowitz, M.; Commerford, P.; Avezum, A.; Pais, P.; Zhu, J.; Jansky, P.; Sigamani, A.; Morillo, C.A.; et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15 400 emergency department patients in 46 countries: The RE-LY atrial fibrillation registry. Circulation 2014, 129, 1568–1576. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Li, G.; Brumback, B.D.; Huang, L.; Zhang, D.M.; Yin, T.; Lipovsky, C.E.; Hicks, S.C.; Jimenez, J.; Boyle, P.M.; Rentschler, S.L. Acute Glycogen Synthase Kinase-3 Inhibition Modulates Human Cardiac Conduction. JACC Basic Transl. Sci. 2022, 7, 1001–1017. [Google Scholar] [CrossRef]
- Veldhuis, M.G. A Little Too Much: Cardiac Electrophysiological Effects of Elevated Inward Rectifying Current Carried by the K_(IR)2.1 Ion Channel Protein. Adapt. Med. 2015, 7, 1–8. [Google Scholar] [CrossRef][Green Version]
- Makiyama, T.; Akao, M.; Shizuta, S.; Doi, T.; Nishiyama, K.; Oka, Y.; Ohno, S.; Nishio, Y.; Tsuji, K.; Itoh, H.; et al. A Novel SCN5A Gain-of-Function Mutation M1875T Associated With Familial Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 52, 1326–1334. [Google Scholar] [CrossRef]
- Roberts, J.D.; Gollob, M.H. A Contemporary Review on the Genetic Basis of Atrial Fibrillation. Methodist Debakey Cardiovasc. J. 2014, 10, 18–24. [Google Scholar] [CrossRef]
- Fatkin, D.; Santiago, C.F.; Huttner, I.G.; Lubitz, S.A.; Ellinor, P.T. Genetics of Atrial Fibrillation: State of the Art in 2017. Heart Lung Circ. 2017, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dong, J.; Ma, C. Is Atrial Fibrillation a Preventable Disease? J. Am. Coll. Cardiol. 2017, 69, 1968–1982. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef]
- Imazio, M.; Lazaros, G.; Picardi, E.; Vasileiou, P.; Orlando, F.; Carraro, M.; Tsiachris, D.; Vlachopoulos, C.; Georgiopoulos, G.; Tousoulis, D.; et al. Incidence and prognostic significance of new onset atrial fibrillation/flutter in acute pericarditis. Heart 2015, 101, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.E.; DiMarco, J.P. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012, 125, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-S.; Lin, C.-L. Risk of Atrial Fibrillation in Patients with Congenital Heart Disease: Results of a Propensity Score-Matched, Nationwide Cohort Study. J. Atheroscler. Thromb. 2019, 26, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Heist, E.K.; Ruskin, J.N. Atrial Fibrillation and Congestive Heart Failure: Risk Factors, Mechanisms, and Treatment. Prog. Cardiovasc. Dis. 2006, 48, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Barbisan, J.N.; Fuchs, F.D.; Schaan, B.D. Prevalence of thyroid dysfunction in patients with acute atrial fibrillation attended at a cardiology emergency room. Sao Paulo Med. J. 2003, 121, 159–162. [Google Scholar] [CrossRef]
- Knollmann, B.C.; Roden, D.M. A genetic framework for improving arrhythmia therapy. Nature 2008, 451, 929–936. [Google Scholar] [CrossRef]
- Wilhelms, M.; Hettmann, H.; Maleckar, M.M.; Koivumäki, J.T.; Dössel, O.; Seemann, G. Benchmarking electrophysiological models of human atrial myocytes. Front. Physiol. 2013, 3, 487. [Google Scholar] [CrossRef]
- Kaese, S.; Verheule, S. Cardiac electrophysiology in mice: A matter of size. Front. Physiol. 2012, 3, 345. [Google Scholar] [CrossRef]
- De Sousa Lopes, S.M.C.; Hassink, R.J.; Feijen, A.; Van Rooijen, M.A.; Doevendans, P.A.; Tertoolen, L.; De La Rivière, A.B.; Mummery, C.L. Patterning the heart, a template for human cardiomyocyte development. Dev. Dyn. 2006, 235, 1994–2002. [Google Scholar] [CrossRef]
- Zanella, F.; Lyon, R.C.; Sheikh, F. Modeling heart disease in a dish: From somatic cells to disease-relevant cardiomyocytes. Trends Cardiovasc. Med. 2014, 24, 32–44. [Google Scholar] [CrossRef]
- Anderson, R. The Gross Physiology of the Cardiovascular System; Racquet Press: Tucson, AZ, USA, 2008; ISBN 9780961752811. [Google Scholar]
- Dobrev, D.; Wehrens, X.H.T. Calcium-mediated cellular triggered activity in atrial fibrillation. J. Physiol. 2017, 595, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Priest, B.T.; McDermott, J.S. Cardiac ion channels. Channels 2015, 9, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Krummen, D.E.; Hebsur, S.; Salcedo, J.; Narayan, S.M.; Lalani, G.G.; Schricker, A.A. Mechanisms Underlying AF: Triggers, Rotors, Other? Curr. Treat. Options Cardiovasc. Med. 2015, 17, 14. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chan, P.; Chang, M.S.; Lin, C.I. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation 2001, 104, 2849–2854. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+ Leak and Increased Na+-Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients With Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Trausch, A.; Mintert-Jancke, E.; Pott, L.; Ravens, U.; Dobrev, D. Impaired Na+-dependent regulation of acetylcholine-activated inward-rectifier K+ current modulates action potential rate dependence in patients with chronic atrial fibrillation. J. Mol. Cell. Cardiol. 2013, 61, 142–152. [Google Scholar] [CrossRef]
- Burashnikov, A.; Antzelevitch, C. Reinduction of Atrial Fibrillation Immediately After Termination of the Arrhythmia Is Mediated by Late Phase 3 Early Afterdepolarization–Induced Triggered Activity. Circulation 2003, 107, 2355–2360. [Google Scholar] [CrossRef]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Chen, P.-S.; Qu, Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 2010, 7, 1891–1899. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Wakili, R.; Qi, X.Y.; Chartier, D.; Boknik, P.; Kääb, S.; Ravens, U.; Coutu, P.; Dobrev, D.; Nattel, S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ. Arrhythm. Electrophysiol. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients With Paroxysmal Atrial Fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Beavers, D.L.; Wang, W.; Ather, S.; Voigt, N.; Garbino, A.; Dixit, S.S.; Landstrom, A.P.; Li, N.; Wang, Q.; Olivotto, I.; et al. Mutation E169K in Junctophilin-2 Causes Atrial Fibrillation Due to Impaired RyR2 Stabilization. J. Am. Coll. Cardiol. 2013, 62, 2010–2019. [Google Scholar] [CrossRef]
- Waks, J.W.; Josephson, M.E. Mechanisms of Atrial Fibrillation—Reentry, Rotors and Reality. Arrhythm. Electrophysiol. Rev. 2014, 3, 90. [Google Scholar] [CrossRef]
- Nattel, S.; Shiroshita-Takeshita, A.; Brundel, B.J.J.M.; Rivard, L. Mechanisms of Atrial Fibrillation: Lessons From Animal Models. Prog. Cardiovasc. Dis. 2005, 48, 9–28. [Google Scholar] [CrossRef]
- Cheniti, G.; Vlachos, K.; Pambrun, T.; Hooks, D.; Frontera, A.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Martin, C.; et al. Atrial fibrillation mechanisms and implications for catheter ablation. Front. Physiol. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Antoons, G.; Sipido, K.R. Targeting calcium handling in arrhythmias. Europace 2008, 10, 1364–1369. [Google Scholar] [CrossRef]
- Nishida, K.; Michael, G.; Dobrev, D.; Nattel, S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. EP Eur. 2010, 12, 160–172. [Google Scholar] [CrossRef]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef]
- Kato, T.; Iwasaki, Y.K.; Nattel, S. Connexins and atrial fibrillation: Filling in the gaps. Circulation 2012, 125, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.M. An Introduction to Cardiovascular Disease. In Social Support and Cardiovascular Disease; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Fast, V.G.; Kléber, A.G. Role of wavefront curvature in propagation of cardiac impulse. Cardiovasc. Res. 1997, 33, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, E.J.; Coromilas, J.; Wit, A.L.; Peters, N.S.; Garan, H. Source-Sink Mismatch Causing Functional Conduction Block in Re-Entrant Ventricular Tachycardia. JACC Clin. Electrophysiol. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spector, P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ. Arrhythm. Electrophysiol. 2013, 6, 655–661. [Google Scholar] [CrossRef]
- Rohr, S.; Kucera, J.P.; Fast, V.G.; Kleber, A.G. Paradoxical Improvement of Impulse Conduction in Cardiac Tissue by Partial Cellular Uncoupling. Science 1997, 275, 841–844. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yeh, Y.H.; Xiao, L.; Burstein, B.; Maguy, A.; Chartier, D.; Villeneuve, L.R.; Brundel, B.J.J.M.; Dobrev, D.; Nattel, S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ. Res. 2008, 103, 845–854. [Google Scholar] [CrossRef]
- Chi, X.; Chatterjee, P.K.; Wilson, W.; Zhang, S.-X.; Demayo, F.J.; Schwartz, R.J. Complex cardiac Nkx2-5 gene expression activated by noggin-sensitive enhancers followed by chamber-specific modules. Proc. Natl. Acad. Sci. USA 2005, 102, 13490–13495. [Google Scholar] [CrossRef]
- Qi, X.Y.; Diness, J.G.; Brundel, B.J.J.M.; Zhou, X.B.; Naud, P.; Wu, C.T.; Huang, H.; Harada, M.; Aflaki, M.; Dobrev, D.; et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 2014, 129, 430–440. [Google Scholar] [CrossRef]
- Özgen, N.; Dun, W.; Sosunov, E.A.; Anyukhovsky, E.P.; Hirose, M.; Duffy, H.S.; Boyden, P.A.; Rosen, M.R. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc. Res. 2007, 75, 758–769. [Google Scholar] [CrossRef]
- Igarashi, T.; Finet, J.E.; Takeuchi, A.; Fujino, Y.; Strom, M.; Greener, I.D.; Rosenbaum, D.S.; Donahue, J.K. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 2012, 125, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Pellman, J.; Lyon, R.C.; Sheikh, F. Extracellular matrix remodeling in atrial fibrosis: Mechanisms and implications in atrial fibrillation. J. Mol. Cell. Cardiol. 2010, 48, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.J.; Zhao, J.; Csepe, T.A.; Moore, B.T.; Li, N.; Jayne, L.A.; Kalyanasundaram, A.; Lim, P.; Bratasz, A.; Powell, K.A.; et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur. Heart J. 2015, 36, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, L.J.M.E.; Starreveld, R.; Kharbanda, R.K.; Knops, P.; Kik, C.; Bogers, A.J.J.C.; de Groot, N.M.S. Detection of Endo-epicardial Asynchrony in the Atrial Wall Using One-Sided Unipolar and Bipolar Electrograms. J. Cardiovasc. Transl. Res. 2021, 14, 902–911. [Google Scholar] [CrossRef]
- Van der Does, L.J.M.E.; Kik, C.; Bogers, A.J.J.C.; Allessie, M.A.; de Groot, N.M.S. Dynamics of Endo- and Epicardial Focal Fibrillation Waves at the Right Atrium in a Patient With Advanced Atrial Remodelling. Can. J. Cardiol. 2016, 32, 1260.e19–1260.e21. [Google Scholar] [CrossRef]
- Pellman, J.; Sheikh, F. Atrial fibrillation: Mechanisms, therapeutics, and future directions. Compr. Physiol. 2015, 5, 649–665. [Google Scholar] [CrossRef]
- Youn, J.Y.; Zhang, J.; Zhang, Y.; Chen, H.; Liu, D.; Ping, P.; Weiss, J.N.; Cai, H. Oxidative stress in atrial fibrillation: An emerging role of NADPH oxidase. J. Mol. Cell. Cardiol. 2013, 62, 72–79. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Burashnikov, A.; Antzelevitch, C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE—Pacing Clin. Electrophysiol. 2006, 29, 290–295. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The Clinical Profile and Pathophysiology of Atrial Fibrillation. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Savelieva, I.; Lip, G.Y.H. Rate control in the medical management of atrial fibrillation. BMJ 2007, 93, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.P.; Bernard, M.L.; Madias, C.; Rogers, P.A.; Thihalolipavan, S.; Estes, N.A.M. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin. Proc. 2016, 91, 1778–1810. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef]
- Nattel, S. Experimental evidence for proarrhythmic mechanisms of antiarrhythmic drugs. Cardiovasc. Res. 1998, 37, 567–577. [Google Scholar] [CrossRef]
- Ravens, U.; Poulet, C.; Wettwer, E.; Knaut, M. Atrial selectivity of antiarrhythmic drugs. J. Physiol. 2013, 591, 4087–4097. [Google Scholar] [CrossRef]
- Milnes, J.T.; Madge, D.J.; Ford, J.W. New pharmacological approaches to atrial fibrillation. Drug Discov. Today 2012, 17, 654–659. [Google Scholar] [CrossRef]
- Savelieva, I.; Graydon, R.; Camm, A.J. Pharmacological cardioversion of atrial fibrillation with vernakalant: Evidence in support of the ESC Guidelines. Europace 2014, 16, 162–173. [Google Scholar] [CrossRef]
- Woods, C.E.; Olgin, J. AF Therapy Now and in the Future: Drugs, Biologicals, and Ablation. Circ. Res. 2014, 114, 1532–1546. [Google Scholar] [CrossRef]
- Nattel, S.; Bourne, G.; Talajic, M. Insights into mechanisms of antiarrhythmic drug action from experimental models of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1997, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Melnyk, P.; Gaspo, R.; Wang, Z.; Nattel, S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ. Res. 1999, 84, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, D.; Bapat, A.; Kääb, S.; Lee, K.; Tomsits, P.; Clauss, S.; Hucker, W.J. Animal Models of Atrial Fibrillation. Circ. Res. 2020, 127, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; McDonald, A.D.; Donahue, J.K. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc. Res. 2004, 61, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, J.G.; Alfonso-Almazán, J.M.; Pérez-Castellano, N.; Pandit, S.V.; Jalife, J.; Pérez-Villacastín, J.; Filgueiras-Rama, D. Instantaneous Amplitude and Frequency Modulations Detect the Footprint of Rotational Activity and Reveal Stable Driver Regions as Targets for Persistent Atrial Fibrillation Ablation. Circ. Res. 2020, 125, 609–627. [Google Scholar] [CrossRef]
- Kishihara, J.; Niwano, S.; Niwano, H.; Aoyama, Y.; Satoh, A.; Oikawa, J.; Kiryu, M.; Fukaya, H.; Masaki, Y.; Tamaki, H.; et al. Effect of carvedilol on atrial remodeling in canine model of atrial fibrillation. Cardiovasc. Diagn. Ther. 2014, 4, 28–35. [Google Scholar] [CrossRef]
- Nakatani, Y.; Nishida, K.; Sakabe, M.; Kataoka, N.; Sakamoto, T.; Yamaguchi, Y.; Iwamoto, J.; Mizumaki, K.; Fujiki, A.; Inoue, H. Tranilast Prevents Atrial Remodeling and Development of Atrial Fibrillation in a Canine Model of Atrial Tachycardia and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2013, 61, 582–588. [Google Scholar] [CrossRef]
- Remes, J.; van Brakel, T.J.; Bolotin, G.; Garber, C.; de Jong, M.M.; van der Veen, F.H.; Maessen, J.G. Persistent atrial fibrillation in a goat model of chronic left atrial overload. J. Thorac. Cardiovasc. Surg. 2008, 136, 1005–1011. [Google Scholar] [CrossRef]
- Nyns, E.C.A.; Poelma, R.H.; Volkers, L.; Plomp, J.J.; Bart, C.I.; Kip, A.M.; van Brakel, T.J.; Zeppenfeld, K.; Schalij, M.J.; Zhang, G.Q.; et al. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl. Med. 2019, 11, eaau6447. [Google Scholar] [CrossRef]
- Ausma, J.; Van der Velden, H.M.W.; Lenders, M.H.; Van Ankeren, E.P.; Jongsma, H.J.; Ramaekers, F.C.S.; Borgers, M.; Allessie, M.A. Reverse Structural and Gap-Junctional Remodeling After Prolonged Atrial Fibrillation in the Goat. Circulation 2003, 107, 2051–2058. [Google Scholar] [CrossRef]
- Anné, W.; Willems, R.; Holemans, P.; Beckers, F.; Roskams, T.; Lenaerts, I.; Ector, H.; Heidbüchel, H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J. Mol. Cell. Cardiol. 2007, 43, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, C.; Battaglia, E.; Young, R.; Suzuki, G. Lkb1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J. Am. Heart Assoc. 2015, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.U.; Lewin, G.; Baba, H.A.; Bokník, P.; Fabritz, L.; Kirchhefer, U.; Kirchhof, P.; Loser, K.; Matus, M.; Neumann, J.; et al. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J. Biol. Chem. 2005, 280, 6906–6914. [Google Scholar] [CrossRef]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef]
- Nygren, A.; Fiset, C.; Firek, L.; Clark, J.W.; Lindblad, D.S.; Clark, R.B.; Giles, W.R. Mathematical model of an adult human atrial cell: The role of K+ currents in repolarization. Circ. Res. 1998, 82, 63–81. [Google Scholar] [CrossRef]
- Moe, G.K.; Rheinboldt, W.C.; Abildskov, J. A computer model of atrial fibrillation. Am. Heart J. 1964, 67, 200–220. [Google Scholar] [CrossRef]
- Majumder, R.; De Coster, T.; Kudryashova, N.; Verkerk, A.O.; Kazbanov, I.V.; Ördög, B.; Harlaar, N.; Wilders, R.; de Vries, A.A.F.; Ypey, D.L.; et al. Self-restoration of cardiac excitation rhythm by anti-arrhythmic ion channel gating. eLife 2020, 9, e55921. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zahid, S.; Trayanova, N.A. Using personalized computer models to custom-tailor ablation procedures for atrial fibrillation patients: Are we there yet? Expert Rev. Cardiovasc. Ther. 2017, 15, 339–341. [Google Scholar] [CrossRef]
- Heijman, J.; Sutanto, H.; Crijns, H.J.G.M.; Nattel, S.; Trayanova, N.A. Computational models of atrial fibrillation: Achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Protze, S.I.; Laksman, Z.; Backx, P.H.; Keller, G.M. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 2017, 21, 179–194.e4. [Google Scholar] [CrossRef] [PubMed]
- Schwach, V.; Cofiño-Fabres, C.; ten Den, S.A.; Passier, R. Improved Atrial Differentiation of Human Pluripotent Stem Cells by Activation of Retinoic Acid Receptor Alpha (RARα). J. Pers. Med. 2022, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Laksman, Z.; Wauchop, M.; Lin, E.; Protze, S.; Lee, J.; Yang, W.; Izaddoustdar, F.; Shafaattalab, S.; Gepstein, L.; Tibbits, G.F.; et al. Modeling Atrial Fibrillation using Human Embryonic Stem Cell-Derived Atrial Tissue. Sci. Rep. 2017, 7, 5268. [Google Scholar] [CrossRef]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Liu, J.; Volkers, L.; Jangsangthong, W.; Bart, C.I.; Engels, M.C.; Zhou, G.; Schalij, M.J.; Ypey, D.L.; Pijnappels, D.A.; de Vries, A.A.F. Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity. Cardiovasc. Res. 2018, 114, 1848–1859. [Google Scholar] [CrossRef]
- Harlaar, N.; Dekker, S.O.; Zhang, J.; Snabel, R.R.; Veldkamp, M.W.; Verkerk, A.O.; Fabres, C.C.; Schwach, V.; Lerink, L.J.S.; Rivaud, M.R.; et al. Conditional immortalization of human atrial myocytes for the generation of in vitro models of atrial fibrillation. Nat. Biomed. Eng. 2022, 6, 389–402. [Google Scholar] [CrossRef]
- Van Gorp, P.R.R.; Trines, S.A.; Pijnappels, D.A.; de Vries, A.A.F. Multicellular In vitro Models of Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 43. [Google Scholar] [CrossRef]
- Hamers, J.; Sen, P.; Merkus, D.; Seidel, T.; Lu, K.; Dendorfer, A. Preparation of Human Myocardial Tissue for Long-Term Cultivation. J. Vis. Exp. 2022, 184, e63964. [Google Scholar] [CrossRef]
- Amesz, J.H.; Zhang, L.; Everts, B.R.; De Groot, N.M.S.; Taverne, Y.J.H.J. Living myocardial slices: Advancing arrhythmia research. Front. Physiol. 2023, 14, 17. [Google Scholar] [CrossRef]
- Meki, M.H.; Miller, J.M.; Mohamed, T.M.A. Heart Slices to Model Cardiac Physiology. Front. Pharmacol. 2021, 12, 617922. [Google Scholar] [CrossRef]
- Amesz, J.H.; de Groot, N.M.S.; Langmuur, S.J.J.; el Azzouzi, H.; Tiggeloven, V.P.C.; van Rooij, M.M.M.M.; Knops, P.; Bogers, A.J.J.C.; Taverne, Y.J.H.J. Biomimetic cultivation of atrial tissue slices as novel platform for in-vitro atrial arrhythmia studies. Sci. Rep. 2023, 13, 3648. [Google Scholar] [CrossRef]
- Kang, C.; Qiao, Y.; Li, G.; Baechle, K.; Camelliti, P.; Rentschler, S.; Efimov, I.R. Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials. Sci. Rep. 2016, 6, 28798. [Google Scholar] [CrossRef]
- Pertsov, A.M.; Davidenko, J.M.; Salomonsz, R.; Baxter, W.T.; Jalife, J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ. Res. 1993, 72, 631–650. [Google Scholar] [CrossRef]
- Watanabe, M.; Feola, I.; Majumder, R.; Jangsangthong, W.; Teplenin, A.S.; Ypey, D.L.; Schalij, M.J.; Zeppenfeld, K.; de Vries, A.A.F.; Pijnappels, D.A. Optogenetic manipulation of anatomical re-entry by light-guided generation of a reversible local conduction block. Cardiovasc. Res. 2017, 113, 354–366. [Google Scholar] [CrossRef]
- Devalla, H.D.; Passier, R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci. Transl. Med. 2018, 10, eaah5457. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, J.; Han, P.; Yuan, Q.; Zhang, J.; Zhang, X.; Xu, Y.; Cao, H.; Meng, Q.; Chen, L.; et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011, 21, 579–587. [Google Scholar] [CrossRef]
- Schwach, V.; Verkerk, A.O.; Mol, M.; Monshouwer-Kloots, J.J.; Devalla, H.D.; Orlova, V.V.; Anastassiadis, K.; Mummery, C.L.; Davis, R.P.; Passier, R. A COUP-TFII Human Embryonic Stem Cell Reporter Line to Identify and Select Atrial Cardiomyocytes. Stem Cell Rep. 2017, 9, 1765–1779. [Google Scholar] [CrossRef]
- Veerman, C.C.; Kosmidis, G.; Mummery, C.L.; Casini, S.; Verkerk, A.O.; Bellin, M. Immaturity of human stem-cell-derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev. 2015, 24, 1035–1052. [Google Scholar] [CrossRef]
- Nakanishi, H.; Lee, J.K.; Miwa, K.; Masuyama, K.; Yasutake, H.; Li, J.; Tomoyama, S.; Honda, Y.; Deguchi, J.; Tsujimoto, S.; et al. Geometrical patterning and constituent cell heterogeneity facilitate electrical conduction disturbances in a human induced pluripotent stem cell-based platform: An in vitro disease model of atrial arrhythmias. Front. Physiol. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.; Rivera-Arbeláez, J.M.; Cofiño-Fabres, C.; Schwach, V.; Slaats, R.H.; ten Den, S.A.; Vermeul, K.; van den Berg, A.; Pérez-Pomares, J.M.; Segerink, L.I.; et al. A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. J. Pers. Med. 2022, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef]
- Lemoine, M.D.; Lemme, M.; Ulmer, B.M.; Braren, I.; Krasemann, S.; Hansen, A.; Kirchhof, P.; Meyer, C.; Eschenhagen, T.; Christ, T. Intermittent Optogenetic Tachypacing of Atrial Engineered Heart Tissue Induces Only Limited Electrical Remodelling. J. Cardiovasc. Pharmacol. 2020, 77, 291–299. [Google Scholar] [CrossRef]
- Lemme, M.; Braren, I.; Prondzynski, M.; Aksehirlioglu, B.; Ulmer, B.M.; Schulze, M.L.; Ismaili, D.; Meyer, C.; Hansen, A.; Christ, T.; et al. Chronic intermittent tachypacing by an optogenetic approach induces arrhythmia vulnerability in human engineered heart tissue. Cardiovasc. Res. 2019, 116, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Yi, B.A.; Ellinor, P.T. Genetics of Atrial Fibrillation. Heart Fail. Clin. 2010, 6, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Marczenke, M.; Piccini, I.; Mengarelli, I.; Fell, J.; Röpke, A.; Seebohm, G.; Verkerk, A.O.; Greber, B. Cardiac Subtype-Specific Modeling of Kv1.5 Ion Channel Deficiency Using Human Pluripotent Stem Cells. Front. Physiol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef]
- Ravenscroft, S.M.; Pointon, A.; Williams, A.W.; Cross, M.J.; Sidaway, J.E. Cardiac Non-myocyte Cells Show Enhanced Pharmacological Function Suggestive of Contractile Maturity in Stem Cell Derived Cardiomyocyte Microtissues. Toxicol. Sci. 2016, 152, 99–112. [Google Scholar] [CrossRef]
- Vunjak Novakovic, G.; Eschenhagen, T.; Mummery, C. Myocardial tissue engineering: In vitro models. Cold Spring Harb. Perspect. Med. 2014, 4, a014076. [Google Scholar] [CrossRef]
- Heijman, J.; Algalarrondo, V.; Voigt, N.; Melka, J.; Wehrens, X.H.T.; Dobrev, D.; Nattel, S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: A critical analysis. Cardiovasc. Res. 2016, 109, 467–479. [Google Scholar] [CrossRef] [PubMed]



 In vivo models |  In silico models |  In vitro models | |
| PROS | |||
| CONS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofiño-Fabres, C.; Passier, R.; Schwach, V. Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines 2023, 11, 2355. https://doi.org/10.3390/biomedicines11092355
Cofiño-Fabres C, Passier R, Schwach V. Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines. 2023; 11(9):2355. https://doi.org/10.3390/biomedicines11092355
Chicago/Turabian StyleCofiño-Fabres, Carla, Robert Passier, and Verena Schwach. 2023. "Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation" Biomedicines 11, no. 9: 2355. https://doi.org/10.3390/biomedicines11092355
APA StyleCofiño-Fabres, C., Passier, R., & Schwach, V. (2023). Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines, 11(9), 2355. https://doi.org/10.3390/biomedicines11092355







